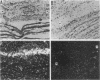
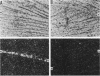
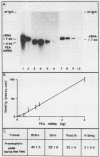
Abstract
Proenkephalin A (PEA), a neuropeptide-encoding gene, is widely expressed in the nervous and endocrine systems. Recently, we demonstrated that in addition to its abundance in fetal brain tissue; PEA is markedly expressed in nondifferentiated mesodermal cells of developing fetuses. To evaluate the implication of these findings for the normal development of tissues of mesodermal origin, we examined the expression of PEA in rat mesenchymal tissues during pre- and postnatal development. Using in situ hybridization analysis combined with RNA blots and a Met-enkephalin-specific radioimmunoassay, we showed that (i) PEA mRNA levels in embryonic and newborn mesenchymal derivative tissues were as high as in the developing brain, (ii) PEA mRNA concentrations in these tissues dropped to undetectable levels shortly after birth, and (iii) this mRNA was translated and processed differentially among different mesenchymal tissues, yielding a tissue-specific pattern of PEA-derived peptides. Our results demonstrate multilevel regulation of PEA gene expression during ontogenic development of mesenchymal derivative tissues. The transient expression and the correlation between PEA mRNA and tissue maturation support the notion that peptides encoded by PEA play a significant role in normal development of these tissues. These findings provide a framework for examination of the mechanisms and roles of PEA gene expression during mesenchymal ontogeny.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akil H., Watson S. J., Young E., Lewis M. E., Khachaturian H., Walker J. M. Endogenous opioids: biology and function. Annu Rev Neurosci. 1984;7:223–255. doi: 10.1146/annurev.ne.07.030184.001255. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Civelli O., Douglass J., Goldstein A., Herbert E. Sequence and expression of the rat prodynorphin gene. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4291–4295. doi: 10.1073/pnas.82.12.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comb M., Seeburg P. H., Adelman J., Eiden L., Herbert E. Primary structure of the human Met- and Leu-enkephalin precursor and its mRNA. Nature. 1982 Feb 25;295(5851):663–666. doi: 10.1038/295663a0. [DOI] [PubMed] [Google Scholar]
- Cossu G., Cusella-De Angelis M. G., Senni M. I., De Angelis L., Vivarelli E., Vella S., Bouchè M., Boitani C., Molinaro M. Adrenocorticotropin is a specific mitogen for mammalian myogenic cells. Dev Biol. 1989 Feb;131(2):331–336. doi: 10.1016/s0012-1606(89)80006-5. [DOI] [PubMed] [Google Scholar]
- Dandekar S., Sabol S. L. Cell-free translation and partial characterization of mRNA coding for enkephalin-precursor protein. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1017–1021. doi: 10.1073/pnas.79.4.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglass J., Civelli O., Herbert E. Polyprotein gene expression: generation of diversity of neuroendocrine peptides. Annu Rev Biochem. 1984;53:665–715. doi: 10.1146/annurev.bi.53.070184.003313. [DOI] [PubMed] [Google Scholar]
- Gubler U., Seeburg P., Hoffman B. J., Gage L. P., Udenfriend S. Molecular cloning establishes proenkephalin as precursor of enkephalin-containing peptides. Nature. 1982 Jan 21;295(5846):206–208. doi: 10.1038/295206a0. [DOI] [PubMed] [Google Scholar]
- Howells R. D., Kilpatrick D. L., Bailey L. C., Noe M., Udenfriend S. Proenkephalin mRNA in rat heart. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1960–1963. doi: 10.1073/pnas.83.6.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J., Smith T. W., Kosterlitz H. W., Fothergill L. A., Morgan B. A., Morris H. R. Identification of two related pentapeptides from the brain with potent opiate agonist activity. Nature. 1975 Dec 18;258(5536):577–580. doi: 10.1038/258577a0. [DOI] [PubMed] [Google Scholar]
- Imura H., Kato Y., Nakai Y., Nakao K., Tanaka I., Jingami H., Koh T., Yoshimasa T., Tsukada T., Suda M. Endogenous opioids and related peptides: from molecular biology to clinical medicine. The Sir Henry Dale lecture for 1985. J Endocrinol. 1985 Nov;107(2):147–157. doi: 10.1677/joe.0.1070147. [DOI] [PubMed] [Google Scholar]
