Abstract
Purpose
Osteoporosis is primarily evident in postmenopausal women, but its roots are traceable to periods of growth, including during adolescence. Depression, anxiety, and smoking are associated with lower bone mineral density (BMD) in adults. These associations have not been studied longitudinally across adolescence when more than 50% of bone accrual occurs.
Methods
To determine the impact of depressive and anxiety symptoms, smoking, and alcohol use on bone accrual in girls 11–19 years, 262 healthy girls were enrolled in age cohorts of 11, 13, 15, and 17 years. Using a cross-sequential design, girls were seen for 3 annual visits. Outcome measures included total body bone mineral content (TB BMC) and BMD of the total hip and lumbar spine using dual energy x-ray absorptiometry. Depressive and anxiety symptoms and smoking and alcohol use were by self-report.
Results
Higher-frequency smoking was associated with a lower rate of lumbar spine and total hip BMD accrual from age 11–19. Higher depressive symptoms were associated with lower lumbar spine BMD across all ages. There was no effect of depressive symptoms on TB BMC, and there was no effect of alcohol intake on any bone outcome.
Conclusion
Adolescent smokers are at higher risk for less than optimal bone accrual. Even in the absence of diagnosable depression, depressive symptoms may influence adolescent bone accrual. These findings have import for prevention of later osteoporosis and fractures.
Keywords: bone accrual, depression, smoking, adolescent, substance use
Introduction
Osteoporosis is a costly health problem. The National Osteoporosis Foundation (NOF) estimates that 10 million Americans already have osteoporosis and an additional 34 million are at risk. Further, there were an estimated 2 million fractures likely resulting from osteoporosis and as a result, $19 billion in costs incurred for 2005. These numbers are expected to rise to 3 million fractures and $25.3 billion in costs each year by 2025 presenting a significant public health burden (http://www.nof.orgaccessed9/22/11 accessed 9/22/11).1 Osteoporosis is primarily evident in postmenopausal women, but its roots are traceable to periods of growth, including adolescence. Despite awareness that more than 50% of bone mineral accrual occurs in puberty,2–4 2 there is little effort to identify important modifiable factors that affect bone health in adolescence beyond exercise and nutrition. Maximizing adolescent bone accrual ensures fewer deficits in the postmenopausal “bone bank”,5 and in turn may lower rates of osteoporosis and fracture.
Modifiable factors affecting bone health, beyond exercise and nutrition, have been identified among adults. Meta-analyses report adult depression is associated with osteoporosis and lower bone mineral density (BMD), with effect sizes generally moderate.6–8 Smoking also has a negative impact on bone health, with adult smokers exhibiting lower BMD compared to non-smokers,9,10 likely increasing lifetime fracture risk by as much as 31%.10,11 For adult alcohol use, findings are mixed: chronic or high use has a negative impact,12 whereas moderate use may be advantageous to adult men or postmenopausal women13–15 but not always.16
There is a dearth of information on whether modifiable factors, such as depressive symptoms and substance use, affect bone accrual in adolescence. Adolescence is a critical period when depression and anxiety increase, particularly in girls and affecting nearly 20% of youth,17 and smoking and alcohol use are initiated.18 Globally, neuropsychiatric disorders (including depression and alcohol use) are the main cause of disability in 10- to 24-year-olds, accounting for 45% of disease burden.19 Further, both depression and substance use often become chronic post-adolescence. Nearly 43% of those with child or adolescent depression had recurring episodes later in life,20 and nearly 80% of adults addicted to tobacco began smoking as adolescents.21 Knowing these disorders might become chronic enhances their potential long-term impact on health, and in turn, their importance for understanding early roots of chronic disease. To our knowledge, this is the first study to longitudinally examine the impact of substance use and depressive symptoms on bone health in pubertal-age girls. We previously reported cross-sectional findings showing higher depressive symptoms, and in some cases anxiety, were associated with lower total body bone mineral content (TB BMC). Further, the combination of regular users of both cigarettes and alcohol showed stronger negative associations between depressive symptoms and TB BMC compared to nonusers/experimental smokers and regular users of alcohol.22,23 Goals of the current study were to determine the longitudinal impact of substance use and depressive and anxiety symptoms on bone accrual in girls aged 11–19 years. We hypothesized greater substance use (smoking, alcohol) and higher depressive or anxiety symptoms would negatively predict bone accrual across adolescence.
Method
Study Design and Participants
Two hundred sixty-two healthy girls were recruited from a teen health clinic in a large Midwestern children’s hospital and its surrounding community and enrolled in 4 age cohorts (11, 13, 15, and 17 years). They were recruited to represent typically developing adolescents and not a clinical group. They attended a total of 3 annual on-site visits. Phone interviews were conducted at 3-month intervals between annual visits; these time-points were used to assess smoking as described below. Girls were enrolled by five levels of smoking (1=never, not even a puff, to 5= ≥100 lifetime cigarettes and smoking > 20 of the last 30 days) using a self-reported questionnaire. Our goal was to have each age cohort reflect the number of smokers proportional to national statistics (e.g., 17 year cohort had many more smokers than 11 year cohort). Exclusion criteria were (1) pregnancy or breastfeeding within 6 months, (2) primary amenorrhea (menarche >16 years), (3) secondary amenorrhea (<6 cycles/year; not due to hormonal contraception), (4) body mass index <1st percentile or weight greater than 300 pounds (limit for dual energy x-ray absorptiometry [DXA]), (5) medications or medical disorder influencing bone health, and (6) psychological or developmental disorders impairing comprehension or compliance. Baseline visits were conducted from December 2003 through October 2007. Retention rates varied; 90% were present for at least twice. The cross-sequential24 design (i.e., accelerated longitudinal) is characterized by following multiple age cohorts over time to examine both cross-sectional and longitudinal effects in development, allowing us to examine broad swaths of development across relatively short periods of time.
