Abstract
Spinocerebellar ataxia type 2 (SCA 2) is caused by triple nucleotide repeat (CAG) expansion in the coding region of the ATAXN2 gene on chromosome 12, which produces an elongated, toxic polyglutamine tract, leading to Purkinje cell loss. There is currently no effective therapy. One of the main obstacles that hamper therapeutic development is lack of an ideal disease model. In this study, we have generated and characterized SCA2 induced pluripotent stem (iPS) cell lines as an in vitro cell model. Dermal fibroblasts (FBs) were harvested from primary culture of skin explants obtained from a SCA2 subject and a healthy subject. For reprogramming, hOct4, hSox2, hKlf4, and hc-Myc were transduced to passage-3 FBs by retroviral infection. Both SCA2 iPS and control iPS cells were successfully generated and showed typical stem cell growth patterns with normal karyotype. All iPS cell lines expressed stem cell markers and differentiated in vitro into cells from three embryonic germ layers. Upon in vitro neural differentiation, SCA2 iPS cells showed abnormality in neural rosette formation but successfully differentiated into neural stem cells (NSCs) and subsequent neural cells. SCA2 and normal FBs showed a comparable level of ataxin-2 expression; whereas SCA2 NSCs showed less ataxin-2 expression than normal NSCs and SCA2 FBs. Within neural lineage, neurons have the most abundant expression of ataxin-2. Time-lapsed neural growth assay indicated terminally differentiated SCA2 neural cells were short-lived compared to control neural cells. The expanded CAG repeats of SCA2 were stable throughout reprogramming and neural differentiation. In conclusion, we have established the first disease-specific human SCA2 iPS cell line. These mutant iPS cells have the potential for neural differentiation. The differentiated neural cells harboring mutations are invaluable for the study of SCA2 pathogenesis and therapeutic drug development.
Keywords: Induced pluripotent stem cells, spinocerebellar ataxia, ataxin- polyglutamine
Introduction
Spinocerebellar ataxia type 2 (SCA2 OMIM #183090) is caused by a tri-nucleotide (CAG) repeat expansion in the coding region of the ATAXN2 gene on chromosome 12. Worldwide, it is the second most common SCAs after SCA3. Clinically, SCA2 belongs to a group of SCAs known as autosomal dominant cerebellar ataxia type 1 (ADCA-1), in which cerebellar ataxia (the core phenotype) is associated with extracerebellar neurological abnormalities. Like other disorders of the ADCA-1, the extracerebellar manifestations of SCA2 may include parkinsonism, progressive cognitive impairments, palatal and generalized myoclonus, optic atrophy, distal sensory loss, and adult-onset spinomuscular atrophy (Eto, 1990; Auburger, 2012). However, progressively slow saccadic eye movements that start at an early stage of the disease and areflexia which is often confined to the upper extremities in the beginning distinguish SCA2 from other SCAs. Brains from an SCA2 patients showed cytoplasmic (but not nuclear) microaggregates containing expanded polyglutamines with loss of cerebellar Purkinje cell dendritic arbor and eventual cell loss (Huynh, 2000). The expansion of CAG repeats result in the lengthening of polyglutamine stretch in the encoded protein ataxin-2. However, the normal biological function of ataxin-2 and the exact pathogenesis of expanded polyglutamine in ataxin-2 remain largely unknown (Lastres-Becker, 2008a). Ataxin-2 is a widely expressed protein and may play a role in mRNA homeostasis through its interaction with ataxin-2 binding protein 1 (A2BP1/Fox-1) (Shibata, 2000), and may regulate plastin-associated actin filament organization and endocytosis by interacting with endorphin proteins (Ralser, 2005). Ataxin-2 deficient mice are viable although Atxn2−/− mice showed reduced fertility (Lastres-Becker, 2008b)and there was a significant reduction in the number of female Atxn2 +/− and Atxn2 −/− mice born (Kiehl, 2006). They also show adult-onset obesity (Kiehl, 2006), dissociated fear and spatial learning (Huynh, 2009), locomotion hyperactivity and liver steatosis (Lastres-Becker, 2008b). Transgenic mice expressing ataxin-2 with a 58-glutamine (58Q) track showed progressive functional deficits with loss of the Purkinje cell dendritic arbor and eventual loss of Purkinje cells accompanied by cytoplasmic polyglutamine-containing microaggregates, closely mimicking human SCA2. Another transgenic mice expressing 75Q under the regulation of the ATXN2 promoter, which has been shown to preferentially drive the expression in the cerebellum and olfactory bulb, showed specific Purkinje cell degeneration (Aguiar, 2006; Scoles, 2012). However, such animal models still have limitations as a faithful model of human neurological diseases such as SCA2 due to the interspecies difference between mouse and human. The emergence of induced pluripotent stem (iPS) cells, which are generated by direct reprogramming of human somatic cells, offers an alternative cellular model for mechanistic study (Takahashi, 2007; Yu, 2007; Kastenberg, 2008). Patient-derived iPS cells will preserve the genetic mutation carried by the patient on a functional human genomic background, which cannot be accomplished in animal models. Furthermore, they can be differentiated into human cells of neural lineage, which are not readily available. In this study, we have generated a human SCA2 iPS cell line and characterized it as an in vitro disease model.
Materials and Methods
Reagents and Cells
iPS culture media and reagents: DMEM/F12, 20% KSR(#108281), Glutamax (35050,1X), 2-mercaptoethanol(#21985, 1X), sodium pyruvate (1mM), MEM NEAA(#11140, 1X), penicillin/streptomycin (#15140, 1X), recombinant human fibroblast growth factor-basic (FGFb) (#PHG0021) (10ng/ml) and recombinant human epidermal growth factor (Hu EGF) (#PHG0311L), collagenase IV and AFF 488, AFF 555 are from Invitrogen/Gibco. Defined cryopreservation medium for hESC and hiPSCs (#05854), accutase (#07920), anti-Oct-3/4 (#01550), anti-SSEA-4 (#01554), FITC-conjugated goat anti-mouse IgG (#10210), heparin (#07980), Y-2763 ROCK inhibitor (07171), AggreWell™ 800(#27865), STEMdiff™ Neural Induction Medium (#05831), STEMdiff Neural Rosette Selection Reagent (# 05832), NeuroCult NS-A proliferation kit (#05751) and NeuroCult NS-A Differentiation kit (# 05752) are from StemCell Technologies. Poly-L-ornithine (#P4957), laminin (#L2020), and mitomycin C (M0503) are from Sigma.
Antibodies: nestin (#MAB 1259) (R&D), AFP (R&D), neurofilament H (Cell Signaling #2836), GFAP (Novus #NB300-41A), beta-tubulin III (fluorescence-conjugated, Millipore # CBL 412 X), calbindin (Sigma # 078K4768), ubiqitin (Cell Signaling #3936), ataxin-2 (Novus Biologicals #NBP1-90063), and desmin (Lab-Vision). IbidiTreat μ-Slides are from Ibidi GmbH (Martinsried, Germany).
