Abstract
Graft-versus-host disease (GVHD) reflects an exaggerated inflammatory allogeneic T-cell response in hosts receiving allogeneic hematopoietic stem cell transplantation (HSCT). Inhibition of pan-Notch receptor signaling in donor T cells causes reduction of GVHD. However, which Notch ligand(s) in what antigen-presenting cells are important for priming GVH reaction remains unknown. We demonstrate that δ-like ligand-4 (Dll4) and Dll4-positive (Dll4hi) inflammatory dendritic cells (i-DCs) play important roles in eliciting allogeneic T-cell responses. Host-type Dll4hi i-DCs occurred in the spleen and intestine of HSCT mice during GVHD induction phase. These Dll4hi i-DCs were CD11c+B220+PDCA-1+, resembling plasmacytoid DCs (pDCs) of naïve mice. However, as compared to unstimulated pDCs, Dll4hi i-DCs expressed higher levels of costimulatory molecules, Notch ligands Jagged1 and Jagged2 and CD11b and, produced more Ifnb and Il23 but less Il12. In contrast, Dll4-negative (Dll4lo) i-DCs were CD11c+B220−PDCA-1−, and had low levels of Jagged1. In vitro assays showed that Dll4hi i-DCs induced significantly more IFN-γ- and IL-17-producing effector T cells (3- and 10-fold, respectively) than Dll4lo i-DCs. This effect could be blocked by anti-Dll4 antibody. In vivo administration of Dll4 antibody reduced donor alloreactive effector T cells producing IFN-γ and IL-17 in GVHD target organs, leading to reduction of GVHD and improved survival of mice after allogeneic HSCT. Our findings indicate that Dll4hi i-DCs represent a previously uncharacterized i-DC population distinctive from steady state DCs and Dll4lo i-DCs. Furthermore, Dll4 and Dll4hi i-DCs may be beneficial targets for modulating allogeneic T-cell responses, and could facilitate the discovery of human counterparts of mouse Dll4hi i-DCs.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is potentially a curative therapy for hematopoietic malignancies and non-malignant disorders.(1–8) However, graft-versus-host disease (GVHD) remains a major barrier to the success of allogeneic HSCT. GVHD is mediated by donor T cells that recognize and react to histocompatibility differences of the host. These alloreactive T cells and their recruited inflammatory cells attack epithelial organs such as intestine, liver and skin.(2–4) In both human patients and experimental mouse models, severe damage to the gastrointestinal (GI) tract is associated with the high mortality after allogeneic HSCT.(2, 3, 9, 10)
The Notch signaling pathway is important for cell-cell communication, which controls multiple cell processes, including proliferation, differentiation and survival.(11, 12) Notch receptors (Notch 1, 2, 3, and 4) interact with Notch ligands of the δ-like (Dll1, Dll3 and Dll4) and Jagged families (J1 and J2).(11–13) Binding of a Notch ligand to its receptor results in the cleavage of the receptor by γ-secretase complex and the subsequent release of intracellular Notch.(11–13) The intracellular Notch domain translocates to the nucleus where it activates the transcription of Notch target genes.(11–13) Using a genetic approach, we have recently shown that inhibition of pan-Notch receptor signaling in donor T cells markedly reduced GVHD severity and mortality in mouse models of allogeneic HSCT.(14) Donor T cells lacking Notch receptor signaling proliferated and expanded in response to alloantigens in vivo, but showed markedly reduced capability of producing inflammatory cytokines such as IFN-γ, IL-17 and TNF-α.(14) These Notch-deprived donor T cells were incapable to mediate GVHD, in particular of the damage to the GI tract.(14) However, pharmacological blockade of Notch receptors may lead to severe toxicity to the host.(15) Identifying which Notch ligand is critical for activation of the Notch signaling pathway to elicit allogeneic T-cell responses may have significant implications in modulation of GVHD.
Emerging evidence indicate that Notch ligands are important for antigen-presenting cells (APCs) to direct differentiation of distinct lineages of effector T cells.(16–18) For example, Dll4 is required for the development of CD4+ T helper (Th)1 cells(19–21) and Th17 cells,(21–23) whereas Dll1 regulates CD8+ cytotoxic T cell-mediated anti-tumor activity.(17, 24, 25) In contrast, the Jagged family has been found to mainly influence Th2 cell response.(18) Different inflammatory stimuli may stimulate APCs to express different types of Notch ligands.(16–18, 26–28) Toll-like receptor (TLR) ligands or TLR agonists stimulate APCs expression of both δ-like ligands and Jagged ligands, whereas other inflammatory stimuli such as helminthes, allergens and toxins may increase expression of Jagged ligands.(16–18, 26–28) These observations suggest that the expression of Notch ligands may differentiate the capability of APCs to direct effector differentiation of alloreactive T cells. However, which Notch ligands in what APCs play important roles in the regulation of allogeneic T-cell responses and GVHD remains unknown.
In the present study, we identified a previously uncharacterized population of inflammatory dendritic cells (i-DCs) that expressed high levels of Dll4. These Dll4-positive (Dll4hi) i-DCs were mainly located in the spleen and GI tract in mice early after allogeneic HSCT, and possessed a greater ability than Dll4-negative (Dll4lo) i-DCs to induce the generation of alloreactive effector T cells. This effect of Dll4hi i-DCs was abrogated by anti-Dll4 antibody (Ab). Importantly, in vivo treatment with Dll4 Ab markedly reduced the generation of alloreactive effector T cells producing IFN-γ and IL-17 in GVHD target organs, leading to inhibition of GVHD and significantly improved survival of mice after allogeneic HSCT. Our findings indicate that Dll4 and Dll4hi i-DCs are important for eliciting allogeneic T-cell responses and may be beneficial targets for modulating GVHD.
Materials and Methods
Mice
C57BL/6 (H-2b), BALB/c (H-2d), and B6×DBA/2 F1 (BDF1, H-2b/d) mice were purchased from Taconic (Rockville, Maryland). Experimental protocols were approved by the University of Michigan’s Committee on Use and Care of Animals.
Antibodies (Abs), flow cytometry analysis and cell lines
Neutralizing antibodies specific to mouse Dll1, Dll4, J1 and J2 were prepared as previously described.(29–32) All other antibodies used for immunofluorescence staining were purchased from eBioscience, BioLegend, or BD Biosciences. Microbead-conjugated Abs and streptavidin were purchased from Miltenyi-Biotech (Auburn, CA), and recombinant human IL-2 was from R&D Systems (Minneapolis, MN). Flow cytometry analyses were performed using FACScan and Canto cytometer (Becton Dickinson) as described.(33)
Cell preparations
T cell–depleted bone marrow (TCD-BM) was prepared by depleting T cells with microbead-conjugated anti-CD4/CD8 antibodies.(33) CD4+ and CD8+ lymphocytes were isolated from spleens and lymph nodes using microbead-conjugated antibodies (MiniMACS; Miltenyi Biotech). Purity was consistently > 92%. The preparation of lumina propria mononuclear cells was performed as previously described.(14)
Induction of GVHD
Mice underwent BMT as described.(33) The GVHD score and severity was assessed as described(34). GVHD severity was also assessed by histopathological analysis.(35) For the B6 anti-BALB/c mouse model, BALB/c recipients were irradiated using 800 rads from a 137Cs source. Donor B6 TCD-BM (5.0 × 106) with B6 CD4+ T cells (1.0 × 106) were transplanted into irradiated BALB/c recipients. In some experiments, we transplanted donor B6 TCD-BM (5.0 × 106) together with B6 CD4+ T cells (1.0 × 106) plus CD8+ T cells (1.0 × 106) to lethally irradiated (1200 rads) BDF1 recipients.
RT-PCR
Total RNA was extracted from the sorted donor CD4+ T cell subsets and DC subsets using TRIzol (Invitrogen Life Technologies). cDNA was quantified through the quantitative real-time polymerase chain reaction (PCR) technique. Real-time PCR was performed with a SYBR Green PCR mix on a Mastercycler realplex (Eppendorf). Thermocycler conditions included an initial holding at 95°C for 2 min; this was followed by a three-step PCR program, as follows: 95°C for 30s, 55°C for 30s, and 72°C for 30s for 40 cycles. Transcript abundance was calculated using the ΔΔCt method (normalization with GAPDH). The primer sequences are listed in Table 1.
Table 1.
