Abstract
Background
Adenosine A2A receptor antagonists reduce or prevent the development of dyskinesia in animal models of levodopa-induced dyskinesia.
Methods
We examined the association between self-reported intake of the A2A receptor antagonist caffeine and time to dyskinesia in the Comparison of the Agonist Pramipexole with Levodopa on Motor Complications of Parkinson’s Disease (CALM-PD) and CALM Cohort extension studies, using a Cox proportional hazards model adjusting for age, baseline Parkinson’s severity, site, and initial treatment with pramipexole or levodopa.
Results
For subjects who consumed > 12 ounces of coffee/day, the adjusted hazard ratio for the development of dyskinesia was 0.61 (95% confidence interval, 0.37–1.01) compared to subjects who consumed < 4 ounces/day. For subjects who consumed between 4 and 12 ounces/day, the adjusted hazard ratio was 0.73 (C.I. 0.46–1.15) (test for trend, p = 0.05).
Conclusions
These results support the possibility that caffeine may reduce the likelihood of developing dyskinesia.
Keywords: Caffeine, adenosine, Parkinson’s disease, PD, dyskinesia
Introduction
Adenosine A2A receptors may play a role in the development of drug-induced dyskinesia. Co-administration of an A2A receptor antagonist with repeated dopamine agonist administration in MPTP-lesioned primates effectively blocked dyskinesia formation1. Similarly, A2A antagonist co-administration with levodopa in unilaterally 6-OHDA-lesioned mice attenuated the development of sensitized rotational and dyskinetic responses in a model of levodopa-induced dyskinesia 2. In addition, genetic depletion of A2A receptors attenuated the development of levodopa-induced rotational sensitization and dyskinesia in hemiparkinsonian mice 2,3, 4. Increased expression of A2A receptors has been shown in PD patients with dyskinesias, but it is not known whether the association is causal5–7. Although several human studies have suggested a slight increase in the frequency of dyskinesia after treatment with A2A antagonists in relatively advanced PD 8, 9,10–12, no trial has yet tested whether chronic A2A receptor antagonism prior to initiation of levodopa therapy will prevent the development of dyskinesia in PD. Prevention of levodopa-induced dyskinesia is a clinically important goal, as the treatment options to reduce established dyskinesia are limited13. In this current study we tested the hypothesis that self-reported use of caffeine (a non-specific adenosine A2A receptor antagonist) in subjects who enrolled in the Comparison of the Agonist Pramipexole with Levodopa on Motor Complications of Parkinson’s Disease (CALM-PD) study would be associated with a reduced risk of developing dyskinesia.
Methods
Subjects
The detailed methods and results of the multicenter randomized, double-blind, placebo-controlled CALM-PD study 14, 15 and CALM Cohort follow-up study 16 have been reported elsewhere. In brief, 301 patients with early PD requiring initial treatment with dopaminergic therapy were randomized to either pramipexole or levodopa between October 1996 and August 1997 (CALM-PD Trial)14. After an initial dose escalation period of 10 weeks, participants were allowed to take open label levodopa. Sixty-eight percent of all participants (including those randomized to pramipexole and to levodopa) required open-label levodopa during the CALM-PD trial a median of 17 months after study initiation (Supplementary Table 2)17. Of the initial trial cohort, 183 completed 4 years of follow-up as part of the CALM-PD trial. A total of 222 subjects, including subjects who had withdrawn from the initial study, agreed to extended follow-up for an additional two years in an extension study (CALM Cohort) for a maximum of up to 6 years from enrollment in CALM-PD. Data from both studies were combined for the current analysis.
Caffeine consumption: a questionnaire assessing both current (“in the past week”) and prior (“on average over the past 5 years”) caffeine intake was provided to all participants in the CALM-PD study a mean of 4.6 (S.D. 0.3) years after enrollment. The daily number of servings of caffeinated coffee (estimated to be 85 mg caffeine/5 oz. serving), tea (36 mg/5 oz. serving) and soda (45 mg/12 oz. serving) were queried. For our analysis, we used self-reported prior use of caffeine based upon our hypothesis that chronic blockade of adenosine A2A receptors by caffeine coincident with initial dopamine replacement therapy would reduce the long-term risk of dyskinesia. Seventy-six percent of CALM-PD participants (228) completed the survey. A follow-up survey of current (“in the past week”) and prior caffeine use (“on average over the past year”) was sent to participants in CALM Cohort approximately one year later and was completed by 73% (163) of CALM Cohort participants. Data from this second survey were compared to the CALM-PD survey results.