Study Protocol
The study was approved by the Institutional Review Board of the associated children’s hospital. Parents provided informed consent and adolescents provided assent. Annual visits were conducted in the Clinical Translational Research Center (CTRC) and included a battery of questionnaires and interviews, anthropometry, a physical examination, phlebotomy, and bone density measurements.
Measures
The outcome measures of bone health included total body bone mineral content (TB BMC), which has been advocated as the appropriate measure to use during growth.25 BMD of the total hip and lumbar spine also were used because these are common sites of osteoporotic fractures later in life. TB BMC and BMD were measured annually by DXA using a Hologic QDR4500 bone densitometer (Hologic, Inc., Bedford, MA) and analyzed using Hologic software release 12.4. Predictor variables included depressive symptoms determined using the Children’s Depression Inventory,26 given annually. The Spielberger State-Trait Anxiety Inventory,27,28 given annually, measured trait anxiety. Smoking was determined by questionnaire based on Mayhew and collegues,29 during annual and 3-month phone interviews by asking, “Which of the following best describes your smoking?” and “During the past 30 days, how many days did you smoke one or more cigarettes?” Responses were coded as follows: don’t smoke (0); smoke, but not in the last 30 days (1); smoked 1–2 days (2); smoked 3–5 days (3); smoked 6–9 days (4); smoked 10–19 days (5); smoked 20–29 days (6); or smoked all 30 days (7). Scores were averaged across the 3-month intervals to characterize typical monthly smoking frequency over the year. This is standard methodology for measuring adolescent smoking and smoking uptake; “pack years” is not yet relevant, and number of cigarettes per time period is inconsistent. Lifetime alcohol use was determined from the Diagnostic Interview Schedule for Children30 and was coded as 0–5 drinks (0) or ≥6 drinks (1).
Covariates reported in the literature as relevant to bone health were included. Such covariates often account for a significant portion of the variance in bone mass, and an unequal distribution of these variables across groups of interest could introduce confounding. Covariates were measured annually and included: race by parent report (recoded as 1 = black and 0 = non-black); puberty (Tanner breast stages I-V)31 by examination by a clinician trained in the procedures; height using a wall-mounted stadiometer (Holtain Ltd., Crosswell, United Kingdom); weight by digital scale (Scaletronix, Carol Stream, IL); age at menarche (year,months); lifetime duration of hormonal contraceptive use by clinician interview (separating effects of depo-medroxyprogesterone [DMPA] versus other contraceptive methods containing estradiol and progestin); weight-bearing physical activity using the Physical Activity Questionnaire for Older Children (PAQ-C),32 whereby participants recalled performance of moderate to vigorous physical activities within the last 7 days (1 [low; did not do] to 5 [high; 7+ times] with weight-bearing activities specifically being used; and calcium intake by a modified food frequency questionnaire (FFQ),33 where girls reported the frequency of consuming calcium-rich foods in the last month. Vitamin D status was assessed by measurement of serum 25-hydroxy Vitamin D [25(OH)D] at baseline by a radioimmunoassay. Inter- and intra-assay coefficients of variation ranged from 3.5%– 4.4% and 11.1%–16.9%, respectively.
Statistical Analyses
Analyses were conducted using PROC MIXED in SAS, version 9.2 (SAS Institute, Cary, NC) using 2-tailed tests of significance (P <.05). Theoretically relevant covariates [race, puberty, height, weight, hormonal contraceptives, activity, calcium intake, and serum 25 (OH)D] were evaluated in all analyses. Due to the correlation between smoking and depressive symptoms (r = 0.25; P <.01), smoking could be a confounder in the assessment of the effects of depressive symptoms (and the reverse). To address this issue, the average level of smoking was used as a covariate in models in which depressive symptoms were examined as a predictor of bone accrual; similarly, average level of depressive symptoms was used as a covariate in models in which smoking was examined. Race, lifetime hormonal contraceptive exposure, age at menarche, and serum 25 (OH)D were time-invariant covariates. All other covariates were time-varying and with the exception of smoking, were used annually.
Hierarchical linear modeling (HLM)34 was used to estimate BMC and BMD trajectories over the ages of 11–19 years. The degree to which independent predictor variables (smoking, alcohol use, depressive and anxiety symptoms) could account for variation in trajectories of bone measures, were then evaluated. HLM and its maximum likelihood estimation methods accommodate missing data, thereby making use of all available data to estimate the entire age trajectory. Using Bayes’ estimation, individuals with more data as well as ages that are more highly represented are given more weight in calculation of parameter estimates. Thus, HLM is ideal for cross-sequential designs in which individuals represent differing portions of the developmental curve and all individuals are not necessarily tested contiguously at every age represented. Restricted maximum likelihood estimation with an estimated degrees of freedom procedure35 was used to arrive at valid parameter estimates under the assumption of ignorable missing data.36
Results
Mean age of the sample was 14.4 years. The majority were black (32%) or white (62%) with 6% of mixed or other races. For the purpose of analysis, the 6% were placed in a non-black category to be congruent with descriptive findings of racial differences in bone.2 See Table 1.
Table 1.