Primers for Real-time RT-PCR.
| Gene name | Primer sequence | |
|---|---|---|
| Gapdh | Forward | 5’- AGGTCGGTGTGAACGGATTTG |
| Reverse | 5’- TGTAGACCATGTAGTTGAGGTCA | |
| Dll4 | Forward | 5’- AGGTGCCACTTCGGTTACACAG |
| Reverse | 5’- CAATCACACACTCGTTCCTCTCTTC | |
| Ifnb | Forward | 5’- TGGGTGGAATGAGACTATTGTT |
| Reverse | 5’- CTCCCACGTCAATCTTTCCTC | |
| IL12p35 | Forward | 5’- CCAGCACATTGAAGACCTGT |
| Reverse | 5’- CAGGGTCATCATCAAAGACG | |
| IL23 | Forward | 5’- GACAACAGCCAGTTCTGCTT |
| Reverse | 5’- AGGGAGGTGTGAAGTTGCTC | |
| Arginase 1 | Forward | 5’- CAAGACAGGGCTCCTTTCAG |
| Reverse | 5’- AAGCAAGCCAAGGTTAAAGC | |
| Id2 | Forward | 5’- ATGAAAGCCTTCAGTCCGGTG |
| Reverse | 5’- AGCAGACTCATCGGGTCGT | |
| Xcr1 | Forward | 5’- CCTACGTGAAACTCTAGCACTGG |
| Reverse | 5’- AAGGCTGTAGAGGACTCCATCTG | |
| Pou2f2 | Forward | 5’- GCTCAACGACGCAGAGACTA |
| Reverse | 5’- GTCTCGATGCTGGTCCTCTT | |
| Tcf4 | Forward | 5’- TTTGCCGTCTTCAGTCTACG |
| Reverse | 5’- GCATGAAGAAGGAGCTAGGG | |
| Runx2 | Forward | 5’- CCAAGAAGGCACAGACAGAA |
| Reverse | 5’- ATACTGGGATGAGGAATGCG | |
| Irf8 | Forward | 5’- TACAATCAGGAGGTGGATGC |
| Reverse | 5’- TTCAGAGCACAGCGTAACCT | |
| Flt3 | Forward | 5’- CCCTACTTTCCAGGCACATT |
| Reverse | 5’- CATTGAACCCTGAGAGCTGA | |
| Dtx1 | Forward | 5’- GACGCCCAGCTGGTGCCCTACATC |
| Reverse | 5’- CGCCCCGCCGTCGTTCTCC | |
Mixed lymphocyte reaction (MLR)
Donor (B6)-derived CD4+ T cells (1–2 × 105 cells/well) in 96-well U-bottom plates with complete medium were stimulated with or without DCs (1–4 × 104 cells/well) from BALB/c mice. Cells were cultured for four or five days to assess intracellular cytokines as described.(33, 36–38)
Statistical analysis
Survival in different groups was compared using the log-rank test. Comparison of 2 means was analyzed using the 2-tailed unpaired Student t test.
Results
Host DCs upregulate Notch ligands early during GVHD induction
To determine the role of Notch ligands in regulating allogeneic T cell responses, we examined the expression of Notch ligands on the surface of APCs after transplantation. B6 TCD-BM plus CD4+ T cells were injected into lethally irradiated BALB/c mice to induce GVHD. As expected, GVHD occurred in these allogeneic recipients, with all of them dying of the disease between days 7 and 35 after transplantation (Fig. 1A). Given the importance of host APCs in eliciting GVH reaction,(9, 35, 37, 39–44) we first assessed the expression of Notch ligands on host CD11c+ DCs. On days 1 and 3 after transplantation CD11c+ cells were all of host origin (Fig.1B). By 7 days after transplantation, host CD11c+ cells were reduced about 20-fold in the spleen of these allogeneic HSCT mice compared to day 1 (Fig. 1B), which coincides with previous studies.(37, 45, 46) Notch ligand Dll4, J1 and J2 were dramatically upregulated on the surface of host CD11c+ DCs from the spleen of allo-HSCT recipients by 3 days after transplantation and declined by 7 days (Fig. 1C,D). Interestingly, there were only few host CD11c+ DCs expressing low levels of Dll1 (Fig. 1C,D), although Dll1 has been implicated in other types of antigen-driven T cell responses.(17, 25) These host CD11c+ DCs expressed high levels of MHC class II molecule Ia and costimulatory molecules CD80 and CD86 (Fig. 1E), resembling the phenotype of i-DCs.(47–50) Donor-derived CD11c+ cells did not occur by 7 days after transplantation (Fig.1B). They expressed low levels of Dll4, J1 and moderate levels of J2 (Fig. 1F). These results suggest that host DCs upregulate the expression of Dll4, J1 and J2 during early phase of GVHD induction.
Fig.1. Notch ligands are up-regulated on the surface of CD11c+ DCs in the recipient mice early during GVHD induction.
Lethally irradiated (8Gy) BALB/c mice were injected with B6 TCD-BM (5.0×106) mixed with or without CD4 T cells (1.0×106). Cells were isolated from the spleens of these recipients at various time points after transplantation. (A) Survival of animals was monitored over time. Data shown here are pooled from three independent experiments. (B) Dot plots and graphs show the percentage and number of host (H2-Kd+) or donor (H2-Kd−) origin CD11c+ cells (mean ± SD, n=6 to 8 mice per group). (C) Histograms show the expression of Notch ligands on the surface of host CD11c+ cells which were recovered from the spleens of normal BALB/c mice and allogeneic HSCT BALB/c mice at the time point as indicated. Representative histograms from three independent experiments are shown. (D) Graphs show the percentage and mean fluorescent intensity (MFI) of Notch ligand expression on the surface of host CD11c+ cells (mean ± SD, n=6 to 8 mice per group). (E) Histograms show the expression of tested markers on the surface of host CD11c+ cells. Representative histograms from three independent experiments are shown. (F) Histograms show the expression of Notch ligands on the surface of donor CD11c+ cells that were recovered from the spleens of BALB/c recipients 7 days after HSCT. Representative histograms from three independent experiments are shown. *: P<0.05, **: p<0.01.
Dll4 derived from host type DCs promotes production of IFN-γ and TNF-α in alloantigen-activated CD4+ T cells
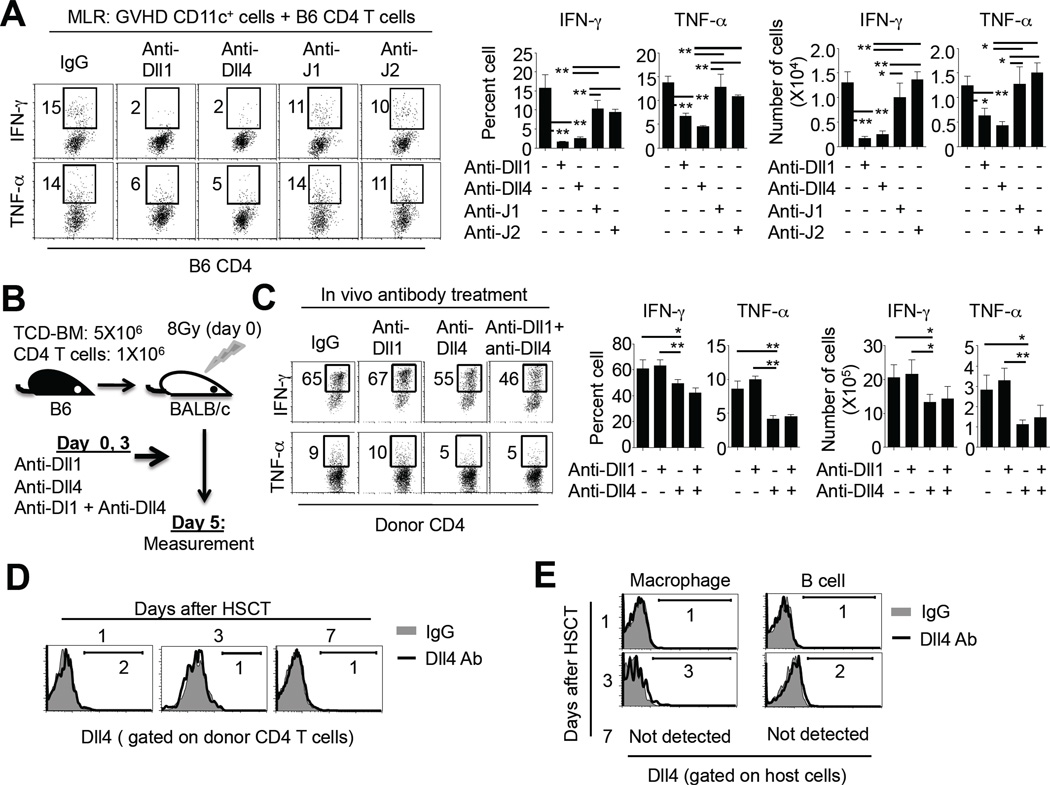
We next used in vitro MLR assays to examine if Notch ligands expressed by DCs were important for effector differentiation of alloantigen-activated T cells. CD11c+ DCs were isolated from BALB/c mice receiving HSCT 3 days after transplantation and cultured ex vivo with normal B6 mouse-derived CD4+ T cells, with or without addition of Ab specific to individual Notch ligand. Blocking Dll1 and Dll4 led to a significant reduction of effector T cells producing IFN-γ and TNF-α compared to control IgG (Fig. 2A). Inhibition of either J1 or J2 had less effect on production of IFN-γ and TNF-α in alloantigen-activated T cells compared to blockade of either Dll1 or Dll4 (Fig. 2A). These data suggest that Dll1 and Dll4 may play important roles in regulating the generation of alloreactive effector T cells.