Statistical Analysis
Time of dyskinesia onset was defined using the completion date of a dopaminergic event form that captured whether participants had experienced any dyskinesia since the prior visit. The distribution of time to onset of dyskinesia was analyzed using the Kaplan-Meier method and log-rank test with caffeine use stratified into tertiles (< 67.5 mg/day, 67.5–206.0 mg/day and > 206.0 mg/day). A Cox proportional hazards model was also used to examine the association between prior caffeine use and time to onset of dyskinesia adjusting for relevant covariates. Gender, age, PD duration, baseline Unified Parkinson’s Disease Rating Scale (UPDRS) total score, baseline weight, site and treatment (initial randomization to pramipexole versus levodopa) were entered into the model, and a stepwise selection process, with significance levels for both entry and retention in the model set at 0.05, was used to select the most significant predictors. Prior caffeine use was then added to the selected model, with a test performed for a linear trend in the log-hazard ratio across the tertiles. To assess the test-retest reliability of the self-reported survey results, an intraclass correlation coefficient was used to summarize the association between “current” caffeine consumption in CALM-PD and “prior” caffeine consumption in CALM Cohort (referring to approximately the same time period). All analyses were performed using SAS statistical software version 9.2 (SAS Institute, Cary, NC).
Results
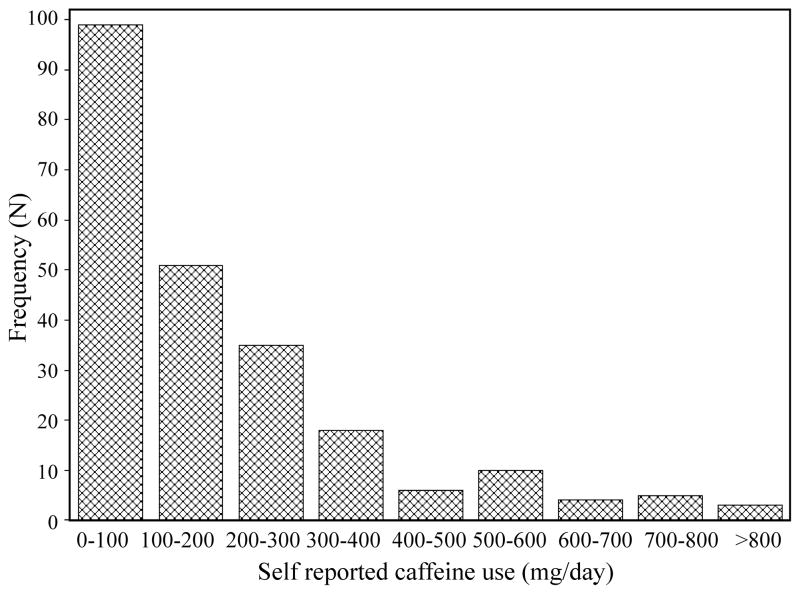
A total of 111 participants experienced dyskinesia during the course of the two studies. The mean follow-up time for the 228 participants with self-reported caffeine data was 5.5 years (S.D. 1.3 years). The median (25th and 75th percentiles) of self-reported prior caffeine consumption from the CALM-PD study was 138 mg (45 mg, 265 mg), and median current use was 85 mg (21 mg, 192 mg). The median (25th and 75th percentiles) prior caffeine consumption from the CALM Cohort questionnaire was 111 mg (36 mg, 210 mg). The distribution of self-reported caffeine intake was highly skewed to the right (Figure 1), consistent with other studies of caffeine consumption in PD 18. The estimated intraclass correlation coefficient between current (in the past week) caffeine consumption in CALM-PD and prior (on average over the last year) caffeine consumption in CALM Cohort, referring to approximately the same time period, was 0.62 (lower 95% confidence bound = 0.52) which indicates moderate agreement.