Descriptive characteristics of study sample at Time 1 in 262 adolescent girls
| Variable | Mean (SD) | % (N) |
|---|---|---|
| Age (yr) | 14.35 (2.16) | |
| Black race | 31.8% (83) | |
| Calcium intake (mg/day) | 736 (433) | |
| Serum 25(OH)D (g/mL) | 20.44 (9.21) | |
| Physical activity* | 2.04 (0.49) | |
| Height (m) | 1.60 (0.08) | |
| Weight (kg) | 62.15 (18.20) | |
| BMI z-score | 0.73 (1.00) | 16.0% (42) overweight |
| 16.4% (43) obese | ||
| Tanner Breast stage | ||
| I | 1.5% (4) | |
| II | 1.9% (5) | |
| III | 10.3% (27) | |
| IV | 14.9% (39) | |
| V | 71.4% (187) | |
| DMPA exposure** | 0.88 (1.96) | 79.0% (207) never exposed |
| OCP exposure** | 1.92 (2.59) | 53.8% (141) never exposed |
| Depressive Sx (T score) | 46.27 (10.77) | |
| Trait Anxiety (T score) | 46.37 (10.64) | |
| Smoking*** | 1.69 (2.72) | 65.3% (171) not smokers |
| 14.9% (39) smoke daily in past 30 days | ||
| Lifetime alcohol intake | 91.2% (239) ≤ 5 drinks | |
| Total body BMC (g) | 1912 (416) | |
| Total hip BMD (g/cm2) | 0.960 (0.156) | |
| Lumbar spine BMD (g/cm2) | 0.974 (0.170) |
-
-25 Hydroxy Vitamin D [25(OH)D]
-
-* Weight-bearing physical activity assessed by Physical Activity Questionnaire32 where scores are 1 (did not do in last 7 days) to 5 (did 7+ times in last 7 days)
-
-Depo-medroxyprogesterone (DMPA)
-
-**Oral contraceptive pills (OCPs) (total exposure for DMPA and OCPs range from 0 = no exposure [79% DMPA/54% OCP], 3 = exposure for 6–9 months [2% DMPA/3% OCP], 7 = consistent exposure for last 6y [3% DMPA/11% OCP]
-
-T-scores use a mean of 50 and SD of 10; clinical cutoff is 65.
-
-***Smoking (0 = Don’t smoke [65%], 3 = 3–5 days in the last 30 days [2%], 7 = all 30 days in last 30 [15%])
-
-Lifetime Alcohol (0–5 drinks vs. ≥ 6)
-
-Bone mineral content (BMC)
-
-Bone mineral density (BMD).
Descriptive Trajectories
Bone accrual trajectories from ages 11–19 were estimated for TB BMC and lumbar spine and total hip BMD. For TB BMC, a random linear slope coefficient (B = 214.87; P <.01) and fixed quadratic slope coefficient (B = –16.05; P <.01) were identified, indicating that gains in TB BMC with age were best characterized by positive linear accrual with a significant tapering off in later adolescence. Lumbar spine and total hip BMD were best characterized by a positive linear slope from ages 11–19 without a significant quadratic coefficient. For lumbar spine BMD, a significant linear slope coefficient (B = 0.087; P <.01) was modeled. A quadratic slope coefficient was estimated and found non-significant (B = –0.001; P = .10). Similarly, for total hip BMD a significant linear slope coefficient (B = 0.066; P <.01) was modeled. A quadratic slope coefficient was estimated and was not significant (B= –0.006; P = .06). For total hip and lumbar spine BMD, non-significant quadratic terms were excluded in subsequent analyses. Independent variables (e.g., smoking, alcohol, depressive or anxiety symptoms with covariates) were then added to each of these HLM models. For depressive symptoms and smoking, interactions with the linear slope effect were also included to examine whether smoking and depressive symptoms predict the intercept and linear slope of TB BMC and BMD of the total hip and lumbar spine.
Effects of Smoking
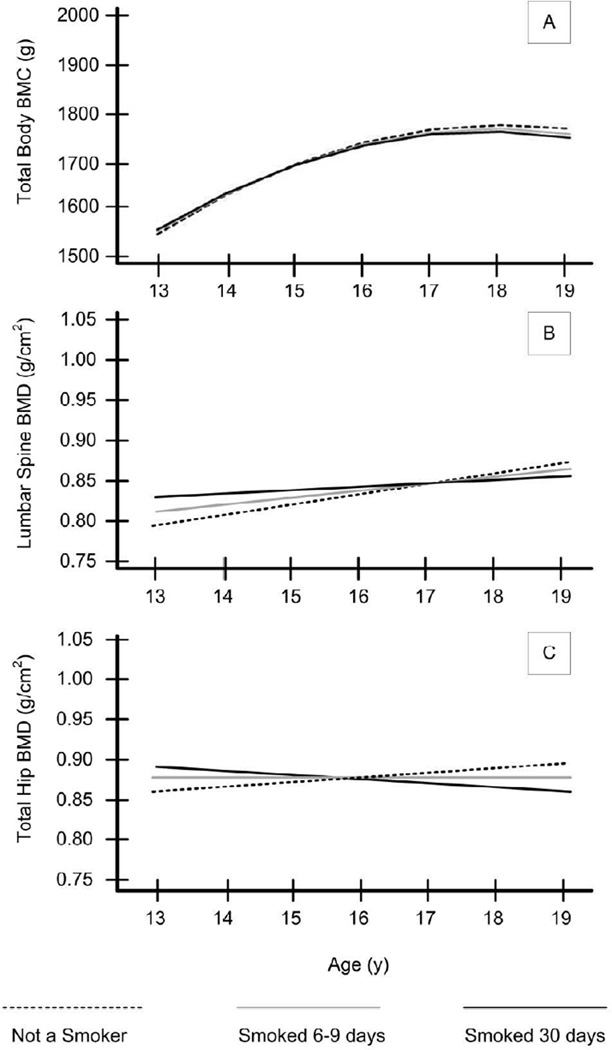
Figure 1a–1c and Table 2 depict how the frequency of smoking impinges on bone accrual. There was little smoking in this cohort prior to age 13, so the analyses were limited to participants ages 13–19. For TB BMC, no significant main effect of smoking or smoking × age effect was detected.
