Abstract
Background
Millions of individuals worldwide have used anabolic-androgenic steroids (AAS) to gain muscle or improve athletic performance. Recently, in vitro investigations have suggested that supraphysiologic AAS doses cause apoptosis of neuronal cells. These findings raise the possibility, apparently still untested, that humans using high-dose AAS might eventually develop cognitive deficits.
Methods
We administered five cognitive tests from the computerized CANTAB battery (Pattern Recognition Memory, Verbal Recognition Memory, Paired Associates Learning, Choice Reaction Time, and Rapid Visual Information Processing) to 31 male AAS users and 13 non-AAS-using weightlifters age 29-55, recruited and studied in May 2012 in Middlesbrough, UK. Testers were blinded to participants’ AAS status and other historical data.
Results
Long-term AAS users showed no significant differences from nonusers on measures of response speed, sustained attention, and verbal memory. On visuospatial memory, however, AAS users performed significantly more poorly than nonusers, and within the user group, visuospatial performance showed a significant negative correlation with total lifetime AAS dose. These were large effects: on Pattern Recognition Memory, long-term AAS users underperformed nonusers by almost one standard deviation, based on normative population scores (adjusted mean difference in z-scores = 0.89; p = 0.036), and performance on this test declined markedly with increasing lifetime AAS dose (adjusted change in z-score = −0.13 per 100g of lifetime AAS dose; p = 0.002). These results remained stable in sensitivity analyses addressing potential confounding factors.
Conclusions
These preliminary findings raise the ominous possibility that long-term high-dose AAS exposure may cause cognitive deficits, notably in visuospatial memory.
Keywords: Anabolic-androgenic steroids, Testosterone, Apoptosis, Substance abuse, Cognitive deficits, Visuospatial memory
1. INTRODUCTION
The anabolic-androgenic steroids (AAS) are a group of hormones, comprising testosterone and its synthetic relatives, which permit users to greatly increase their muscle mass and improve athletic performance (Kanayama et al., 2010; Sjoqvist et al., 2008). Prior to the 1980s, AAS use was largely restricted to elite athletes, but in recent decades these drugs have spread to the general population, and have now emerged as a major new form of substance abuse throughout the Western world (Kanayama et al., 2008). Importantly, recent studies suggest that as many as 30% of AAS abusers may develop an AAS dependence syndrome, characterized by prolonged use of these drugs, sometimes for many years, at doses 10-100 times the normal endogenous male output of testosterone (Kanayama et al., 2009a). The eventual public health consequences of such high-dose AAS exposure are still largely unknown, because most AAS users in the general population did not begin using these drugs until after 1980. Thus the oldest of these users, those who started AAS as youths in the 1980s, are only now reaching middle age and entering the age of risk for adverse effects of long-term use (Kanayama et al., 2008).
Pending larger clinical studies of this first wave of aging AAS users, one can look to laboratory data for evidence as to where AAS toxicity might manifest itself in humans. Among these data are findings that supraphysiologic concentrations of testosterone and other AAS can induce apoptosis in many types of mammalian cells, including myocardial (Fanton et al., 2009; Riezzo et al., 2011; Zaugg et al., 2001), skeletal muscle (Abu-Shakra et al., 1997), endothelial (D’Ascenzo et al., 2007), and neuronal cells (Estrada et al., 2006). Of particular concern among these studies is one report demonstrating apoptosis of human neuroblastoma cells in vitro after only 6-12 hours of exposure to testosterone concentrations as low as 1μM (Estrada et al., 2006). This study demonstrated decreased cell viability induced by testosterone-induced activation of the apoptotic program, as evidenced by increased numbers of annexin V-positive cells, DNA fragmentation, and caspase activation. These changes were likely initiated by a marked and sustained increase in intracellular calcium which in turn appeared to be mediated by testosterone’s effects on inositol 1,4,5-triphosphate-sensitive calcium release channels. Since testosterone concentrations of 1μM and above are within the range plausibly attainable by human AAS abusers, the investigators speculated that long-term AAS abuse might lead to irreversible cognitive deficits.
More recently, two other groups have also demonstrated neurotoxic effects in mammalian neuronal cells exposed to AAS concentrations within the probable human-abuse range (Caraci et al., 2011; Cunningham et al., 2009). One of these groups (Caraci et al., 2011) additionally demonstrated that nandrolone and methandrostenolone, two widely abused AAS, appeared to potentiate the apoptotic stimulus provided by beta-amyloid, the likely principal culprit in Alzheimer’s disease. These investigators also speculated that AAS abuse might facilitate the onset or progression of neurodegenerative diseases.
Although AAS can certainly precipitate acute psychiatric effects in some individuals (Hall et al., 2005; Kanayama et al., 2010), we are not aware of any reports of AAS-induced neurodegenerative diseases in humans. If supraphysiologic AAS exposure can cause such diseases, why would this not already have been witnessed? In response, it must be remembered that most of the world’s illicit AAS users are still under age 50, as just explained. Therefore, these men might have incurred neurotoxic effects, but still be too young to exhibit gross cognitive or motor deficits. Admittedly, there are some men over age 50 who used AAS when competing in elite athletics or bodybuilding before the 1980s. However, the AAS doses used in that era were typically lower than those used today (Duchaine, 1981), and thus perhaps less likely to induce neurotoxicity. Therefore, it remains plausible that human AAS-induced neurotoxicity could be a genuine phenomenon that has simply not yet emerged into view.