Fig.2. The effect of each Notch ligand on cytokine production by donor T cells activated by allogeneic DCs.
Lethally irradiated (8Gy) BALB/c mice (n=12) were injected with B6 TCD-BM (5.0×106) mixed with CD4+ T cells (1.0×106). CD11c+ DCs were isolated from these recipients 3 days after HSCT and cultured ex vivo with donor CD4+ T cells (2.0×105) derived from normal B6 mice (DC and CD4 T cell ratio was 1 : 5). Cells were plated in the U-bottom of 96-well plates. Neutralizing Ab (20 µg/ml) specific to Notch ligand Dll1, Dll4, J1 and J2 were individually added into the culture. Five days later, cells were collected to measure their production of IFN-γ and TNF-α. (A) Dot plots (left panel) and graphs (right panel) show the percentage and number of cytokine-producing cells. Data show mean ± SD. Representative results from two independent experiments are shown. (B–E) Lethally irradiated (8Gy) BALB/c mice were transplanted with B6 TCD-BM (5.0×106) with CD4+ T cells (1.0×106), followed by treatment with anti-Dll1Ab (250 µg/mouse/injection), anti-Dll4 Ab (250 µg/mouse/injection), and anti-Dll1 Ab + anti-Dll4 Ab, respectively, at day 0 and 3 after transplantation (3 mice per group). At day 5, cells were recovered from spleen to measure cytokine production by donor CD4 T cells (B). Dot plots (left panel) and graphs (right panel) show the percentage of cytokine-producing cells. Data show mean ± SD. Representative results from two independent experiments are shown (C). Cells were isolated from the spleen of HSCT recipients without receiving anti-Dll4 Ab 1, 3, and 7 days after transplantation. Histograms show the expression of Dll4 on the surface of donor CD4+ T cells at the time points as indicated (D). Histograms show the expression of Dll4 on the surface of host-type CD11b+CD11c− macrophages and B220+CD11c− B cells at the indicated time points (E). Representative results from two independent experiments are shown. *: P<0.05, **: p<0.01.
To ask if blocking Dll1 and/or Dll4 could reduce production of IFN-γ and TNF-α by CD4+ T cells activated in vivo, we transplanted B6 donor T cells with TCD-BM into lethally irradiated BALB/c mice and administered two doses of anti-Dll1 Ab, anti-Dll4 Ab and anti-Dll1 Ab + anti-Dll4 Ab at day 0 and 3 after transplantation (Fig. 2B). Donor T cells were recovered at day 5 after transplantation from the spleens of these recipients. In vivo blockade of Dll4 resulted in more profound reduction of alloreactive effector T cells producing IFN-γ and TNF-α than blocking Dll1 (Fig. 2C). To rule out the possibility that reduction of alloreactive effector T cells might result from the binding of anti-Dll4 Ab to activated T cells, we examined the expression of Dll4 in donor T cells. As shown in Fig. 2D, donor CD4+ T cells derived from GVHD mice did not express Dll4 protein on their surface, which is in agreement with previous observations.(28, 51) Further assessment showed that neither host-type B cells nor macrophages expressed Dll4 (Fig. 2E). Thus, it is likely that i-DCs expressing Dll4 could be important for promoting the generation of alloreactive effector CD4+ T cells.
DCs expressing high levels of Dll4 represent a unique i-DC subset distinct from Dll4lo i-DCs and steady state DCs
DCs are heterogeneous cell populations.(52, 53) In naïve mice under steady state conditions, two major types of DCs have been defined based on their surface phenotype, anatomical location and function, including plasmacytoid DCs (pDCs, CD11c+PDCA-1+B220+) and conventional DCs (cDCs, CD11c+PDCA-1−B220−).(48, 53) Under inflammatory conditions, i-DCs occur and have properties different from steady state DCs, as evidenced by profound phenotypic changes, enhanced antigen-presenting capacity and altered migration capability.(47–50, 54) However, whether distinct i-DC subsets may have differential effects on inducing alloreactive effector T cells was not well defined.
To examine if Dll4 could be useful for identifying distinct i-DC subsets that have differential capacities to prime allogeneic T-cell responses, we isolated cells from the spleens of GVHD BALB/c mice 3 days after transplantation. As shown in Fig. 3A, about 64% of Dll4hi CD11c+ DCs were PDCA-1+B220+ cells, suggesting that they resemble pDCs.(53) When gating on CD11c+PDCA-1+B220+ cells, we noted that approximately 85% of them expressed high levels of Dll4 (Fig. 3B). However, as compared to steady state pDCs derived from naïve mice (Fig. 3B,C), GVHD mouse-derived Dll4hi CD11c+PDCA-1+B220+ cells expressed higher levels of Ia, CD40, CD80 and CD86, a typical phenotype of activated DCs.(48, 49, 54) Intriguingly, these Dll4hi DCs expressed CD11b (Fig. 3B), which is normally not expressed on the surface of steady state pDCs (Fig. 3C).(53) Furthermore, Dll4hi i-DCs did not express Siglec-H (Fig.3B) and had higher levels of J1 and J2 compared to pDCs (Fig. 3D). Thus, Dll4hi i-DCs resemble but are distinct from pDCs. In addition, Dll4hi i-DCs did not express other lineage markers such as NK1.1, CD19 and surface IgM (data not shown).
Fig.3. Characterization of host DCs expressing Dll4.
Lethally irradiated (8Gy) BALB/c mice were injected with B6 TCD-BM (5.0×106) mixed with CD4 T cells (1.0×106). Cells were recovered from the spleens of allogeneic HSCT BALB/c mice 3 days after HSCT to assess their biological properties. (A, B) Histograms and plots showed the expression of indicated markers on the surface of host DCs. Representative results from four independent experiments are shown. (C) Histograms showed the expression of indicated markers on the surface of DCs isolated from the spleen of normal BALB/c mice. Representative histograms from three independent experiments are shown. (D) Histograms showed the expression of Notch ligands on the surface of DCs isolated from the spleen of allogeneic HSCT recipient mice and normal BALB/c mice. Representative histograms from two independent experiments are shown.
In contrast, approximately 56% of Dll4lo CD11c+ cells derived from GVHD mice were PDCA-1−B220− cells (Fig. 3A), resembling cDCs from naïve mice.(47, 48, 50, 53, 55) When gating on these CD11c+PDCA-1−B220− cDC-like cells derived from GVHD mice, we found that while they had higher levels of costimulatory molecules (CD40, CD80 and CD86) than steady state cDCs (Fig. 3B,C), only less than 10% of them expressed low levels of Dll4 (Fig. 3B). This suggests that these CD11c+PDCA-1−B220− DCs are activated i-DCs and distinct from steady state cDCs. As compared to steady state cDCs, Dll4lo i-DCs also upregulated the expression of J2 (Fig. 3D). Collectively, these data indicate that Dll4hi i-DCs and Dll4lo i-DCs are distinct entities.
Dll4hi i-DCs have greater ability than Dll4lo i-DCs to promote the development of effector T cells producing IFN-γ and IL-17
To further define the biological properties of these Dll4hi i-DCs and Dll4lo i-DCs, we purified these two DC subsets from GVHD mice based on their characteristic phenotype of CD11c+PDCA-1+B220+ and CD11c+PDCA-1−B220−, respectively (Fig.4A). This allowed us to evaluate the functional activity of Dll4 in these i-DC subsets without using neutralizing anti-Dll4 Ab during the process. Morphological examination showed Dll4hi i-DCs showed irregular shape and contained more cytoplasmic vacuoles compared to pDCs and cDCs (Fig. 4B). Unlike cDCs and Dll4lo i-DCs, Dll4hi i-DCs did not display long processes on the cell periphery (Fig. 4B). Real-time RT-PCR analysis showed that Dll4hi i-DCs expressed higher levels of Dll4, Ifnb and Il23 but lower levels Il12 than Dll4lo i-DCs (Fig. 4C). In addition, compared to pDCs, Dll4hi i-DCs produced more Dll4, Ifnb and Il23 but less Il12 and Arginase 1 (Arg1) (Fig. 4C), further confirming that Dll4hi i-DCs are distinct from Dll4lo i-DCs and those pDCs and cDCs at steady state conditions.
Fig.4. Morphology, gene expression and function of host Dll4hi i-DCs.
