Abstract
Children with neurofibromatosis type 1 (NF1) are prone to learning and behavioral abnormalities, including problems with spatial learning and attention. The molecular etiology for these deficits is unclear, as previous studies have implicated defective dopamine, cAMP and Ras homeostasis. Using behavioral, electrophysiological and primary culture, we now demonstrate that reduced dopamine signaling is responsible for cAMP-dependent defects in neuron function and learning. Collectively, these results establish defective dopaminergic function as one contributing factor underlying impaired spatial learning and memory in children and adults with NF1, and support the use of treatments that restore normal dopamine homeostasis for select individuals.
Introduction
Neurofibromatosis type 1 is a common neurogenetic disorder in which 60–80% of affected children exhibit impairments in learning, memory, and attention1, 2. However, the molecular and neurochemical bases for these deficits are unclear, as revealed by studies using Nf1 genetically-engineered mouse (GEM) strains. Whereas spatial learning and memory defects in Nf1 mutant mice can result from abnormalities in NF1 protein (neurofibromin) Ras/ERK regulation3, selective and nonselective attention deficits have been shown to reflect both Ras-dependent and Ras-independent signaling4–6. In this regard, Ras-dependent abnormalities in Nf1 GEM are corrected by Lovastatin administration4, while Ras-independent deficits result from abnormal dopamine homeostasis and are not corrected by Lovastatin5, 6. Since neurofibromin also positively controls cyclic AMP levels7, 8 and olfactory learning and short term memory deficits in Nf1 mutant flies result from impaired G-protein-activated adenylyl cyclase activity9, 10 and dopamine receptor function11, we sought to determine whether cAMP/dopaminergic dysfunction also causes the impaired hippocampal learning in preclinical Nf1 GEM. Using a complementary combination of behavioral, electrophysiological and primary neuronal culture methods, we establish that reduced dopamine/cAMP signaling is responsible for the defects in hippocampal neuron function as well as spatial learning and memory in Nf1 mutant mice.