Figure 1. Self-reported prior caffeine use from the CALM-PD study.
Total milligrams of caffeine use per day from the CALM-PD questionnaire are divided into 100 mg/day increments. Frequency refers to the number of participants. The distribution is highly skewed to the right with a large number of participants reporting less than 100 mg of caffeine use per day. The number of participants reporting 0 mg of caffeine use/day was 19.
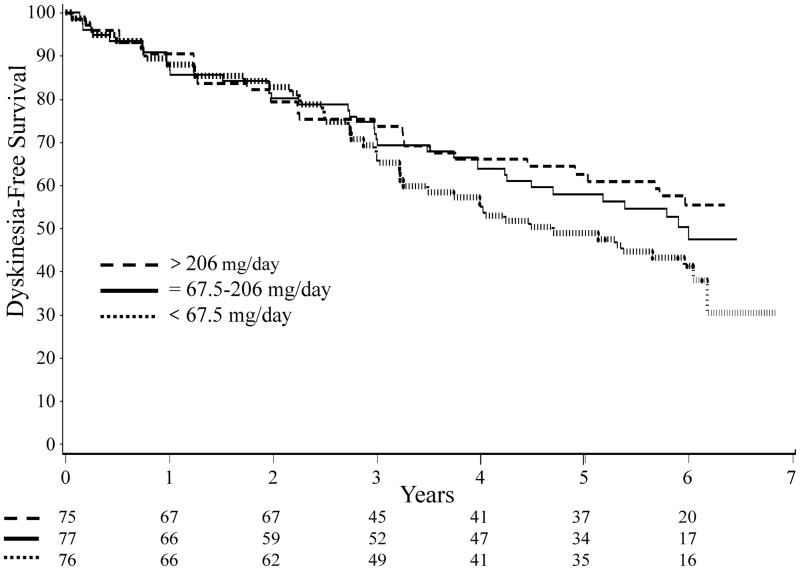
The Kaplan-Meier curves for dyskinesia-free survival based upon tertiles of self-reported caffeine use are shown in Figure 2. The three groups appear to diverge after approximately 4 years of follow-up. The estimated 5-year dyskinesia-free survival rate in the highest tertile (comparable to > 12 ounces of coffee/day) was 63%, while the rate in the lowest tertile (comparable to < 4 ounces of coffee/day) was 49%. However the log-rank test was not significant (p=0.19).
Figure 2. Kaplan-Meier curves for time to dyskinesia stratified by tertile of prior caffeine use.
Total number at risk in each tertile is shown at the bottom of the graph. Dashed line: > 206 mg/day of caffeine; solid line: 67.5–206 mg/day of caffeine; dotted line: < 67.5 mg/day of caffeine (log-rank test, p=0.19).
The baseline characteristics of study participants based upon tertiles of self-reported caffeine use are shown in Supplementary Table 1. The only variable that was significantly different between the tertiles was age (higher caffeine consumers tended to be younger, p=0.002). After stepwise selection, the final variables retained in the Cox proportional hazards model were age, baseline UPDRS total score, site and treatment assignment (to levodopa or pramipexole). Prior caffeine use (based on tertiles) was then added to the selected multivariate model. The hazard ratio for subjects in the highest tertile (comparable to > 12 ounces of coffee/day) was 0.61 (95% CI 0.37–1.01) and for subjects in the middle tertile was 0.73 (95% CI 0.46–1.15), compared with the lowest tertile (comparable to < 4 ounces of coffee/day) (test for trend, p = 0.050).
Given the fact that the caffeine survey was retrospective, we performed a sensitivity analysis of current caffeine use and time to dyskinesia onset after the date of the first survey (excluding the 77 subjects who experienced dyskinesia prior to the date of the survey). Consistent with the primary analysis, the estimated 1.5-year dyskinesia-free survival rate in the highest tertile of current caffeine consumption was slightly higher than the rate in the lowest tertile (78% and 70%), which was not significant (log-rank test, p=0.23).
