Abstract
The Dicer nuclease generates small RNAs that regulate diverse biological processes through post-transcriptional gene repression and epigenetic silencing of transcription and recombination. Dicer-deficient cells exhibit impaired differentiation, activity, proliferation, and survival. Dicer inactivation in developing mouse lymphocytes impairs their proliferation and survival and alters antigen receptor gene repertoires for largely undefined reasons. To elucidate functions of Dicer in lymphocyte development and antigen receptor locus transcription and recombination, we analyzed mice with conditional Dicer deletion in thymocytes containing un-rearranged or pre-rearranged TCRβ loci. Expression of either a pre-assembled functional TCRβ gene (Vβ1NT) or the pro-survival BCL2 protein inhibited death and partially rescued proliferative expansion of Dicer-deficient thymocytes. Notably, combined expression of Vβ1NT and BCL2 completely rescued proliferative expansion of Dicer-deficient thymocytes and revealed that Dicer promotes survival of cells attempting TCRβ recombination. Finally, inclusion of an endogenous pre-assembled DJβ complex that enhances Vβ recombination increased death and impaired proliferative expansion of Dicer-deficient thymocytes. These data demonstrate a critical role for Dicer in promoting survival of thymocytes experiencing DNA double strand breaks (DSBs) during TCRβ recombination. Since DSBs are common and ubiquitous in cells, our findings indicate that impaired cellular survival in response to DSBs should be considered when interpreting Dicer-deficient phenotypes.
Introduction
Dicer promotes biogenesis of small RNAs that control post-transcriptional gene expression and epigenetic silencing. In fission yeast, Dicer is required for production of short interfering RNAs (siRNAs) that promote epigenetic silencing of repetitive DNA transcription and mating type locus recombination (1). In animals, Dicer is essential for generation of microRNAs (miRNAs) that block translation or induce turnover of target mRNAs (2). Dicer-deficient mice are embryonic lethal (3), while mice with tissue-specific deletion of Dicer often exhibit pathological conditions (2). Dicer−/− mouse cells exhibit loss of miRNAs, gene expression changes, altered differentiation, and impaired activity (2), suggesting that Dicer-dependent miRNAs are critical for control of lineage-specific functions. Consistent with this notion, altered expression of individual miRNAs can recapitulate Dicer-deficient phenotypes (4). However, these interpretations are complicated by miRNA-independent Dicer functions and impaired proliferation and survival of Dicer−/− cells. For example, Dicer−/− oocytes and embryonic stem (ES) cells exhibit increased transcripts from retrotransposons and other repetitive sequences that correlate with reduced siRNA levels and impaired epigenetic silencing (5, 6). Thus, Dicer−/− phenotypes could arise from loss of miRNA-mediated gene repression, processing of repetitive transcripts, and/or siRNA-dependent epigenetic silencing.
Mouse αβ T- and B-cell differentiation involves stage specific regulation of gene expression and genomic recombination or epigenetic silencing of antigen receptor gene segments (7). Transcription and recombination of TCRβ and IgH loci occurs in CD4−/CD8− double-negative (DN) thymocytes and bone marrow pro-B cells, respectively. The RAG1/RAG2 (RAG) endonuclease induces DSBs at transcribing TCRβ or IgH variable (V), diversity (D), and joining (J) gene segments in G1 phase cells, and nonhomologous end-joining (NHEJ) proteins repair these DSBs to form V(D)J coding exons upstream of constant (C) exons. Assembly and expression of functional VβDβJβCβ or VHDHJHCH genes leads to β-or H-selection signals that silence germline Vs and drive proliferative expansion of cells as they differentiate into CD4+/CD8+ double-positive (DP) thymocytes or pre-B cells, respectively. Assembly and expression of in-frame VαJαCα or VLJLCL genes in DP or pre-B cells can lead to TCRα/TCRβ or IgH/IgL antigen receptors. Upon their positive selection, antigen receptors signal differentiation into CD4+ or CD8+ “single-positive” (SP) thymocytes or immature B-cells. These cells emigrate from the thymus or bone marrow as CD4+ or CD8+ αβ T-cells, or transitional B-cells, and can differentiate into effector lineages in peripheral tissues.
Dicer promotes B and αβ T-cell differentiation through generation of small RNAs that direct miRNA-mediated gene repression and possibly siRNA-mediated control of V(D)J recombination (8-10). Conditional Dicer deletion in pro-B cells caused absence of miRNAs (including one that represses the Bim pro-apoptotic protein), blocked pro-B to pre-B cell differentiation, and increased pro-B cell apoptosis (10). Development of Dicer−/− pro-B cells was partially rescued by Bim deletion, expression of the BCL2 pro-survival protein, or co-expression of pre-assembled IgH and IgL transgenes that repress Bim, indicating that Dicer-dependent post-transcriptional repression of Bim is important for normal B-cell development (10). However, since IgH transgenes bypass necessity of IgH recombination for pro-B to pre-B cell development, this finding also suggests additional potential roles of Dicer in control of V(D)J recombination. Bi-directional transcription of V(D)J segments and flanking repetitive sequences has been proposed to generate siRNAs that direct epigenetic silencing (11, 12). Consistent with these models, Dicer−/− B-lineage cells exhibit increased transcription and usage of DH segments flanked by DNA repeats (10, 13). Yet, since Dicer−/− B-cells exhibit altered VHDHJH repertoires due to the loss of Dicer-dependent miRNAs that modulate IgH/IgL selection (10, 13), further studies are needed to directly test whether Dicer-dependent siRNAs control IgH recombination. Dicer deletion in DN cells led to loss of miRNAs, impaired DN-to-DP proliferative expansion, and increased apoptosis of thymocytes cycling in vitro, but no effect upon DN-to-DP thymocyte differentiation per se (8, 9), suggesting that Dicer-dependent RNAs regulate thymocyte survival directly and/or during cell division (8). However, since defects in TCRβ recombination or β-selection also impair DN-to-DP proliferative expansion (14-17), additional functions of Dicer may help promote thymocyte development.
We have used mice expressing pre-assembled functional endogenous TCRβ genes to elucidate V(D)J recombination control mechanisms that were impossible to discover using TCRβ transgenes (18-21). Pre-assembled functional TCRβ transgenes/genes facilitate study of mechanisms that silence transcription and recombination of germline Vβs on un-rearranged TCRβ alleles (19). However, only pre-assembled TCRβ genes enable study of mechanisms that control transcription and recombination of Vβs located on VDJβ-recombined alleles (21). Our studies with a pre-assembled Vβ1Dβ1Jβ1.4Cβ1 (Vβ1NT) gene showed that repetitive sequences within Vβ regions correlate with boundaries between chromatin domains that are differentially silenced in response to β-selection signals (20, 21). Bi-directional transcription of Vβs within these domains precedes their epigenetic silencing (20, 21). To elucidate potential roles of Dicer in control of TCRβ germline transcription and recombination, we generated and analyzed mice with Dicer deletion initiating in DN thymocytes that contain un-rearranged TCRβ alleles or Vβ1NT alleles.
Materials and Methods
Mice
Lck-cre (22), Dicerlox/lox (8), Vβ1NT/NT (21), Vβ14NT/NT (18), Rag1−/− (15), EμBCL2 transgenic (23), and DJ/DJ (24) mice were bred to generate the animals in this study. The background strain of these mice was mixed 129SvEv and C57BL/6. All experimental mice were littermates or age-matched mice between 4-6 weeks of age. All experiments were conducted in accordance with national guidelines and approved by the Children’s Hospital of Philadelphia IACUC committee.
Flow Cytometry
Single cell suspensions were stained with antibodies in PBS containing 2% BSA and 1mM EDTA as described (21). All antibodies were purchased from BD Pharmigen: anti-Vβ4 (553364), anti-Vβ5 (553189), anti-Vβ6 (553192), anti-Vβ8 (553862), anti-Vβ10 (553285), anti-Vβ14 (553258), anti-CD4 (553051), anti-CD8α (553033), anti-TCRβ (553172), anti-B220 (553090), anti-CD19 (553786), anti-CD11b (553311), anti-CD11c (557401), anti-Cδ (553178), anti-NK1.1 (553165), anti-CD8α (553033), anti-Ter119 (553673), anti-CD25 (552880), and anti-CD117 (553356). Data was acquired on a FACSCalibur (BD Biosciences, San Jose, CA) using CellQuest software (BD Biosciences) and analyzed using FlowJo software (Tree Star).
Analysis of Vβ Rearrangements, Transcription, and CpG Methylation
Analyses of Vβ rearrangements, transcription, and CpG methylation were conducted as described (21).