Others: Hygromycin-resistant primary mouse embryonic fibroblasts (MEFs) (StemCell Technologies #00324) and AmpliTaq (Life technologies #4398901).
Identification and Recruitment of Study Subjects
The study was approved by the University of Florida Institutional Review Board. The SCA2 subject was a 30-year-old male with 20/44 CAG repeats. A 51-year-old male with no medical problems was biopsied as a control. All human subjects were provided with the approved informed consent.
Skin Biopsy and Culture of Human Dermal Fibroblasts
Skin biopsy was performed by punch biopsy (6mm in diameter) under local anesthesia. The skin specimens were placed in sterile DMEM culture medium supplemented with 20% FBS and penicillin/streptomycin at room temperature for transport to the lab. Biopsy specimens were processed into 0.5mm cubes. Fifteen pieces were placed into duplicate 25cm2 flasks. The explants were allowed to air-dry for 30 min, and 12 ml of primary culture medium (DMEM with 20% FBS) was added to the flask. The flasks were placed into a 37°C, 5% CO2 humidified circulating incubator. Medium was removed and fresh primary culture medium was replenished after 7 days. When fibroblasts (FBs) from adjacent explants started merging, the flasks were treated with 0.05% Trypsin/EDTA and passed to a 75cm2 flask. These cells were designated as passage 1. Passage 3 FBs were used for reprogramming.
Generation of iPS cells
Reprogramming was performed with the four traditional Yamanaka factors using retroviral vectors. Retroviruses, one for each of the four reprogramming factors (i.e. Oct4, Sox2, Klf4, and c-Myc) and enhanced green fluorescent protein (EGFP), was individually packaged using 293FT human embryonic kidney cell line (Invitrogen). After 72 hours of virus production, cell supernatant was filtered through a 0.45µM pore size filter, and the virus was concentrated by centrifugation for 16 hours at 8,000 ×g at 4 °C. Oct4, Sox2, Klf4, and c-Myc viruses were combined and used for transduction. Transduction efficiency was monitored using additional fibroblasts retrovirally transduced with EGFP virus alone. One day prior to transduction, fibroblasts were plated at a density of 1×105 cells per 9.5cm2 cell culture dish in DMEM containing 15% FBS. On day 5 after transduction, each 9.5cm2 plate was passed to a 56cm2 dish with feeders (6.7×105 mitomycin-C mitotically inactivated MEFs). On Day 6, the medium was changed from DMEM containing 15% FBS to iPS medium. Half of the medium was removed and replaced with fresh iPS medium every other day (Day 8, 10, 12, etc.) until colonies were ready for harvesting. Propagation and freezing of iPS cells were performed according to published protocols (Baharvand; Mollamohammadi, 2009). MFreSR freezing medium (StemCell Technologies) was used to prepare iPSC frozen stocks.
Characterization of iPS cells
Karyotyping
Karyotyping was performed by G-banding to examine the integrity of the chromosomes by the Cytogenetics Laboratory at University of Florida.
RT-PCR of stem cell markers
Total RNA was extracted using RNaeasy Micro Kit (Qiagen, Valencia, CA). cDNA was synthesized using Invitrogen/Gibco kit (SuperScript III Reverse Transcriptase, #18080) from 1µg total RNA in a final volume of 20µl. 1µl was used for subsequent PCR. Stem cell marker RT-PCRs was performed using published primers (Takahashi, 2007) targeting endogenous human OCT4, SOX2, NANOG and MYC. hOCT 4: F 5'-GAC AGG GGG AGG GGA GGA GCT AGG-3', R 5'-CTT CCC TCC AAC CAG TTG CCC CAA AC-3'; hSOX2: F 5'-GGG AAA TGG GAG GGG TGC AAA AGA GG-3', R 5'- TTG CGT GAG TGT GGA TGG GAT TGG TG-3'; hNANOG: F 5'-CAG CCC TGA TTC TTC CAC CAG TCC C-3', R 5'- GG AAG GTT CCC AGT CGG GTT CAC C-3'; hMYC: F 5'- GCG TCC TGG GAA GGG AGA TCC GGA GC-3', R 5'- TTG AGG GGC ATC GTC GCG GGA GGC TG-3'; beta-Actin was amplified as an internal control. PCR was carried out in a final volume of 20 µl containing 0.5 µM forward and reverse primers, using AmpliTaqGold 360 master mix. The initial denaturation at 95 C for 3 min was followed by 36 cycles of denaturation at 95 C for 30s, annealing 60°C (except for beta-actin 55 °C) for 30s and elongation at 72°C for 1min, and a final elongation step of 10min at 72°C. Electrophoresis of the PCR products was carried out on 1.6% agarose gel.
Alkaline phosphatase activity assay and Immunocytofluorescence staining of stem cell markers
The assays were performed on IbidiTreat μ-Slides. 3–5 iPS clumps (around 100 cells) were seeded into chambers with feeders and cultured for 2–3 days to allow the colonies to expand. For the alkaline phosphatase activity assay, the chamber was incubated with Liquid Fast-Red Substrate System (Thermo Scientific, #TA-060-AC) overnight at 4 °C. Nuclear transcription factor Oct4 and cell surface marker SSEA4 were examined by immunocytofluorescence staining. The slides were washed with PBS and fixed in 4% paraformaldhyde for 5 minutes, permeabilized with 0.3% Triton X-100 for 15 minutes, blocked with 10 % normal goat serum (Vector Lab) for 30 minutes and incubated with Oct 4 or SSEA4 (1:100) overnight at 4 °C. The slides were washed and incubated with goat anti-mouse FITC-conjugated secondary antibody (1:500) (StemCell Technologies) for 30 minutes. Afterwards slides were washed and mounted with Vectashield mounting medium with DAPI (Vector Laboratories, #H-1200). Pictures were taken under confocal microscope (Olympus IX81-DSU Spining Disk).
Prolonged self-renewal
iPS cells were passed at 1:4 ratios approximately every 4–5 days and were maintained to a passage 35 at the time of this submission. Over this time, iPS cell growth pattern and morphology were recorded.
Embryoid body (EB) formation
Undifferentiated colonies (P21 of SCA2 iPS cells and P8 of normal control iPS cells) were detached with Collagenase IV (1mg/ml) and Trypsin-EDTA (0.05%) for 20 minutes; cells were flushed with 1ml large bore tips and passed through a 70µm cell strainer to remove single cells and MEFs. Next, the strainer was turned over to collect all the colonies by washing with DMEM/F12 medium. Colonies were centrifuged at 192 ×g for 3 minutes, resuspended in 3 ml Accutase and incubated for 25 min at 37°C, pipetting every 5 minutes to make a single cell suspension. Cells were passed through a 37 µm cell strainer and 7 ml of DME/F-12 medium was added, and then centrifuged at 300g for 5 minutes. Cell pellets were resuspended in EB medium to generate 1×106 cells/ml and 2 ml was added to each well of an AggreWell 800 plate (StemCell Technologies). The plate was centrifuged at 100g for 3 minutes to capture the cells in the microwells. 3/4 of the medium was changed daily for 4 days. EBs were then detached by flushing the microwells with induction medium and transferred to a low attachment 6-well plate for further culture for 5 days. Some EB were transferred to IbidiTreat μ-Slides for differentiation. EBs growing in 6-well plates were collected and embedded in agarose, fixed in 4% paraformaldhyde overnight, and embedded in paraffin for subsequent staining.