Figure 1.
Data portrayal for effect of smoking frequency on bone accrual at three categories of smoking: not smoking, smoking an average of 6–9 days in the past 30 days over the past year, and smoking an average of 30 days in the past 30 days over the past year (See Table 2 for corresponding statistical values). Smoking × intercept effects are all non-significant (P ≥ .05), indicating that there are no differences in BMC or BMD by smoking status at age 13. Smoking × slope effects for total hip and lumbar spine were significant. A. Trajectories of Total Body BMC. B. Trajectories of Lumbar Spine BMD. C. Trajectories of Total Hip BMD. All effects are controlling for relevant covariates (see Table 2).
Table 2.
Effect of Smoking on Bone Accrual using Hierarchical Linear Modeling in 262 Adolescent Girls
| TB BMC | Lumbar Spine BMD | Total Hip BMD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect | B | SE | CI | B | SE | CI | B | SE | CI | |||
| Intercept | 1583.10** | 45.52 | 1493.88 | 1672.32 | 0.779** | 0.024 | 0.732 | 0.826 | 0.863** | 0.022 | 0.820 | 0.906 |
| Slope (Age) |
77.12** | 6.87 | 63.65 | 90.59 | 0.013** | 0.002 | 0.009 | 0.017 | 0.006** | 0.002 | 0.002 | 0.010 |
| Quadratic (Age2) |
−7.64** | 1.14 | −9.87 | −5.41 | - | - | - | - | - | - | - | - |
| Smoking | 1.03 | 4.87 | −8.52 | 10.58 | 0.004 | 0.002 | 0.001 | 0.008 | 0.004** | 0.002 | 0.001 | 0.008 |
| Smoking × Age |
−0.57 | 1.11 | −2.75 | 1.61 | −0.001* | 0.001 | −0.003 | 0.001 | −0.002** | 0.000 | −0.002 | −0.002 |
| Depressive Symptoms |
−0.56 | 0.41 | −1.36 | 0.24 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Race | 189.04** | 27.59 | 134.96 | 243.12 | 0.063** | 0.014 | 0.036 | 0.09 | 0.074** | 0.015 | 0.045 | 0.103 |
| Height | 2268.23** | 173.68 | 1927.82 | 2608.64 | 0.583** | 0.089 | 0.409 | 0.757 | 0.438** | 0.088 | 0.266 | 0.610 |
| Weight | 3.15** | 0.47 | 2.23 | 4.07 | 0.001** | 0.000 | 0.001 | 0.001 | 0.001** | 0.000 | 0.001 | 0.001 |
| Tanner Stage |
36.01** | 9.22 | 17.94 | 54.08 | 0.035** | 0.005 | 0.025 | 0.045 | 0.015** | 0.004 | 0.007 | 0.023 |
| DMPA exposure |
−14.51* | 6.65 | −27.54 | −1.48 | −0.013** | 0.003 | −0.019 | −0.007 | −0.007* | 0.004 | −0.015 | 0.001 |
| OCP exposure |
12.76** | 4.98 | 3 | 22.52 | 0.007** | 0.003 | 0.001 | 0.013 | 0.006* | 0.003 | 0.000 | 0.012 |
p < .05,
p < .01.
NOTE: Total body bone mineral content (TB BMC), bone mineral density (BMD), depo-medroxyprogesterone (DMPA); oral contraceptive pills (OCP). Variables were centered as Age at 11, Depressive Symptoms at 50, Smoking at 0 (none), Race at 0 (black), Height at 1.6m, Weight at 60 kg, Tanner at 4, DMPA and OCP exposure at 0 (no exposure). DMPA and OCP total exposure ranges from 0 = no exposure [79% DMPA/54% OCP], 3 = exposure for 6–9 months [2% DMPA/3% OCP], 7 = consistent exposure for last 6y [3% DMPA/11% OCP] for last 6y. All estimates in this table are unstandardized. Interaction effects between smoking and potential confounders were tested and found to be non-significant (P > .05) with the exception of smoking and Tanner stage in predicting total hip BMD (B = −0.005, SE = 0.002, P = 0.009), indicating an additional negative impact of smoking on total hip BMD for girls in higher Tanner stages.
For lumbar spine BMD, there was no significant main effect of smoking. However, a significant smoking × age effect was identified whereby as smoking increased, the rate of accrual decreased as girls got older (see Figure 1b). Similarly, for total hip BMD there was a significant smoking-by- age effect whereby as smoking increased, the rate of accrual decreased as girls got older (see Figure 1c). Thus smokers entered adolescence with equivalent levels of lumbar spine and total hip BMD (intercept effects) but that overall BMD accrual across adolescence was significantly lower as smoking frequency increased. Importantly, these findings are based on multivariate models controlling for race, Tanner stage, height, weight, and exposure to hormone contraceptives. Age at menarche, physical activity, calcium intake, and exposure to serum 25(OH)D were tested as potential covariates and found to be non-significant predictors (P > .05). For parsimony these items were excluded from the analyses.