Laboratory test data from an ongoing study at our center raise further concern about the vulnerability of AAS abusers. In 11 sequential men currently injecting testosterone, we found a mean (SD) serum testosterone level of 6401 (5448) ng/dL, with one man reaching 20300 ng/dL (normal range in our laboratory 175-781 ng/dL). Importantly, these levels substantially underestimated the men’s total burden of AAS, since most were taking other AAS simultaneously with testosterone at the time of evaluation. These observations demonstrate that human abusers can achieve total serum AAS levels at least 50 times average physiologic levels. Given that the above in vitro studies found some neuronal apoptotic effects of AAS at even 10-20 times physiologic concentrations, after only 6-48 hours of exposure, the possibility of clinically significant neurotoxicity in long-term human AAS abusers cannot be dismissed.
In a pilot study to explore this possibility, we administered a battery of cognitive tests to male AAS users and to comparison weightlifters reporting no AAS use.
2. METHODS
2.1. Study participants
We recruited male weightlifters age 29-55 in Middlesbrough, England, a city with a high prevalence of AAS users where we have previously conducted research (Pope et al., 2010). Participants were recruited by one of the investigators (JK) from among clients of Lifeline Middlesbrough, a charitable organization providing needle exchange facilities and counseling for drug users, and by advertising in local gymnasia for experienced weightlifters, using methods previously described (Kanayama et al., 2009b, 2003; Pope et al., 2012, 2010). We chose a minimum age of 29 in order to favor individuals with long-term AAS use, and hence presumably at greater risk for AAS-induced cognitive effects if they existed. We imposed no other formal inclusion or exclusion criteria, but for the purposes of this pilot investigation, the recruiter attempted to enrich the sample with men likely to fall at opposite ends of the distribution of AAS exposure (i.e., men with very long-term AAS use and men with no AAS use at all). Participants were compensated £50 (approximately $80 US). All participants were recruited and evaluated within the month of May 2012.
2.2. Study evaluation
Upon arriving for evaluation, all participants signed written informed consent for the study, which was approved by the McLean Hospital Institutional Review Board. Participants then received 1) an interview administered by one of the investigators (JK) and 2) a computerized battery of cognitive tests administered by two of the other investigators (GK and HGP). The interviewer and the testers remained blinded to each others’ findings until both evaluations were completed. Thereafter, blindness was broken, and the interview results were reviewed with the participant by the senior investigator (HGP) to clarify any questions about history of AAS and other substance use, as well as medical or psychiatric conditions that might have influenced cognitive performance.
2.2.1 Interview evaluation
The interviewer administered a semi-structured instrument to each participant, similar to that used in our prior studies (Kanayama et al., 2003; Pope et al., 2012, 2010), covering 1) demographic data; 2) weightlifting history; 3) history of treatment for medical or psychiatric disorders; 4) history of tobacco, alcohol, and classical illicit substance use; 5) history of AAS use; and 6) use of any other performance- or image-enhancing drugs such as human growth hormone, clenbuterol, and insulin (Baker et al., 2006; Skarberg et al., 2009). In men reporting AAS use, the interviewer determined as accurately as possible their a) age at onset of AAS use; b) maximum weekly dose of AAS, expressed as mg of testosterone equivalent, calculated as we (Kanayama et al., 2009b, 2003; Pope and Katz, 1994) and others (Pope and Katz, 2003) have done in past studies; c) lifetime average weekly dose of AAS; d) total lifetime weeks of AAS exposure; and e) time of most recent AAS use.
2.2.2 Cognitive evaluation
The cognitive testers first administered the New Adult Reading Test (NART) to estimate verbal IQ (Crawford et al., 2001; Willshire et al., 1991), followed by five tests from the CANTAB battery (Cambridge Cognition, Cambridge, UK), a widely used collection of computerized cognitive tests (Robbins et al., 1998, 1994). When administering this battery, the testers first oriented participants to use of the touchscreen computer, and then provided brief verbal instructions for each successive test, using verbatim scripts from the CANTAB test administration manual. The five selected tests, lasting a total of approximately 40 minutes, began with Pattern Recognition Memory, which assessed visual memory by serially presenting 12 visual patterns, followed by a recognition phase where participants were shown two patterns and asked to touch the one that they had previously seen. The recognition phase was administered both immediately and again after a 30-minute delay. Second, Verbal Recognition Memory assessed verbal memory by serially presenting 18 words, followed immediately by a) a free-recall phase, where participants were asked to recall as many words as possible without cues, and then b) a recognition phase, where participants were shown two words and asked to touch the word that they had previously seen. The recognition phase was administered both immediately and after a 20-minute delay. Third, Paired Associates Learning assessed both visuospatial memory and new learning by presenting several white boxes that each briefly “opened” to reveal underlying patterns. Participants were subsequently shown each pattern and asked to touch the box covering that pattern. Fourth, Choice Reaction Time assessed motor speed and general alertness by asking participants to rapidly press left or right buttons upon seeing left- or right-pointing arrows on the screen. Fifth, Rapid Visual Information Processing assessed sustained attention by rapidly displaying serial digits on the screen and asking participants to press a button when they saw a particular sequence (e.g., 3-5-7). All tests were administered in their standard Clinical Modes except Paired Associates Learning, which was administered in the Parallel pa2 Six-Attempt Mode, with five stages extending up to eight patterns. These tests are detailed in online materials from Cambridge Cognition (www.cantab.com) and in numerous published studies (Ersche et al., 2006; Fillmore and Rush, 2002; Fishbein et al., 2007; Morgan et al., 2009; Robbins et al., 1997; Sahakian et al., 1988).