Quantification of Dicer Deletion
DN thymocytes were isolated by FACS purification of lineage-negative cells and genomic DNA isolated as described (22). DNA was used as a template for qPCR measurement of Cre-mediated Dicer deletion on an Applied Biosystems Viia 7 Fast Real Time PCR System by quantifying “floxed” Dicer Exon 20 sequences and “non-floxed” Dicer Exon 19 sequences and calculating the ratio of Exon 20 to Exon 19 sequences. Primers: Exon 19Forward: 5′-TACATCCAATCCCAGCATCA-3′, Exon 19Reverse: 5′-TCTGAGCTCTTAGTTCCTCTGC-3′, Exon 20Forward: 5′-AACTCCTCGTTGGCTGAGAG-3′, Exon 20Reverse: 5′-TCATGGTTTTCTAAGGAGGGTCT-3′.
Results
Pre-assembled Functional TCRβ Genes Partially Rescue Development of Dicer−/− Thymocytes
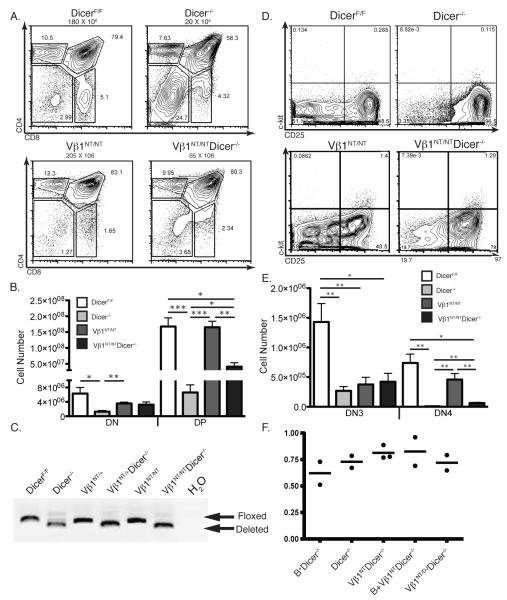
The Lck-cre transgene and “floxed” Dicer (Dicerlox) alleles were previously used to create mice with conditional Dicer deletion initiating in DN thymocytes (8, 9). Thus, we generated and analyzed in parallel Dicerlox/lox (DicerF/F), Lck-cre:Dicerlox/lox (Dicer−/−), Vβ1NT/NTDicerlox/lox (Vβ1NT/NT), and Lckcre:Vβ1NT/NTDicerlox/lox (Vβ1NT/NTDicer−/−) mice. Since expression of pre-assembled functional TCRβ transgenes/genes rescues the blocks in αβ T-lymphocyte development caused by defects in TCRβ recombination (21, 25), we first conducted flow cytometry (FACS) analysis with anti-CD4 and anti-CD8 antibodies to quantify the numbers of DN and DP cells in mice of each genotype. Consistent with published observations (8, 9), we found that Dicer−/− mice contained ~5-fold lower numbers of DN cells and ~9-fold lower numbers of DP cells as compared to DicerF/F mice (Fig. 1A,B). Yet, as compared to Vβ1NT/NT mice, we found that Vβ1NT/NTDicer−/− mice contained equivalent numbers of DN cells and only ~3-fold fewer numbers of DP cells (Fig. 1A,B). In addition, the numbers of DN and DP thymocytes in Vβ1NT/NTDicer−/− mice were higher than in Dicer−/− mice (Fig. 1A,B). Notably, we detected similar extents of Dicer deletion in total thymocytes from mice of each genotype (Fig. 1C), indicating that Vβ1NT did not increase DP thymocyte numbers solely by pushing cells through DN-to-DP differentiation prior to Dicer inactivation. Thus, our finding that Vβ1NT expression partially rescues DN-to-DP proliferative expansion of Dicer-deficient thymocytes supports the notion that Dicer is required for normal TCRβ recombination. Yet, it is important to note that the inability of Vβ1NT to completely rescue Dicer−/− DP thymocyte cellularity is consistent with the additional proposed role of Dicer in promoting thymocyte survival during cell division (8).
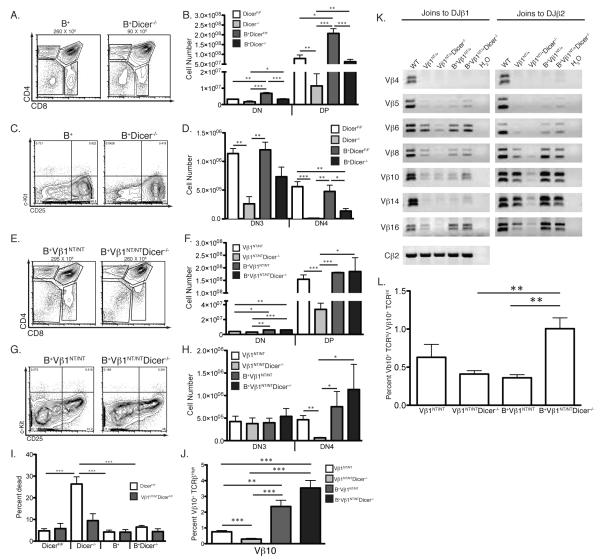
FIGURE 1.
A pre-assembled functional TCRβ gene partially rescues early αβ T-cell development. A, Representative CD4/CD8 FACS data of thymocytes from DicerF/F, Dicer−/−, Vβ1NT/NT, and Vβ1NT/NTDicer−/− mice. The average numbers of total thymocytes, the DN, DP, CD4+ SP and CD8+ SP cell gates, and the percentage of thymocyte in each gate are indicated. B, Graph showing the average numbers of DN and DP thymocytes from mice of the indicated genotypes. Error bars are standard error. Significant differences: *p<0.05, **p≤0.01, and ***p≤ 0.001. This experiment was independently performed five times with at least one mouse of each genotype in each experimental replicate. C, PCR analysis showing equivalent deletion of Dicer in total thymocytes of mice of the indicated genotypes. D, Representative c-Kit/CD25 FACS data of DN thymocytes from DicerF/F, Dicer−/−, Vβ1NT/NT, and Vβ1NT/NTDicer−/− mice. The DN1, DN2, DN3, and DN4 thymocyte quadrants and the percentage of DN cells in each quadrant are indicated. E, Graph showing the average numbers of DN3 and DN4 cells from mice of the indicated genotypes. Error bars are standard error. Significant differences: *p<0.05 and **p≤0.01. This experiment was independently performed five times with at least one mouse of each genotype in each experimental replicate. F, qPCR analysis showing equivalent deletion of Dicer in DN thymocytes sort-purified from mice of the indicated genotypes (mice expressing the EμBCL2 transgene are designated B+ in this figure). The dots represent data from individual mice and the bars indicate the average values from mice of each genotype.
To further investigate the potential requirement of Dicer for normal TCRβ recombination, we next assayed the effects of Vβ1NT expression on the thymocyte development stages in which TCRβ genes are assembled and selected. Assembly and selection of functional TCRβ genes in c-Kit−/CD25+ DN3 cells promotes their concomitant proliferation and differentiation into c-Kit−/CD25− DN4 and then DP cells (20, 25, 26). We conducted cell counting and FACS analysis of CD4−/CD8− thymocytes with anti-c-Kit and anti-CD25 antibodies. Consistent with published findings (8, 9), we observed a ~5-fold decrease in the numbers of DN3 thymocytes and a ~90-fold reduction in DN4 cell numbers in Dicer−/− mice relative to DicerF/F mice (Fig. 1D,E). Mice expressing pre-assembled TCRβ transgenes/genes exhibit decreased numbers of DN3 thymocytes, yet normal numbers of DN4 cells, due to enhanced β-selection (18, 21). We detected similar numbers of DN3 cells in Vβ1NT/NT and Vβ1NT/NTDicer−/− mice, but ~8-fold fewer DN4 cells in Vβ1NT/NTDicer−/− mice as compared to Vβ1NT/NT mice (Fig. 1D,E). The numbers of DN4 cells in Vβ1NT/NTDicer−/− mice were lower than in DicerF/F mice, but higher than in Dicer−/− mice (Fig. 1D,E). These data indicate that Vβ1NT expression partially rescues DN4 cellularity and the DN3-to-DN4 developmental transition of Dicer-deficient thymocytes, providing further support to our interpretation that Dicer is required for normal TCRβ recombination. We found equivalent deletion of Dicer in DN thymocytes of Dicer−/− and Vβ1NT/NTDicer−/− mice (Fig. 1F), indicating that Vβ1NT expression does not increase DN4 thymocyte numbers solely by pushing cells through DN3-to-DN4 development prior to Dicer inactivation. However, the inability of Vβ1NT expression to completely rescue Dicer−/− DN4 cell numbers and the DN3-to-DN4 developmental transition also is consistent with the additional proposed role of Dicer in promoting thymocyte survival during cell division (8) and a potential requirement of Dicer for normal survival of DN3 cells during β-selection.