Depressive Symptoms Effects
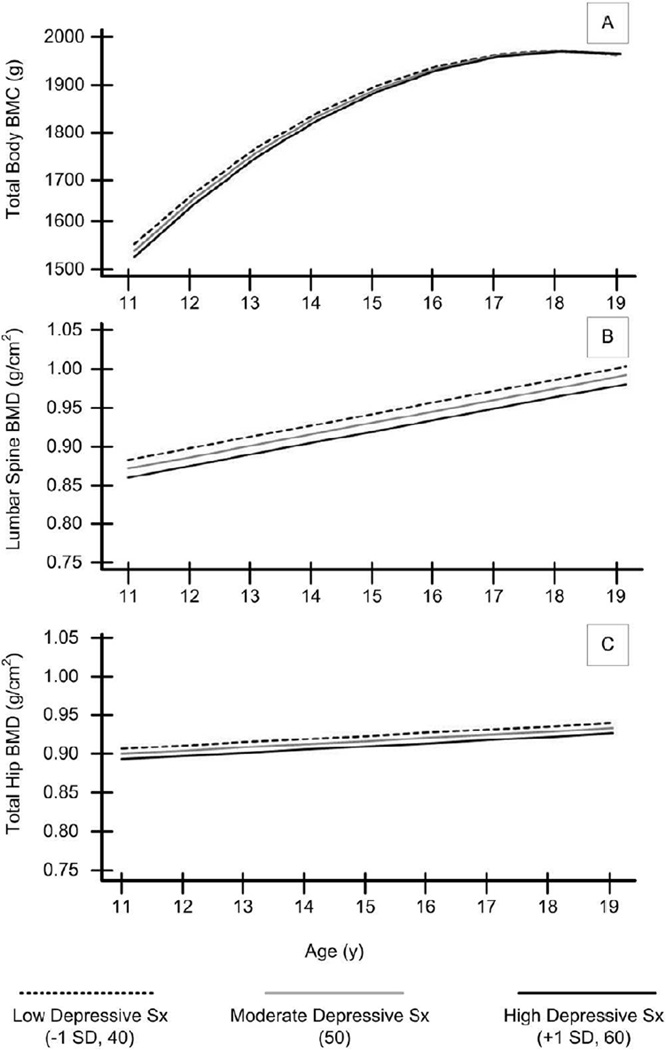
Figure 2a–2c and Table 3 depict how levels of depressive symptoms impinge on bone accrual. For TB BMC, there was no main effect of depressive symptoms or a significant interaction with age (see Figure 2a). For lumbar spine BMD, there was a significant main effect of depressive symptoms: higher depressive symptoms were related to lower BMD, but there was no interaction between depressive symptoms and age (see Figure 2b). Thus, lower spine BMD persists across ages 11–19 for those with higher depressive symptoms. Finally, for total hip BMD, there was no main effect of depressive symptoms, and no interaction between depressive symptoms and age (see Figure 2c). As with the model for smoking, depressive symptoms findings were based on multivariate models controlling for race, Tanner stage, height, weight, and exposure to hormone contraceptives. Age at menarche, physical activity, calcium intake, and exposure to serum 25(OH)D were tested as potential covariates and found to be non-significant predictors (P > .05). For parsimony these items were excluded from the analyses.
Figure 2.
Data portrayal for the effect of depressive symptoms on bone accrual (see Table 3 for corresponding statistical values). Average depressive sx = 50, low and high represent 1 SD below and above the mean (40 and 60, respectively). Depressive symptoms × intercept effects significant for lumbar spine BMD only. All depressive symptoms × slope effects were non-significant (P ≥ .05). A. Trajectories of Total Body BMC. B. Trajectories of Lumbar Spine BMD. C. Trajectories of Total Hip BMD. All effects are controlling for relevant covariates (see Table 3).
Table 3.
Effect of Depressive Symptoms on Bone Accrual using Hierarchical Linear Modeling in 262 Adolescent Girls
| Effect | TB BMC | Lumbar Spine BMD | Total Hip BMD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect | B | SE | CI | B | SE | CI | B | SE | CI | |||
| Intercept | 1560.96** | 22.99 | 1515.90 | 1606.02 | 0.883** | 0.011 | 0.861 | 0.905 | 0.903** | 0.011 | 0.881 | 0.925 |
| Slope (Age) |
110.25** | 8.55 | 93.49 | 127.01 | 0.014** | 0.002 | 0.010 | 0.018 | 0.003 | 0.002 | −0.001 | 0.007 |
| Quadratic (Age2) |
−7.80** | 0.84 | −9.45 | −6.15 | - | - | - | - | - | - | - | - |
| Depressive Symptoms |
−1.26 | 0.92 | −3.06 | 0.54 | −0.001* | 0.001 | −0.003 | 0.001 | −0.001 | 0.000 | −0.001 | −0.001 |
| Depressive Symptoms × Age |
0.17 | 0.18 | −0.18 | 0.52 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Smoking | −1.40 | 2.86 | −7.01 | 4.21 | 0.000 | 0.002 | −0.004 | 0.004 | −0.002 | 0.001 | −0.004 | 0.000 |
| Race | 163.58** | 26.56 | 111.52 | 215.64 | 0.058** | 0.014 | 0.031 | 0.085 | 0.068** | 0.015 | 0.039 | 0.097 |
| Height | 1789.44** | 143.10 | 1508.96 | 2069.92 | 0.817** | 0.067 | 0.686 | 0.948 | 0.737** | 0.064 | 0.612 | 0.862 |
| Weight | 3.71** | 0.49 | 2.75 | 4.67 | 0.001** | 0.000 | 0.001 | 0.001 | 0.001** | 0.000 | 0.001 | 0.001 |
| Tanner Stage |
19.32** | 7.02 | 5.56 | 33.08 | 0.028** | 0.004 | 0.020 | 0.036 | 0.018** | 0.003 | 0.012 | 0.024 |
| DMPA Exposure |
−10.87 | 6.82 | −24.24 | 2.50 | −0.011** | 0.003 | −0.017 | −0.005 | −0.004 | 0.004 | −0.012 | 0.004 |
| OCP Exposure |
14.49** | 5.06 | 4.57 | 24.41 | 0.008** | 0.003 | 0.002 | 0.014 | 0.008** | 0.003 | 0.002 | 0.014 |
p < .05,
p < .01
NOTE: Total body bone mineral content (TB BMC), bone mineral density (BMD), depo-medroxyprogesterone (DMPA); oral contraceptive pills (OCP). Variables were centered as Age at 11, Depressive Symptoms at 50, Smoking at 0 (none), Race at 0 (black), Height at 1.6m, Weight at 60 kg, Tanner at 4, DMPA and OCP exposure at 0 (no exposure). DMPA and OCP total exposure ranges from 0 = no exposure [79% DMPA/54% OCP], 3 = exposure for 6–9 months [2% DMPA/3% OCP], 7 = consistent exposure for last 6y [3% DMPA/11% OCP] for last 6y. All estimates in this table are unstandardized. Interaction effects between depressive symptoms and potential confounders were tested and found to be non-significant (P > .05) with the exception of depressive symptoms and Tanner stage in predicting TB BMC (B = −1.38, SE = 0.58, P = 0.02), indicating an additional negative impact of depressive symptoms on TB BMC for girls in higher Tanner stages.