Immunohistochemical staining of EB
Pluripotency was assayed by immunohistochemical staining of AFP (endoderm), desmin (mesoderm) and neural differentiation (ectoderm) (see Neural Differentiation below). Four-µm sections from paraffin-embedded EB were used for immunohistochemical staining of AFP and desmin. Sections were de-paraffinized and incubated with 3% H2O2/methanol to block endogenous peroxidase activity, and then treated with Antigen Retrieval Citra Solution (BioGenex, # HK086-9K) for 30 minutes. Background Sniper (Biocare Medical, l# BS966M, Walnut Creek, CA) was used to reduce nonspecific background staining. Sections were incubated with AFP (1:100) or desmin (1:100) antibody overnight at 4°C. After incubation with secondary antibody, sections were developed using diaminobenzidine (DAB) chromogen (Biocare Medical, Walnut Creek, CA) and lightly counterstained with Hematoxylin (Biocare Medical, Walnut Creek, CA).
Neural differentiation
Neural differentiation using AggreWell 800: AggreWell 800 was prepared following manufacture's protocol. Single cell suspension was prepared as described above in EB formation but with STEMdiff Induction Medium (StemCell Technologies) supplemented with ROCK inhibitor to final concentration of 10 µM. 1.2 to 2.5 ×106 cells were seeded in each well of the AggreWell 800 in a total volume of 2 ml. The Plate was centrifuged at 100×g for 3 minutes to capture the cells in the microwells, and 3/4 of the medium was changed daily for 5 days. Over this time, the iPS cells collected at the bottom of the well aggregated to form neurospheres. The spheres were flushed from the microwells using neural induction medium and were transferred to a Poly-Ornithine-Laminin coated 6-well plate (one well of the AggreWell 800 well to one well of a 6-well plate). Neurospheres attached and medium was changed daily. On day 7, neural rosettes were detached using STEMdiff Neural Rosette Selection Reagent and transferred to one well of 6-well plate coated with Poly-Ornithine-Laminin. Neural stem cells (NSCs) start growing out of neural rosettes in 12 hours. Cells were cultured for 7 days. NSCs were detached with accutase to make a single cells suspension and transferred to a 6-well plate at a density of 1–5×105 per well as NSC passage 1. 4×104/ml cells were seeded to IbidiTreat μ-Slides and cultured in Neural Induction medium for observation and staining of neural markers. For further propagation and differentiation, NSC were detached using accutase and grown either in suspension or as attached cells using NeuroCult NS-A proliferation medium.
Neural differentiation with EB method was performed as reported (Hu, 2010) but commercial induction medium (STEMdiff Neural Induction Medium, StemCell Technologies) was used.
Immunocytofluroscence staining of ataxin-2 and neural cell markers
The studies were performed on IbidiTreat μ-Slides. Forty µl of neural stem cell at density of 1×106/ml were seeded to IbidiTreat μ-Slides and cultured in Neural Induction Medium for observation and staining of neural markers. Cells were cultured for 3–5 days to allow for spontaneous differentiation. The slides were then washed twice with PBS, fixed in 4% paraformaldhyde for 5 minutes, washed once with PBS, permeabilized with 0.3% Triton X-100 for 15 minutes, washed once with PBS, blocked with 10% normal goat serum (Vector Lab # S-1000) for 30 minutes, and incubated with ataxin-2 (1:100), nestin (1:100), neurofilament H (1:50), beta-tubulin III (1:250), GFAP (1:500), or calbindin (1:250) overnight at 4°C. The following day, slides were washed three times with PBS, incubated with the appropriate secondary antibody conjugated with AF 555 or AF 488 (Invitrogen) (1:500) for 30 minutes, washed with PBS and mounted with Vectashield Mounting Medium with DAPI. Pictures were taken under confocal microscope (Olympus IX81-DSU Spining Disk). For double immunocytofluroscence staining, ataxin-2 and nestin or neurofilament H antibodies were co-incubated overnight at 4°C. AF488 goat anti-rabbit and AF 555 goat anti-mouse secondary were co-incubated at room temperature for 30 minutes.
Western blot
Western blot was performed as previously described (Xia, 2005). Briefly, 20 µg whole cell lysate was run on 4% to 20% Tris-glycine gradient gel. Membranes were first probed with ataxin-2 antibody (1:1000) overnight, washed and probed with the secondary antibody and developed. The membranes were then stripped with Restore Western Blot Stripping Buffer (Pierce) and reprobed with β-actin to confirm equivalent loading and transfer of protein. Signal was detected using SuperSignal West Femto Maximum Sensitivity Substrate (Pierce).
Time-lapsed study of neural differentiation
4×104 neural stem cells were seeded in IbidiTreat μ-Slides and cultured in STEMdiff Neural Induction Medium (StemCell Technologies). Three fields were observed every 12 hours and successive photos were taken for analysis of cell survival. Only neurons were tracked and counted. The survival rates at 50 hours were compared between SCA2 and control neurons.
SCA 2 CAG expansion PCR
Genomic DNA was isolated from cultured cells using Qiagen DNeasy Blood & Tissue kit #69506). The region containing the SCA2 CAG repeat was PCR amplified using previously published primers: F, 5′- GGGCCCCTCACCATGTCG-3′; R, 5′- CGGGCTTGCGGACATTGG 3′ (Pulst, 1996). PCR was carried out in a final volume of 20 µl, containing 100ng genomic DNA, 0.5 µM forward and reverse primer, and AmpliTaq master mix with denaturation at 95 C for 3min, followed by 36 cycles of denaturation at 95°C for 30 sec, annealing at 56 C for 30sec, and elongation at 72 C for 1min, and a final elongation step of 10min at 72°C. Electrophoresis of the PCR products was carried out on a 2% agarose gel. For repeat stability analysis, the precise sizes of the fluorescent PCR product were determined by DNA Analyzer 3720XL (Applied Biosystems).
Statistical Analysis
Time-lapsed neuronal survival study between SCA2 neurons and control neurons was compared by Fisher's Exact Test using IBM SPSS Statistics 20.
Results
1. Reprogramming efficiency of skin fibroblast is similar during SCA 2 and normal iPS generation
The growth patterns of SCA2 and normal fibroblasts were not different (Supplement Figure 1). Cells from passage 3 were used for iPS cell generation. The traditional retroviral method had similar transduction efficiency between SCA2 and control cells (Supplement Figure 2). Colonies with typical hESC morphology started appearing between days 17–19 and were ready to isolate around day 30 for both SCA2 and control iPS cells. The method yields a reprogramming efficiency of roughly 0.01%. 4–10 individual clones were further propagated. Clone number 2 of SCA2 iPS and clone number 10 of control iPS were used below.