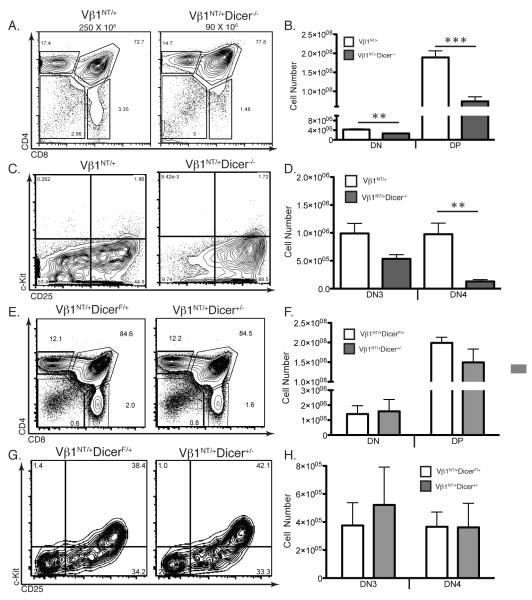
We observed identical phenotypes in mice with Dicer−/− thymocytes containing only one Vβ1NT allele (Vβ1NT/+)(Fig. 2A-D), or the pre-assembled functional endogenous Vβ14NT gene (data not shown). The ability of two different pre-assembled functional TCRβ genes to partially rescue DN-to-DP and DN3-to-DN4 development of Dicer−/− thymocytes provides more support for our interpretation that Dicer is required for normal TCRβ recombination. We also observed no differences in thymocyte development between Vβ1NT/+Dicerlox/+ (Vβ1NT/+DicerF/+) and Lck-cre:Vβ1NT/+Dicerlox/+ (Vβ1NT/+Dicer+/−) mice (Fig. 2E-H), indicating that Cre expression in Dicer-deficient thymocytes does not impair early αβ T cell development.
FIGURE 2.
Vβ1NT Expresssion Partially Rescues Development of Dicer Deficient Thymocytes. A, Representative CD4/CD8 FACS analysis of thymocytes from Vβ1NT/+ and Vβ1NT/+Dicer−/− mice. The average numbers of total thymocytes, the DN, DP, CD4+ SP and CD8+ SP cell gates, and the percentage of thymocytes in each gate are indicated. B, Graph showing average numbers of DN and DP thymocytes from Vβ1NT/+ and Vβ1NT/+Dicer−/− mice. Error bars are standard error. No significant differences were observed. This experiment was done three independent times on at least one mouse of each genotype. C, Representative c-Kit/CD25 FACS data of thymocytes from Vβ1NT/+ and Vβ1NT/+Dicer−/− mice. The DN1, DN2, DN3, and DN4 quadrants and the percentage of DN cells in each quadrant are indicated. D, Graph showing average numbers of DN3 and DN4 thymocytes from Vβ1NT/+ and Vβ1NT/+Dicer−/− mice. Error bars are standard error. Significant differences: **p<0.01. This experiment was done three independent times on at least one mouse of each genotype. E, Representative CD4/CD8 FACS analysis of thymocytes from Vβ1NT/+DicerF/+ and Vβ1NT/+Dicer+/− (LckCreVβ1NT/+DicerF/+) mice. The DN, DP, CD4+ SP and CD8+ SP cell gates and the percentages of thymocytes in each gate are indicated. F, Graph showing average numbers of DN and DP thymocytes from Vβ1NT/+DicerF/+ and Vβ1NT/+Dicer+/− mice. Error bars are standard error. No significant differences were observed. This experiment was done three independent times on at least one mouse of each genotype. G, Representative c-Kit/CD25 FACS data of thymocytes from Vβ1NT/+DicerF/+ and Vβ1NT/+Dicer+/− mice. The DN1, DN2, DN3, and DN4 quadrants and the percentage of DN cells in each quadrant are indicated. H, Graph showing average numbers of DN3 and DN4 thymocytes from Vβ1NT/+DicerF/+ and Vβ1NT/+Dicer+/− mice. Error bars are standard error. No significant differences were observed. This experiment was done three independent times on at least one mouse of each genotype.
Dicer is Required for Normal TCRβ Recombination
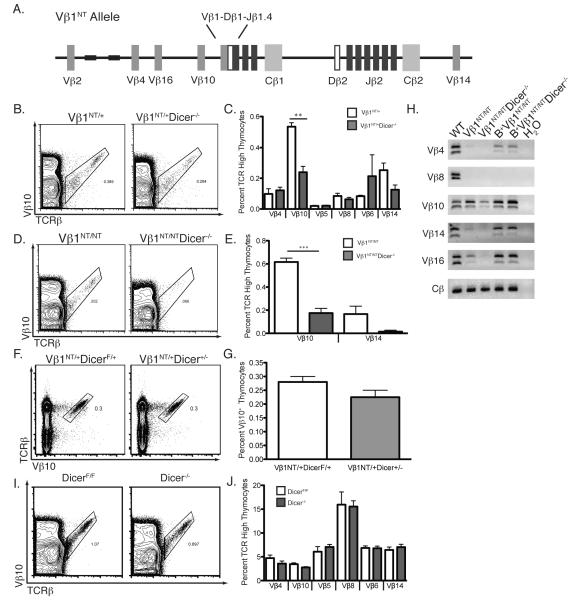
To directly assess whether Dicer is required for normal TCRβ recombination, we characterized Vβ rearrangement and expression in Vβ1NT/+, Vβ1NT/NT, and wild-type αβ T-lineage cells expressing or lacking Dicer. We conducted cell counting and FACS analysis of thymocytes with anti-Vβ and anti-Cβ antibodies to quantify the percentages of cells expressing particular Vβs. We compared the percentages, rather than absolute numbers, of thymocytes expressing particular Vβs since mice of Dicer-deficient backgrounds harbor reduced thymic cellularity relative to Dicer-sufficient backgrounds. Most Vβs rearrange at low levels on wild-type alleles in Vβ1NT/+ DN3 thymocytes and are expressed in small fractions of Vβ1NT/+ DP thymocytes and splenic αβ T-lymphocytes (21). Vβ10, which resides just upstream of the Vβ1NT gene (Fig. 3A), rearranges at a higher frequency than other Vβs on the Vβ1NT allele, and is expressed in ~1% of Vβ1NT/+ and Vβ1NT/NT αβ T-lineage cells (21). On Vβ1NT alleles, repetitive sequences mark a boundary between Vβ10 segments that recombine and upstream Vβ4/Vβ16 segments that do not recombine (21). Although Dicer deletion had no effect upon the percentages of Vβ1NT/+ thymocytes that expressed Vβs other than Vβ10 (Fig. 3B,C), we detected ~2-fold decreases in the percentages of Vβ10+ thymocytes upon deletion of Dicer in Vβ1NT/+ and Vβ1NT/NT mice (Fig. 3B-E). We found no difference in the percentages of Vβ10+ thymocytes between Vβ1NT/+DicerF/+ and Vβ1NT/+Dicer+/− mice (Fig. 3F,G), indicating that Cre expression in Dicer-deficient thymocytes does not affect Vβ10 rearrangement and expression. Notably, we did not notice any significant effect of Dicer deletion upon Vβ4, Vβ16, or Vβ14 expression Vβ1NT/+ or Vβ1NT/NT thymocytes (Fig. 3C,E). PCR with specific Vβ and Jβ primers to quantify Vβ rearrangements detected decreases in levels of Vβ rearrangements upon deletion of Dicer in Vβ1NT/+ and Vβ1NT/NT mice (Fig. 3H). Since Vβ10 recombination on Vβ1NT alleles occurs only in DN3 cells (20), the reduced rearrangement and expression of Vβ10 in Vβ1NT/+Dicer−/− and Vβ1NT/NTDicer−/− thymocytes provides functional evidence that Dicer is inactivated in these DN3 cells. In contrast to mice containing the Vβ1NT allele, we observed no differences in Vβ10 expression between DicerF/F and Dicer−/− mice (Fig. 3I,J). These data indicate that Dicer is required for normal recombination of Vβ10 segments on Vβ1NT alleles, but not on wild-type TCRβ alleles; they also provide no evidence that Dicer controls heterochromatin formation over Vβ4 and Vβ16 on Vβ1NT alleles.
FIGURE 3.