To explore other potential sources of confounding, we tested whether participants who experienced dyskinesia were more likely to reduce their intake of caffeine after the onset of dyskinesia (for example, if caffeine were felt to increase their dyskinesia). Both dyskinetic and non-dyskinetic subjects reported a reduction in caffeine use between the first and second surveys, which did not differ between the groups (Kruskal-Wallis test, p=0.94). We also examined whether the observed association could be due to a lower requirement for levodopa in subjects with higher levels of caffeine consumption, due to a symptomatic effect of caffeine or to a synergistic effect of caffeine on levodopa response. However, the frequency of open-label levodopa use and the time to initiation of open-label levodopa did not differ significantly between the caffeine tertiles (Supplementary Table 2).
Discussion
These results lend support to the hypothesis posed originally on preclinical evidence 2, 3 that chronic administration of caffeine (or more specific adenosine A2A receptor antagonists) early in the course of PD may reduce the long-term risk of dyskinesia induced by levodopa therapy. As in animal models, chronic A2A receptor blockade may prevent the maladaptive plasticity triggered by repeated dopaminergic stimulation 1–4. Interestingly, pairing of levodopa with an A2A antagonist 3, 4 or genetic depletion of the A2A receptor 2, 3 in mouse models of levodopa-induced dyskinesia prevented the late but not initial development of abnormal responses to levodopa, suggesting a potential biological basis for the apparent lag in the manifestation of a caffeine-dyskinesia association until after 3–4 years of dopaminergic treatment (Figure 2). An alternative explanation for the prevention of dyskinesia formation might be that caffeine indirectly lowers the risk of levodopa-induced dyskinesia by reducing the need for dopaminergic medication because of a symptomatic effect on PD 8–12, 19, 20. In addition to adjusting for randomization to levodopa, we tested this hypothesis by examining the use of open-label levodopa in each of the caffeine tertiles and found no association. Therefore we do not believe this to be the primary explanation of our results.
There are multiple limitations to this study, including the fact that caffeine use was surveyed an average of 4.6 years after the start of the study, after the onset of dyskinesia in the majority of the participants. It is theoretically possible that the presence of dyskinesia might affect the reporting of prior caffeine consumption, although participants were not aware of this hypothesis when completing the questionnaires. We attempted to address this problem in three ways: first, by assessing the test-retest reliability of prior caffeine reporting compared to current caffeine reporting; second, by comparing the difference between self-reported prior and current caffeine use in participants with and without dyskinesia; third, we performed a sensitivity analysis limited to subjects who had not yet experienced dyskinesia at the time of the survey, and the results of this analysis were consistent with our main findings, although not significant.
We should emphasize that this was an observational study, only designed to explore an association between caffeine use and time to onset of dyskinesia. Residual confounding may have occurred because of unmeasured genetic, pharmacologic (such as caffeine intolerance leading to gastric discomfort or insomnia), dietary or other environmental factors, which may have been associated with both caffeine use and dyskinesia in PD 21. Similarly, reverse causation cannot be ruled out. Nevertheless, when taken together with preclinical data, the present findings strengthen the rationale for investigating caffeine and more specific adenosine antagonists as early adjunctive therapy, prior to or at the time of initiation of dopaminergic medication, in an effort to prevent the development of dyskinesia.
Supplementary Material
Acknowledgments
Funding agencies: The Michael J. Fox Foundation for Parkinson’s Research Fast Track 2001 Program, the Parkinson’s Disease Foundation, and NIH grants R01NS054978 and K24NS60991.
Footnotes
Documentation of Author Roles. The authors contributed to the following specific roles in the project and manuscript preparation as indicated.
1. Research project: A. Conception, B. Organization, C. Execution;
2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique;
3. Manuscript Preparation: A. Writing of the first draft, B. Review and Critique;
Anne-Marie A. Wills (1B,1C,2A,2C,3A,3B), Shirley Eberly (2A,2B,2C,3B), Marsha Tennis (1B,1C,3B), Cornelia Kamp (1B,1C,3B), Susan Messing (2B,3B), Daniel Togasaki (1B,3B), Alberto Ascherio (2C,3B), Robert Holloway (1B,3B), Caroline M. Tanner (1B,2C,3B), Anthony E. Lang (1B,3B), Michael P. McDermott (2A,2C,3B), David Oakes (2A,3B), Jiang-Fan Chen (1A,3B), Michael A. Schwarzschild (1A,1B,1C,2A,2C,3A,3B)
Data Access and Responsibility. Drs. Wills, Eberly and Schwarzschild take responsibility for the integrity of the data and the accuracy of the data analysis.