- Keshet E., Polakiewicz R. D., Itin A., Ornoy A., Rosen H. Proenkephalin A is expressed in mesodermal lineages during organogenesis. EMBO J. 1989 Oct;8(10):2917–2923. doi: 10.1002/j.1460-2075.1989.tb08441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick D. L., Borland K., Jin D. F. Differential expression of opioid peptide genes by testicular germ cells and somatic cells. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5695–5699. doi: 10.1073/pnas.84.16.5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick D. L., Howells R. D., Noe M., Bailey L. C., Udenfriend S. Expression of preproenkephalin-like mRNA and its peptide products in mammalian testis and ovary. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7467–7469. doi: 10.1073/pnas.82.21.7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick D. L., Millette C. F. Expression of proenkephalin messenger RNA by mouse spermatogenic cells. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5015–5018. doi: 10.1073/pnas.83.14.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebisch D. C., Weber E., Kosicka B., Gramsch C., Herz A., Seizinger B. R. Isolation and structure of a C-terminally amidated nonopioid peptide, amidorphin-(8-26), from bovine striatum: a major product of proenkephalin in brain but not in adrenal medulla. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1936–1940. doi: 10.1073/pnas.83.6.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston D. R., Vanderhaeghen J. J., Rossier J. Presence in brain of synenkephalin, a proenkephalin-immunoreactive protein which does not contain enkephalin. Nature. 1983 Mar 3;302(5903):62–65. doi: 10.1038/302062a0. [DOI] [PubMed] [Google Scholar]
- Liston D., Rossier J. Distribution and characterization of synenkephalin immunoreactivity in the bovine brain and pituitary. Regul Pept. 1984 Jan;8(1):79–87. doi: 10.1016/0167-0115(84)90031-4. [DOI] [PubMed] [Google Scholar]
- Miller R. P., Husain F., Svensson M., Lohin S. Enhancement of [3H-methyl]thymidine incorporation and replication of rat chondrocytes grown in tissue culture by plasma, tissue extracts and vasopressin. Endocrinology. 1977 May;100(5):1365–1375. doi: 10.1210/endo-100-5-1365. [DOI] [PubMed] [Google Scholar]
- Nilsson J., von Euler A. M., Dalsgaard C. J. Stimulation of connective tissue cell growth by substance P and substance K. Nature. 1985 May 2;315(6014):61–63. doi: 10.1038/315061a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Furutani Y., Takahashi H., Toyosato M., Hirose T., Inayama S., Nakanishi S., Numa S. Cloning and sequence analysis of cDNA for bovine adrenal preproenkephalin. Nature. 1982 Jan 21;295(5846):202–206. doi: 10.1038/295202a0. [DOI] [PubMed] [Google Scholar]
- Pfaus J. G., Gorzalka B. B. Opioids and sexual behavior. Neurosci Biobehav Rev. 1987 Spring;11(1):1–34. doi: 10.1016/s0149-7634(87)80002-7. [DOI] [PubMed] [Google Scholar]
- Rosen H., Douglass J., Herbert E. Isolation and characterization of the rat proenkephalin gene. J Biol Chem. 1984 Nov 25;259(22):14309–14313. [PubMed] [Google Scholar]
- Rosen H., Polakiewicz R. Postnatal expression of opioid genes in rat brain. Brain Res Dev Brain Res. 1989 Mar 1;46(1):123–129. doi: 10.1016/0165-3806(89)90149-1. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Sinnett-Smith J. W. Bombesin induction of c-fos and c-myc proto-oncogenes in Swiss 3T3 cells: significance for the mitogenic response. J Cell Physiol. 1987 May;131(2):218–225. doi: 10.1002/jcp.1041310211. [DOI] [PubMed] [Google Scholar]
- Udenfriend S., Kilpatrick D. L. Biochemistry of the enkephalins and enkephalin-containing peptides. Arch Biochem Biophys. 1983 Mar;221(2):309–323. doi: 10.1016/0003-9861(83)90149-2. [DOI] [PubMed] [Google Scholar]
- Vilijn M. H., Vaysse P. J., Zukin R. S., Kessler J. A. Expression of preproenkephalin mRNA by cultured astrocytes and neurons. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6551–6555. doi: 10.1073/pnas.85.17.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa K., Aizawa T. Enkephalin precursor gene expression in postmeiotic germ cells. Biochem Biophys Res Commun. 1988 Mar 15;151(2):664–671. doi: 10.1016/s0006-291x(88)80332-2. [DOI] [PubMed] [Google Scholar]
- Zachary I., Woll P. J., Rozengurt E. A role for neuropeptides in the control of cell proliferation. Dev Biol. 1987 Dec;124(2):295–308. doi: 10.1016/0012-1606(87)90483-0. [DOI] [PubMed] [Google Scholar]
- Zurawski G., Benedik M., Kamb B. J., Abrams J. S., Zurawski S. M., Lee F. D. Activation of mouse T-helper cells induces abundant preproenkephalin mRNA synthesis. Science. 1986 May 9;232(4751):772–775. doi: 10.1126/science.2938259. [DOI] [PubMed] [Google Scholar]