Dicer is required for expression and accessibility of Vβ10 segments on Vβ1NT alleles. A, Schematic representation of the Vβ1NT TCRβ locus showing the relative locations of the pre-assembled Vβ1NT coding join and the germline Vβ and Dβ2-Jβ2 segments. B, Representative TCRβ/Vβ10 FACS data from Vβ1NT/+ and Vβ1NT/+Dicer−/− mice. The Vβ10+ cell gate and the percentage of thymocytes in this gate are indicated. C, Graph showing the average percentages of TCRβhigh thymocytes expressing the indicated Vβ segments in Vβ1NT/+ and Vβ1NT/+Dicer−/− mice. Error bars are standard error. Significant differences: **p≤0.01. This experiment was independently performed three times with at least one mouse of each genotype in each experimental replicate. D, Representative TCRβ/Vβ10 FACS data from Vβ1NT/NT and Vβ1NT/NTDicer−/− mice. The Vβ10+ cell gate and the percentages of thymocytes within these gates are indicated. E, Graph showing the average percentages of TCRβhigh thymocytes expressing the indicated Vβ segments in Vβ1NT/NT and Vβ1NT/NTDicer−/− mice. Error bars are standard error. Significant differences: ***p≤ 0.001. This experiment was independently performed three times with at least one mouse of each genotype in each experimental replicate. F, Representative TCRβ/Vβ10 FACS data from Vβ1NT/+DicerF/+ and Vβ1NT/+Dicer+/− mice. The Vβ10+ cell gate and the percentage of thymocytes in this gate are indicated. G, Graph showing average percentage of Vβ10+ thymocytes from Vβ1NT/+DicerF/+ and Vβ1NT/+Dicer+/− mice. Error bars are standard error. No significant differences were observed. This experiment was done three independent times on at least one mouse of each genotype. H, Representative PCR analysis of rearrangements involving the indicated Vβ segments to DJβ1.1/DJβ1.2 or DJβ2.1/DJβ2.2 complexes in thymocytes of Vβ1NT/+ and Vβ1NT/+Dicer−/− mice. A Cβ PCR control for genomic DNA content is also shown. This experiment was done three independent times on one mouse of each genotype. I, Representative TCRβ/Vβ10 FACS data from DicerF/F and Dicer−/− mice. The Vβ10+ cell gate and the percentage of thymocytes in this gate are indicated. J, Graph showing average percentages of TCRβhigh thymocytes expressing the indicated Vβ segments in DicerF/F and Dicer−/− mice. Error bars are standard error. No significant differences were detected. This experiment was done three independent times on at least one mouse of each genotype.
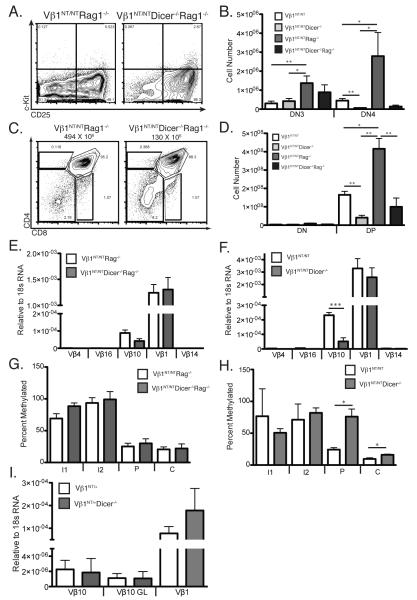
Since V(D)J recombination is controlled by modulating accessibility of gene segments to RAG proteins (7), the requirement of Dicer for normal recombination of Vβ10 segments on Vβ1NT alleles could be in promoting Vβ10 accessibility. RAG accessibility is assayed by quantifying steady-state germline transcripts and CpG methylation of gene segments (7). On Vβ1NT alleles, Vβ10 segments are transcribed and exhibit low DNA CpG methylation in both DN and DP thymocytes (20, 21), enabling assessment of Vβ10 accessibility through analysis of total thymocytes isolated from mice on a Vβ1NT/NT background (21). To assess whether Dicer promotes accessibility of Vβ10 segments on Vβ1NT alleles, we analyzed Vβ1NT/NTRag1−/− and Vβ1NT/NTDicer−/−Rag1−/− thymocytes (Fig. 4A-D) to avoid potential compounding effects from recombination of transcribing Vβ10 segments. In parallel, we also analyzed Vβ1NT/NT and Vβ1NT/NTDicer−/− thymocytes to evaluate whether cells with accessible Vβ10 segments are lost while attempting recombination. We found that Dicer deletion had no significant effect on relative levels of germline Vβ10 transcripts in Vβ1NT/NTRag1−/− thymocytes (Fig. 4E), however Dicer deletion led to ~4-fold lower relative levels of germline Vβ10 transcripts in Vβ1NT/NT cells (Fig. 4F). We also found that Dicer deletion had no effect on Vβ10 CpG methylation in Vβ1NT/NTRag1−/− cells (Fig. 4G), but led to significantly higher Vβ10 CpG methylation in Vβ1NT/NT cells (Fig. 4H). The simplest explanation for decreased germline transcription and increased CpG methylation of germline Vβ10 segments in Vβ1NT/NTDicer−/−Rag1−/− thymocytes compared to Vβ1NT/NTDicer−/− thymocytes is increased apoptosis of Dicer-deficient Vβ1NT/NT DN cells attempting TCRβ recombination.
FIGURE 4.
Dicer is required for survival of DN cells attempting Vβ10 rearrangements on Vβ1NT alleles. A, Representative c-Kit/CD25 FACS data of DN thymocytes from Vβ1NT/NTRag1−/− and Vβ1NT/NTDicer−/−Rag1−/− mice. The DN1, DN2, DN3, and DN4 thymocyte quadrants and the percentages of DN cells within each of these quadrants are indicated. B, Graph showing the average numbers of DN3 and DN4 cells from Vβ1NT/NTRag1−/− and Vβ1NT/NTDicer−/−Rag1−/− mice. Error bars are standard error. Significant differences: *p<0.05 and **p≤0.01. This experiment was done three independent times on at least one mouse of each genotype. C, Representative CD4/CD8 FACS data of thymocytes from Vβ1NT/NTRag1−/− and Vβ1NT/NTDicer−/−Rag1−/− mice. The average numbers of total thymocytes, the DN, DP, CD4+ SP and CD8+ SP cell gates, and the percentages of thymocytes within each of these gates are indicated. D, Graph showing the average numbers of DN and DP thymocytes from Vβ1NT/NTRag1−/− and Vβ1NT/NTDicer−/−Rag1−/− mice. Error bars are standard error. Significant differences: *p<0.05 and **p≤0.01. This experiment was done three independent times on at least one mouse of each genotype. E and F, Graphs showing average levels of Vβ1NT gene transcripts or germline transcripts of the other indicated Vβ segments in thymocytes of (E) Vβ1NT/NTRag1−/− and Vβ1NT/NTDicer−/−Rag1−/− mice or (F) Vβ1NT/NT and Vβ1NT/NTDicer−/− mice. Error bars are standard error. Significant differences: ***p≤ 0.001. This experiment was independently performed three times with at least one mouse of each genotype in each experimental replicate. G and H, Graphs showing average percent Vβ10 DNA CpG methylation at restriction enzyme sites within the intron upstream of Vβ10 (I1 and I2), the Vβ10 promoter (P), or the Vβ10 coding sequence (C) in thymocytes of (G) Vβ1NT/NTRag1−/− and Vβ1NT/NTDicer−/−Rag1−/− mice or (H) Vβ1NT/NT and Vβ1NT/NTDicer−/− mice. Error bars are standard error. Significant differences: *p<0.05. This experiment was independently performed three times with at least one mouse of each genotype in each experimental replicate. I, Graph showing the average levels of transcripts for rearranged Vβ10 segments, germline (GL) Vβ10 segments, or the Vβ1NT gene in splenic αβ T-cells from Vβ1NT/NT and Vβ1NT/NTDicer−/− mice. Error bars are standard error. No significant differences were detected.
On Vβ1NT alleles, bi-directional transcription of germline Vβ10s in thymocytes precedes their heterochromatin-mediated silencing in mature αβ T-cells (21). We observed no difference in the levels of germline Vβ10 transcripts between Vβ1NT/NT and Vβ1NT/NTDicer−/− splenic αβ T-cells (Fig. 4I), consistent with normal silencing. This observation indicates that epigenetic silencing of germline Vβ10s on Vβ1NT alleles does not require Dicer-dependent processing of potential Vβ10 siRNAs.
Dicer is Required for Survival of Thymocytes Attempting TCRβ Recombination
Expression of BCL2 sustains lymphocyte survival in response to DSBs induced during V(D)J recombination (27). Therefore, as an initial means to assess whether Dicer is required for survival of cells attempting TCRβ recombination, we analyzed thymocyte development in DicerF/F, Dicer−/−, Vβ1NT/NT, and Vβ1NT/NTDicer−/− mice lacking or expressing the EμBCL2 transgene (the latter are designated B+ within the figures). We found that EμBCL2 expression partially rescued DN3-to-DN4 and DN-to-DP development of Dicer−/− thymocytes (Fig. 5A-D). We also found that combined expression of EμBCL2 and Vβ1NT completely restored DN3-to-DN4 and DN-to-DP development of Dicer−/− thymocytes to levels observed in Dicer+/+ thymocytes (Fig. 5E-H). We found similar deletion of Dicer in DN and total thymocytes of Dicer−/− mice and Dicer−/− mice containing EμBCL2 and/or Vβ1NT (Fig. 1C,F), indicating that EμBCL2 expression does not increase DN4 and DP thymocyte numbers solely by enabling thymocytes to escape Dicer deletion during the DN3-to-DN4 and DN-to-DP developmental transitions. Although the ability of BCL2 to rescue DN3-to-DN4 and DN-to-DP development of Dicer−/− and Vβ1NT/NTDicer−/− thymocytes is consistent with a requirement for Dicer in survival of DN cells attempting TCRβ recombination, this phenotype also could be due to the ability of BCL2 to prevent death of dividing thymocytes.