Lethally irradiated (8Gy) BALB/c mice (n=20) were injected with B6 TCD-BM (5.0×106) mixed with CD4 T cells (1.0×106). Cells were recovered from the spleens of allogeneic HSCT BALB/c mice 3 days after transplantation. Cells purified from the spleens of normal BALB/c mice were also accessed as controls. (A) Dll4hi i-DCs and Dll4lo i-DCs were highly purified by using FACS Sorter. Histogram and dot plots show representative results of DC purification from the spleen of 20 mice receiving allogeneic HSCT. Approximately 0.5 to 0.7 × 105 cells of each DC subset were acquired. (B) Pictures show the morphology of FACS sorted subsets of DCs after Wright-Giemsa staining. Cells were photographed with an OlympusBX41 microscope (10/0.3 NA lens, 400× magnification, digital DP70 camera). (C) Graphs show the relative expression of indicated genes in each DC subset (mean ± SD). Results shown are representative of three independent experiments. (D, E) DC subsets purified from the allogeneic recipient mice were cultured ex vivo with donor CD4+ T cells (1.0 × 105) derived from normal B6 mice in the presence of IL-2 (DC and CD4+ T cell ratio was 1:10). Neutralizing anti-Dll4 Ab (20 µg/ml) was added into the culture. Four days later, cells were collected to measure their production of IFN-γ and IL-17. Data show mean ± SD of the percentage and number of cytokine producing cells. Results shown are representative of two independent experiments.*: P<0.05, **: p<0.01.
We then used MLR assays to examine the difference in function between Dll4hi i-DCs and Dll4lo i-DCs in vitro. Highly purified Dll4hi i-DCs and Dll4lo i-DCs were added to cultures containing B6 CD4+ T cells, respectively, with or without addition of neutralizing anti-Dll4 Ab (Fig. 4D,E). Dll4hi i-DCs induced approximately 3-fold and 10-fold more IFN-γ- and IL-17-producing T cells, respectively, than Dll4lo i-DCs (Fig. 4D,E). Addition of anti-Dll4 Ab abrogated the ability of Dll4hi i-DCs to promote effector differentiation, but had little effect on cytokine production in T cells stimulated by Dll4lo i-DCs (Fig. 4D,E). IL-2 was added to the culture to enhance the proliferation and differentiation of alloantigen-activated T cells.(36) However, IL-2 alone did not induce production of high levels of IFN-γ and IL-17 by donor T cells cultured in the absence of allogeneic DCs (Fig. 4D,E). Thus, Dll4hi i-DCs may represent a unique i-DC subset and have greater capability than Dll4lo i-DCs to promote the development of Th1 and Th17 cells.
Time kinetics in generation of Dll4hi i-DCs and their transcriptional signature
We next determined the time kinetics in generation of Dll4hi i-DCs and Dll4lo i-DCs during GVH response by assessing the occurrence of Dll4hiCD11c+PDCA-1+B220+ and Dll4loCD11c+PDCA-1−B220− cells. As shown in Fig. 5A, host-type Dll4lo i-DCs were a dominant DC population in the spleen of these GVHD recipients one day after transplantation and markedly declined 3 days after transplantation. This is in agreement with previous observations that host DCs may finally diminish during the GVHD process.(37, 38, 45, 46) In contrast, host origin Dll4hi i-DCs occurred by day 3 after transplantation and declined by day 7 (Fig. 5A). Interestingly, mice receiving TCD-BM plus donor T cells had approximately 3-fold more number of Dll4hi i-DCs than those mice receiving TCD-BM alone at day 3 after transplantation (Fig. 5B). However, there was no difference in expression of Dll4 on the surface of i-DCs between these two groups (Fig. 5C). These results suggest that infusion of donor T cells is not necessary to the generation of Dll4hi i-DCs in mice receiving lethal irradiation and transfer of TCD-BM, but can enhance the production of Dll4hi i-DCs.
Fig.5. Time kinetics in generation of Dll4hi i-DCs and their transcriptional signature.
(A) Lethally irradiated (8Gy) BALB/c mice were injected with B6 TCD-BM (5.0×106) mixed with CD4+ T cells (1.0×106). Graphs show the number of host Dll4hi and Dll4lo i-DCs in the spleen of BALB/c recipients at different time points after transplantation. Data show mean ± SD (3 mice per group). Results shown are representative of three independent experiments. (B, C) Lethally irradiated (8Gy) BALB/c mice were injected with B6 TCD-BM (5.0×106) mixed with or without CD4+ T cells (1.0×106). Cells were recovered from the spleen of allogeneic BALB/c recipients 3 days after transplantation. Graphs show the number of host Dll4hi i-DCs. Data show mean ± SD (3 mice per group) *: P<0.05 (B). Histograms showed the expression of Dll4 on the surface of host CD11c+ B220+ PDCA1+ DCs. Results shown are representative of two independent experiments. (D) Graphs show the relative expression of indicated genes in each DC subset (mean ± SD). Results shown are representative of two independent experiments.
The delayed occurrence of Dll4hi i-DCs relative to Dll4lo i-DCs during GVHD induction suggests that they could be de novo generated from DC precursors. To assess the relationship in developmental origin between Dll4hi i-DCs and other DC subsets, we highly purified Dll4hi i-DCs from GVHD mice to compare their transcriptional signature to different DC subsets as described by The Immunological Genome Consotium.(56) As shown in Fig. 5D, Dll4hi i-DCs expressed a transcriptional signature that differed from pDCs, as evidenced by expressing drastically lower levels of Tcf4, Runx2 and Irf8. Id2 and Xcr1, which are two signature genes of CD8+ cDCs, were also absent in Dll4hi i-DCs. In addition, as compared to CD8− cDCs, Dll4hi i-DCs expressed lower levels of Id2 and Flt3.(56) These data suggest that Dll4hi i-DCs express a transcriptional signature distinct from each of these tested DC subsets. Further studies are required to determine the developmental origin of Dll4hi i-DCs.
Spleen and GI tract contain more Dll4hi i-DCs than other tested tissues
Data from our studies and others suggest that tissue resident APCs play important roles in mediating GVHD.(10, 38, 41, 57) To understand the potential effect of Dll4hi i-DCs on GVH reaction, we examined their tissue distribution during the GVHD process. Mononuclear cells were isolated from various tissues of lethally irradiated BALB/c mice 3 days after receiving B6 TCD-BM and CD4+ T cells. Both the spleen and intestine contained more Dll4hi i-DCs than other GVHD target organs such as the mesenteric lymph node (MLN), liver, BM and lung (Fig. 6A). Like spleen Dll4hi i-DCs (Fig. 3B), intestinal Dll4hi i-DCs expressed high levels of Dll4, Ia, CD80, CD86 and CD11b (Fig. 6B,C). We could not recover Peyer’s patch from these recipients due to lethal irradiation-mediated severe lymphopenia 3 days after transplantation.
Fig.6. Tissue distribution of Dll4hi i-DCs in allogeneic recipient mice.
Lethally irradiated (8Gy) BALB/c mice (n=4) were injected with B6 TCD-BM (5.0×106) mixed with CD4 T cells (1.0×106). Cells were recovered from GVHD target organs 3 days after transplantation. (A) Graphs show the number of host Dll4hi i-DCs in different organs. Data show mean ± SD of cell number. Results shown are representative of two independent experiments. (B, C) Histograms and plots show the expression of Dll4 and other makers on the surface of host CD11c+ B220+ PDCA1+ i-DCs isolated from intestinal lamina propria. Representative histograms of three independent experiments are shown.
In vivo blocking Dll4 reduces the production of alloreactive effector T cells and GVHD
To test the impact of blocking Dll4 on alloreactive T-cell response in vivo, we transplanted B6 donor CD4+ T cells with TCD-BM into lethally irradiated BALB/c mice. Anti-Dll4 Ab was given to these recipients at day 0, 2 and 4 after transplantation. Donor T cells were recovered 5 days after transplantation (Fig. 7A). We observed that in vivo blockade of Dll4 resulted in a marked reduction of donor effector T cells producing high levels of IFN-γ and IL-17 in the spleen and intestine (Fig. 7B,C). Anti-Dll4 treatment reduced the expression of the Notch target gene Dtx1 in donor T cells (Fig. 7D), but had no effect on the recovery of Dll4hi i-DCs in the spleen compared to IgG control (0.98 ± 0.2 ×104 versus 0.83 ± 0.1×104, respectively). This is in agreement with previous studies showing that in vivo administration of antibody to any single Notch ligand could not deplete DCs.(58) These results suggest that Dll4 may play an important role in the regulation of alloreactive effector T cells in GVHD target organs.
Fig.7. In vivo blocking of Dll4 reduces effecter differentiation of alloreactive CD4+ T cells.