Relevant conflicts of interest/financial disclosures: The present study is a secondary analysis of the database of a clinical trial, CALM-PD, and its extension study, CALM Cohort, which were sponsored by Pharmacia Corporation (Peapack, NJ) and Boehringer Ingelheim (Ingelheim, Germany). In January 2001 Pharmacia approved an investigator (M.A.S.)-initiated proposal to introduce an add-on caffeine questionnaire to CALM-PD subjects to test our pre-specified hypotheses that higher caffeine consumption is associated with lower rates of dyskinesia development and/or disease progression. Neither Pharmacia nor Boehringer Ingelheim provided funding for, or otherwise supported or participated in, the present study. A.M. Wills participates in the Schering-Plough sponsored clinical trials of Preladenant and has served as a consultant for Accordant (a CVS/Caremark disease management company), for Asubio Pharmaceuticals, and for NanoDerma Ltd. S. Eberly has received research support from NIH, DOD, Michael J Fox Foundation, Parkinson’s Disease Foundation, Cephalon, and Lundbeck. A.E. Lang has served as an advisor for Abbott, Allon Therapeutics, Astra Zenica, Avanir Pharmaceuticals, Biovail, Boerhinger-Ingelheim, Cephalon, Ceregene, Eisai, GSK, Lundbeck A/S, Medtronic, Merck Serono, Novartis, Santhera, Solvay, and Teva; received honoraria from Teva; received grants from Canadian Institutes of Health Research, Dystonia Medical Research Foundation, Michael J. Fox Foundation, National Parkinson Foundation, and Ontario Problem Gambling Research Centre; received publishing royalties from Saunders, Wiley-Blackwell, Johns Hopkins Press, and Cambridge University Press; and has served as an expert witness in cases related to the welding industry. D.M. Togasaki participated in the Schering-Plough-sponsored open-label extension study of Preladenant. C.M. Tanner is an employee of the Parkinson’s Institute. She serves on the Scientific Advisory Boards of the Michael J. Fox Foundation and the National Spasmodic Dystonia Association, and has provided consulting services to Impax Pharmaceuticals and Adamas Pharmaceuticals. She received grant support from the Michael J. Fox Foundation, the Brin Foundation, James and Sharron Clark, the Parkinson’s Institute and Clinical Center, the Parkinson’s Disease Foundation, USAMRAA (TATRC managed NETRP Program), National Institute of Neurological Disorders and Stroke (NINDS) and the Agency for Healthcare and Research Quality (AHRQ). C. Kamp is supported by research grants from NINDS, Michael J. Fox Foundation, and the Friedreich’s Ataxia Research Alliance. J.-F. Chen has served as a consultant for Kyowa Pharmaceutical, Inc. and Adenosine Therapeutics. D. Oakes has received support from the NIH, the Department of Defense and the Michael J. Fox Foundation for his research into Parkinson’s disease. He has received an honorarium from Novo Nordisk Inc. for consulting about an unrelated topic. M. P. McDermott received research grants from NIH, FDA, CDC, Muscular Dystrophy Association, SMA Foundation, Michael J. Fox Foundation, Boehringer-Ingelheim Pharmaceuticals, Inc., NeuroSearch Sweden AB, and Medivation, Inc., and served as a consultant for the NY State Department of Health, Pfizer, Inc., Smith and Nephew, Inc., Synosia, Inc., Teva Pharmaceutical Industries, Ltd., Impax Pharmaceuticals, and Bioness, Inc. M.T. and S.M. have nothing to report.