Alcohol and Trait Anxiety
There was no effect of alcohol use or anxiety symptoms or their interactions with age on any bone measure.
Summary
These novel longitudinal findings support our hypotheses regarding both smoking and depressive symptoms as negatively influencing bone accrual in adolescent girls. We found effects on BMD of the lumbar spine and total hip, which are common sites of osteoporotic fractures later in life, but not for TB BMC, which is an aggregate measure of bone health throughout the skeleton. To our knowledge, our longitudinal findings are the first delineated in the adolescent population. Specifically, higher-frequency smokers entered adolescence with the same total hip and lumbar spine BMD as lower-rate smokers (or non-smokers), yet a higher frequency of smoking was associated with a lower rate of bone accrual across adolescence. For example, at age 19, the clinical relevance of the difference in the lumbar spine BMD of a non-smoker versus a daily smoker approximates the amount an adolescent gains in BMD in 1 year. With continued smoking behavior, one could surmise that lumbar spine BMD could become dramatically lower throughout adulthood. Smoking uptake across time was typical of adolescents in which levels of smoking are generally low and highly variable across 30 day periods, and pack years cannot be ascertained. However, it is concerning that even relatively low levels of smoking have a negative impact on bone accrual. Alternatively, smoking may be a marker for other unmeasured behaviors that negatively impact bone. Further research is needed to confirm findings in girls with higher rates of smoking.
The associations between depressive symptoms and bone were somewhat different. Lower lumbar spine BMD persisted across ages 11–19 for those with higher compared to lower depressive symptoms, and there was no interaction with age. An increase of 1 SD (10 points) in depressive symptoms was associated with lower BMD of the lumbar spine; similar to what a girl might typically gain in BMD in 1 year2. Importantly, in a nonclinical sample (i.e., not depressed), it is worrisome that effects in bone accrual were evident even at these lower levels of depressive symptoms. The magnitude of effects of diagnosable depression on bone accrual may be even greater.
In addition to its novelty, the attention to a wide range of potential covariates that may have confounded previous adult studies, was another strength of our study. Few studies have accounted for this array of relevant covariates. Importantly, even after accounting for significant covariates, the two key predictors—depressive symptoms and smoking— still played a role in lower bone accrual.
In spite of the strengths of the study, future research is essential and limitations should be addressed. First, further exploration should focus on whether anxiety and alcohol play a role in accrual of bone as reported in animal models and adult human studies. Anxiety is common in pubertal-age girls37 and often is comorbid with and precedes depression; thus, we hypothesized that anxiety might have a similar impact on bone accrual as did depressive symptoms. Our prior cross-sectional findings revealed negative associations with anxiety and bone.22,23 Second, alcohol might not have been significant in this study because of low use, particularly in the younger cohorts, but also because alcohol was not the focus of the study and its measurement was limited. Third, the study may not be generalizable to all adolescent girls; it may only represent the sample at hand. However, characteristics of our sample with respect to BMI, calcium intake, physical activity, serum 25 (OH)D, and bone health are similar to published studies on those measures. Notably, our sample fell below recommended guidelines for calcium intake and physical activity, and findings may not generalize to girls meeting those national standards. Importantly, future studies should expand to other geographical regions and include other races beyond black and white girls.