2. Both SCA 2 iPS cells and normal control iPS have pluripotent stem cell features
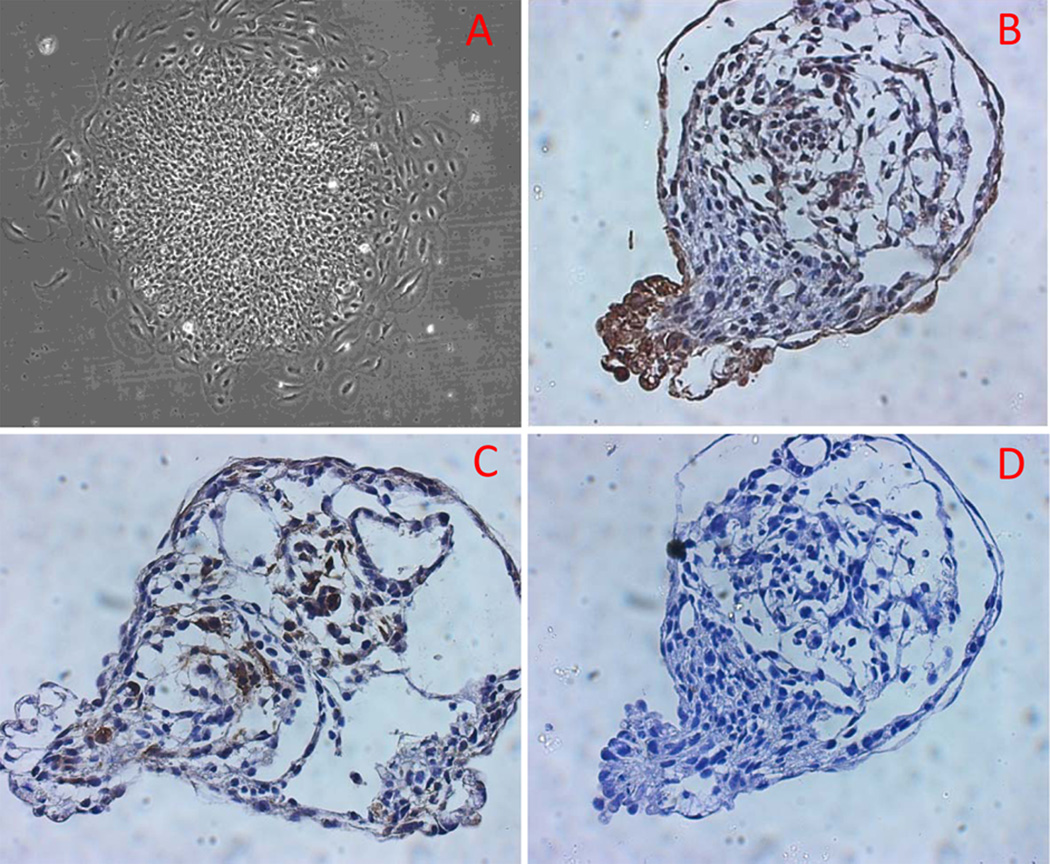
Both SCA2 and control iPS cells grew as flat, well-circumscribed colonies on feeder cells in iPS culture medium. The colonies contained tightly compacted cells with a high nuclear/cytoplasm ratio (Figure 1, only SCA2 iPS cells are shown). The iPS colonies maintained similar growth rates and pattern through subsequent passages. Karyotyping and genetic analysis showed normal male karyotype (Metaphase number of chromosome =46) without clonal structural or numerical aberrations for SCA 2 and normal iPS cells (Supplement Figure 3). Agarose gel electrophoresis of RT-PCR-amplified Oct4, Nanog and Sox2 mRNA demonstrated that SCA2 iPS cells, but not the original fibroblasts, expressed these stem cell markers. In contrast c-Myc is ubiquitously expressed in both somatic and stem cells (Supplement Figure 4). A similar expression pattern was also found in control iPS cells (data not shown). Immunocytofluorescence analysis of stem cell markers in SCA2 and control iPS colonies stained positive for alkaline phosphatase, Oct4, and SSEA4 while the surrounding feeder cells served as a negative control (Figure 2). To analyze the pluripotency, we performed EB formation assay. SCA2 EBs showed normal morphology and differentiation in culture chambers (Figure 3A–B). Cell differentiation was confirmed by immunohistochemical staining of AFP (for endoderm) (Figure 3C) and desmin (for mesoderm) (Figure 3D) on paraffin-embedded EBs. Ectoderm differentiation was evaluated by neural differentiation.
Fig. 1.
Typical SCA2 iPS colony growing on MEFs is shown to be flat, well-circumscribed (A, B). High magnification shows tightly compacted cells with high nuclear: cytoplasm ratio (C). H&E staining after cytospin shows the relatively small cell size compared to MEF feeder cells (arrow) (D).
Fig. 2.
SCA2 iPS cells stained positive for alkaline phosphatase (A), cell surface marker SSEA4 (C, DAPI; D, FITC; E, Merge) and nuclear marker Oct4 (F, DAPI; G, FITC; H, Merge) while the surrounding feeder cells were negative. The same staining pattern was detected in control iPS cells: Alkaline phosphatase (B), SSEA4 (I, DAPI; J, FITC; K, Merge) and nuclear marker Oct4 (L, DAPI; M, FITC; N, Merge).
Fig. 3.
SCA2 EBs underwent differentiation into cells with different morphology in culture chambers (A, 7 days after attachment). Endoderm and mesoderm cell differentiation was confirmed by immunohistochemical staining of AFP (endoderm) (B) and desmin (mesoderm) (C) on paraffin-embed EB. Notice the staining cells have no overlap. D: negative control with normal mouse IgG.
3. SCA2 iPS neural differentiation is deviated from normal process
Neural differentiation of SCA2 iPS cells was compared to control iPS cells. Both SCA2 and control iPS cells aggregated and formed neurospheres in the microwells (Figure 4A, Figure 4E). Control iPS cells had subsequent normal neural differentiation. Neural rosettes started forming 3 days after neurospheres attachment (Figure 4B). NSCs egressed from attached neural rosettes and re-grouped into small, loosely arranged rosettes (Figure 4C–D) from which spontaneous differentiation occurred (Figure 4D, white arrow); However, neural rosette formation from SCA2 iPS cells was abnormal after transfer to poly-ornithine-laminin coated plates, as compared to rosettes from control iPS cells. Instead of forming neural rosettes, SCA2 neurospheres evolved into a cyst-like structure with a pedicle from which NSCs (white arrow) emerged (Figure 4F). SCA2 NSCs also had a tendency to spontaneously differentiate at early passages. Neural differentiation using the EB method yielded similar differences.
Fig. 4.