2.3. Statistical methods
We performed two primary analyses. First, we compared long-term AAS users against nonusers on cognitive performance. We defined long-term AAS users, prior to inspection of the cognitive testing data, as individuals reporting at least two years of cumulative lifetime AAS exposure (i.e., at least 104 total weeks during which they were using AAS). We compared long-term users with nonusers by linear regression, where the outcome variables were scores on the various CANTAB tests, and with adjustment for age and level of education, both measured in years. We chose level of education, as opposed to NART scores, to adjust for premorbid intellectual ability because several participants reported they had dyslexia and mispronounced virtually every word on the NART, thus yielding estimated IQ scores in the 70s and low 80s that almost certainly understated their true intellectual ability. Despite these concerns, we subsequently repeated the analyses involving our principal findings while adjusting for NART scores instead of level of education to assess the stability of these results.
For the second primary analysis, we assessed the association between participants’ total lifetime dose of AAS (i.e., lifetime weeks of AAS use multiplied by lifetime average weekly dose of AAS) and the cognitive outcome measures. We chose lifetime dose of AAS as the primary predictor variable, rather than simply lifetime duration of AAS use, because we hypothesized that neurotoxic effects, if any, would likely be associated with both dose and duration of exposure. We initially analyzed these associations using product-moment correlations and by linear regression assessing the effect of lifetime AAS dose on test scores, again adjusting for age and years of education. However, given that we lacked a priori knowledge of the appropriate functional forms of the predictor and outcome variables (e.g., whether to model a linear effect, a threshold effect, or a more complicated relationship), we repeated these analyses using nonparametric methods. These included Spearman correlations between lifetime AAS dose and the outcome variables, together with regression analyses using ranks of lifetime AAS dose, rather than numerical dose, as a predictor variable.
Finally, for two of the five tests (Rapid Visual Information Processing and Pattern Recognition Memory) we possessed normative data from the adult UK population. Therefore we were able to transform results on these two tests into z-scores, allowing us to evaluate test performance relative to population norms.
For both of the primary analyses above, we performed Kolmogorov-Smirnov tests on all outcome variables to test whether their distribution violated the assumption of normality. None of the variables significantly departed from normality on these tests. We also performed several sensitivity analyses to assess the stability of the cognitive findings. These included analyses 1) excluding participants who displayed confounding features that might affect cognitive performance (e.g., acute AAS withdrawal, history of classical polysubstance dependence, recent ingestion of prescribed or illicit psychoactive substances, or history of head injury with prolonged loss of consciousness); 2) comparing current and past AAS users; 3) comparing AAS users with and without a history of using other performance- and image-enhancing drugs; and 4) assessment of current or recent stimulant drug use by participants.
All analyses were performed using Stata 9.2 (Stata Corporation, College Station, Texas), with alpha set at 0.05, 2-tailed. Note that the study generated multiple comparisons, increasing the likelihood of type I errors. However, it appears likely that Bonferroni and similar correction procedures for these comparisons would be too conservative and would potentially inflate type II error rates (Rothman and Greenland, 1998). Thus, we present the results without correction. Although there are reasons to favor this uncorrected reporting approach (Feise, 2002; Savitz and Olshan, 1995), one must consider the greater possibility of chance associations when evaluating the tests of significance reported below.
3. RESULTS
We evaluated 45 men, of whom 31 reported a history of AAS use and 14 denied use. However, one man was strikingly muscular, yet denied any history of AAS or other performance- or image-enhancing drug use. Both the interviewer and the cognitive testers independently suspected that this man had actually used AAS, but had not disclosed this information. We therefore excluded his data, leaving 13 nonusers for analysis. The 31 AAS users reported a lifetime duration of AAS use ranging from 8-640 weeks. Twenty-two of these men met our definition of “long-term AAS users,” in that they reported at least two years of lifetime use. AAS users and nonusers were similar in mean age and years of lifting weights, but nonusers reported substantially more years of education (Table 1).
Table 1. Demographic Features of AAS Users and Nonusers.
| Attribute | AAS nonusers N = 13 |
All AAS users N = 31 |
Long-term AAS users N = 22 |
Short-term AAS users N = 9 |
|---|---|---|---|---|
|
| ||||
| Mean (SD) | ||||
| Age | 37.0 (7.1) | 37.1 (7.0) | 37.3 (6.5) | 36.6 (8.4) |
| Years of education | 17.8 (4.3) | 12.8 (2.8)a | 12.9 (2.7) | 12.4 (3.3) |
| Age began regular weightlifting | 17.5 (4.8) | 17.5 (5.4) | 16.8 (4.6) | 19.4 (6.9) |
| Years of regular weightlifting | 17.8 (8.3) | 17.1 (7.9) | 19.2 (7.5) | 11.9 (6.5)b |
| Age began AAS use | 24.3 (5.9) | 23.0 (5.0) | 27.6 (6.9)b | |
| Lifetime weeks of AAS use | 271 (201) | 364 (161) | 44 (35)c | |
p < 0.001 vs. nonusers by t test, two-tailed
p < 0.05 vs. long-term users by t test, two-tailed
p < 0.001 vs. long-term users by t test, two-tailed
Comparing long-term users with nonusers on the test results (Table 2), we found no significant differences between groups on Choice Reaction Time and Rapid Visual Information Processing, suggesting that motor speed, alertness, and ability to maintain attention were not associated with AAS exposure. Verbal Recognition Memory also revealed no significant differences between groups on number of words generated on immediate free recall, errors on immediate recognition, and errors on delayed recognition of the 18 words. However, on immediate Pattern Recognition Memory, long-term users committed significantly more errors than nonusers when attempting to recognize patterns that they had previously seen. In the delayed presentation of this test, long-term users also committed somewhat more errors than nonusers, but this difference did not reach significance. On Paired Associates Learning, a test also tapping visuospatial memory, long-term users again performed significantly more poorly than nonusers, both in adjusted total errors on the full test up through the stage presenting eight patterns, and in adjusted total errors only up through the stage of six patterns. Finally, examining the two measures for which we possessed z-scores, the user and nonuser groups showed no clinically significant difference in sensitivity to the target on Rapid Visual Information Processing (estimated mean difference in z-scores [95% confidence interval]: 0.18 [-0.77, 1.12]), but a marked difference in errors on immediate Pattern Recognition Memory (estimated mean difference: 0.89 [0.06, 1.71]). To appreciate the magnitude of this latter effect, one could standardize this result to a mean of 100 and standard deviation of 15, in the manner of the Wechsler Adult Intelligence Scale (Wechsler, 1939). On this hypothetical “visuospatial IQ” scale, long-term AAS users scored an average of 13.4 points below nonusers.