Materials and Methods
Methods and relevant references are available in the online version of the paper.
Mice
Nf1+/−GFAPCKO (CKO) with one non-functional Nf1 allele in all somatic cells and complete Nf1 loss in GFAP+ (glial) cells and littermate control Nf1flox/flox (CTL) mice were maintained on an inbred C57BL/6 background with ad libitum access to food and water. All experiments were performed on 3–4 month old mice, unless otherwise stated, using approved protocols established by the Washington University Animal Studies Committee.
Results
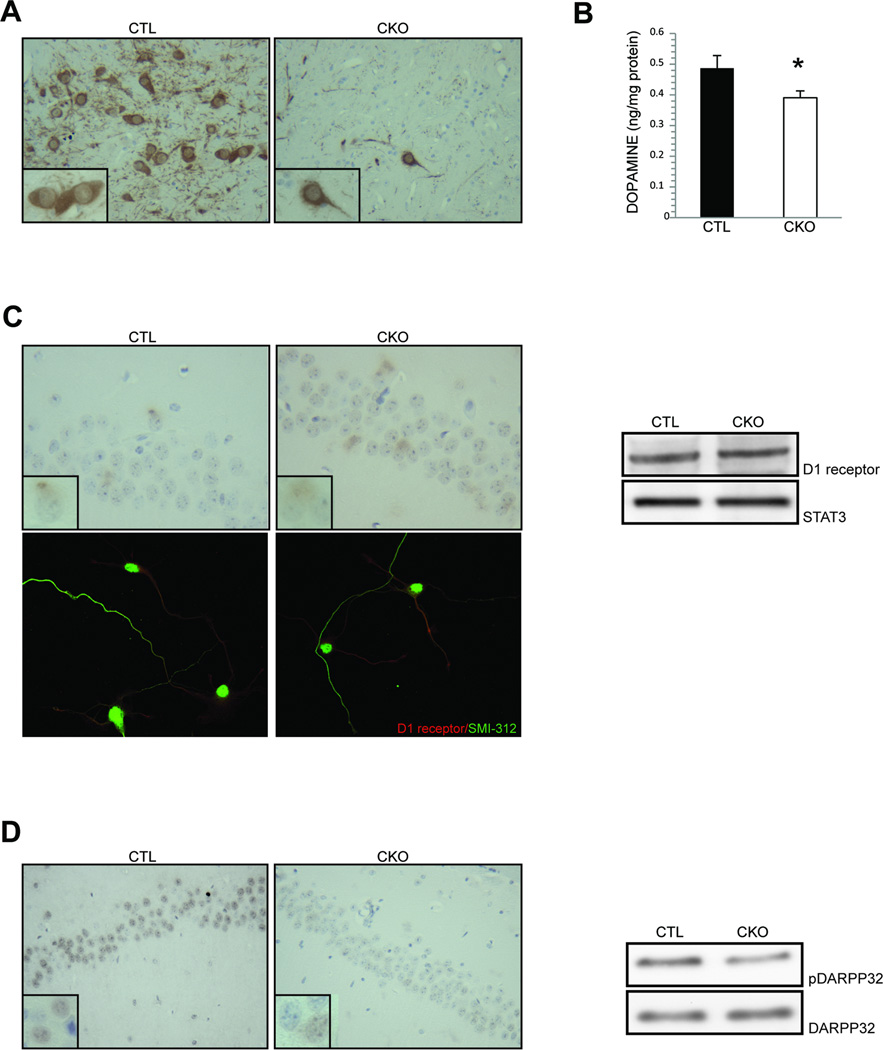
The ventral tegmental area (VTA) is a component of the dopaminergic mesolimbic pathway that projects to CA1 region of the hippocampus. As previously reported in the striatum of Nf1 mutant (CKO) mice5, we now show that tyrosine hydroxylase (TH) expression (Fig. 1A) is also reduced in the VTA compared to littermate controls. Consistent with the reduced TH expression, direct measurements of hippocampal dopamine levels (Fig. 1B) and DARPP32 phosphorylation (Fig. 1D) reveal a 20% and 61% reduction in CKO mice relative to control mice, respectively. In contrast, post-synaptic D1 receptor expression is similar in hippocampal neurons from CKO and wild-type mice both in vitro and in vivo (Fig. 1C), indicating a presynaptic dopaminergic defect, similar to our previous findings in the striatum6.
Figure 1. Reduced hippocampal dopamine signaling is observed in Nf1 mutant mice.
(A) Reduced numbers of tyrosine hydroxylase (TH)-expressing neurons are detected in the ventral tegmental area (VTA) of CKO mice compared to controls. (B) Dopamine levels in the hippocampus of CKO mice are 20% lower than littermate controls (CTL). (C) Hippocampal D1 receptor expression is similar in CKO and control (CTL) mice in vivo and in vitro (1.0 v. 1.1 relative fold change). (D) Hippocampal DARPP32 phosphorylation is reduced by 3-fold in the CA1 region of CKO mice relative to littermate controls (CTL). Insets are representative 600x high-power field magnification images of individual cells. Asterisk denotes p<0.05.
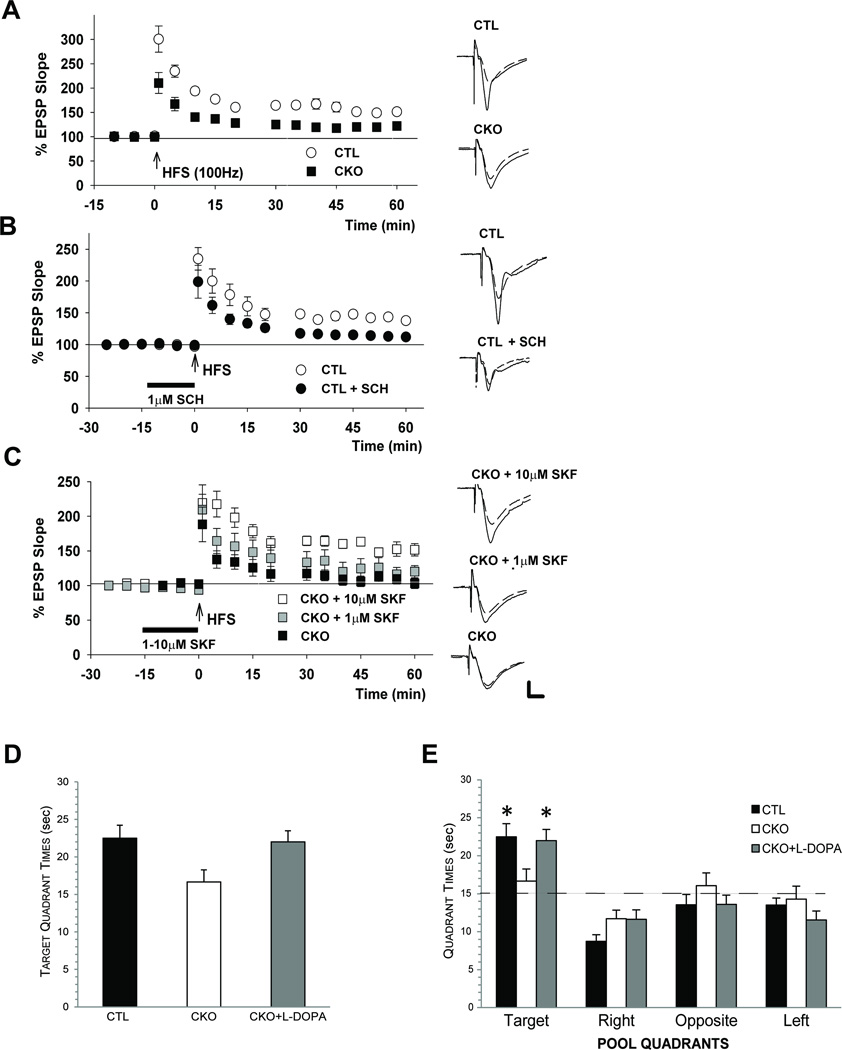