Both SCA2 (A) and control iPS cells (E) aggregated and formed neurospheres normally in the microwells. During control iPS cell neural differentiation, neural rosettes began forming 3 days following neurosphere attachment (B). NSCs egressed from re-plated neural rosettes and still maintained the tendency to form small loosely arranged rosettes (C–D), from which spontaneous differentiation occurred (D, arrow). In contrast, neural rosettes never formed during SCA2 iPS cell neural differentiation. After attaching to poly-ornithine-laminin coated plate, SCA2 neurospheres (E) formed a cyst-like structure with a pedicle, from which neural stem cells (arrow) egressed (F).
SCA2 NSCs expressed GFAP and Nestin (Figure 5A–B) and could further differentiate into neurons and astrocytes (Figure 5C–E). Some of these neurons expressed calbindin (Figure 5F). During subsequent passages, NCSs cultured in suspension were able to form spheres. Once these spheres were seeded onto poly-ornithine-laminin coated plates, they attached and grew out of the sphere in a bipolar shape (Figure 5G–H). However, when NSCs grew to confluence, they tended to form spheres again (Figure 5I). Similar finding were seen in control NSCs (data not shown). These NSCs (the latest passage of P7) maintained their potential of further neural differentiation and formed a neural network (Figure 6A–B).
Fig. 5.
SCA2 NSCs expressed GFAP (A) and Nestin (B) and further differentiated into neurons and astrocytes (C, D: double staining with neurofilament H (red) and GFAP (green); E:Beta-tubulin III). Some of the neurons expressed calbindin (F). During subsequent passage, NSCs cultured in suspension formed spheres. When these spheres were seeded to poly-ornithine-laminin coated plates, they attached and NSCs grew out of the sphere in a bipolar shape (G, H). However, when NSCs grew to confluent, they tended to form spheres again (I). Similar finding were observed in control NSCs (data not shown).
Fig. 6.
Passage 7 of control NSCs (A) and SCA2 NSCs (B) still maintained the potential of further neural differentiation and formed a neural network (36 hours after neural differentiation).
4. SCA 2 NSCs express less ataxin-2 compared to normal NSCs
Western blot was performed to compare the expression of ataxin-2 between neural lineage cells (NSCs) and non-neural lineage cells (fibroblasts) derived from SCA2 and control iPS cells. Specific bands around 150kD (predicted size for ataxin-2) were detected. We found SCA2 NSCs expressed less ataxin-2 than normal NSCs and SCA2 FB. In SCA2 NSCs both mutant and wild type protein appear to be equally expressed (Figure 7). The differential expression of ataxin-2 in subtype of neural lineages cells was compared by immunofluorescence (IF) staining below.
Fig. 7.
Western blot of ataxin-2 expression in normal and SCA2 cells. SCA 2 NSC showed less ataxin-2 expression compared to normal NSCs. SCA2 NSCs had lower expression compared to SCA2 FBs.
5. Neuron cells express more ataxin-2 than glias
Newly generated NSCs had the tendency of spontaneous differentiation, which resulted in coexistence of NSCs, neurons and glias in culture. Ataxin-2 expression in neural lineage cells was examined by double IF staining of ataxin-2 and specific neural markers. We found ataxin-2 was expressed most abundantly in newly differentiated neurons. Nestin and Neu H negative cells (glias-like) had the least expression. No cytoplasmic or nuclear inclusion was detected (Figure 8).
Fig. 8.
Immunocytofluorescence double staining of ataxin-2 and neural markers in neural lineage cells. Ataxin-2 was expressed homogenously but neurons had the most abundant expression. A. Normal control: Nestin positive cells (NSCs) had strong Ataxin-2 expression. B. Normal control: Neu H positive cells (neurons) had strong ataxin-2 expression. The expression of ataxin-2 in Neurofilament H-negative cells varied. Strong expression ones (arrow) are more likely NSC and weak expression ones are likely glias (arrow head). C.SCA2. Strong ataxin-2 expressions were found in newly differentiated neurons which were still expressing nestin (arrow). Nestin-negative cells have low expression of ataxin-2 (arrow head). D: SCA2/Strong expression of ataxin-2 in Neurofilament H positive cells (arrow). Neurofilament H negative cells have low ataxin-2 expression (arrow heads).
6. SCA2 neural cells have shorter life span compared to normal control neural cells
Neuronal growth in culture was compared by time-lapse imaging. Imaging of the same group of neurons over successive days showed that the mean survival rate for SCA2 neurons (total 21 cells) was shorter compared to normal control neuronal cells (total 64 cells) (33.3%±17% vs 100%±0%, P=0.00) within 50 hours after differentiation calculated from 3 different fields (Figure 9).
Fig. 9.
Time-lapse imaging of neurons over successive days. Within 50 hours, all control neurons were viable (upper panel, low right hand corner numbers are change of neuron number over time). In contrast, SCA2 neurons were short-lived (lower panel).
7. CAG repeats remained stable during reprogramming and neural differentiation
Expansion of the CAG repeats in Ataxin-2 gene was verified by genomic PCR and sequencing in fibroblasts and the established SCA2 iPS clone. The normal allele had two CAA interruptions in the (CAG)8CAA(CAG)4CAA(CAG)8 configuration, while the expanded allele had pure (CAG)44 without interruption. The expanded allele showed no detectable differences in blood, FB and iPS cells (Supplement Figure 5A). Serial passage of SCA2 FBs (P0–P19) (Supplement Figure 5B), iPS cells (P9 and P26) (Supplement Figure 5C), and NSCs (P2) (Supplement Figure 5C) all showed stable CAG repeat. The CAG expansion in the ATXN2 gene was further stably maintained throughout the reprogramming and neural differentiation processes (Figure Supplement 5C). All repeat sizes were further confirmed by PCR with Fluorescent-labeled primer and capillary electrophoresis.
Discussion
The main result of the present study was the successful generation of a line of mutant iPS cells from an SCA2 subject and the identification of a neuronal defect in SCA2 during neural development. To date, the SCA2 iPS cells have been propagated to passage 37 with stable expanded CAG repeat size and growth morphology. We have also demonstrated in vitro pluripotency, self-renewability and neural differentiation of these cells. Furthermore, neural stem cells (neural progenitor cells) derived from the SCA2 iPS cell line underwent multiple passages without losing neural differentiation capability. Thus, the SCA2 iPS cells can serve as an unlimited resource for an ideal in vitro cellular model for SCA2. Since the first reprogramming of human somatic cell to iPS cell using retroviral vectors (Takahashi, 2007), many integration-free methods have been developed to enhance the safety in future clinical cell-based therapy (Kim, 2009; Yu, 2009; Warren, 2010). There are also successful reports of reprogramming using different combinations of core transcription factors (Yu, 2007; Huangfu, 2008; Nakagawa, 2008). However, reprogramming with the four traditional Yamanaka factors using retroviral vectors remains the optimal method for high reprogramming efficiency. As our primary purpose for this study was to establish a cellular model for the study of pathogenesis and drug screening, we justified the use of Yamanaka’s method although our iPS cells are not suitable to clinical cell-based therapy.