Financial Disclosures. All submissions require two entries that cover financial disclosure of all authors:
Full financial disclosure for the previous 12 months:
Anne-Marie A. Wills (research grants from the Muscular Dystrophy Association, NIH/NINDS and consultant payments from Asubio pharmaceuticals, NanoDerma Ltd. and Accordant, a CVS/Caremark disease management company).
Shirley Eberly (support from NIH, DOD, Michael J Fox Foundation, Parkinson’s Disease Foundation, Cephalon, and Lundbeck.)
Marsha Tennis (nothing to report)
Cornelia Kamp (research grants from NINDS, Michael J. Fox Foundation, and the Friedreich’s Ataxia Research Alliance)
Susan Messing (nothing to report)
Daniel Togasaki (research funding from Schering-Plough, Teva, Abbot, Allon, Solvay, NIH) Caroline M. Tanner has provided consulting services to Impax Pharmaceuticals and Adamas Pharmaceuticals. She received grant support from the Michael J. Fox Foundation, the Brin Foundation, James and Sharron Clark, the Parkinson’s Institute and Clinical Center, the Parkinson’s Disease Foundation, USAMRAA (TATRC managed NETRP Program), National Institute of Neurological Disorders and Stroke (NINDS) and the Agency for Healthcare and Research Quality (AHRQ)
Anthony E. Lang (support from Canadian Institutes of Health Research, Dystonia Medical Research Foundation, Michael J. Fox Foundation, National Parkinson Foundation, and Ontario Problem Gambling Research Centre)
Michael P. McDermott (research grants from NIH, FDA, CDC, SMA Foundation, and Medivation, Inc.; consultant payments from NY State Department of Health, Teva Pharmaceutical Industries, Ltd., Impax Pharmaceuticals, and Bioness, Inc.),
David Oakes (support from the NIH, the Department of Defense and the Michael J. Fox Foundation. He has received an honorarium from Novo Nordisk Inc. for consulting about an unrelated topic.)
Jiang-Fan Chen (nothing to report)
Michael A. Schwarzschild (research grants from NIH, DoD, Michael J. Fox Foundation and RJG Foundation; consultant payments from Harvard Univ.)
References
- 1.Bibbiani F, Oh JD, Petzer JP, et al. A2A antagonist prevents dopamine agonist-induced motor complications in animal models of Parkinson’s disease. Exp Neurol. 2003;184(1):285–294. doi: 10.1016/s0014-4886(03)00250-4. [DOI] [PubMed] [Google Scholar]
- 2.Xiao D, Bastia E, Xu YH, et al. Forebrain adenosine A2A receptors contribute to L-3,4-dihydroxyphenylalanine-induced dyskinesia in hemiparkinsonian mice. J Neurosci. 2006;26(52):13548–13555. doi: 10.1523/JNEUROSCI.3554-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fredduzzi S, Moratalla R, Monopoli A, et al. Persistent behavioral sensitization to chronic L-DOPA requires A2A adenosine receptors. J Neurosci. 2002;22(3):1054–1062. doi: 10.1523/JNEUROSCI.22-03-01054.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao D, Cassin JJ, Healy B, et al. Deletion of adenosine A or A(A) receptors reduces L-3,4-dihydroxyphenylalanine-induced dyskinesia in a model of Parkinson’s disease. Brain Res. 2010;1367:310–318. doi: 10.1016/j.brainres.2010.08.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calon F, Dridi M, Hornykiewicz O, Bedard PJ, Rajput AH, Di Paolo T. Increased adenosine A2A receptors in the brain of Parkinson’s disease patients with dyskinesias. Brain. 2004;127(Pt 5):1075–1084. doi: 10.1093/brain/awh128. [DOI] [PubMed] [Google Scholar]