FIGURE 5.
EμBCL2 expression prevents the death of Dicer-deficient thymocytes attempting TCRβ recombination. A, Representative CD4/CD8 FACS analysis of thymocytes from B+ and B+Dicer−/− mice. The average numbers of total thymocytes, the DN, DP, CD4+ SP and CD8+ SP cell gates, and the percentage of thymocytes in each gate are indicated. B, Graph showing average numbers of DN and DP thymocytes from mice of the indicated genotypes. Error bars are standard error. Significant differences: *p<0.05, **p≤0.01, and ***p≤ 0.001. C, Representative c-Kit/CD25 FACS data of thymocytes from B+ and B+Dicer−/− mice. The DN1, DN2, DN3, and DN4 quadrants and the percentage of DN cells in each quadrant are indicated. D, Graph showing average numbers of DN3 and DN4 thymocytes from mice of the indicated genotypes. Error bars are standard error. Significant differences: *p<0.05, **p≤0.01, and ***p≤ 0.001. E, Representative CD4/CD8 FACS analysis of thymocytes from B+Vβ1NT/NT and B+Vβ1NT/NTDicer−/− mice. The average numbers of total thymocytes, the DN, DP, CD4+ SP and CD8+ SP cell gates, and the percentage of thymocytes in each gate are indicated. F, Graph showing average numbers of DN and DP thymocytes from mice of the indicated genotypes. Error bars are standard error. Significant differences: *p<0.05 and **p≤0.01. G, Representative c-Kit/CD25 FACS data of thymocytes from B+Vβ1NT/NT and B+Vβ1NT/NTDicer−/− mice. The DN1, DN2, DN3, and DN4 quadrants and the percentage of DN cells in each quadrant are indicated. H, Graph showing average numbers of DN3 and DN4 thymocytes from mice of the indicated genotypes. Error bars are standard error. Significant differences: *p<0.05, **p≤0.01, and ***p≤ 0.001. I, Graph showing the average percentage of dead thymocytes in mice of the indicated genotypes. Error bars are standard error. Significant differences: ***p≤ 0.001. J, Graph showing average percentages of Vβ10+ thymocytes from mice of the indicated genotypes. Error bars are standard error. Significant differences: **p≤0.01, and ***p≤ 0.001. K, Representative PCR analysis of rearrangements involving the indicated Vβ segments to DJβ1.1/DJβ1.2 or DJβ2.1/DJβ2.2 complexes in thymocytes of mice of the indicated genotypes. A Cβ PCR control for genomic DNA content is also shown. This experiment was done three independent times on one mouse of each genotype. L, Graph showing the average ratios of the percentages of Vβ10+ TCRβhi and Vβ10+ TCRβlo thymocytes from mice of the indicated genotypes. Error bars are standard error. Significant differences: **p≤0.01. Each experiment in this figure was independently performed three times with at least one mouse of each genotype in each replicate.
We next evaluated the effect of EμBCL2 upon the survival of Dicer−/− and Vβ1NT/NTDicer−/− thymocytes. Since death of Dicer−/− thymocytes cannot be detected ex vivo by increased Annexin V staining or reduced mitochondrial potential (8), we quantified dead thymocytes by FACS analysis using forward and side scatter to distinguish between live and dead cells. We compared the percentages, rather than numbers, of dead thymocytes since mice of Dicer-deficient backgrounds harbor reduced thymic cellularity relative to Dicer-sufficient backgrounds. We detected a ~5-fold increase in the percentage of dead thymocytes in Dicer−/− mice relative to DicerF/F mice (Fig. 5I). We found that EμBCL2 expression in Dicer−/− mice lowered the percentage of dead cells to levels observed in DicerF/F and EμBCL2 mice (Fig. 5I). We also found that Vβ1NT expression in Dicer−/− mice lowered the percentage of dead thymocytes to levels slightly above those detected in DicerF/F and Vβ1NT/NT mice (Fig. 5I), while combined EμBCL2 and Vβ1NT expression in Dicer−/− mice lowered the percentage of dead thymocytes to levels found in DicerF/F, Vβ1NT/NT, and EμBCL2 mice (Fig. 5I). Since expression of pre-assembled TCRβ transgenes/genes inhibits RAG activity in DN cells and bypasses necessity of TCRβ recombination for thymocyte development, the ability of Vβ1NT to decrease the percentage of dead Dicer−/− thymocytes supports the idea that Dicer is required for survival of cells attempting TCRβ recombination. Yet, it is important to note that since expression of both Vβ1NT and EμBCL2 further decreases the percentage of dead Dicer−/− thymocytes, our findings are consistent with an additional role of Dicer in promoting thymocyte survival during cell division (8). The notion that Dicer promotes survival of proliferating thymocytes is also supported by our data that Dicer deletion in Vβ1NT/NTRag1−/− mice leads to reduced numbers of DN4 and DP cells (Fig. 4A-D).
To evaluate whether Dicer is required for survival of DN cells attempting TCRβ recombination, we analyzed Vβ10 expression and rearrangement in thymocytes of Vβ1NT/NT and Vβ1NT/NTDicer−/− mice lacking or expressing the EμBCL2 transgene. We detected a ~12-fold increase in the percentages of Vβ10 thymocytes in Vβ1NT/NTDicer−/− mice expressing EμBCL2 as compared to Vβ1NT/NTDicer−/− mice lacking EμBCL2 (Fig. 5J). The elevated percentages of Vβ10+ thymocytes in Vβ1NT/NTDicer−/− mice expressing EμBCL2 were comparable to those in Vβ1NT/NT mice expressing EμBCL2 (Fig. 5J). The increased percentages of Vβ10+ cells in Vβ1NT/NTDicer−/− and Vβ1NT/NT mice expressing EμBCL2 corresponded with higher levels of Vβ10 recombination (Fig. 5K). The ability of BCL2 to rescue Vβ10+ thymocytes in Vβ1NT/NTDicer−/− mice is consistent with the notion that Dicer is required for survival of cells attempting TCRβ recombination. Since BCL2 affects αβ TCR selection (28), and αβ TCR selection can alter Vβ repertoire and levels of Vβ rearrangements between non-selected TCRβint DP thymocytes and selected TCRβhigh SP thymocytes (29), these phenotypes also could be caused by differences in selection of Vβ10+ cells among mice of these genotypes. Although EμBCL2 expression caused a ~2-fold increase in the ratio of TCRβhighVβ10+ to TCRβintVβ10+ thymocytes in Vβ1NT/NTDicer−/− mice (Fig. 5L), this change was lower than the ~4-fold increase in the percentage of Vβ10+ thymocytes observed between these mice. In addition, the ratio of TCRβhighVβ10+ to TCRβintVβ10+ thymocyte numbers in Vβ1NT/NT mice was not altered by EμBCL2 expression (Fig. 5L). Collectively, these data indicate that the increased frequency of Vβ10+ cells in Vβ1NT/NTDicer−/− and Vβ1NT/NT mice expressing EμBCL2 compared to lacking EμBCL2 resulted from both greater survival of DN cells attempting Vβ10 rearrangements and increased selection of Vβ10+ DP thymocytes.
We sought another means to show that Dicer is required for survival of cells attempting TCRβ recombination. We previously showed that TCRβ alleles containing a pre-assembled DJβ1 complex (DJ alleles) exhibit increased Vβ rearrangement frequencies relative to wild-type alleles (24). Despite the ability of pre-assembled functional TCRβ genes to activate β-selection signals that inhibit RAG activity and Vβ recombination, ~5% of Vβ1NT/+ DN3 cells assemble VβDJβ1 joins on wild-type alleles (21). Thus, we reasoned that comparison of DN3-to-DN4 and DN-to-DP thymocyte development between Vβ1NT/+ and Vβ1NT/DJ mice on wild-type and Dicer-deficient backgrounds would enable us to determine whether Dicer is required for survival of cells attempting TCRβ recombination. To validate this approach, we first needed to show that Vβ rearrangement frequencies are increased on DJ alleles in Vβ1NT/DJ mice relative to on wild-type alleles in Vβ1NT/+ mice. We observed greater percentages of TCRβhigh thymocytes expressing each Vβ assayed in Vβ1NT/DJ mice relative to Vβ1NT/+ mice (Fig. 6A-B). We also detected higher levels of Vβ-to-DJβ rearrangements involving DJβ1.1 or DJβ1.2 complexes of all Vβs assayed in Vβ1NT/DJ cells as compared to in Vβ1NT/+ cells (Fig. 6C). Since β-selection does not alter Vβ repertoire during DN3-to-DN4 and DN-to-DP development (30), these data confirm that Vβ recombination frequencies are higher on DJ alleles in Vβ1NT/DJ mice as compared to on wild-type alleles in Vβ1NT/+ mice.