(A) Lethally irradiated (8Gy) BALB/c mice were transplanted with B6 TCD-BM (5.0×106) with CD4+ T cells (1.0×106), followed treatment with or without anti-Dll4 Ab (250 µg/mouse/injection) at day 0, 2 and 4 after transplantation (3 mice per group). Control IgG was given as control. At day 5, cells were recovered from the spleen and intestine lamina propria to assess cytokine production and gene expression. (B,C) Dot plots and graphs show the percentage and number of donor CD4+ T cells producing IFN-γ and IL-17 in the spleen and intestine. Data show mean ± SD. (D) Graphs show relative expression of Notch target gene Dtx1 in donor CD4+ T cells (mean ± SD). Results shown are representative of two independent experiments. *: P<0.05, **: p<0.01.
We next asked if blockade of Dll4 could prevent production of GVHD in BALB/c mice receiving donor B6 CD4 T cells. Nine doses of anti-Dll4 Ab were administered to these recipients once every three days from day 0 to day 24 after transplantation. In vivo administration of Dll4 Ab significantly attenuated GVHD in mice receiving high dose of donor CD4+ T cells, with markedly prolonged survival and reduced clinical signs of GVHD (Fig. 8A,B). Histology examination showed markedly reduced inflammation in the intestine of these recipients treated by anti-Dll4 Ab (Fig. 8C,D). This decrease of donor effector T cells was accompanied with significant reduction of serum IFN-γ at day 7 after HSCT (Fig. 8E). Interestingly, administration of 6 doses of anti-Dll4 Ab from day 0 to day 10 after transplantation effectively reduced GVHD in these BALB/c recipients (Fig. 8F,G). These data suggest that a short-term blockade of Dll4 during early phase of GVHD is sufficient to reduce the disease, which coincides with the occurrence of Dll4hi i-DCs during this period of GVH reaction.
Fig.8. In vivo administration of anti-Dll4 reduces GVHD in MHC mismatched recipient mice.
Lethally irradiated (8Gy) BALB/c mice were injected with B6 TCD-BM (5.0×106) mixed with CD4+ T cells (1.0×106). (A,B) Nine doses of anti-Dll4 antibody (250 µg/mouse) were given to BALB/c recipients once every three days from day 0 to day 24 after HSCT. BALB/c recipients treated with anti-Hamster IgG were used as controls. Survival and GVHD clinical score of the recipients were monitored over time. (C) Representative images show the tissues from one of 6 recipients in each group at day 7 after transplantation. Images were obtained with an OlympusBX41 microscope (10/0.3 NA lens, 200× magnification, digital DP70 camera). (D) Pathological scores of GVHD 7 days after HSCT (6 mice per group). (E) ELISA assays show the serum IFNγ in allogeneic BALB/c recipients (4 mice for each group) 7 days after HSCT. Data show mean ± SD. (F,G) Six doses of anti-Dll4 antibody (250 µg/mouse) were administered once every other day from day 0 to day 10 after HSCT. BALB/c recipients treated with anti-Hamster IgG were used as controls. Survival and GVHD clinical score of the recipients were monitored over time.*: P<0.05, **: p<0.01.
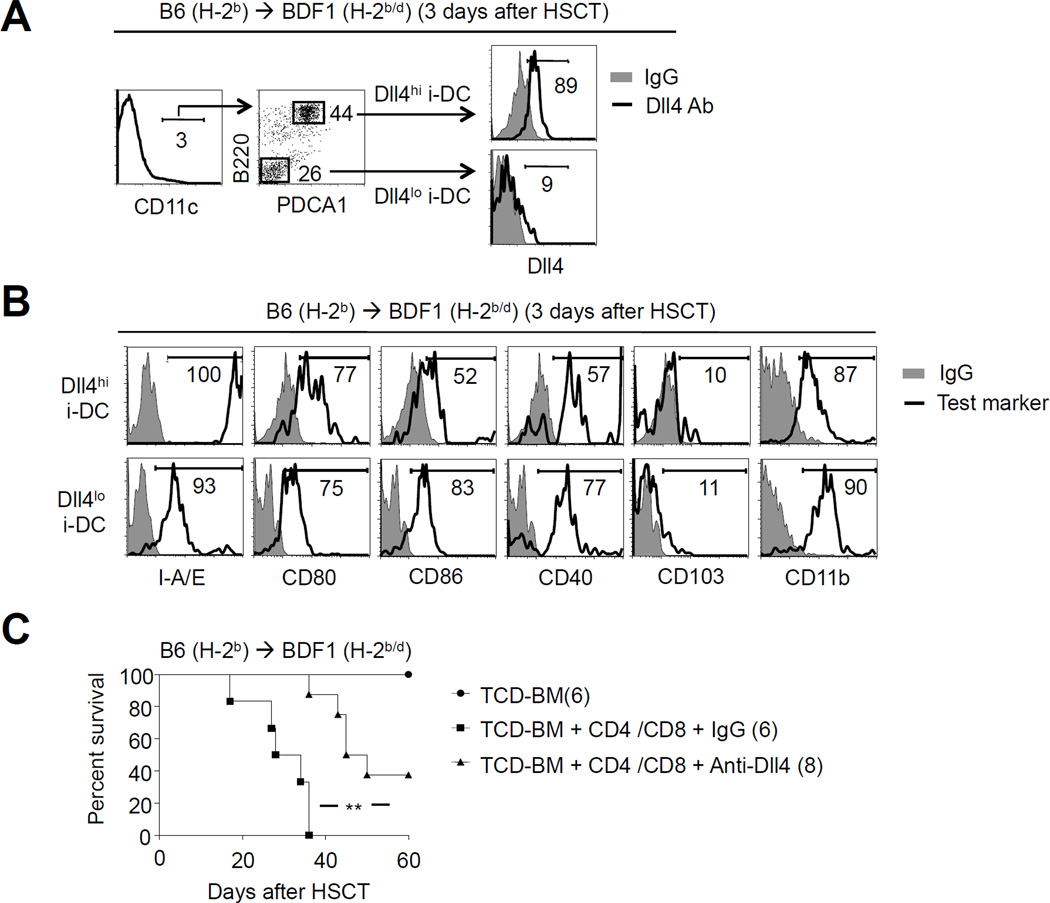
Dll4hi i-DCs occur in a second mouse model of GVHD
To rule out the possibility that Dll4hi i-DCs might be model-specific, we finally tested if they could be detected in a different mouse model. TCD-BM and T cells derived from B6 mice were transplanted into lethally irradiated BDF1 mice (Fig. 9). We found a similar population of host Dll4hi i-DCs in these BDF1 recipients (Fig. 9A,B). Notably, short-term treatment with anti-Dll4 Ab reduced GVHD in irradiated BDF1 mice receiving both CD4+ and CD8+ T cells (Fig. 9C). These data indicate that induction of Dll4+ i-DCs can be generalized to other mouse models.
Fig.9. Identification of Dll4hi i-DCs in second mouse model of GVHD.
Lethally irradiated (12Gy) BDF1 mice were injected with B6 TCD-BM (5.0×106) mixed with B6 CD4+ T cells (1.0×106) and CD8+ T cells (1.0×106). (A, B) Cells were recovered from the spleens of allogeneic HSCT BDF1 recipients 3 days after transplantation. Dot plots and histograms show the expression of Dll4 and other markers on the surface of different DC subsets. Results shown are representative of three independent experiments. (C) Six doses of anti-Dll4 antibody (250 µg/mouse) were administered once every other day from day 0 to day 10 after transplantation. Control recipients were treated with anti-Hamster IgG. Survival of animals was monitored over time for BDF1 mice. *: P<0.05, **: p<0.01.
Discussion
The regulation of GVHD pathogenesis requires the generation of alloreactive effector T cells capable of mediating host tissue injury.(1–3) In the present study, we provide evidence for a function of Dll4 and Dll4hi i-DCs in eliciting allogeneic T-cell responses. Upon preparative conditioning for allogeneic HSCT, Notch ligands Dll4, J1 and J2 were markedly upregulated on the surface of host origin i-DCs. Importantly, based on the expression of Dll4, i-DCs could be divided into two subsets: Dll4hi i-DCs and Dll4lo i-DCs. These cells had different entities in the context of their surface phenotype, expression of cytokine transcripts and capability to promote the production of alloreactive effector T cells. As compared to Dll4lo i-DCs, Dll4hi i-DCs had greater ability to stimulate the generation of alloreactive effector T cells producing IFN-γ and IL-17. Neutralizing Dll4 abrogated this effect of Dll4hi i-DCs in vitro. In vivo administration of anti-Dll4 Ab caused a marked reduction of alloreactive effector T cells in GVHD target organs, leading to reduction of GVHD and significantly improved survival of mice after allogeneic HSCT. Our findings indicate that Dll4hi i-DCs and Dll4 could be beneficial targets for improving the efficacy of allogeneic HSCT.
i-DCs occur as a result of inflammation and may have altered phenotype and function that are not normally present in steady state DCs.(47, 48, 50, 59) This includes the upregulation of antigen-presenting molecules and costimulatory molecules.(47, 48, 50, 53) Prior studies suggested that i-DCs can be derived from monocytes transferred into mice with vigorous inflammation, but not in clean and non-irradiated mice.(55, 60) Some other studies suggested that upon infection and activation by inflammatory stimuli, BM derived pDCs differentiated into conventional i-DC-like cells and acquired potent ability to prime naive T cells.(49, 54, 59) We found that host origin Dll4hi i-DCs occurred in the spleen and GI tract by day 3 after preparative irradiation and HSCT. As compared to pDCs and cDCs, Dll4hi i-DCs increased expression of Ia, CD80 and CD86, showed higher levels of Notch ligands Dll4, J1 and J2 on their surface, and produced more abundant IFN-β and IL-23 transcripts. Thus, Dll4hi i-DCs may represent a unique and previously uncharacterized inflammatory APC subset.