Consistent with a known role for hippocampal dopamine signaling in long term potentiation (LTP), CKO slices display diminished LTP (closed circles, EPSP slope; 128.8+ 7.6%, n=8) in response to high frequency stimulation (HFS) (open circles, EPSP slope; 165.2 + 6.8%, n=8) relative to controls (Fig. 2A). This defective CKO LTP response was rescued by treatment with a dopamine D1 receptor agonist (SKF38393). In untreated CKO slices, HFS induced short term, but not long term, potentiation (Fig. 2C, triangles, EPSP slope; 118.6 + 8.7%, n=8). While low dose SKF38393 (1 µM) treatment elicited a partial effect, (Fig. 2C, diamonds, 132.1+13.1%, n=8), 10 µM SKF38393 restored normal LTP in CKO slices (Fig. 2C, circles, 158.5+7.7%, n=8, p<0.01 vs. untreated). Conversely, inhibiting dopamine receptor function with a dopamine D1 antagonist SCH23390 (1 µM) impaired LTP in control slices, phenocopying the observed CKO mouse LTP deficits (closed circles, 120.1 + 5.8%, n=9, p<0.05 vs. untreated) (Fig. 2B). Together, these data demonstrate that LTP deficits in Nf1 mutant mice result from reduced dopamine-mediated hippocampal neuronal function.
Figure 2. Dopamine treatment rescues LTP and spatial learning deficits in Nf1 mutant mice.
(A) In control (CTL) hippocampal slices, high frequency stimulation (HFS) induces long term potentiation (LTP). In contrast, reduced LTP is observed in CKO slices. (B) D1 receptor blockade with the antagonist SCH23390 (1 µM SCH) reduces LTP in control (CTL) slices. (C) D1 receptor activation by SKF38393 (SKF) in CKO slices induces a partial LTP rescue at 1 µM and a complete rescue at 10 µM. (D) During the second probe trial in the water maze, both control (CTL) and CKO mice treated with L-DOPA (50 mg/kg; CKO + L-DOPA) spent significantly more time in the target quadrant compared to the untreated CKO group (p=0.01, p=0.02, respectively). (E) Both control (CTL) and L-DOPA-treated (CKO + L-DOPA) mice spent more time in the target quadrant compared to each of the other quadrants (p<0.002 for target vs. other quadrant comparisons; dashed line represents chance) relative to untreated CKO mice (no spatial bias). Traces depict EPSPs before (dashed lines) and 60 min after HFS (solid lines). Scale = 1mV, 5 ms. Asterisks denote p<0.05.