Table 2. Cognitive Test Performance in Long-Term AAS users vs. Non-users.
| AAS users (N = 22) Mean (SD) |
AAS Non-users (N = 13) Mean (SD) |
Estimated mean differencea (95% confidence interval) |
P | |
|---|---|---|---|---|
| Choice Reaction Time | ||||
| Mean latency, millisec | 318 (65) | 299 (33) | 0.73 (−47.6, 49.0) | 0.98 |
| Rapid Visual Information Processing | ||||
| Sensitivity to target (RVP A’) | 0.91 (0.061) | 0.92 (0.037) | 0.0088 (−0.038, 0.056) | 0.71 |
| Verbal Recognition Memory | ||||
| Words remembered, immediate free recall | 7.8(1.7) | 9.1 (2.8) | −1.03 (−2.97, 0.91) | 0.29 |
| Recognition errors, immediate presentationb | 1.7 (1.8) | 1.5 (1.0) | 0.58 (−0.72, 1.89) | 0.37 |
| Recognition errors, delayed presentation | 3.1 (3.5) | 1.6 (1.6) | 2.34 (−0.25, 4.93) | 0.075 |
| Pattern Recognition Memory | ||||
| Recognition errors, immediate presentation | 4.0 (2.8) | 1.5 ( 1.5) | 2.33 (0.16, 4.51) | 0.0 36 |
| Recognition errors, delayed presentation | 3.7 (1.9) | 2.7 (1.8) | 1.14 (−0.46, 2.75) | 0.16 |
| Paired Associates Learninga | ||||
| Total errors, adjusted | 2.9 (0.7) | 2.2 (0.9) | 0.76 (0.12, 1.41) | 0.0 21 |
| Total errors, 6 shapes, adjusted | 1.4 (0.9) | 0.9 (0.7) | 0.78 (0.10, 1.45) | 0.026 |
Represents AAS users minus nonusers
N = 21 long-term AAS users because of missing data
Log-transformed values
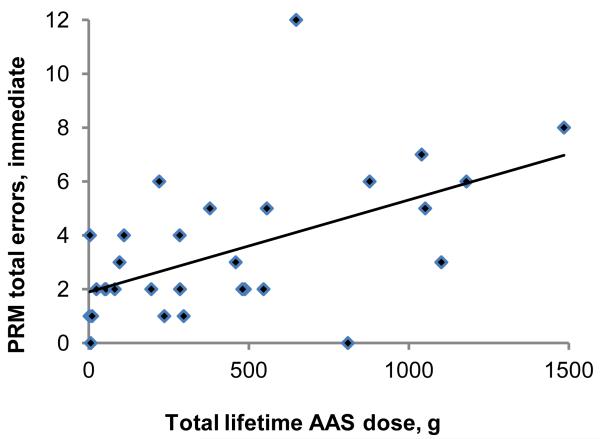
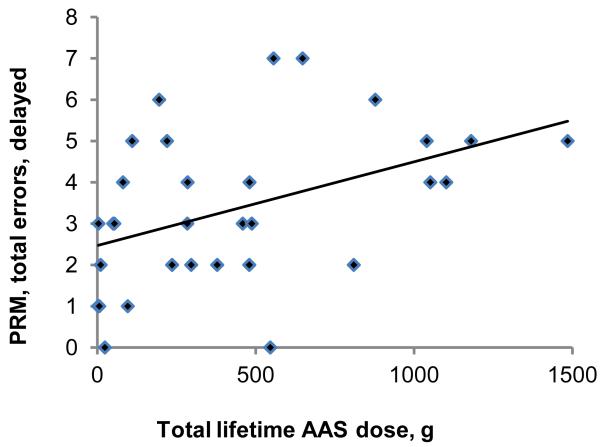
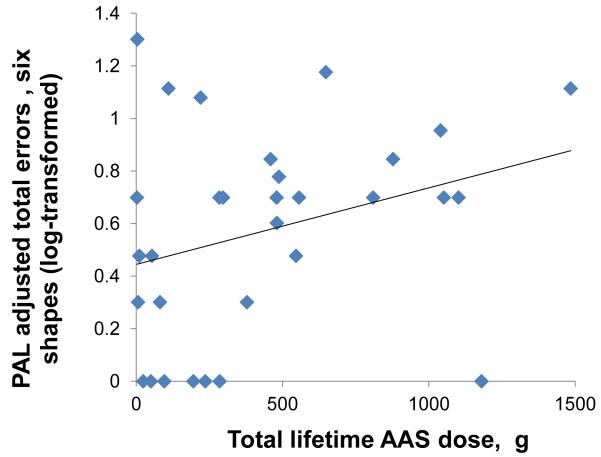
The second primary analysis, assessing associations between lifetime AAS dose and the outcome variables among all 31 AAS users, yielded findings generally consistent with those of the first. Specifically, Choice Reaction Time, Rapid Visual Information Processing, and Verbal Recognition Memory scores showed little or no significant association with AAS dose on regression analyses (Table 3). However, the number of errors on Pattern Recognition Memory, both in the immediate and delayed presentations (Figure 1, panels A and B), was strongly and significantly associated with lifetime AAS dose. Paired Associates Learning showed a slight but nonsignificant association with lifetime AAS dose (Figure 1 panel C). These findings remained virtually identical on repeat analyses using the nonparametric methods described above. Looking at the tests with available z-scores, Rapid Visual Information Processing showed essentially no correlation with lifetime AAS dose (estimated change in z-scores per 100g of lifetime AAS use: −0.01 [−0.12, 0.10]), but z-scores on Pattern Recognition Memory declined markedly with increasing lifetime AAS exposure (change in z-scores per 100g of lifetime AAS use: −0.13 [−0.22, −.05]; change in z-scores per year of lifetime AAS use: −0.11 [−0.21, −0.02]). To illustrate the magnitude of this latter effect on our hypothetical “visuospatial IQ” scale described above, AAS users showed a 1.7-point decline in visuospatial IQ for every year of continued AAS use.
Table 3. Associations of Cognitive Test Performance with Lifetime AAS Dose in 31 AAS Users.
| Coefficienta (95% confidence interval) |
p | |
|---|---|---|
| Choice Reaction Time | ||
| Latency, millisec | −0.42 (−5.82, 4.99) | 0.88 |
| Rapid Visual Information Processing | ||
| Sensitivity to target (RVP A’) | −0.00049 (−0.0058, 0.0048) | 0.85 |
| Verbal Recognition Memory | ||