Delineating the developmental origin of Dll4hi i-DCs will be important for better understanding their function. Our studies and others have shown that a large proportion of host-type pDCs and cDCs were depleted within 24 hours following HSCT.(37, 38, 45, 46) In contrast, host origin Dll4hi i-DCs were detected as later as three days after transplantation. Thus, it is possible that Dll4hi i-DCs could be de novo generated during GVHD induction. To understand the relationship in development between Dll4hi i-DCs and other DC subsets, we performed PCR-based gene profiling analysis. We found that Dll4hi i-DCs expressed a transcriptional signature distinct from pDCs, CD8+ cDCs and CD8− cDCs.(56) However, since immature DCs may alter their phenotype and gene expression upon inflammatory stimulation,(48, 50) we do not rule out the possibility that Dll4hi i-DCs could be derived from those immature DCs with altered phenotype during GVHD induction. Further studies are needed to determine whether and how Dll4 may be upregulated in immature DCs and how Dll4hi i-DCs are induced in vivo during the GVHD process.
Prior studies have shown that Dll4 may play multiple roles in regulating T-cell responses during inflammation. Dll4 may directly regulate the production of effector T cells during infection and autoimmune responses.(16, 17) Activation of CD4+ T cells with Dll4 enhanced the generation of Th1 cells in cultures, whereas competitively reducing the binding of endogenous Dll4 to Notch receptors by soluble Dll4 led to a decrease in IFN-γ production by activated CD4+ T cells in vivo.(19) Dll4 directed the development of Th1 cell differentiation in the absence of IL-12.(19) Recent studies suggested that Dll4 promoted CD4+ T cells to secrete IL-17 via a mechanism of upregulating Rorc and Il17 transcripts.(21–23) On the other hand, Dll4 may influence inflammatory T-cell response via modulating chemokine receptor expression in activated T cells during experimental autoimmune encephalomyelitis, thereby regulating migration of effector T cells.(28) Thus, although our in vitro MLR assays clearly showed that blocking Dll4 led to a marked reduction of alloreactive effector T cells producing IFN-γ and IL-17, we don’t not rule out the possibility that in vivo blocking Dll4 could impair the migration of alloreactive effector T cells and consequently reduced GVH reaction.
Tissue resident APCs play critical roles in inducing organ-specific GVHD.(9, 10, 38, 41, 57) Depletion of APCs in the spleen and liver reduced GVHD in the liver but had no effect on GVHD in skin.(38) Other studies suggest that deletion of Langerhan’s cells in skin caused inhibition of cutaneous GVHD.(41) We observed that Dll4hi i-DCs represented a majority population of CD11c+ cells in the intestine 3 days after allogeneic HSCT. In vivo administration of anti-Dll4 Ab reduced GVH reactions in the GI tract, coincident with our recent observations that donor T cells lacking Notch signaling failed to mediate intestinal GVHD.(14) We proposed that Dll4 and intestinal Dll4hi i-DCs could play important roles in mediating GVH reaction in these tissues. However, some studies suggested that Dll4 was expressed in inflammatory macrophages and endothelial cells in other models.(12, 17, 28, 61, 62) The impact of Dll4 blockade on T-cell responses in vivo should consider the potential contribution of Dll4-expressing non-hematopoietic APCs (such as endothelial cells and epithelial cells).
One major arguable point in the field of allogeneic HSCT is whether hematopoietic-derived APCs are essentially required for CD4 T cell-mediated GVHD. Some studies revealed that chimeric mice, where host hematopoietic APCs lacking MHC class II (therefore incapable of priming donor T cells), developed severe GVHD,(9) suggesting the importance of nonhematopoietic APCs in GVHD induction. In contrast, many other studies using chimeric mice wherein host nonhematopoietic APCs lack MHC class II revealed that hematopoietic-derived APCs were sufficient to cause GVHD mediated by CD4 T cells.(35, 40–42, 63) Although how to reconcile these observations remains controversial, data from previous studies indicate that the function of APCs in regulating allogeneic T cell responses in vitro may closely reflect their ability to mediate GVHD.(43, 44, 64–66) For example, deletion of immature pDCs that had suppressive effect on T cells in vitro induced amelioration of GVHD.(66) Adoptive transfer of regulatory DCs capable of repressing allogeneic T cell responses in vitro caused GVHD inhibition.(64, 65) In contrast, adoptive transfer of DCs, which showed potent ability to stimulate allogeneic T cell responses, resulted in lethal GVHD in MHC class II-deficient mice wherein recipient APCs cannot elicit GVHD.(43, 44) We found that Dll4hi i-DCs had potent effect on stimulating the generation of alloreactive effector T cells producing high levels of effector cytokines (e.g., TNF-α, IFN-γ and IL-17) essential for mediating GVHD. This effect could be abrogated by neutralizing Dll4. Furthermore, in vivo blockade of Dll4 reduced GVHD, leading to significantly improved survival. These data suggest that Dll4hi i-DCs play important roles in eliciting allogeneic T cell responses, although novel approaches are needed to formally assess if Dll4hi i-DCs are essentially required for GVHD induction.
In summary, we have identified previously uncharacterized Dll4hi i-DCs that may have important roles in the regulation of GVHD pathogenesis. Most notably, Dll4 derived from these Dll4hi i-DCs was required for them to direct effector differentiation of alloantigen-activated donor T cells. Given the central role of DCs in adaptive immunity, our findings may have broad implications in the development of novel strategies to modulate T-cell responses for controlling GVHD and other inflammatory disorders such as graft-rejection of transplanted solid organs, autoimmune diseases and other immune cell-mediated blood diseases. Dll4 may facilitate the discovery of human counterparts of mouse Dll4hi i-DCs.
Footnotes
This work was supported by a Damon Runyon-Rachleff Innovation Award (YZ), the American Cancer Society (YZ), Department of Defense (YZ) and NIH (R01-CA-172106-01, YZ). This research is supported in part by a grant from the National Institutes of Health through the University of Michigan’s Cancer Center Support Grant (5P30CA46592).
Author contributions
K. M. and Y. Z. conceived and designed the project. K.M., F.X., S.H., Q.T., Y.L., I.M., Y.G., K.K., H.Y., S.M., and Y.Z. performed experiments, analyzed the data. K. M. and Y. Z. wrote the manuscript.
Conflict of interest disclosure
The authors declare that they have no conflicts of interest in relation to this manuscript.