Next, we sought to determine whether dopamine (L-DOPA) treatment could rescue the retention deficits that we and others have observed in Nf1 GEM during probe trials in the Morris water maze3, 5. In the present study, L-DOPA treatment ameliorated post-acquisition probe trial deficits (probe trial 2) in CKO mice, as demonstrated by the amount of time spent in the target quadrant by control and L-DOPA-treated CKO mice compared to the untreated CKO group (p=0.01 and 0.02, respectively). In these experiments, L-DOPA-treated CKO mice demonstrate control-like performance levels (Fig. 2D). Importantly, L-DOPA treatment also rescued the impaired spatial bias in CKO mice, such that L-DOPA-treated CKO spend similar amounts of time in the target quadrant as control littermates (p<0.002 for target vs. all other quadrants). In contrast, CKO mice showed no quadrant bias (Fig. 2E). As we reported previously, the probe trial impairments in CKO mice are selective, as they do not exhibit performance deficits during the cued (Supplementary Fig. 1A) or place (Supplementary Fig. 1B) trials, and their performance during these trials was unaltered by L-DOPA treatment.
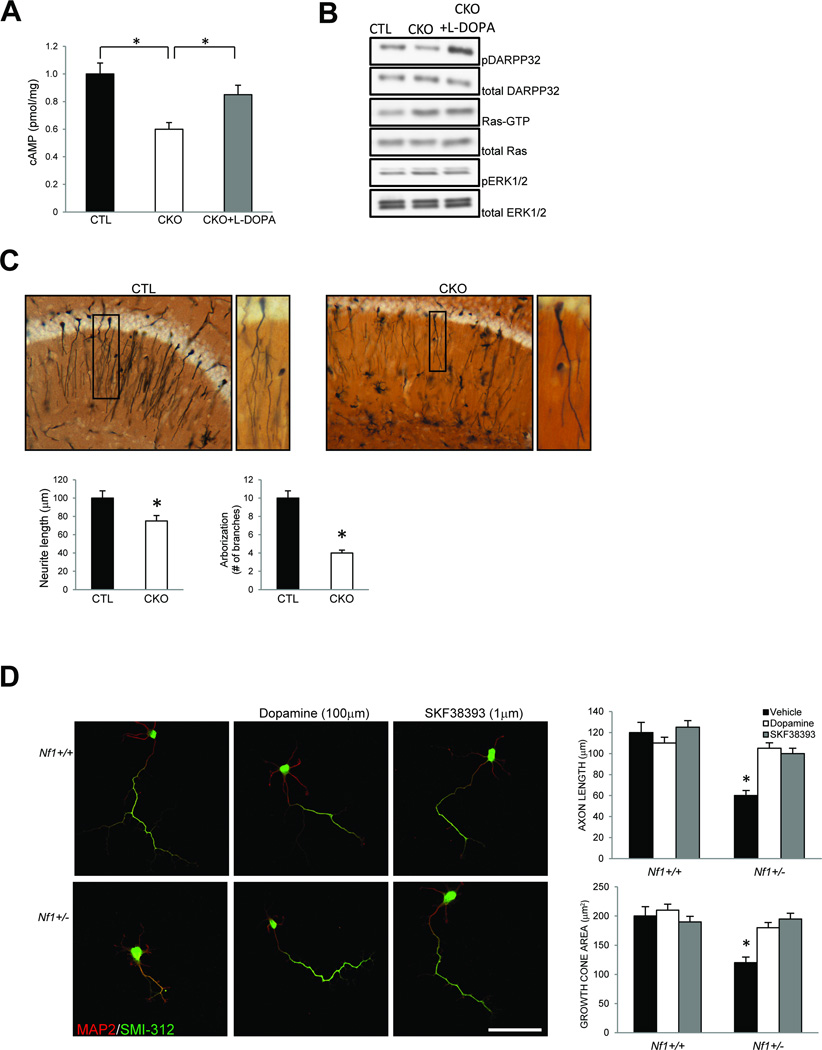
To elucidate the mechanism responsible for the dopamine-mediated hippocampal dysfunction in Nf1 mutant mice, we first measured the activity of known neurofibromin downstream signaling intermediates (Ras/ERK and cAMP) in hippocampal tissue. CKO mice exhibit reduced cAMP (Fig. 3A) and increased Ras/ERK activity (Fig. 3B) relative to littermate controls. However, systemic L-DOPA treatment (sufficient to rescue the behavioral and learning deficits) increased cAMP levels (Fig. 3A) and DARPP32 phosphorylation (Fig. 3B) in the hippocampus, but had no effect on Ras/ERK activity (Fig. 3B). These results establish that the impaired hippocampal function resulting from low dopamine levels in Nf1 CKO mice are mediated by cAMP, and not Ras, signaling.