SCA2 is caused by an expansion of polyglutamine-coding CAG repeat in the ATAXN2 gene. This mutation produces the ataxin-2 protein containing an elongated polyglutamine tract. Normal individuals have 13 to 31 CAG repeats whereas individuals with SCA2 have 32 to 79 repeats, with some in the range greater than 200 repeats (Pulst, 1996; Babovic-Vuksanovic, 1998). Late-onset, L-DOPA-responsive Parkinson’s disease, either in isolation or association with the SCA2 phenotype, has been found in patients with 32 to 39 SCA2 CAG repeats. Recent studies have shown 29 or more CAG repeats are associated with ALS, designated ALS13 (OMIM #183090) (Elden, 2010), and small full expansion SCA2 allele may cause ALS phenotype (Van Damme, 2011). The pathogenic mechanism of SCA2 has been attributed to polyglutamine toxicity rather than loss of ataxin-2 function as Atxn2 knockout mice have normal neural development (Kiehl, 2006). In current study, we found SCA 2 NSCs express less ataxin-2 compared to normal control. Although additional studies are needed to confirm these findings, the reduced ataxin-2 expression level in SCA2 NSCs may be relevant to the pathophysiological mechanism of SCA2. The ATXN2 promoter is located in a typical CpG island in exon 1 of the ATXN2 gene and is usually partially methylated. Using a methyl-specific PCR(Laffita-Mesa, 2012), Laffita-Mesa et al. found that alleles with pathogenic CAG expansions were preferentially hypermethylated and hypermethylation at the promoter leads to partial or complete epigenetic silencing. They also noted that hypermethylation was associated with longer expansions of the ATXN2 repeat. While these findings may represent part of the cellular defense mechanism to reduce the burden of cytotoxic mutant ATXN2, ataxin-2 deficiency has been implicated to be detrimental during the embryonic development (Kiehl, 2006; Lastres-Becker, 2008b). The decreased expression of ATXN2 in our SCA2 NSCs suggests that cells at early differentiation may be particularly susceptible to hypermethylation. In a recent infantile SCA2 patient with 92 CAG repeats presented with facial dysmorphism, dystonia, developmental delay and retinitis pigmentosa (Di Fabio, 2012), the developmental phenotype may be relevant to the gene silencing mechanism. However, we found differentiated neurons have a higher expression level of ataxin-2, which may explain why neurons are more susceptible to degeneration based on polyglutamine toxicity. Thus, we propose that both decreased expression of ATXN2 and polyglutamine toxicity play roles in SCA 2 pathogenesis. Ataxin-2 loss of function may cause early depletion of NSC while polyglutamine gain of function may contribute to degenerative neuron loss at later ages.
The main pathological characteristics of SCA2 brain are cytoplasmic polyglutamine inclusion bodies and PC loss with poor dendritic arborization and torpedo-like axons, which are accompanied by neuronal losses in the pontocerebellar nucleus, inferior olive, substantia nigra, and in some cases, thalamus and reticulotegmental nucleus of pons (Geschwind, 1997; Huynh, 2000; Lastres-Becker, 2008a). Reduced size and number of spinal motor neurons and Clarke’s column and demyelination of dorsal roots and dorsal columns have also been reported. The cytoplasmic location of polyglutamine aggregates on histopathology, a combination of cerebellar ataxia, loss of ocular saccades and areflexia on clinical phenotype, and associations with Parkinson’s disease and ALS distinguish SCA2 from other SCAs (Lastres-Becker, 2008a). Interestingly, ataxin-2, a cytoplasmic RNA binding protein and constituent protein of stress granules, is a potent modifier of the toxicity of TDP-43, which is one of the key molecules in ALS and other neurodegenerative disorders (Ito, 2011). Furthermore, ataxin-2 is a key “hub” protein in the ataxia interactome, a molecular network involved in pathogenic mechanism of degenerative ataxias (Lim, 2006). While the neuronal cell culture model derived from the SCA2 iPS cells will not be able to address tissue specificity of the SCA2 pathology until technological advances allow us to differentiate iPS cells into specific types of neurons, they will be useful for providing fundamental cellular pathophysiology involved in SCA 2 and, potentially, in Parkinson’s disease, ALS and other degenerative ataxias.
Little is known when the mutant protein with elongated polyglutamines starts to accumulate and how it is chronologically related with neuronal loss in SCA2. We did not find cytoplasmic or intranuclear inclusions in NSCs or newly differentiated neural cells. The study of neural tissues in the teratoma derived from SCA2 iPS cells may provide further insights into understanding the timing of polyglutamine micro-aggregation during the neuronal differentiation. Existing evidence from animal studies suggests that neurological phenotype may emerge prior to neuronal loss in polyglutamine expansion disorders (Clark, 1997; Lorenzetti, 2000; Chen, 2008; Liu, 2009; Shakkottai, 2011). Thus, both neuronal dysfunction and neuronal loss are likely to play roles in phenotypic expression in these disorders. The neuronal cells derived from our SCA2 iPS cells appeared to have a shorter life span in culture compared to those derived from control iPS cells. Further studies of cell death mechanisms and electrophysiological properties of ion channels in SCA2 iPS cells would provide insights in the pathophysiology behind the neuronal dysfunction leading to development of the clinical phenotype of ataxia.
In polyglutamine expansion disorders, expanded alleles are prone to change in repeat length (Jodice, 1997). This happens during both meiosis and mitosis (Chong, 1995). In SCA2, most normal alleles are interrupted by CAA trinucleotides but the expanded alleles have longer than 35 pure CAG units (Imbert, 1996). In our SCA2 iPS cells, both the expanded allele and normal allele remained stable in size during reprogramming, proliferation and differentiation from stem cells to terminally differentiated neurons. There has been only one research paper addressed the trinucleotide repeat stability during reprogramming. Ku et al. described GAA triplet repeats in the FXN gene showed both expansions and contractions in iPS cells from two patients with Friedreich’s ataxia (Liu, 2011). Since the degree of repeat instability is known to depend on the length of the pure repeat tract, the sequence of the repeat unit and the cellular environment (Wells RD, 2006), the remarkable stability of the expanded allele in our cells is not surprising. However, we expect the expanded SCA2 allele would show some degree of instability in further somatic and germline differentiation of our iPS cells, especially if sensitive assays, such as small-pool PCR (Monckton, 1995) are employed. The relative stability of the CAG expansion size during the establishment and subsequent neuronal differentiation of our SCA2 iPS cells assures the consistency and integrity of these cells as a cellular model.
Effective disease modeling with human iPS cells has been demonstrated in neurodegenerative and neurodevelopmental disorders, such as spinal muscular atrophy (Ebert, 2009), familial dysautonomia (Lee, 2009) and familial Parkinson’s disease (Soldner, 2009; Hargus, 2010; Seibler, 2011). In these studies, iPS cells recapitulated some disease features and showed potential to become a platform for drug development (Ebert, 2009; Lee, 2011). Successful generation of iPS cells has also been reported in ataxic disorders, such as Friedreich’s ataxia and ataxia telangiectasia (Ku, 2010; Kinoshita, 2011). At the writing of this manuscript, SCA7 iPS cells have been reported and showed the capability of neural differentiation (Luo, 2012). In addition to the current SCA2 iPS cells, iPS cell repositories for SCAs (SCA1, SCA3, SCA5, SCA6, SCA8, SCA10 and SCA13) are all in line for generation in our center. These disease-specific iPS cells are promising in future studies for: (1) further differentiation to specific types of neurons and glias including those in the cerebellum, (2) the pathogenic mechanism of SCAs, (3) the development and screening of therapeutic drugs, (4) correcting expansion mutations to normal repeats, and (5) cell engrafting studies in mutant mouse models to gain knowledge for future cell-based therapy.