- 6.Ramlackhansingh AF, Bose SK, Ahmed I, Turkheimer FE, Pavese N, Brooks DJ. Adenosine 2A receptor availability in dyskinetic and nondyskinetic patients with Parkinson disease. Neurology. 2011;76(21):1811–1816. doi: 10.1212/WNL.0b013e31821ccce4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mishina M, Ishiwata K, Naganawa M, et al. Adenosine A(2A) receptors measured with [C]TMSX PET in the striata of Parkinson’s disease patients. PLoS One. 6(2):e17338. doi: 10.1371/journal.pone.0017338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mizuno Y, Hasegawa K, Kondo T, Kuno S, Yamamoto M. Clinical efficacy of istradefylline (KW-6002) in Parkinson’s disease: a randomized, controlled study. Mov Disord. 2010;25(10):1437–1443. doi: 10.1002/mds.23107. [DOI] [PubMed] [Google Scholar]
- 9.Hauser RA, Shulman LM, Trugman JM, et al. Study of istradefylline in patients with Parkinson’s disease on levodopa with motor fluctuations. Mov Disord. 2008;23(15):2177–2185. doi: 10.1002/mds.22095. [DOI] [PubMed] [Google Scholar]
- 10.Hauser RA, Cantillon M, Pourcher E, et al. Preladenant in patients with Parkinson’s disease and motor fluctuations: a phase 2, double-blind, randomised trial. Lancet Neurol. 2011;10(3):221–229. doi: 10.1016/S1474-4422(11)70012-6. [DOI] [PubMed] [Google Scholar]
- 11.LeWitt PA, Guttman M, Tetrud JW, et al. Adenosine A2A receptor antagonist istradefylline (KW-6002) reduces “off” time in Parkinson’s disease: a double-blind, randomized, multicenter clinical trial (6002-US-005) Ann Neurol. 2008;63(3):295–302. doi: 10.1002/ana.21315. [DOI] [PubMed] [Google Scholar]
- 12.Shoulson I, Chase T. Caffeine and the antiparkinsonian response to levodopa or piribedil. Neurology. 1975;25(8):722–724. doi: 10.1212/wnl.25.8.722. [DOI] [PubMed] [Google Scholar]
- 13.Gottwald MD, Aminoff MJ. Therapies for dopaminergic-induced dyskinesias in Parkinson disease. Ann Neurol. 2011;69(6):919–927. doi: 10.1002/ana.22423. [DOI] [PubMed] [Google Scholar]
- 14.Parkinson Study Group. Pramipexole vs levodopa as initial treatment for Parkinson disease: A randomized controlled trial. JAMA. 2000;284(15):1931–1938. doi: 10.1001/jama.284.15.1931. [DOI] [PubMed] [Google Scholar]
- 15.Parkinson Study Group. A randomized controlled trial comparing pramipexole with levodopa in early Parkinson’s disease: design and methods of the CALM-PD Study. Clin Neuropharmacol. 2000;23(1):34–44. doi: 10.1097/00002826-200001000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Parkinson Study Group. Long-term effect of initiating pramipexole vs levodopa in early Parkinson disease. Arch Neurol. 2009;66(5):563–570. doi: 10.1001/archneur.66.1.nct90001. [DOI] [PubMed] [Google Scholar]
- 17.Constantinescu R, Romer M, McDermott MP, Kamp C, Kieburtz K. Impact of pramipexole on the onset of levodopa-related dyskinesias. Mov Disord. 2007;22(9):1317–1319. doi: 10.1002/mds.21292. [DOI] [PubMed] [Google Scholar]
- 18.Simon DK, Swearingen CJ, Hauser RA, et al. Caffeine and progression of Parkinson disease. Clin Neuropharmacol. 2008;31(4):189–196. doi: 10.1097/WNF.0b013e31815a3f03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Postuma RB, Lang AE, Munhoz RP, et al. Caffeine for treatment of Parkinson disease: A randomized controlled trial. Neurology. 2012;79(7):651–8. doi: 10.1212/WNL.0b013e318263570d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Altman RD, Lang AE, Postuma RB. Caffeine in Parkinson’s disease: a pilot open-label, dose-escalation study. Mov Disord. 2011;26(13):2427–2431. doi: 10.1002/mds.23873. [DOI] [PubMed] [Google Scholar]
- 21.Hamza TH, Chen H, Hill-Burns EM, et al. Genome-wide gene-environment study identifies glutamate receptor gene GRIN2A as a Parkinson’s disease modifier gene via interaction with coffee. PLoS Genet. 7(8):e1002237. doi: 10.1371/journal.pgen.1002237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.