| Words remembered, immediate free recall | −0.079 (−0.022, 0.070) | 0.29 |
| Recognition errors, immediate presentationb | 0.055 (−0.087, 0.20) | 0.43 |
| Recognition errors, delayed presentation | 0.14 (−0.16, 0.43) | 0.36 |
| Pattern Recognition Memory | ||
| Recognition errors, immediate presentation | 0.35 (0.14, 0.57) | 0.002 |
| Recognition errors, delayed presentation | 0.20 (0.028, 0.37) | 0.023 |
| Paired Associates Learningc | ||
| Total errors, adjusted | 0.010 (−0.061, 0.081) | 0.78 |
| Total errors, 6 shapes, adjusted | 0.045 (−0.035, 0.13) | 0.26 |
Coefficient represents adjusted mean change in the outcome variable for each increase of 100 grams in total lifetime AAS dose.
N = 30 because of missing data
Log-transformed values
Figure 1.
Association between lifetime dose of anabolic-androgenic steroids (AAS) and cognitive test measures in 31 AAS users. (A) Pattern Recognition Memory Processing, errors on immediate presentation: r = 0.54; p =0.002. (B) Pattern Recognition Memory Processing, errors on delayed presentation: r = 0.44; p =0.014. (C) Paired Associates Learning, adjusted total errors, six shapes: r = 0.30; p =0.10.
The sensitivity analyses suggested that the findings were quite stable. We first repeated our analyses while excluding the eight individuals (seven AAS users and one nonuser) who displayed one or more of the following attributes that might affect cognitive performance: 1) having discontinued AAS within the last six months, thus possibly precipitating AAS-withdrawal hypogonadism (Tan and Scally, 2009); 2) history of past polydrug dependence (involving stimulants, cocaine, sedative-hypnotics, opioids, and/or cannabis); 3) consumption of a psychoactive drug of abuse or prescription sedative medication within the past 24 hours; and 4) history of head injury with prolonged loss of consciousness. In these analyses, all results remained little changed, with most coefficients remaining within 15% of their original values. Sensitivity analyses comparing the 18 current AAS users with the 13 past users, while adjusting for lifetime AAS dose, age, and education, showed no suggestion of differences in cognitive performance. Similarly, no suggestion of differences was found when comparing the 19 AAS users reporting a history of using any additional performance- or image-enhancing drugs (e.g., human growth hormone, insulin, etc.) with the 12 users who had not. We also considered that weightlifters sometimes use stimulants (e.g. amphetamine, methamphetamine, or clenbuterol) to improve performance or body appearance (Hildebrandt et al., 2011), and that these drugs might affect cognition. However, upon exploring this possibility, we found that no participant reported use of an amphetamine derivative currently or within two weeks prior to evaluation, and only one reported current use of clenbuterol.
Finally, upon reanalyzing the results of the two visuospatial tests while adjusting for NART scores as opposed to years of education, differences between long-term AAS users and nonusers were slightly attenuated on both tests, with adjusted mean differences reduced to about two thirds of their previous values on most measures. However, on associations between the test measures and lifetime AAS dose, the coefficients remained virtually identical, and the p-values remained unchanged or slightly decreased with the NART adjustment.