Supplementary Material
Acknowledgement
Dr. Guangbin Xia was a trainee of the Clinical Research Consortium for Spinocerebellar Ataxias (CRC-SCA), which was supported by NIH NS068897 (TA).
Footnotes
Conflict of Interest
The authors declare to have no conflict of interest.
The original publication is available at www.springerlink.com.
References
- 1.Eto K, Sumi SM, Bird TD, McEvoy-Bush T, Boehnke M, Schellenberg G. Family with dominantly inherited ataxia, amyotrophy, and peripheral sensory loss. Spinopontine atrophy or Machado-Joseph Azorean disease in another non-Portuguese family? Arch Neurol. 1990;47:968–974. doi: 10.1001/archneur.1990.00530090038011. [DOI] [PubMed] [Google Scholar]
- 2.Auburger GW. Spinocerebellar ataxia type 2. Handb Clin Neurol. 2012;103:423–436. doi: 10.1016/B978-0-444-51892-7.00026-7. [DOI] [PubMed] [Google Scholar]
- 3.Huynh DP, Figueroa K, Hoang N, Pulst SM. Nuclear localization or inclusion body formation of ataxin-2 are not necessary for SCA2 pathogenesis in mouse or human. Nat Genet. 2000;26:44–50. doi: 10.1038/79162. [DOI] [PubMed] [Google Scholar]
- 4.Lastres-Becker I, Rub U, Auburger G. Spinocerebellar ataxia 2 (SCA2) Cerebellum. 2008a;7:115–124. doi: 10.1007/s12311-008-0019-y. [DOI] [PubMed] [Google Scholar]
- 5.Shibata H, Huynh DP, Pulst SM. A novel protein with RNA-binding motifs interacts with ataxin-2. Hum Mol Genet. 2000;9:1303–1313. doi: 10.1093/hmg/9.9.1303. [DOI] [PubMed] [Google Scholar]
- 6.Ralser M, Nonhoff U, Albrecht M, et al. Ataxin-2 and huntingtin interact with endophilin-A complexes to function in plastin-associated pathways. Hum Mol Genet. 2005;14:2893–2909. doi: 10.1093/hmg/ddi321. [DOI] [PubMed] [Google Scholar]
- 7.Lastres-Becker I, Brodesser S, Lutjohann D, et al. Insulin receptor and lipid metabolism pathology in ataxin-2 knock-out mice. Hum Mol Genet. 2008b;17:1465–1481. doi: 10.1093/hmg/ddn035. [DOI] [PubMed] [Google Scholar]
- 8.Kiehl TR, Nechiporuk A, Figueroa KP, Keating MT, Huynh DP, Pulst SM. Generation and characterization of Sca2 (ataxin-2) knockout mice. Biochem Biophys Res Commun. 2006;339:17–24. doi: 10.1016/j.bbrc.2005.10.186. [DOI] [PubMed] [Google Scholar]
- 9.Huynh DP, Maalouf M, Silva AJ, Schweizer FE, Pulst SM. Dissociated fear and spatial learning in mice with deficiency of ataxin-2. PLoS One. 2009;4:e6235. doi: 10.1371/journal.pone.0006235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aguiar J, Fernandez J, Aguilar A, et al. Ubiquitous expression of human SCA2 gene under the regulation of the SCA2 self promoter cause specific Purkinje cell degeneration in transgenic mice. Neurosci Lett. 2006;392:202–206. doi: 10.1016/j.neulet.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 11.Scoles DR, Pflieger LT, Thai KK, Hansen ST, Dansithong W, Pulst SM. ETS1 regulates the expression of ATXN2. Hum Mol Genet. 2012;21:5048–5065. doi: 10.1093/hmg/dds349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 13.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 14.Kastenberg ZJ, Odorico JS. Alternative sources of pluripotency: science, ethics, and stem cells. Transplant Rev (Orlando) 2008;22:215–222. doi: 10.1016/j.trre.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Baharvand H, Totonchi M, Taei A, Seifinejad A, Aghdami N, Salekdeh GH. Human-induced pluripotent stem cells: derivation, propagation, and freezing in serum- and feeder layer-free culture conditions. Methods Mol Biol. 584:425–443. doi: 10.1007/978-1-60761-369-5_23. [DOI] [PubMed] [Google Scholar]
- 16.Mollamohammadi S, Taei A, Pakzad M, et al. A simple and efficient cryopreservation method for feeder-free dissociated human induced pluripotent stem cells and human embryonic stem cells. Hum Reprod. 2009;24:2468–2476. doi: 10.1093/humrep/dep244. [DOI] [PubMed] [Google Scholar]
- 17.Hu BY, Zhang SC. Directed differentiation of neural-stem cells and subtype-specific neurons from hESCs. Methods Mol Biol. 2010;636:123–137. doi: 10.1007/978-1-60761-691-7_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xia G, Kumar SR, Masood R, et al. EphB4 expression and biological significance in prostate cancer. Cancer Res. 2005;65:4623–4632. doi: 10.1158/0008-5472.CAN-04-2667. [DOI] [PubMed] [Google Scholar]
- 19.Pulst SM, Nechiporuk A, Nechiporuk T, et al. Moderate expansion of a normally biallelic trinucleotide repeat in spinocerebellar ataxia type 2. Nat Genet. 1996;14:269–276. doi: 10.1038/ng1196-269. [DOI] [PubMed] [Google Scholar]
- 20.Kim D, Kim CH, Moon JI, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4:472–476. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu J, Hu K, Smuga-Otto K, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warren L, Manos PD, Ahfeldt T, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huangfu D, Osafune K, Maehr R, et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol. 2008;26:1269–1275. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- 24.Nakagawa M, Koyanagi M, Tanabe K, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 25.Babovic-Vuksanovic D, Snow K, Patterson MC, Michels VV. Spinocerebellar ataxia type 2 (SCA 2) in an infant with extreme CAG repeat expansion. Am J Med Genet. 1998;79:383–387. [PubMed] [Google Scholar]
- 26.Elden AC, Kim HJ, Hart MP, et al. Ataxin-2 intermediate-length polyglutamine expansions are associated with increased risk for ALS. Nature. 2010;466:1069–1075. doi: 10.1038/nature09320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Damme P, Veldink JH, van Blitterswijk M, et al. Expanded ATXN2 CAG repeat size in ALS identifies genetic overlap between ALS and SCA2. Neurology. 2011;76:2066–2072. doi: 10.1212/WNL.0b013e31821f445b. [DOI] [PubMed] [Google Scholar]
- 28.Laffita-Mesa JM, Bauer PO, Kouri V, et al. Epigenetics DNA methylation in the core ataxin-2 gene promoter: novel physiological and pathological implications. Hum Genet. 2012;131:625–638. doi: 10.1007/s00439-011-1101-y. [DOI] [PubMed] [Google Scholar]