In sum, adolescence is a crucial period of development that lays the foundation for women’s health across the lifespan. To our knowledge, our study is the first to test and demonstrate that smoking behavior and depressive symptoms in girls have a negative impact on bone accrual in adolescence. For several decades, such studies have been conducted in adults but not in adolescents, in whom nearly 50% of bone is accrued. Importantly, as much bone is accrued in the 2 years surrounding menarche as is lost in the last 4 decades of life.3,38 Thus, adolescence is paramount when considering factors that interfere with optimum accrual. In addition to the reported gender difference in osteoporosis in adults,39 reproductive-age women generally exhibit higher rates of depression than men40 and differing outcomes of substance use. Because depression and substance use often become chronic, their deleterious effects on bone might result in long-term and costly public health problems. Based on our study, it may be premature to advocate DXA screening for BMD in adolescents with depressive symptoms or those who smoke. Our study should be replicated to determine whether greater vigilance in monitoring bone mineral status is necessary in those with smoking behavior and higher depressive symptoms. While adolescent depressive symptoms already signal a red flag for future adult depressive episodes, they also may become a red flag for a future constrained by low bone mass or osteoporosis and higher fracture rates in postmenopausal years.
Panel: Research in Context
Systematic Review
Systematic review and meta analyses reveal that depression or its symptoms have a negative association with bone density in adults. Fewer studies have been conducted regarding the impact of smoking. No such longitudinal studies have been reported in adolescence; a time when nearly 50% of bone is accrued.
Interpretation
Higher-frequency smokers entered adolescence with the same total hip and lumbar spine BMD as lower-rate smokers, yet a higher frequency of smoking was associated with a lower rate of bone accrual across adolescence. Also lower lumbar spine BMD persisted across ages 11–19 for those with higher compared to lower depressive symptoms. It is worrisome that effects in bone accrual were evident even at these lower levels of smoking and depressive symptoms.
Acknowledgements
This research was supported in part by Grant Number R01 DA 16402, National Institute of Drug Abuse, NIH, PI: Dorn and by USPHS # UL1RR026314 from the National Center for Research Resources, NIH and by funds from the Bureau of Health Professions (BHPr), Health Resources and Services Administration (HRSA), Department of Health and Human Services (DHHS), under # T32HP10027.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Home page statistics. [Accessed 15 Sep, 2011];National Osteoporosis Foundation. 2012 at http://www.nof.org.
- 2.Kalkwarf HJ, Zemel BS, Gilsanz V, et al. The Bone Mineral Density in Childhood Study: Bone mineral content and density according to age, sex, and race. J Clin Endocrinol Metab. 2007;92:2087–2099. doi: 10.1210/jc.2006-2553. [DOI] [PubMed] [Google Scholar]
- 3.Bailey DA, Faulkner RA, McKay HA. Growth, physical activity, and bone mineral acquisition. Exerc Sport Sci Rev. 1996;24:233–266. [PubMed] [Google Scholar]
- 4.Magarey AM, Boulton TJ, Chatterton BE, Schultz C, Nordin BE, Cockington RA. Bone growth from 11 to 17 years: Relationship to growth, gender and changes with pubertal status including timing of menarche. Acta Paediatr. 1999;88:139–146. doi: 10.1080/08035259950170286. [DOI] [PubMed] [Google Scholar]
- 5.Loud KJ, Gordon CM. Adolescent bone health. Arch Pediatr Adolesc Med. 2006;160:1026–1032. doi: 10.1001/archpedi.160.10.1026. [DOI] [PubMed] [Google Scholar]
- 6.Cizza G, Primma S, Coyle M, Gourgiotis L, Csako G. Depression and osteoporosis: A research synthesis with meta-analysis. Horm Metab Res. 2010;42:467–482. doi: 10.1055/s-0030-1252020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Q, Liu J, Gallegos-Orozco JF, Hentz JG. Depression, fracture risk, and bone loss: A meta-analysis of cohort studies. Osteoporos Int. 2010;21:1627–1635. doi: 10.1007/s00198-010-1181-x. [DOI] [PubMed] [Google Scholar]
- 8.Yirmiya R, Bab I. Major depression is a risk factor for low bone mineral density: A meta-analysis. Biol Psychiatry. 2009;66:423–432. doi: 10.1016/j.biopsych.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 9.Hollenbach KA, Barrett-Connor E, Edelstein SL, Holbrook T. Cigarette smoking and bone mineral density in older men and women. Am J Public Health. 1993;83:1265–1270. doi: 10.2105/ajph.83.9.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ward KD, Klesges RC. A meta-analysis of the effects of cigarette smoking on bone mineral density. Calcif Tissue Int. 2001;68:259–270. doi: 10.1007/bf02390832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hopper JL, Seeman E. The bone density of female twins discordant for tobacco use. N Engl J Med. 1994;330:387–392. doi: 10.1056/NEJM199402103300603. [DOI] [PubMed] [Google Scholar]
- 12.Crilly RG, Anderson C, Hogan D, Delaquerrière-Richardson L. Bone histomorphometry, bone mass, and related parameters in alcoholic males. Calcif Tissue Int. 1988;43:269–276. doi: 10.1007/BF02556634. [DOI] [PubMed] [Google Scholar]
- 13.Feskanich D, Korrick SA, Greenspan SL, Rosen HN, Colditz GA. Moderate alcohol consumption and bone density among postmenopausal women. J Womens Health. 1999;8:65–73. doi: 10.1089/jwh.1999.8.65. [DOI] [PubMed] [Google Scholar]