Figure 3. Dopamine treatment restores normal hippocampal cAMP signaling in vivo and rescues Nf1+/− hippocampal neuron abnormalities in vitro.
(A) L-DOPA treatment of CKO mice (CKO + L-DOPA) increases hippocampal cAMP levels to control (CTL) levels. (B) L-DOPA treatment of CKO mice (CKO + L-DOPA) increases hippocampal DARPP32 phosphorylation without affecting Ras activity or ERK phosphorylation. (C) Golgi staining of CKO hippocampal sections (CKO) reveals shorter axons and reduced neuronal branching (arborization) relative to littermate controls (CTL). (D) Primary cultured Nf1+/− hippocampal neurons have reduced axon lengths (SMI-312) and growth cone areas relative to wild-type (Nf1+/+) littermate controls. No differences in dendritic length were observed in Nf1+/− hippocampal neurons (MAP2). The neurite length and growth cone area deficits in Nf1+/− hippocampal neurons are rescued by dopamine (100 µM) treatment and D1 receptor activation (SKF38393, 1 µM). Scale bar = 50µm. Asterisks denote p<0.05.
Neurofibromin controls central nervous system neuronal length, growth cone diameter, and survival in a cAMP-dependent manner in vitro and in vivo8. Consistent with these findings, neurons in the hippocampus of CKO mice have shorter processes and reduced neuronal arborization compared to their littermate counterparts (Fig. 3C). This cell autonomous defect is observed in primary hippocampal neuronal cultures from both CKO and Nf1+/− mice, which exhibit reduced axon lengths and growth cone areas relative to wild-type neurons (Fig. 3D, Supplementary Fig. 2). Importantly, these cAMP-dependent hippocampal neuron defects are completely rescued by either dopamine (100 µM) or D1 receptor agonist (SKF38393, 1 µM) treatment in vitro (Fig. 3D, Supplementary Fig. 2), establishing defective dopaminergic function as the primary abnormality underlying both spatial learning/memory and attention system function in these Nf1 mutant mice.
Discussion
The findings in this study coupled with previous reports on Ras/ERK-dependent defects in Nf1 mutant flies and mice have important implications for our understanding and treatment of children and adults with NF1-associated learning and memory deficits. Examination of children with NF1 have revealed a left-shift on tests of intellectual and executive function12, yet the frequency of specific learning deficits can vary greatly for each patient. This suggests that children with NF1 and learning disabilities do not represent a homogeneous group2. In this regard, the spectrum of NF1-associated cognitive and behavioral problems likely reflect an admixture of distinct diseases defined by the underlying molecular abnormality, the specific neuronal populations affected, and other co-morbid factors (sex, modifying genomic loci). This re-conceptualization might explain why Ras-targeted therapies (e.g., Simvastatin) demonstrated limited efficacy in improving cognitive performance in children with NF1 as a group, but enhanced functioning on specific tasks in a subset of these individuals13. Similarly, some children with NF1 exhibited learning improvements following treatment with dopamine-elevating drugs, like Methylphenidate1.
Nf1 mutant mice and flies provide unique opportunities to define these subgroups and to develop targeted therapies relevant to the clinical management of cognitive and behavioral problems in children with NF1. The original studies reporting NF1-associated cognitive deficits employed Nf1+/− mice, which have been instructive for revealing the importance of Ras/ERK regulation in spatial learning and memory3, 4, 14. Further analysis of these Ras-mediated defects has shown that the primary abnormality results from selective impairment of neurofibromin function in inhibitory hippocampal interneurons3. Additional evidence supporting a critical role for Ras regulation in these behaviors derives from mice with hyperactive Ras/ERK signaling resulting from impaired spred1 gene function. Similar to individuals with the NF1-like Legius syndrome, spred1 mutant mice have decreased learning and memory performance and reduced LTP15.