4. DISCUSSION
Recent laboratory evidence suggests that supraphysiologic doses of testosterone and other AAS may induce apoptotic effects on neuronal cells, raising the possibility that long-term high-dose AAS exposure might cause cognitive deficits in human users. In a preliminary attempt to explore this issue, we administered a battery of neuropsychological tests to 31 AAS users and 13 non-AAS-using weightlifters. Long-term AAS users did not differ significantly from nonusers on tests of reaction time, alertness, sustained attention, or verbal memory. However, AAS users showed substantial and statistically significant deficits compared to nonusers on both of the two visuospatial tests, Pattern Recognition Memory and Paired Associates Learning. Moreover, within the AAS-using group, deficits in both immediate and delayed Pattern Recognition Memory were highly correlated with total lifetime AAS dose. Sensitivity analyses addressing several potential confounding variables suggested that these factors were unlikely to explain the differences observed.
Although we are not aware of prior human studies of cognitive function in AAS abusers, it is interesting to note that a recent study using the Morris water maze demonstrated spatial memory deficits in rats following supraphysiologic AAS exposure (Pieretti et al., 2012). These deficits were perhaps mediated by AAS-induced depletion of nerve growth factor in the basal forebrain, thus compromising cholinergic neuronal function and impairing spatial memory.
Several limitations of our study must be considered. First, the sample was small (31 AAS users and 13 non-users). Second, we attempted to enrich the sample for participants at the opposite extremes of AAS exposure (i.e. men reporting long-term AAS use versus men reporting no use), and our comparison of AAS users versus nonusers in Table 2 was also specifically restricted to users reporting at least 2 years of cumulative lifetime AAS use. Thus, these findings likely would not generalize to individuals reporting lesser degrees of AAS exposure. Third, our cross-sectional design does not permit inferences of causality; for example, AAS users’ lower visuospatial scores might simply have reflected innate cognitive deficits that antedated AAS use, rather than a toxic effect of AAS exposure itself. This possibility gains credence from the observation that the AAS users showed substantially lower educational attainment than nonusers. Although we cannot exclude level of education as a contributor to visuospatial cognitive test performance, several observations argue against this hypothesis. First, AAS-associated visuospatial cognitive deficits were found even after adjusting for level of education. Second, if the AAS users had lower baseline intellectual ability with consequent lower educational attainment, then one might particularly expect them to underperform nonusers on verbal skills. However, the users’ deficits were almost entirely in visuospatial domains. Third, a hypothesis of baseline intellectual differences could not easily explain the strong associations between lifetime AAS dose and scores on Pattern Recognition Memory within the group of AAS users. Educational attainment was similar across levels of AAS use (in fact, educational level was slightly positively correlated with lifetime AAS dose), so these associations would seem unlikely to reflect baseline differences.
Our findings are also limited by reliance on participants’ retrospective reports of their AAS use, which may have yielded inaccurate estimates of their total lifetime AAS exposure. These inaccurate estimates probably included both overstatements and understatements of lifetime AAS exposure, thus likely yielding non-differential measurement error, which would bias the results towards the null in our tests of associations between lifetime AAS exposure and the cognitive outcome measures. Another possibility is that some AAS users might have failed to disclose their AAS use, as indeed we suspected in one case, noted above. However, if we erroneously included any surreptitious AAS users, misclassified as nonusers, this also would have biased results towards the null. Finally, the participants selected for the study were not representative of the entire underlying source population of users and nonusers. But the study selection procedures would still not have biased comparisons between groups or comparisons across levels of AAS use, barring the seemingly unlikely possibility of differential selection effects (say, that the selection process was biased towards individuals with lower cognitive ability from within the pool of AAS users, but free of this same bias within the pool of nonusers).
In summary, despite the possible limitations listed above, our findings offer preliminary evidence that long-term AAS abuse may cause neurotoxicity, particularly perhaps in brain regions associated with visuospatial memory. Further studies in this area, with larger samples of participants, careful attention to potential confounding variables, and expanded neuropsychological test batteries, are needed to assess this potentially ominous effect of AAS use. Structural and functional neuroimaging studies, focusing particularly on regions involved in visuospatial processing, such as medial temporal areas (Barton, 2011), may also be informative.
Acknowledgments
Role of funding source: Funding for this study was provided in part by NIDA Grant DA029141 to Drs. Pope, Kanayama, and Hudson.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author disclosures:
Conflict of interest: Dr. Pope has provided expert testimony in legal cases involving anabolic-androgenic steroids on five occasions in the last three years. Mr. Kean is employed by Lifeline Middlesbrough, a charitable organization that provides services to substance users, including anabolic-androgenic steroid users. Dr. Pope and Mr. Kean declare no other conflicts of interest. The other two authors declare that they have no conflicts of interest.
Contributors: All authors have materially participated to the research and manuscript preparation. Drs. Kanayama and Pope and Mr. Kean recruited and evaluated the study participants and performed the data collection. Drs. Kanayama and Pope drafted the manuscript. Dr. Hudson performed statistical analyses of the data and drafted the statistical portions of the manuscript. All four authors contributed to successive revisions of the entire manuscript and have approved the final manuscript.