Reference
- 1.Goker H, Haznedaroglu IC, Chao NJ. Acute graft-vs-host disease: pathobiology and management. Exp Hematol. 2001;29:259–277. doi: 10.1016/s0301-472x(00)00677-9. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol. 2012;12:443–458. doi: 10.1038/nri3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teshima T, Maeda Y, Ozaki K. Regulatory T cells and IL-17-producing cells in graft-versus-host disease. Immunotherapy. 2011;3:833–852. doi: 10.2217/imt.11.51. [DOI] [PubMed] [Google Scholar]
- 5.Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 2008;112:4371–4383. doi: 10.1182/blood-2008-03-077974. [DOI] [PubMed] [Google Scholar]
- 6.Porter DL. Allogeneic Immunotherapy to Optimize the Graft-versus-Tumor Effect: Concepts and Controversies. Hematology Am Soc Hematol Educ Program. 2011;2011:292–298. doi: 10.1182/asheducation-2011.1.292. [DOI] [PubMed] [Google Scholar]
- 7.Socie G, Blazar BR. Acute graft-versus-host disease: from the bench to the bedside. Blood. 2009;114:4327–4336. doi: 10.1182/blood-2009-06-204669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu CJ, Ritz J. Induction of tumor immunity following allogeneic stem cell transplantation. Adv Immunol. 2006;90:133–173. doi: 10.1016/S0065-2776(06)90004-2. [DOI] [PubMed] [Google Scholar]
- 9.Koyama M, Kuns RD, Olver SD, Raffelt NC, Wilson YA, Don AL, Lineburg KE, Cheong M, Robb RJ, Markey KA, Varelias A, Malissen B, Hammerling GJ, Clouston AD, Engwerda CR, Bhat P, Macdonald KP, Hill GR. Recipient nonhematopoietic antigen-presenting cells are sufficient to induce lethal acute graft-versus-host disease. Nat Med. 2011;18:135–142. doi: 10.1038/nm.2597. [DOI] [PubMed] [Google Scholar]
- 10.Beilhack A, Schulz S, Baker J, Beilhack GF, Nishimura R, Baker EM, Landan G, Herman EI, Butcher EC, Contag CH, Negrin RS. Prevention of acute graft-versus-host disease by blocking T-cell entry to secondary lymphoid organs. Blood. 2008;111:2919–2928. doi: 10.1182/blood-2007-09-112789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maillard I, Fang T, Pear WS. Regulation of lymphoid development, differentiation, and function by the Notch pathway. Annu Rev Immunol. 2005;23:945–974. doi: 10.1146/annurev.immunol.23.021704.115747. [DOI] [PubMed] [Google Scholar]
- 12.Radtke F, Fasnacht N, Macdonald HR. Notch Signaling in the Immune System. Immunity. 2010;32:14–27. doi: 10.1016/j.immuni.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Sandy AR, Wang J, Radojcic V, Shan GT, Tran IT, Friedman A, Kato K, He S, Cui S, Hexner E, Frank DM, Emerson SG, Pear WS, Maillard I. Notch signaling is a critical regulator of allogeneic CD4+ T-cell responses mediating graft-versus-host disease. Blood. 2011;117:299–308. doi: 10.1182/blood-2010-03-271940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Y, Cain-Hom C, Choy L, Hagenbeek TJ, de Leon GP, Chen Y, Finkle D, Venook R, Wu X, Ridgway J, Schahin-Reed D, Dow GJ, Shelton A, Stawicki S, Watts RJ, Zhang J, Choy R, Howard P, Kadyk L, Yan M, Zha J, Callahan CA, Hymowitz SG, Siebel CW. Therapeutic antibody targeting of individual Notch receptors. Nature. 2010;464:1052–1057. doi: 10.1038/nature08878. [DOI] [PubMed] [Google Scholar]
- 16.Amsen D, Antov A, Flavell RA. The different faces of Notch in T-helper-cell differentiation. Nat Rev Immunol. 2009;9:116–124. doi: 10.1038/nri2488. [DOI] [PubMed] [Google Scholar]
- 17.Mochizuki K, He S, Zhang Y. Notch and inflammatory T-cell response: new developments and challenges. Immunotherapy. 2011;3:1353–1366. doi: 10.2217/imt.11.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amsen D, Blander JM, Lee GR, Tanigaki K, Honjo T, Flavell RA. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 2004;117:515–526. doi: 10.1016/s0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
- 19.Skokos D, Nussenzweig MC. CD8- DCs induce IL-12-independent Th1 differentiation through Delta 4 Notch-like ligand in response to bacterial LPS. J Exp Med. 2007;204:1525–1531. doi: 10.1084/jem.20062305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun J, Krawczyk CJ, Pearce EJ. Suppression of Th2 cell development by Notch ligands Delta1 and Delta4. In J Immunol. 2008:1655–1661. doi: 10.4049/jimmunol.180.3.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takeichi N, Yanagisawa S, Kaneyama T, Yagita H, Jin YH, Kim BS, Koh CS. Ameliorating effects of anti-Dll4 mAb on Theiler's murine encephalomyelitis virus-induced demyelinating disease. Int Immunol. 2010;22:729–738. doi: 10.1093/intimm/dxq059. [DOI] [PubMed] [Google Scholar]
- 22.Agarwal P, Raghavan A, Nandiwada SL, Curtsinger JM, Bohjanen PR, Mueller DL, Mescher MF. Gene regulation and chromatin remodeling by IL-12 and type I IFN in programming for CD8 T cell effector function and memory. J Immunol. 2009;183:1695–1704. doi: 10.4049/jimmunol.0900592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mukherjee S, Schaller MA, Neupane R, Kunkel SL, Lukacs NW. Regulation of T cell activation by Notch ligand, DLL4, promotes IL-17 production and Rorc activation. J Immunol. 2009;182:7381–7388. doi: 10.4049/jimmunol.0804322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maekawa Y, Minato Y, Ishifune C, Kurihara T, Kitamura A, Kojima H, Yagita H, Sakata-Yanagimoto M, Saito T, Taniuchi I, Chiba S, Sone S, Yasutomo K. Notch2 integrates signaling by the transcription factors RBP-J and CREB1 to promote T cell cytotoxicity. Nat Immunol. 2008;9:1140–1147. doi: 10.1038/ni.1649. [DOI] [PubMed] [Google Scholar]
- 25.Sugimoto K, Maekawa Y, Kitamura A, Nishida J, Koyanagi A, Yagita H, Kojima H, Chiba S, Shimada M, Yasutomo K. Notch2 signaling is required for potent antitumor immunity in vivo. J Immunol. 2010;184:4673–4678. doi: 10.4049/jimmunol.0903661. [DOI] [PubMed] [Google Scholar]
- 26.Ito T, Schaller M, Hogaboam CM, Standiford TJ, Sandor M, Lukacs NW, Chensue SW, Kunkel SL. TLR9 regulates the mycobacteria-elicited pulmonary granulomatous immune response in mice through DC-derived Notch ligand delta-like 4. J Clin Invest. 2009;119:33–46. doi: 10.1172/JCI35647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jang S, Schaller M, Berlin AA, Lukacs NW. Notch ligand delta-like 4 regulates development and pathogenesis of allergic airway responses by modulating IL-2 production and Th2 immunity. J Immunol. 2010;185:5835–5844. doi: 10.4049/jimmunol.1000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reynolds ND, Lukacs NW, Long N, Karpus WJ. Delta-like ligand 4 regulates central nervous system T cell accumulation during experimental autoimmune encephalomyelitis. J Immunol. 2011;187:2803–2813. doi: 10.4049/jimmunol.1100160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elyaman W, Bradshaw EM, Wang Y, Oukka M, Kivisakk P, Chiba S, Yagita H, Khoury SJ. JAGGED1 and delta1 differentially regulate the outcome of experimental autoimmune encephalomyelitis. J Immunol. 2007;179:5990–5998. doi: 10.4049/jimmunol.179.9.5990. [DOI] [PubMed] [Google Scholar]
- 30.Moriyama Y, Sekine C, Koyanagi A, Koyama N, Ogata H, Chiba S, Hirose S, Okumura K, Yagita H. Delta-like 1 is essential for the maintenance of marginal zone B cells in normal mice but not in autoimmune mice. Int Immunol. 2008;20:763–773. doi: 10.1093/intimm/dxn034. [DOI] [PubMed] [Google Scholar]
- 31.Kijima M, Iwata A, Maekawa Y, Uehara H, Izumi K, Kitamura A, Yagita H, Chiba S, Shiota H, Yasutomo K. Jagged1 suppresses collagen-induced arthritis by indirectly providing a negative signal in CD8+ T cells. J Immunol. 2009;182:3566–3572. doi: 10.4049/jimmunol.0803765. [DOI] [PubMed] [Google Scholar]
- 32.Yamanda S, Ebihara S, Asada M, Okazaki T, Niu K, Ebihara T, Koyanagi A, Yamaguchi N, Yagita H, Arai H. Role of ephrinB2 in nonproductive angiogenesis induced by Delta-like 4 blockade. Blood. 2009;113:3631–3639. doi: 10.1182/blood-2008-07-170381. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Joe G, Hexner E, Zhu J, Emerson SG. Host-reactive CD8+ memory stem cells in graft-versus-host disease. Nat Med. 2005;11:1299–1305. doi: 10.1038/nm1326. [DOI] [PubMed] [Google Scholar]
- 34.Cooke KR, Kobzik L, Martin TR, Brewer J, Delmonte J, Jr, Crawford JM, Ferrara JL. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood. 1996;88:3230–3239. [PubMed] [Google Scholar]