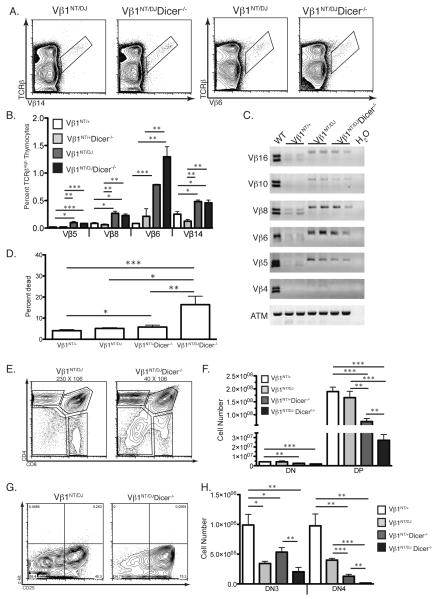
FIGURE 6.
Dicer is required for survival of DN thymocytes attempting Vβ recombination. A, Representative TCRβ/Vβ14 and TCRβ/Vβ6 FACS data from Vβ1NT/DJ and Vβ1NT/DJDicer−/− mice. The Vβ14+ and Vβ6+ cell gates are indicated. B, Graph showing average percentages of TCRβhigh thymocytes expressing the indicated Vβ segments in Vβ1NT/+, Vβ1NT/+Dicer−/−, Vβ1NT/DJ, and Vβ1NT/DJDicer−/− mice. Error bars are standard error. Significant differences: *p<0.05, **p≤0.01, and ***p≤ 0.001. C, Representative PCR analysis of rearrangements involving the indicated Vβ segments to DJβ1.1/DJβ1.2 or DJβ2.1/DJβ2.2 complexes in thymocytes of mice of the indicated genotypes. A Cβ PCR control for genomic DNA content is also shown. D, Graph showing the average percentage of dead thymocytes in mice of the indicated genotypes. Error bars are standard error. Significant differences: *p<0.05, **p≤0.01, and ***p≤ 0.001. E, Representative CD4/CD8 FACS data of thymocytes from Vβ1NT/DJ and Vβ1NT/DJDicer−/− mice. The average numbers of total thymocytes and the DN, DP, CD4+ SP and CD8+ SP cell gates are indicated. F, Graph showing the average numbers of DN and DP thymocytes from mice of the indicated genotypes. Error bars are standard error. Significant differences: *p<0.05, **p≤0.01, and ***p≤ 0.001. G, Representative c-Kit/CD25 FACS data of DN thymocytes from Vβ1NT/DJ and Vβ1NT/DJDicer−/− mice. The DN1, DN2, DN3, and DN4 thymocyte quadrants and the percentages of DN cells within each of these quadrants are indicated. H, Graph showing the average numbers of DN3 and DN4 cells from mice of the indicated genotypes. Error bars are standard error. Significant differences: *p<0.05, **p≤0.01, and ***p≤ 0.001. Each experiment in this figure was independently performed three times with at least one mouse of each genotype in each experimental replicate.
To directly evaluate whether Dicer is required for survival of DN thymocytes attempting TCRβ recombination, we analyzed the effects of Dicer deletion upon Vβ repertoire and DN3-to-DN4 and DN-to-DP thymocyte development in Vβ1NT/DJ and Vβ1NT/+ mice. We observed equivalent percentages of thymocytes expressing each Vβ assayed in Vβ1NT/DJ and Vβ1NT/DJDicer−/− mice (Fig. 6A-B). We also detected similar levels of Vβ-to-DJβ rearrangements involving DJβ1.1 or DJβ1.2 complexes in Vβ1NT/DJ and Vβ1NT/DJDicer−/− thymocytes (Fig. 6C). In addition, we found equivalent deletion of Dicer in DN thymocytes of Vβ1NT/+Dicer−/− mice and Vβ1NT/DJDicer−/− mice (Fig. 1F). At first approximation, these data suggest that Dicer inactivation has no effect upon the survival of Vβ1NT/DJ cells attempting Vβ rearrangements. Yet, we considered that Vβ recombination frequencies on DJ alleles might be elevated high enough to mask death of Vβ1NT/DJDicer−/− cells attempting Vβ rearrangements. Consistent with this idea, Dicer deletion in Vβ1NT/DJ mice led to a more substantial increase of dead thymocytes and a corresponding decrease in DN-to-DP proliferative expansion than in Vβ1NT/+ mice (Fig. 6D-F). We also found that deletion of Dicer in Vβ1NT/+ mice caused ~2-fold decreases in the numbers of DN3 and DN4 thymocytes (Fig. 6G-H); whereas deletion of Dicer in Vβ1NT/DJ mice had no significant effect (albeit a ~2-fold reduction) on DN3 thymocyte numbers, yet caused a ~10-fold decrease in DN4 cell numbers (Fig. 6H). These data indicate that Dicer deletion caused a more pronounced loss of DN4 cells in Vβ1NT/DJ mice as compared to Vβ1NT/+ mice. Since the assembly of functional VDJCβ genes is required for DN3-to-DN4 development and the only phenotypic difference between Vβ1NT/DJ and Vβ1NT/+ mice is the frequency of Vβ recombination, these data provide unequivocal evidence that Dicer is required for survival of DN thymocytes attempting TCRβ recombination.
Discussion
We have shown that Dicer is required for normal survival of DN thymocytes attempting Vβ recombination. TCRβ genes are assembled in G0/G1 phase cells through an ordered process involving Dβ-to-Jβ and then Vβ-to-DJβ recombination (7). Expression of functional TCRβ genes drives DN3 cells into S phase and through many cell cycles as they differentiate into DN4 cells (20, 31). Vβ-to-DJβ recombination is repressed through TCRβ-mediated feedback inhibition signals (7). Yet, functional TCRβ genes can be detected on both alleles in 1-10% of αβ T-cells (7), indicating that DN3 cells can attempt Vβ recombination while experiencing proliferation signals from assembled VβDJβCβ genes. The increased death and decreased proliferative expansion of Vβ1NT/DJDicer−/− thymocytes compared to Vβ1NT/+Dicer−/− thymocytes demonstrates that Dicer is required for survival of DN3 cells that attempt Vβ recombination while experiencing proliferation signals from TCRβ genes assembled first on other allele. Despite TCRβ-mediated feedback inhibition, ~6% of Vβ1NT/+ and Vβ1NT/NT splenic αβ T-cells contain Vβ-to-DJβ2 rearrangements that replace Vβ1NT genes (21), revealing that a significant percentage of DN3 thymocytes that have assembled functional VβDJβCβ1 genes can attempt replacement Vβ-to-DJβ2 rearrangements. The increased death, decreased proliferative expansion, reduced levels of Vβ10-to-DJβ2 rearrangements, and decreased frequency of Vβ10+ thymocytes in Vβ1NT/NTDicer−/− mice relative to Vβ1NT/NT mice demonstrates that Dicer also is required for survival of DN3 cells that attempt Vβ-to-DJβ2 recombination while experiencing proliferation signals from VβDJβCβ1 genes assembled on the same allele.
Although pre-assembled functional TCRβ genes inhibit Vβ recombination, their suppression of other V(D)J recombination events likely contributes to their ability to partially rescue the development of Dicer-deficient thymocytes. In DN cells, Dβ-to-Jβ rearrangements occur on both alleles and are not subject to feedback inhibition (7). Despite this lack of regulation, Dβ-to-Jβ recombination is decreased on wild-type alleles in αβ T-cells of Vβ1NT/+ mice (18, 21), likely because expression of pre-assembled TCRβ genes/transgenes accelerates DN3-to-DN4 thymocyte development (18, 32). The decreased death and proliferative expansion of Dicer−/− thymocytes relative to Dicer+/+ thymocytes may reflect that Dicer is required for survival of DN3 cells that attempt Dβ-to-Jβ recombination while experiencing TCRβ proliferation signals. In DN cells, the RAG proteins also promote TCRγ, TCRδ, and IgH recombination and induce “off-target” DSBs at other genetic loci (7). Since pre-assembled TCRβ genes/transgenes down-regulate RAG activity in DN cells (7), the decreased death and increased proliferative expansion of Vβ1NT/NT Dicer−/− thymocytes as compared to Dicer−/− thymocytes is consistent with a requirement for Dicer in survival of DN3 cells that induce RAG DSBs outside of TCRβ loci while experiencing proliferation signals. Normal DP-to-SP thymocyte development in Dicer−/− mice indicates that Dicer is not required for survival in response to RAG DSBs induced in DP thymocytes that do not experience proliferation signals. We propose that this would be the case in DN3 cells not undergoing β-selection.