REFERENCES
- Abu-Shakra S, Alhalabi MS, Nachtman FC, Schemidt RA, Brusilow WS. Anabolic steroids induce injury and apoptosis of differentiated skeletal muscle. J. Neurosci. Res. 1997;47:186–197. [PubMed] [Google Scholar]
- Baker JS, Graham MR, Davies B. Steroid and prescription medicine abuse in the health and fitness community: a regional study. Eur. J. Intern. Med. 2006;17:479–484. doi: 10.1016/j.ejim.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Barton JJ. Disorders of higher visual processing. Handb. Clin. Neurol. 2011;102:223–261. doi: 10.1016/B978-0-444-52903-9.00015-7. [DOI] [PubMed] [Google Scholar]
- Caraci F, Pistara V, Corsaro A, Tomasello F, Giuffrida ML, Sortino MA, Nicoletti F, Copani A. Neurotoxic properties of the anabolic androgenic steroids nandrolone and methandrostenolone in primary neuronal cultures. J. Neurosci. Res. 2011;89:592–600. doi: 10.1002/jnr.22578. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Deary IJ, Starr J, Whalley LJ. The NART as an index of prior intellectual functioning: a retrospective validity study covering a 66-year interval. Psychol. Med. 2001;31:451–458. doi: 10.1017/s0033291701003634. [DOI] [PubMed] [Google Scholar]
- Cunningham RL, Giuffrida A, Roberts JL. Androgens induce dopaminergic neurotoxicity via caspase-3-dependent activation of protein kinase Cdelta. Endocrinology. 2009;150:5539–5548. doi: 10.1210/en.2009-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ascenzo S, Millimaggi D, Di Massimo C, Saccani-Jotti G, Botre F, Carta G, Tozzi-Ciancarelli MG, Pavan A, Dolo V. Detrimental effects of anabolic steroids on human endothelial cells. Toxicol. Lett. 2007;169:129–136. doi: 10.1016/j.toxlet.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Duchaine D. The Original Underground Steroid Handbook. OEM Publishing; Santa Monica, CA: 1981. [Google Scholar]
- Ersche KD, Clark L, London M, Robbins TW, Sahakian BJ. Profile of executive and memory function associated with amphetamine and opiate dependence. Neuropsychopharmacology. 2006;31:1036–1047. doi: 10.1038/sj.npp.1300889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada M, Varshney A, Ehrlich BE. Elevated testosterone induces apoptosis in neuronal cells. J. Biol. Chem. 2006;281:25492–25501. doi: 10.1074/jbc.M603193200. [DOI] [PubMed] [Google Scholar]
- Fanton L, Belhani D, Vaillant F, Tabib A, Gomez L, Descotes J, Dehina L, Bui-Xuan B, Malicier D, Timour Q. Heart lesions associated with anabolic steroid abuse: comparison of post-mortem findings in athletes and norethandrolone-induced lesions in rabbits. Exp. Toxicol. Pathol. 2009;61:317–323. doi: 10.1016/j.etp.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Feise RJ. Do multiple outcome measures require p-value adjustment? BMC Med. Res. Methodol. 2002;2:8. doi: 10.1186/1471-2288-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT, Rush CR, . Drug Alcohol. Depend. 2002;66:265–273. doi: 10.1016/s0376-8716(01)00206-x. [DOI] [PubMed] [Google Scholar]
- Fishbein DH, Krupitsky E, Flannery BA, Langevin DJ, Bobashev G, Verbitskaya E, Augustine CB, Bolla KI, Zvartau E, Schech B, Egorova V, Bushara N, Tsoy M. Neurocognitive characterizations of Russian heroin addicts without a significant history of other drug use. Drug Alcohol Depend. 2007;90:25–38. doi: 10.1016/j.drugalcdep.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall RC, Hall RC, Chapman MJ. Psychiatric complications of anabolic steroid abuse. Psychosomatics. 2005;46:285–290. doi: 10.1176/appi.psy.46.4.285. [DOI] [PubMed] [Google Scholar]
- Hildebrandt T, Lai JK, Langenbucher JW, Schneider M, Yehuda R, Pfaff DW. The diagnostic dilemma of pathological appearance and performance enhancing drug use. Drug Alcohol Depend. 2011;114:1–11. doi: 10.1016/j.drugalcdep.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama G, Brower KJ, Wood RI, Hudson JI, Pope HG. Anabolic-androgenic steroid dependence: an emerging disorder. Addiction. 2009a;104:1966–1978. doi: 10.1111/j.1360-0443.2009.02734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama G, Hudson JI, Pope HG. Illicit anabolic-androgenic steroid use. Horm. Behav. 2010;58:111–121. doi: 10.1016/j.yhbeh.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama G, Hudson JI, Pope HG., Jr. Long-term psychiatric and medical consequences of anabolic-androgenic steroid abuse: a looming public health concern? Drug Alcohol Depend. 2008;98:1–12. doi: 10.1016/j.drugalcdep.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama G, Hudson JI, Pope HG., Jr. Features of men with anabolic-androgenic steroid dependence: A comparison with nondependent AAS users and with AAS nonusers. Drug Alcohol Depend. 2009b;102:130–137. doi: 10.1016/j.drugalcdep.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanayama G, Pope HG, Cohane G, Hudson JI. Risk factors for anabolic-androgenic steroid use among weightlifters: a case-control study. Drug Alcohol Depend. 2003;71:77–86. doi: 10.1016/s0376-8716(03)00069-3. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Muetzelfeldt L, Curran HV. Ketamine use, cognition and psychological wellbeing: a comparison of frequent, infrequent and ex-users with polydrug and non-using controls. Addiction. 2009;104:77–87. doi: 10.1111/j.1360-0443.2008.02394.x. [DOI] [PubMed] [Google Scholar]