- 29.Di Fabio R, Santorelli F, Bertini E, et al. Infantile childhood onset of spinocerebellar ataxia type 2. Cerebellum. 2012;11:526–530. doi: 10.1007/s12311-011-0315-9. [DOI] [PubMed] [Google Scholar]
- 30.Geschwind DH, Perlman S, Figueroa CP, Treiman LJ, Pulst SM. The prevalence and wide clinical spectrum of the spinocerebellar ataxia type 2 trinucleotide repeat in patients with autosomal dominant cerebellar ataxia. Am J Hum Genet. 1997;60:842–850. [PMC free article] [PubMed] [Google Scholar]
- 31.Ito D, Suzuki N. Conjoint pathologic cascades mediated by ALS/FTLD-U linked RNA-binding proteins TDP-43 and FUS. Neurology. 2011;77:1636–1643. doi: 10.1212/WNL.0b013e3182343365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim J, Hao T, Shaw C, et al. A protein-protein interaction network for human inherited ataxias and disorders of Purkinje cell degeneration. Cell. 2006;125:801–814. doi: 10.1016/j.cell.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 33.Clark HB, Burright EN, Yunis WS, et al. Purkinje cell expression of a mutant allele of SCA1 in transgenic mice leads to disparate effects on motor behaviors, followed by a progressive cerebellar dysfunction and histological alterations. J Neurosci. 1997;17:7385–7395. doi: 10.1523/JNEUROSCI.17-19-07385.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lorenzetti D, Watase K, Xu B, Matzuk MM, Orr HT, Zoghbi HY. Repeat instability and motor incoordination in mice with a targeted expanded CAG repeat in the Sca1 locus. Hum Mol Genet. 2000;9:779–785. doi: 10.1093/hmg/9.5.779. [DOI] [PubMed] [Google Scholar]
- 35.Chen X, Tang TS, Tu H, et al. Deranged calcium signaling and neurodegeneration in spinocerebellar ataxia type 3. J Neurosci. 2008;28:12713–12724. doi: 10.1523/JNEUROSCI.3909-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu J, Tang TS, Tu H, et al. Deranged calcium signaling and neurodegeneration in spinocerebellar ataxia type 2. J Neurosci. 2009;29:9148–9162. doi: 10.1523/JNEUROSCI.0660-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shakkottai VG, do Carmo Costa M, Dell'Orco JM, Sankaranarayanan A, Wulff H, Paulson HL. Early changes in cerebellar physiology accompany motor dysfunction in the polyglutamine disease spinocerebellar ataxia type 3. J Neurosci. 2011;31:13002–13014. doi: 10.1523/JNEUROSCI.2789-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jodice C, Giovannone B, Calabresi V, Bellocchi M, Terrenato L, Novelletto A. Population variation analysis at nine loci containing expressed trinucleotide repeats. Ann Hum Genet. 1997;61:425–438. doi: 10.1046/j.1469-1809.1997.6150425.x. [DOI] [PubMed] [Google Scholar]
- 39.Chong SS, McCall AE, Cota J, et al. Gametic and somatic tissue-specific heterogeneity of the expanded SCA1 CAG repeat in spinocerebellar ataxia type 1. Nat Genet. 1995;10:344–350. doi: 10.1038/ng0795-344. [DOI] [PubMed] [Google Scholar]
- 40.Imbert G, Saudou F, Yvert G, et al. Cloning of the gene for spinocerebellar ataxia 2 reveals a locus with high sensitivity to expanded CAG/glutamine repeats. Nat Genet. 1996;14:285–291. doi: 10.1038/ng1196-285. [DOI] [PubMed] [Google Scholar]
- 41.Liu J, Verma PJ, Evans-Galea MV, et al. Generation of induced pluripotent stem cell lines from Friedreich ataxia patients. Stem Cell Rev. 2011;7:703–713. doi: 10.1007/s12015-010-9210-x. [DOI] [PubMed] [Google Scholar]
- 42.Wells RDAT. Overvies of the field. In: Ashizawa T, Wells RD, editors. Genetic instabilities and neurological disorder. 2nd Edition. Burlington: Elsevier; 2006. p. 17. [Google Scholar]
- 43.Monckton DG, Wong LJ, Ashizawa T, Caskey CT. Somatic mosaicism, germline expansions, germline reversions and intergenerational reductions in myotonic dystrophy males: small pool PCR analyses. Hum Mol Genet. 1995;4:1–8. doi: 10.1093/hmg/4.1.1. [DOI] [PubMed] [Google Scholar]
- 44.Ebert AD, Yu J, Rose FF, Jr, et al. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457:277–280. doi: 10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee G, Papapetrou EP, Kim H, et al. Modelling pathogenesis and treatment of familial dysautonomia using patient-specific iPSCs. Nature. 2009;461:402–406. doi: 10.1038/nature08320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soldner F, Hockemeyer D, Beard C, et al. Parkinson's disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009;136:964–977. doi: 10.1016/j.cell.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hargus G, Cooper O, Deleidi M, et al. Differentiated Parkinson patient-derived induced pluripotent stem cells grow in the adult rodent brain and reduce motor asymmetry in Parkinsonian rats. Proc Natl Acad Sci U S A. 2010;107:15921–15926. doi: 10.1073/pnas.1010209107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seibler P, Graziotto J, Jeong H, Simunovic F, Klein C, Krainc D. Mitochondrial Parkin recruitment is impaired in neurons derived from mutant PINK1 induced pluripotent stem cells. J Neurosci. 2011;31:5970–5976. doi: 10.1523/JNEUROSCI.4441-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee G, Studer L. Modelling familial dysautonomia in human induced pluripotent stem cells. Philos Trans R Soc Lond B Biol Sci. 2011;366:2286–2296. doi: 10.1098/rstb.2011.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ku S, Soragni E, Campau E, et al. Friedreich's ataxia induced pluripotent stem cells model intergenerational GAATTC triplet repeat instability. Cell Stem Cell. 2010;7:631–637. doi: 10.1016/j.stem.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kinoshita T, Nagamatsu G, Kosaka T, et al. Ataxia-telangiectasia mutated (ATM) deficiency decreases reprogramming efficiency and leads to genomic instability in iPS cells. Biochem Biophys Res Commun. 2011;407:321–326. doi: 10.1016/j.bbrc.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 52.Luo Y, Fan Y, Zhou B, Xu Z, Chen Y, Sun X. Generation of induced pluripotent stem cells from skin fibroblasts of a patient with olivopontocerebellar atrophy. Tohoku J Exp Med. 2012;226:151–159. doi: 10.1620/tjem.226.151. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.