While Ras is clearly important for learning and memory in Nf1+/− mice, the current study used Nf1+/−GFAPCKO mice, which also exhibit defects in Ras activation, but additionally harbor abnormalities in cAMP and dopamine homeostasis5, 6. In our previous studies, we have shown that the attention deficits in Nf1+/−GFAPCKO mice are rescued by treatments that elevate dopamine levels, but not by Lovastatin (to inhibit Ras hyperactivation)5, 6. We now show that treatments that target the reduced dopamine levels in Nf1+/−GFAPCKO mice also rescue the learning and memory deficits in vivo, and elevate cAMP levels in Nf1+/− hippocampal neurons in vitro. Coupled with converging data from Drosophila mutants, this study has emphasized an independent role for dopamine and cyclic AMP homeostasis in NF1-associated cognitive performance, and revealed distinct cell populations and phases of learning critical for the overall learning and memory phenotype. As such, long-term memory deficits in Nf1 mutant flies is Ras-mediated, whereas immediate memory impairments result from defective neurofibromin G-protein adenylate cyclase (AC) activation16, 17. Similarly, Rutabaga-AC function is required for acquisition and stability of olfactory memory, where neurofibromin predominantly controls memory acquisition in flies10. Within the structure responsible for Drosophila olfactory learning and memory (mushroom bodies), there are two independent populations of neurons (γ and α/β neurons) both controlled by dopamine receptor function11. AC signaling primarily regulates the γ neurons, while neurofibromin controls α/β neuron function. In mice, dopaminergic system function is also critical for learning and memory, such that disruption of hippocampal dopaminergic innervations and loss of hippocampal D1/D5 dopamine receptor function result in spatial learning defects18–20.
Collectively, the findings in this report establish a causative link between cAMP regulation, dopamine homeostasis, and hippocampal learning and memory function in Nf1 mutant mice. As children with NF1-associated learning, memory, and attention deficits comprise a heterogeneous population of individuals, employing distinct Nf1 mouse strains to study these complex behaviors provides unique opportunities to define the different contributing molecular etiologies for future clinical studies. Together with previous studies emphasizing the role of abnormal Ras/ERK signaling in learning and memory, potential targeted therapies for children with NF1-associated scholastic performance issues should be tailored to the primary molecular and cellular defect.
Supplementary Material
Acknowledgments
We thank Sara Conyers in Animal Behavior Core for technical assistance during the execution of these studies. This work was supported by a National Cancer Center Diversity Supplement Award (to K.D.A.), funding from the Department of Defense (to D.H.G. and D.F.W.), National Institute of Child Health and Human Development Center (P30 HD062171 to D.F.W.), and National Institutes of Mental Health (to C.F.Z.), and support from the Neuroscience Blueprint Grant (NS057105).
Footnotes
Author contributions. K.D.A., K.T., and Y. I. performed the experiments and analysis. K.D.A. and D.H.G. wrote and edited the manuscript with comments from D.F.W. and C.F.Z.
Competing financial interests. The authors declare no competing financial interests.
References
- 1.Mautner VF, Kluwe L, Thakker SD, Leark RA. Treatment of ADHD in neurofibromatosis type 1. Dev Med Child Neurol. 2002;44:164–170. doi: 10.1017/s0012162201001876. [DOI] [PubMed] [Google Scholar]
- 2.Hyman SL, Arthur Shores E, North KN. Learning disabilities in children with neurofibromatosis type 1: subtypes, cognitive profile, and attention-deficit-hyperactivity disorder. Dev Med Child Neurol. 2006;48:973–977. doi: 10.1017/S0012162206002131. [DOI] [PubMed] [Google Scholar]
- 3.Cui Y, Costa RM, Murphy GG, et al. Neurofibromin regulation of ERK signaling modulates GABA release and learning. Cell. 2008;135:549–560. doi: 10.1016/j.cell.2008.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li W, Cui Y, Kushner SA, et al. The HMG-CoA reductase inhibitor lovastatin reverses the learning and attention deficits in a mouse model of neurofibromatosis type 1. Curr Biol. 2005;15:1961–1967. doi: 10.1016/j.cub.2005.09.043. [DOI] [PubMed] [Google Scholar]