Dicer-generated miRNAs are required for normal survival of mammalian cells in response to DSBs (33). We showed that a two-fold reduction in expression levels of histone H2AX leads to impaired DSB responses during V(D)J recombination (34). Since loss of Dicer-dependent miRNAs can lead to two-fold changes in the expression of hundreds of proteins, including factors that function in the same pathways (35, 36), impaired survival of Dicer−/− DN cells that induce RAG DSBs could be due to altered constitutive expression of DNA damage response proteins. Upon induction of DSBs in non-lymphoid cells, the ATM, p53, and/or p38MAPK proteins signal to increase generation of mature miRNAs that promote cellular survival (37, 38). Since RAG DSBs signal through ATM, p53, and p38MAPK to eliminate DN cells that attempt to proliferate with un-repaired TCR loci (39, 40), impaired survival of Dicer−/− thymocytes could be due to their inability to up-regulate expression of pro-survival miRNAs in response to RAG DSBs. Recent studies have revealed a requirement for Dicer in the ability of mammalian cells to respond to and repair DSBs by processing double-stranded RNAs (dsRNAs) formed upon transcription of broken DNA ends (41, 42). Perhaps transcription of RAG-generated hairpin coding ends generates dsRNAs that Dicer processes to promote V(D)J recombination and inhibit apoptosis of recombining cells. The comparison of miRNA, mRNA, and protein expression in among wild-type, Dicer−/−, and Rag1−/− DN3 cells and follow-up functional studies will be required to elucidate the precise mechanisms by which Dicer sustains survival of thymocytes during V(D)J recombination.
Our data also reveal a requirement for Dicer in promoting survival of proliferating thymocytes. The ability of Vβ1NT, alone or in combination with Rag1-deficiency, to only partially rescue DN3-to-DN4 and DN-to-DP development in Dicer−/− mice is consistent with the postulated requirement for Dicer in survival of dividing thymocytes (8). Our data that combined expression of Vβ1NT and BCL2 completely rescued these developmental transitions in Dicer−/− mice provides strong evidence that Dicer is required for the survival of proliferating thymocytes. DNA replication-associated DSBs are common and unavoidable in each S phase. Similar to mice with conditional Dicer deletion in DN cells, mice with thymocyte-specific inactivation of the Brca1, Brca2, or Blm proteins that repair DNA replication-associated DSBs exhibit impaired proliferative expansion and increased apoptosis of thymocytes (43-45). Expression of the EμBCL2 transgene, but not pre-assembled TCRβ/TCRα transgenes, rescued these phenotypes (43-45), revealing that the ability of thymocytes to survive in response to DNA replication-associated DSBs is required for normal DN-to-DP proliferative expansion. Therefore, we conclude that Dicer also promotes αβ T-cell differentiation by controlling cellular survival and death decisions in response to DNA replication-associated DSBs.
Our findings suggest that impaired proliferation and survival of cells in response to replication associated DSBs could contribute to Dicer-deficient phenotypes. Conditional Dicer deletion in DN cells leads to lower numbers of developing and mature αβ T-cells; but not γδ T-cells that develop with less cellular expansion, nor any obvious pathological conditions (8, 9). However, Dicer deletion in DP cells after thymocyte proliferative expansion has minimal impact upon thymocyte numbers; yet leads to lower numbers of peripheral αβ T-cells that exhibit impaired survival during proliferation, and causes lethal inflammatory disease late in life (9, 46-49). Moreover, Dicer deletion in mature αβ T-cells as they differentiate into T-reg cells has no effect upon the development or numbers of these immunosuppressive cells, but ablates T-reg function and causes lethal inflammatory disease by two months of age (49, 50). Considering the requirement for Dicer in survival of proliferating cells, Dicer deletion in DN thymocytes would cause death of cells in all mature αβ T-lymphocyte lineages and thus not disrupt adaptive immune system homeostasis. In contrast, Dicer deletion specifcally in αβ T-regs preceding antigen-dependent proliferation would impair their survival and necessary immuno-regulatory functions. Since DSBs are induced by replication, transcription, and byproducts of metabolism and thus ubiquitous, our findings indicate that impaired DNA damage responses should be considered when interpreting Dicer-deficient phenotypes.
Acknowledgments
This work was supported by the Cell and Molecular Biology Training Grant GM-07229 of the University of Pennsylvania (B.L.B.), the Training Grant in Computational Genomics 5T32H000046-14 of the Perelman School of Medicine of the University of Pennsylvania (L.J.R.), a Leukemia and Lymphoma Society Scholar Award (C.H.B.), and NIH grants CA125195 and CA136470 (C.H.B.).
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Grewal SI, Jia S. Heterochromatin revisited. Nat Rev Genet. 2007;8:35–46. doi: 10.1038/nrg2008. [DOI] [PubMed] [Google Scholar]
- 2.Bushati N, Cohen SM. microRNA functions. Annu Rev Cell Dev Biol. 2007;23:175–205. doi: 10.1146/annurev.cellbio.23.090506.123406. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, Mills AA, Elledge SJ, Anderson KV, Hannon GJ. Dicer is essential for mouse development. Nat Genet. 2003;35:215–217. doi: 10.1038/ng1253. [DOI] [PubMed] [Google Scholar]
- 4.Xiao C, Rajewsky K. MicroRNA control in the immune system: basic principles. Cell. 2009;136:26–36. doi: 10.1016/j.cell.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 5.Tam OH, Aravin AA, Stein P, Girard A, Murchison EP, Cheloufi S, Hodges E, Anger M, Sachidanandam R, Schultz RM, Hannon GJ. Pseudogene-derived small interfering RNAs regulate gene expression in mouse oocytes. Nature. 2008;453:534–538. doi: 10.1038/nature06904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watanabe T, Totoki Y, Toyoda A, Kaneda M, Kuramochi-Miyagawa S, Obata Y, Chiba H, Kohara Y, Kono T, Nakano T, Surani MA, Sakaki Y, Sasaki H. Endogenous siRNAs from naturally formed dsRNAs regulate transcripts in mouse oocytes. Nature. 2008;453:539–543. doi: 10.1038/nature06908. [DOI] [PubMed] [Google Scholar]
- 7.Brady BL, Steinel NC, Bassing CH. Antigen receptor allelic exclusion: an update and reappraisal. J Immunol. 2010;185:3801–3808. doi: 10.4049/jimmunol.1001158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cobb BS, Nesterova TB, Thompson E, Hertweck A, O’Connor E, Godwin J, Wilson CB, Brockdorff N, Fisher AG, Smale ST, Merkenschlager M. T cell lineage choice and differentiation in the absence of the RNase III enzyme Dicer. J Exp Med. 2005;201:1367–1373. doi: 10.1084/jem.20050572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chong MM, Zhang G, Cheloufi S, Neubert TA, Hannon GJ, Littman DR. Canonical and alternate functions of the microRNA biogenesis machinery. Genes & development. 2010;24:1951–1960. doi: 10.1101/gad.1953310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koralov SB, Muljo SA, Galler GR, Krek A, Chakraborty T, Kanellopoulou C, Jensen K, Cobb BS, Merkenschlager M, Rajewsky N, Rajewsky K. Dicer ablation affects antibody diversity and cell survival in the B lymphocyte lineage. Cell. 2008;132:860–874. doi: 10.1016/j.cell.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 11.Chakraborty T, Chowdhury D, Keyes A, Jani A, Subrahmanyam R, Ivanova I, Sen R. Repeat organization and epigenetic regulation of the DH-Cμ domain of the immunoglobulin heavy-chain gene locus. Mol Cell. 2007;27:842–850. doi: 10.1016/j.molcel.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Giallourakis C, Alt FW, Bassing CH. V(D)J recombinational accessibilityheading in the opposite direction? Nat Immunol. 2004;5:561–562. doi: 10.1038/ni0604-561. [DOI] [PubMed] [Google Scholar]
- 13.Belver L, de Yebenes VG, Ramiro AR. MicroRNAs prevent the generation of autoreactive antibodies. Immunity. 2010;33:713–722. doi: 10.1016/j.immuni.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 15.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 16.Xu Y, Davidson L, Alt FW, Baltimore D. Function of the pre-T-cell receptor α chain in T-cell development and allelic exclusion at the T-cell receptor β locus. Proc Natl Acad Sci U S A. 1996;93:2169–2173. doi: 10.1073/pnas.93.5.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fehling HJ, Krotkova A, Saint-Ruf C, von Boehmer H. Crucial role of the pre-T-cell receptor α gene in development of αβ but not γδ T cells. Nature. 1995;375:795–798. doi: 10.1038/375795a0. [DOI] [PubMed] [Google Scholar]