- Pieretti S, Mastriota M, Tucci P, Battaglia G, Trabace L, Nicoletti F, Scaccianoce S. Brain nerve growth factor unbalance induced by anabolic androgenic steroids in rat. Med. Sci. Sports Exerc. 2012 doi: 10.1249/MSS.0b013e31826c60ea. ePub ahead of print. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr., Kanayama G, Hudson JI. Risk factors for illicit anabolic-androgenic steroid use in male weightlifters: a cross-sectional cohort study. Biol. Psychiatry. 2012;71:254–261. doi: 10.1016/j.biopsych.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope HG, Jr., Katz DL. Psychiatric and medical effects of anabolic-androgenic steroid use. A controlled study of 160 athletes. Arch. Gen. Psychiatry. 1994;51:375–382. doi: 10.1001/archpsyc.1994.03950050035004. [DOI] [PubMed] [Google Scholar]
- Pope HG, Katz DL. Psychiatric effects of exogenous anabolic-androgenic steroids. In: Wolkowitz OM, Rothschild AJ, editors. Psychoneuroendocrinology: The Scientific Basis of Clinical Practice. American Psychiatric Press; Washington, DC: 2003. pp. 331–358. [Google Scholar]
- Pope HG, Kean J, Nash A, Kanayama G, Samuel DB, Bickel WK, Hudson JI. A diagnostic interview module for anabolic-androgenic steroid dependence: preliminary evidence of reliability and validity. Exp. Clin. Psychopharmacol. 2010;18:203–210. doi: 10.1037/a0019370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riezzo I, De Carlo D, Neri M, Nieddu A, Turillazzi E, Fineschi V. Heart disease induced by AAS abuse, using experimental mice/rats models and the role of exercise-induced cardiotoxicity. Mini Rev. Med. Chem. 2011;11:409–424. doi: 10.2174/138955711795445862. [DOI] [PubMed] [Google Scholar]
- Robbins TW, James M, Owen AM, Sahakian BJ, Lawrence AD, McInnes L, Rabbitt PM. A study of performance on tests from the CANTAB battery sensitive to frontal lobe dysfunction in a large sample of normal volunteers: implications for theories of executive functioning and cognitive aging. Cambridge Neuropsychological Test Automated Battery. J. Int. Neuropsychol. Soc. 1998;4:474–490. doi: 10.1017/s1355617798455073. [DOI] [PubMed] [Google Scholar]
- Robbins TW, James M, Owen AM, Sahakian BJ, McInnes L, Rabbitt P. Cambridge Neuropsychological Test Automated Battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia. 1994;5:266–281. doi: 10.1159/000106735. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Semple J, Kumar R, Truman MI, Shorter J, Ferraro A, Fox B, McKay G, Matthews K. Effects of scopolamine on delayed-matching-to-sample and paired associates tests of visual memory and learning in human subjects: comparison with diazepam and implications for dementia. Psychopharmacology (Berl.) 1997;134:95–106. doi: 10.1007/s002130050430. [DOI] [PubMed] [Google Scholar]
- Rothman K, Greenland S. Modern Epidemiology. 2nd edition Lipppincott-Raven; Philadelphia: 1998. [Google Scholar]
- Sahakian BJ, Morris RG, Evenden JL, Heald A, Levy R, Philpot M, Robbins TW. A comparative study of visuospatial memory and learning in Alzheimer-type dementia and Parkinson’s disease. Brain. 1988;111:Pt–3. doi: 10.1093/brain/111.3.695. [DOI] [PubMed] [Google Scholar]
- Savitz DA, Olshan AF. Multiple comparisons and related issues in the interpretation of epidemiologic data. Am. J. Epidemiol. 1995;142:904–908. doi: 10.1093/oxfordjournals.aje.a117737. [DOI] [PubMed] [Google Scholar]
- Sjoqvist F, Garle M, Rane A. Use of doping agents, particularly anabolic steroids, in sports and society. Lancet. 2008;371:1872–1882. doi: 10.1016/S0140-6736(08)60801-6. [DOI] [PubMed] [Google Scholar]
- Skarberg K, Nyberg F, Engstrom I. Multisubstance use as a feature of addiction to anabolic-androgenic steroids. Eur. Addict. Res. 2009;15:99–106. doi: 10.1159/000199045. [DOI] [PubMed] [Google Scholar]
- Tan RS, Scally MC. Anabolic steroid-induced hypogonadism--towards a unified hypothesis of anabolic steroid action. Med. Hypotheses. 2009;72:723–728. doi: 10.1016/j.mehy.2008.12.042. [DOI] [PubMed] [Google Scholar]
- Wechsler D. The Measurement of Adult Intelligence. Williams and Wilkins; Baltimore: 1939. [Google Scholar]
- Willshire D, Kinsella G, Prior M. Estimating WAIS-R IQ from the National Adult Reading Test: a cross-validation. J. Clin. Exp. Neuropsychol. 1991;13:204–216. doi: 10.1080/01688639108401038. [DOI] [PubMed] [Google Scholar]
- Zaugg M, Jamali NZ, Lucchinetti E, Xu W, Alam M, Shafiq SA, Siddiqui MA. Anabolic-androgenic steroids induce apoptotic cell death in adult rat ventricular myocytes. J. Cell Physiol. 2001;187:90–95. doi: 10.1002/1097-4652(2001)9999:9999<00::AID-JCP1057>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]