- 5.Brown JA, Emnett RJ, White CR, et al. Reduced striatal dopamine underlies the attention system dysfunction in neurofibromatosis-1 mutant mice. Hum Mol Genet. 2010;19:4515–4528. doi: 10.1093/hmg/ddq382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown JA, Xu J, Diggs-Andrews KA, Wozniak DF, Mach RH, Gutmann DH. PET imaging for attention deficit preclinical drug testing in neurofibromatosis-1 mice. Exp Neurol. 2011;232:333–338. doi: 10.1016/j.expneurol.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tong J, Hannan F, Zhu Y, Bernards A, Zhong Y. Neurofibromin regulates G protein-stimulated adenylyl cyclase activity. Nat Neurosci. 2002;5:95–96. doi: 10.1038/nn792. [DOI] [PubMed] [Google Scholar]
- 8.Brown JA, Gianino SM, Gutmann DH. Defective cAMP generation underlies the sensitivity of CNS neurons to neurofibromatosis-1 heterozygosity. J Neurosci. 2010;30:5579–5589. doi: 10.1523/JNEUROSCI.3994-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo HF, Tong J, Hannan F, Luo L, Zhong Y. A neurofibromatosis-1-regulated pathway is required for learning in Drosophila. Nature. 2000;403:895–898. doi: 10.1038/35002593. [DOI] [PubMed] [Google Scholar]
- 10.Buchanan ME, Davis RL. A distinct set of Drosophila brain neurons required for neurofibromatosis type 1-dependent learning and memory. J Neurosci. 2010;30:10135–10143. doi: 10.1523/JNEUROSCI.0283-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin H, Cressy M, Li W, Coravos JS, Izzi SA, Dubnau J. Gamma Neurons Mediate Dopaminergic Input during Aversive Olfactory Memory Formation in Drosophila. Curr Biol. 2012;22:608–614. doi: 10.1016/j.cub.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Payne JM, Hyman SL, Shores EA, North KN. Assessment of executive function and attention in children with neurofibromatosis type 1: relationships between cognitive measures and real-world behavior. Child Neuropsychology. 2011;17:313–329. doi: 10.1080/09297049.2010.542746. [DOI] [PubMed] [Google Scholar]
- 13.Krab LC, de Goede-Bolder A, Aarsen FK, et al. Effect of simvastatin on cognitive functioning in children with neurofibromatosis type 1: a randomized controlled trial. JAMA. 2008;300:287–294. doi: 10.1001/jama.300.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guilding C, McNair K, Stone TW, Morris BJ. Restored plasticity in a mouse model of neurofibromatosis type 1 via inhibition of hyperactive ERK and CREB. Eur J Neurosci. 2007;25:99–105. doi: 10.1111/j.1460-9568.2006.05238.x. [DOI] [PubMed] [Google Scholar]
- 15.Denayer E, Ahmed T, Brems H, et al. Spred1 is required for synaptic plasticity and hippocampus-dependent learning. J Neurosci. 2008;28:14443–14449. doi: 10.1523/JNEUROSCI.4698-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho IS, Hannan F, Guo HF, Hakker I, Zhong Y. Distinct functional domains of neurofibromatosis type 1 regulate immediate versus long-term memory formation. J Neurosci. 2007;27:6852–6857. doi: 10.1523/JNEUROSCI.0933-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hannan F, Ho I, Tong JJ, Zhu Y, Nurnberg P, Zhong Y. Effect of neurofibromatosis type I mutations on a novel pathway for adenylyl cyclase activation requiring neurofibromin and Ras. Hum Mol Genet. 2006;15:1087–1098. doi: 10.1093/hmg/ddl023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gasbarri A, Sulli A, Innocenzi R, Pacitti C, Brioni JD. Spatial memory impairment induced by lesion of the mesohippocampal dopaminergic system in the rat. Neuroscience. 1996;74:1037–1044. doi: 10.1016/0306-4522(96)00202-3. [DOI] [PubMed] [Google Scholar]
- 19.El-Ghundi M, Fletcher PJ, Drago J, Sibley DR, O'Dowd BF, George SR. Spatial learning deficit in dopamine D(1) receptor knockout mice. Eur J Pharmacol. 1999;383:95–106. doi: 10.1016/s0014-2999(99)00573-7. [DOI] [PubMed] [Google Scholar]
- 20.Xing B, Kong H, Meng X, Wei SG, Xu M, Li SB. Dopamine D1 but not D3 receptor is critical for spatial learning and related signaling in the hippocampus. Neuroscience. 2010;169:1511–1519. doi: 10.1016/j.neuroscience.2010.06.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.