- 18.Yang-Iott KS, Carpenter AC, Rowh MA, Steinel N, Brady BL, Hochedlinger K, Jaenisch R, Bassing CH. TCRβ feedback signals inhibit the coupling of recombinationally accessible Vβ14 segments with DJβ complexes. J Immunol. 2010;184:1369–1378. doi: 10.4049/jimmunol.0900723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinel NC, Brady BL, Carpenter AC, Yang-Iott KS, Bassing CH. Posttranscriptional silencing of VβDJβCβ genes contributes to TCRβ allelic exclusion in mammalian lymphocytes. J Immunol. 2010;185:1055–1062. doi: 10.4049/jimmunol.0903099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brady BL, Bassing CH. Differential Regulation of Proximal and Distal Vβ Segments Upstream of a Functional VDJβ1 Rearrangement upon β-Selection. J Immunol. 2011;187:3277–3285. doi: 10.4049/jimmunol.1101079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brady BL, Oropallo MA, Yang-Iott KS, Serwold T, Hochedlinger K, Jaenisch R, Weissman IL, Bassing CH. Position-dependent silencing of germline Vβ segments on TCRβ alleles containing preassembled VβDJβCβ1 genes. J Immunol. 2010;185:3564–3573. doi: 10.4049/jimmunol.0903098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee PP, Fitzpatrick DR, Beard C, Jessup HK, Lehar S, Makar KW, Perez-Melgosa M, Sweetser MT, Schlissel MS, Nguyen S, Cherry SR, Tsai JH, Tucker SM, Weaver WM, Kelso A, Jaenisch R, Wilson CB. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15:763–774. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- 23.Strasser A, Harris AW, Cory S. bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 1991;67:889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- 24.Carpenter AC, Yang-Iott KS, Chao LH, Nuskey B, Whitlow S, Alt FW, Bassing CH. Assembled DJβ complexes influence TCRβ chain selection and peripheral Vβ repertoire. J Immunol. 2009;182:5586–5595. doi: 10.4049/jimmunol.0803270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shinkai Y, Koyasu S, Nakayama K, Murphy KM, Loh DY, Reinherz EL, Alt FW. Restoration of T cell development in RAG-2-deficient mice by functional TCR transgenes. Science. 1993;259:822–825. doi: 10.1126/science.8430336. [DOI] [PubMed] [Google Scholar]
- 26.Godfrey DI, Kennedy J, Suda T, Zlotnik A. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3-CD4-CD8-triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J Immunol. 1993;150:4244–4252. [PubMed] [Google Scholar]
- 27.Bredemeyer AL, Sharma GG, Huang CY, Helmink BA, Walker LM, Khor KC, Nuskey B, Sullivan KE, Pandita TK, Bassing CH, Sleckman BP. ATM stabilizes DNA double-strand-break complexes during V(D)J recombination. Nature. 2006;442:466–470. doi: 10.1038/nature04866. [DOI] [PubMed] [Google Scholar]
- 28.Strasser A, Harris AW, von Boehmer H, Cory S. Positive and negative selection of T cells in T-cell receptor transgenic mice expressing a bcl-2 transgene. Proc Natl Acad Sci U S A. 1994;91:1376–1380. doi: 10.1073/pnas.91.4.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Boehmer H. Selection of the T-cell repertoire: receptor-controlled checkpoints in T-cell development. Adv Immunol. 2004;84:201–238. doi: 10.1016/S0065-2776(04)84006-9. [DOI] [PubMed] [Google Scholar]
- 30.Wilson A, Marechal C, MacDonald HR. Biased Vβ usage in immature thymocytes is independent of DJβ proximity and pTα pairing. J Immunol. 2001;166:51–57. doi: 10.4049/jimmunol.166.1.51. [DOI] [PubMed] [Google Scholar]
- 31.Sicinska E, Aifantis I, Le Cam L, Swat W, Borowski C, Yu Q, Ferrando AA, Levin SD, Geng Y, von Boehmer H, Sicinski P. Requirement for cyclin D3 in lymphocyte development and T cell leukemias. Cancer Cell. 2003;4:451–461. doi: 10.1016/s1535-6108(03)00301-5. [DOI] [PubMed] [Google Scholar]
- 32.Baldwin TA, Sandau MM, Jameson SC, Hogquist KA. The timing of TCR alpha expression critically influences T cell development and selection. J Exp Med. 2005;202:111–121. doi: 10.1084/jem.20050359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kraemer A, Anastasov N, Angermeier M, Winkler K, Atkinson MJ, Moertl S. MicroRNA-mediated processes are essential for the cellular radiation response. Radiat Res. 2011;176:575–586. doi: 10.1667/rr2638.1. [DOI] [PubMed] [Google Scholar]
- 34.Bassing CH, Suh H, Ferguson DO, Chua KF, Manis J, Eckersdorff M, Gleason M, Bronson R, Lee C, Alt FW. Histone H2AX: a dosage-dependent suppressor of oncogenic translocations and tumors. Cell. 2003;114:359–370. doi: 10.1016/s0092-8674(03)00566-x. [DOI] [PubMed] [Google Scholar]
- 35.Baek D, Villen J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature. 2008;455:64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Selbach M, Schwanhausser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455:58–63. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- 37.Wan G, Mathur R, Hu X, Zhang X, Lu X. miRNA response to DNA damage. Trends Biochem Sci. 2011;36:478–484. doi: 10.1016/j.tibs.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cannell IG, Kong YW, Johnston SJ, Chen ML, Collins HM, Dobbyn HC, Elia A, Kress TR, Dickens M, Clemens MJ, Heery DM, Gaestel M, Eilers M, Willis AE, Bushell M. p38 MAPK/MK2-mediated induction of miR-34c following DNA damage prevents Myc-dependent DNA replication. Proc Natl Acad Sci U S A. 2010;107:5375–5380. doi: 10.1073/pnas.0910015107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pedraza-Alva G, Koulnis M, Charland C, Thornton T, Clements JL, Schlissel MS, Rincon M. Activation of p38 MAP kinase by DNA double-strand breaks in V(D)J recombination induces a G2/M cell cycle checkpoint. Embo J. 2006;25:763–773. doi: 10.1038/sj.emboj.7600972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dujka ME, Puebla-Osorio N, Tavana O, Sang M, Zhu C. ATM and p53 are essential in the cell-cycle containment of DNA breaks during V(D)J recombination in vivo. Oncogene. 2010;29:957–965. doi: 10.1038/onc.2009.394. [DOI] [PubMed] [Google Scholar]
- 41.Francia S, Michelini F, Saxena A, Tang D, de Hoon M, Anelli V, Mione M, Carninci P, d’Adda di Fagagna F. Site-specific DICER and DROSHA RNA products control the DNA-damage response. Nature. 2012;488:231–235. doi: 10.1038/nature11179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei W, Ba Z, Gao M, Wu Y, Ma Y, Amiard S, White CI, Rendtlew Danielsen JM, Yang YG, Qi Y. A role for small RNAs in DNA double-strand break repair. Cell. 2012;149:101–112. doi: 10.1016/j.cell.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 43.Mak TW, Hakem A, McPherson JP, Shehabeldin A, Zablocki E, Migon E, Duncan GS, Bouchard D, Wakeham A, Cheung A, Karaskova J, Sarosi I, Squire J, Marth J, Hakem R. Brcal required for T cell lineage development but not TCR loci rearrangement. Nat Immunol. 2000;1:77–82. doi: 10.1038/76950. [DOI] [PubMed] [Google Scholar]
- 44.Cheung AM, Hande MP, Jalali F, Tsao MS, Skinnider B, Hirao A, McPherson JP, Karaskova J, Suzuki A, Wakeham A, You-Ten A, Elia A, Squire J, Bristow R, Hakem R, Mak TW. Loss of Brca2 and p53 synergistically promotes genomic instability and deregulation of T-cell apoptosis. Cancer Res. 2002;62:6194–6204. [PubMed] [Google Scholar]
- 45.Babbe H, Chester N, Leder P, Reizis B. The Bloom’s syndrome helicase is critical for development and function of the alphabeta T-cell lineage. Mol Cell Biol. 2007;27:1947–1959. doi: 10.1128/MCB.01402-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muljo SA, Ansel KM, Kanellopoulou C, Livingston DM, Rao A, Rajewsky K. Aberrant T cell differentiation in the absence of Dicer. J Exp Med. 2005;202:261–269. doi: 10.1084/jem.20050678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cobb BS, Hertweck A, Smith J, O’Connor E, Graf D, Cook T, Smale ST, Sakaguchi S, Livesey FJ, Fisher AG, Merkenschlager M. A role for Dicer in immune regulation. J Exp Med. 2006;203:2519–2527. doi: 10.1084/jem.20061692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang N, Bevan MJ. Dicer controls CD8+ T-cell activation, migration, and survival. Proc Natl Acad Sci U S A. 2010;107:21629–21634. doi: 10.1073/pnas.1016299107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chong MM, Rasmussen JP, Rudensky AY, Littman DR. The RNAseIII enzyme Drosha is critical in T cells for preventing lethal inflammatory disease. J Exp Med. 2008;205:2005–2017. doi: 10.1084/jem.20081219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou X, Jeker LT, Fife BT, Zhu S, Anderson MS, McManus MT, Bluestone JA. Selective miRNA disruption in T reg cells leads to uncontrolled autoimmunity. J Exp Med. 2008;205:1983–1991. doi: 10.1084/jem.20080707. [DOI] [PMC free article] [PubMed] [Google Scholar]