- 14.Wosje KS, Kalkwarf HJ. Bone density in relation to alcohol intake among men and women in the United States. Osteoporos Int. 2007;18:391–400. doi: 10.1007/s00198-006-0249-0. [DOI] [PubMed] [Google Scholar]
- 15.Williams FM, Cherkas LF, Spector TD, MacGregor AJ. The effect of moderate alcohol consumption on bone mineral density: A study of female twins. Ann Rheum Dis. 2005;64:309–310. doi: 10.1136/ard.2004.022269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holbrook TL, Barrett-Connor E. A prospective study of alcohol consumption and bone mineral density. Br Med J. 1993;306:1506–1509. doi: 10.1136/bmj.306.6891.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Arch Gen Psychiatry. 2003;60:837–844. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- 18.Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the future: National results on adolescent drug use: Overview of key findings 2008. Bethesda, MD: National Institute on Drug Abuse NIH Dept of Health and Human Services; 2009. [Google Scholar]
- 19.Gore FM, Bloem PJN, Patton GC, et al. Global burden of disease in young people aged 10–24 years: A systematic analysis. Lancet. 2011;377:2093–2102. doi: 10.1016/S0140-6736(11)60512-6. [DOI] [PubMed] [Google Scholar]
- 20.Rao U, Hammen CL, Poland RE. Longitudinal course of adolescent depression: Neuroendocrine and psychosocial predictors. J Am Acad Child Adolesc Psychiatry. 2010;49:141–151. doi: 10.1097/00004583-201002000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.U.S. Department of Health and Human Services. Preventing tobacco use among young people: A report of the Surgeon General. Atlanta, GA: U. S. Dept. of HHS, CDC, NCCDPHP, Office on Smoking and Health; 1994. [Google Scholar]
- 22.Dorn LD, Pabst S, Sontag LM, Kalkwarf HJ, Hillman JB, Susman EJ. Bone mass, depressive, and anxiety symptoms in adolescent girls: Variation by smoking and alcohol use. J Adolesc Health. 2011;49:498–504. doi: 10.1016/j.jadohealth.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dorn LD, Susman EJ, Pabst S, Huang B, Kalkwarf H, Grimes S. Association of depressive symptoms and anxiety with bone mass and density in ever-smoking and never-smoking adolescent girls. Arch Pediatr Adolesc Med. 2008;162:1181–1188. doi: 10.1001/archpedi.162.12.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donaldson G, Horn JL. Age, cohort, and time developmental muddles: Easy in practice, hard in theory. Exp Aging Res. 1992;18:213–222. doi: 10.1080/03610739208260360. [DOI] [PubMed] [Google Scholar]
- 25.Heaney RP. Bone mineral content, not bone mineral density, is the correct bone measure for growth studies. Am J Clin Nutr. 2003;78:350–351. doi: 10.1093/ajcn/78.2.350. author reply 1-2. [DOI] [PubMed] [Google Scholar]
- 26.Kovacs M. Children's Depression Inventory (CDI) Manual. North Tonawanda, NY: Multi-Health Systems, Inc.; 1992. [Google Scholar]
- 27.Spielberger CD, Edwards CD, Lushene RE, Montuori J, Platzek D. STAIC Preliminary Manual. Palo Alto, CA: Consulting Psychologists Press; 1973. [Google Scholar]
- 28.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory (Form Y) Palo Alto, CA: Consulting Psychologists Press, Inc.; 1983. [Google Scholar]
- 29.Mayhew KP, Flay BR, Mott JA. Stages in the Development of Adolescent Smoking. Drug Alcohol Depend. 2000;59:S61–S81. doi: 10.1016/s0376-8716(99)00165-9. [DOI] [PubMed] [Google Scholar]
- 30.Fisher PW, Shaffer D, Piacentini JC, et al. Sensitivity of the Diagnostic Interview Schedule for Children, 2nd edition (FISC-2.1) for specific diagnoses of children and adolescents. J Am Acad Child Adolesc Psychiatry. 1993;32:666–673. doi: 10.1097/00004583-199305000-00026. [DOI] [PubMed] [Google Scholar]
- 31.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crocker PR, Bailey DA, Faulkner RA, Kowalski KC, McGrath R. Measuring general levels of physical activity: Preliminary evidence for the Physical Activity Questionnaire for Older Children. Med Sci Sports Exerc. 1997;29:1344–1349. doi: 10.1097/00005768-199710000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Barr SI. Associations of social and demographic variables with calcium intakes of high school students. J Am Diet Assoc. 1994;94:260–266. 9. doi: 10.1016/0002-8223(94)90366-2. [DOI] [PubMed] [Google Scholar]
- 34.Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. 2nd ed. Thousand Oaks, CA: Sage Publications; 2002. [Google Scholar]
- 35.Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53:983–997. [PubMed] [Google Scholar]
- 36.Hofer SM, Hoffman L. Statistical analysis with incomplete data: A developmental perspective. In: Little TD, Bovaird JA, Card NA, editors. Modeling contextual effects in longitudinal studies. Mahwah, NJ: Lawrence Erlbaum Associates Publishers; 2007. pp. 13–32. [Google Scholar]
- 37.Costello EJ, Egger HL, Angold A. Developmental epidemiology of anxiety disorders. In: Ollendick TH, March JS, editors. Phobic and anxiety disorders in children and adolescents. New York, NY: Oxford University Press; 2004. pp. 61–91. [Google Scholar]
- 38.National Institutes of Health. Osteoporosis prevention, diagnosis, and therapy. NIH Consensus Statement Online. 2000;2000:1–34. [PubMed] [Google Scholar]
- 39.Pietschmann P, Rauner M, Sipos W, Kerschan-Schindl K. Osteoporosis: An age-related and gender-specific disease—A mini-review. Gerontology. 2009;55:3–12. doi: 10.1159/000166209. [DOI] [PubMed] [Google Scholar]
- 40.Parker G, Brotchie H. Gender differences in depression. Int Rev Psychiatry. 2010;22:429–436. doi: 10.3109/09540261.2010.492391. [DOI] [PubMed] [Google Scholar]