- 35.Anderson BE, McNiff JM, Jain D, Blazar BR, Shlomchik WD, Shlomchik MJ. Distinct roles for donor- and host-derived antigen-presenting cells and costimulatory molecules in murine chronic graft-versus-host disease: requirements depend on target organ. Blood. 2005;105:2227–2234. doi: 10.1182/blood-2004-08-3032. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Joe G, Zhu J, Carroll R, Levine B, Hexner E, June C, Emerson SG. Dendritic cell-activated CD44hiCD8+ T cells are defective in mediating acute graft-versus-host disease but retain graft-versus-leukemia activity. Blood. 2004;103:3970–3978. doi: 10.1182/blood-2003-09-3135. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Louboutin JP, Zhu J, Rivera AJ, Emerson SG. Preterminal host dendritic cells in irradiated mice prime CD8+ T cell-mediated acute graft-versus-host disease. J Clin Invest. 2002;109:1335–1344. doi: 10.1172/JCI14989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y, Shlomchik WD, Joe G, Louboutin JP, Zhu J, Rivera A, Giannola D, Emerson SG. APCs in the liver and spleen recruit activated allogeneic CD8+ T cells to elicit hepatic graft-versus-host disease. J Immunol. 2002;169:7111–7118. doi: 10.4049/jimmunol.169.12.7111. [DOI] [PubMed] [Google Scholar]
- 39.Shlomchik WD, Couzens MS, Tang CB, McNiff J, Robert ME, Liu J, Shlomchik MJ, Emerson SG. Prevention of graft versus host disease by inactivation of host antigen- presenting cells. Science. 1999;285:412–415. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 40.Teshima T, Ordemann R, Reddy P, Gagin S, Liu C, Cooke KR, Ferrara JL. Acute graft-versus-host disease does not require alloantigen expression on host epithelium. Nat Med. 2002;8:575–581. doi: 10.1038/nm0602-575. [DOI] [PubMed] [Google Scholar]
- 41.Merad M, Hoffmann P, Ranheim E, Slaymaker S, Manz MG, Lira SA, Charo I, Cook DN, Weissman IL, Strober S, Engleman EG. Depletion of host Langerhans cells before transplantation of donor alloreactive T cells prevents skin graft-versus-host disease. Nat Med. 2004;10:510–517. doi: 10.1038/nm1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matte CC, Liu J, Cormier J, Anderson BE, Athanasiadis I, Jain D, McNiff J, Shlomchik WD. Donor APCs are required for maximal GVHD but not for GVL. Nat Med. 2004;10:987–992. doi: 10.1038/nm1089. [DOI] [PubMed] [Google Scholar]
- 43.Duffner UA, Maeda Y, Cooke KR, Reddy P, Ordemann R, Liu C, Ferrara JL, Teshima T. Host dendritic cells alone are sufficient to initiate acute graft-versus-host disease. J Immunol. 2004;172:7393–7398. doi: 10.4049/jimmunol.172.12.7393. [DOI] [PubMed] [Google Scholar]
- 44.Koyama M, Hashimoto D, Aoyama K, Matsuoka K, Karube K, Niiro H, Harada M, Tanimoto M, Akashi K, Teshima T. Plasmacytoid dendritic cells prime alloreactive T cells to mediate graft-versus-host disease as antigen-presenting cells. Blood. 2009;113:2088–2095. doi: 10.1182/blood-2008-07-168609. [DOI] [PubMed] [Google Scholar]
- 45.Li H, Demetris AJ, McNiff J, Matte-Martone C, Tan HS, Rothstein DM, Lakkis FG, Shlomchik WD. Profound depletion of host conventional dendritic cells, plasmacytoid dendritic cells, and B cells does not prevent graft-versus-host disease induction. J Immunol. 2012;188:3804–3811. doi: 10.4049/jimmunol.1102795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Markey KA, MacDonald KP, Hill GR. Recipient plasmacytoid DCs are not required to prime allogeneic T-cell responses after BMT. Blood. 2009;113:6038–6039. doi: 10.1182/blood-2009-03-212944. [DOI] [PubMed] [Google Scholar]
- 47.Naik SH. Demystifying the development of dendritic cell subtypes, a little. Immunol Cell Biol. 2008;86:439–452. doi: 10.1038/icb.2008.28. [DOI] [PubMed] [Google Scholar]
- 48.Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol. 2007;7:19–30. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- 49.Schlitzer A, Loschko J, Mair K, Vogelmann R, Henkel L, Einwachter H, Schiemann M, Niess JH, Reindl W, Krug A. Identification of CCR9- murine plasmacytoid DC precursors with plasticity to differentiate into conventional DCs. Blood. 2011;117:6562–6570. doi: 10.1182/blood-2010-12-326678. [DOI] [PubMed] [Google Scholar]
- 50.Reizis B, Colonna M, Trinchieri G, Barrat F, Gilliet M. Plasmacytoid dendritic cells: one-trick ponies or workhorses of the immune system? Nat Rev Immunol. 2011;11:558–565. doi: 10.1038/nri3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koyanagi A, Sekine C, Yagita H. Expression of Notch receptors and ligands on immature and mature T cells. Biochem Biophys Res Commun. 2012;418:799–805. doi: 10.1016/j.bbrc.2012.01.106. [DOI] [PubMed] [Google Scholar]
- 52.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 53.Watowich SS, Liu YJ. Mechanisms regulating dendritic cell specification and development. Immunol Rev. 2010;238:76–92. doi: 10.1111/j.1600-065X.2010.00949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zuniga EI, McGavern DB, Pruneda-Paz JL, Teng C, Oldstone MB. Bone marrow plasmacytoid dendritic cells can differentiate into myeloid dendritic cells upon virus infection. Nat Immunol. 2004;5:1227–1234. doi: 10.1038/ni1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Naik SH, Metcalf D, van Nieuwenhuijze A, Wicks I, Wu L, O'Keeffe M, Shortman K. Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nat Immunol. 2006;7:663–671. doi: 10.1038/ni1340. [DOI] [PubMed] [Google Scholar]
- 56.Miller JC, Brown BD, Shay T, Gautier EL, Jojic V, Cohain A, Pandey G, Leboeuf M, Elpek KG, Helft J, Hashimoto D, Chow A, Price J, Greter M, Bogunovic M, Bellemare-Pelletier A, Frenette PS, Randolph GJ, Turley SJ, Merad M. Deciphering the transcriptional network of the dendritic cell lineage. Nat Immunol. 2012;13:888–899. doi: 10.1038/ni.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007;7:340–352. doi: 10.1038/nri2000. [DOI] [PubMed] [Google Scholar]
- 58.Sekine C, Moriyama Y, Koyanagi A, Koyama N, Ogata H, Okumura K, Yagita H. Differential regulation of splenic CD8- dendritic cells and marginal zone B cells by Notch ligands. Int Immunol. 2009;21:295–301. doi: 10.1093/intimm/dxn148. [DOI] [PubMed] [Google Scholar]
- 59.Liou LY, Blasius AL, Welch MJ, Colonna M, Oldstone MB, Zuniga EI. In vivo conversion of BM plasmacytoid DC into CD11b+ conventional DC during virus infection. Eur J Immunol. 2008;38:3388–3394. doi: 10.1002/eji.200838282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 61.Mailhos C, Modlich U, Lewis J, Harris A, Bicknell R, Ish-Horowicz D. Delta4, an endothelial specific notch ligand expressed at sites of physiological and tumor angiogenesis. Differentiation. 2001;69:135–144. doi: 10.1046/j.1432-0436.2001.690207.x. [DOI] [PubMed] [Google Scholar]
- 62.Gale NW, Dominguez MG, Noguera I, Pan L, Hughes V, Valenzuela DM, Murphy AJ, Adams NC, Lin HC, Holash J, Thurston G, Yancopoulos GD. Haploinsufficiency of delta-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proc Natl Acad Sci U S A. 2004;101:15949–15954. doi: 10.1073/pnas.0407290101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reddy P, Maeda Y, Liu C, Krijanovski OI, Korngold R, Ferrara JL. A crucial role for antigen-presenting cells and alloantigen expression in graft-versus-leukemia responses. Nat Med. 2005;11:1244–1249. doi: 10.1038/nm1309. [DOI] [PubMed] [Google Scholar]
- 64.Highfill SL, Rodriguez PC, Zhou Q, Goetz CA, Koehn BH, Veenstra R, Taylor PA, Panoskaltsis-Mortari A, Serody JS, Munn DH, Tolar J, Ochoa AC, Blazar BR. Bone marrow myeloid-derived suppressor cells (MDSCs) inhibit graft-versus-host disease (GVHD) via an arginase-1-dependent mechanism that is up-regulated by interleukin-13. Blood. 2010;116:5738–5747. doi: 10.1182/blood-2010-06-287839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reddy P, Sun Y, Toubai T, Duran-Struuck R, Clouthier SG, Weisiger E, Maeda Y, Tawara I, Krijanovski O, Gatza E, Liu C, Malter C, Mascagni P, Dinarello CA, Ferrara JL. Histone deacetylase inhibition modulates indoleamine 2,3-dioxygenase-dependent DC functions and regulates experimental graft-versus-host disease in mice. J Clin Invest. 2008;118:2562–2573. doi: 10.1172/JCI34712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Banovic T, Markey KA, Kuns RD, Olver SD, Raffelt NC, Don AL, Degli-Esposti MA, Engwerda CR, MacDonald KP, Hill GR. Graft-versus-host disease prevents the maturation of plasmacytoid dendritic cells. J Immunol. 2009;182:912–920. doi: 10.4049/jimmunol.182.2.912. [DOI] [PubMed] [Google Scholar]