Abstract
Introduction
CMT1A is a slowly progressive neuropathy in which impairment is length dependent. Fibular nerve conduction studies to the anterior tibialis muscle (AT) may serve as a physiological marker of disease progression in patients with CMT1A.
Objective
Determine whether the AT compound muscle action potential (CMAP) amplitude correlates with impairment in patients with CMT1A.
Methods
We correlated AT CMAP amplitudes and impairment measured by the CMT Neuropathy Score (CMTNS) in a cross-section of 121 patients with CMT1A and a subset of 27 patients with longitudinal data.
Results
AT CMAP amplitudes correlated with impairment as measured by the CMTNS in cross sectional analysis. Longitudinal changes in the AT CMAP showed a strong inverse correlation with leg strength but not other components of the CMTNS.
Discussion
AT CMAP amplitude may serve as a useful outcome measure for physiological changes in natural history studies and clinical trials for patients with CMT1A.
Keywords: Neuropathy, Charcot-Marie-Tooth Disease (CMT), Outcome measure, Charcot-Marie-Tooth Neuropathy Score (CMTNS), Nerve Conduction Studies (NCS)
INTRODUCTION
Charcot-Marie-Tooth disease (CMT), the most common inherited neuromuscular disorder,1 is a progressive, length-dependent neuropathy with a phenotype of distal leg weakness, muscle atrophy, and decreased sensation.2 CMT1A, the most common form, is caused by a 1.4 Mb duplication of chromosome 17p11.2, which contains the peripheral myelin protein 22 kD (PMP22) gene.3,4 Duplication of PMP22 causes dysmyelination [reviewed in 5], but secondary axonal degeneration, as measured by reductions in compound muscle action potential (CMAP) amplitude, correlates best with impairment and disability.6 We developed the CMT Neuropathy Score (CMTNS), which incorporates symptoms, findings on neurological examination, and CMAP and sensory nerve action potential (SNAP) amplitudes to measure impairment in all forms of CMT.7,8 The CMTNS has been used to measure disease progression in CMT1A9 and as a primary outcome measure in clinical trials of ascorbic acid treatment of CMT1A,10 but it has been limited in part by relative insensitivity to change.10 The CMTNS utilizes upper extremity nerve conduction studies, as fibular CMAP amplitudes when recorded at the distal extensor digitorum brevis (EDB) muscle in the foot are usually absent in CMT1A.6 However, the anterior compartment of leg muscles is often preferentially affected in patients with CMT, and ankle dorsiflexors are often weak while plantar flexion strength is well preserved. In fact, muscle MRI studies in patients with CMT confirm this observation.11,12 Thus, the anterior tibialis muscle is a particularly attractive recording site for an outcome measure. We evaluated CMAPs from a proximal fibular innervated muscle, the Anterior Tibialis (AT), to determine whether their amplitudes were recordable in most patients with CMT1A and whether AT CMAP amplitudes correlated with impairment.
METHODS
This was an analysis of chart data collected from patients with clinically or genetically diagnosed CMT1A who were evaluated at the Wayne State University CMT clinic between 2001 and 2011. Demographic data, clinical evaluations, including the Charcot-Marie-Tooth Neuropathy Score (CMTNS), and electrophysiologic data including fibular nerve conduction studies to the AT muscle were recorded at the time of the patient visit. We utilized a standardized approach to record the fibular nerve conductions to the AT, in which the recording electrode is placed 9–10 cm below the fibular head on the bulk of the muscle belly. 13
Clinical disability was assessed through the Charcot Marie Tooth Neuropathy Score (CMTNS), which is routinely calculated on all patients evaluated in our clinic. Since the CMTNS was not available prior to 2005, scores from patients evaluated prior to 2005 were calculated retrospectively. The CMTNS is a validated composite score that converts historical, physical examination and nerve conduction data into a summary score between 0–36; higher scores indicate worsening function, and the scores can distinguish between mild (0–10), moderate (11–20) and severe (21–36) impairment and disability.7 Clinical components of this score consist of pin sensibility, vibration perception, arm strength, and leg strength. Electrophysiologic components include ulnar SNAP and CMAP amplitudes (original version) and radial SNAP with ulnar motor CMAP amplitudes (revised version).8
Descriptive statistics were calculated to characterize the study sample using Stata-IC 11 (College station, TX). Pearson correlation coefficients were computed to examine associations between the standardized measures with AT CMAP amplitudes. Finally, using generalized estimating equations (GEE) analysis, 2 models were tested: cross-sectional associations between AT CMAP amplitudes and standardized measures, and longitudinal associations in a subset of those patients. This study was approved by the Institutional Review Board (IRB) at Wayne State University.
RESULTS
Cross-sectional data was collected on 121 patients with CMT1A, aged 16 to 94, who had fibular CMAP amplitudes attempted at the AT between 2001 and 2011. A subset, 27 patients in total, had additional longitudinal evaluations. The characteristics of the entire patient sample are summarized in Table 1.
Table 1.
Patient Demographics and Characteristics (n = 121
| Mean Age (SD) | 50.9 (15.7) |
|
| |
| Gender (%) | 56 Males (46%); 65 Females (54%) |
|
| |
| Race (%) | |
| Caucasian | 118 |
| African American | 0 |
| Asian | 1 |
| Unknown | 3 |
Abbreviations: SD = standard deviation
Cross-sectional Data
AT CMAP amplitudes were recordable initially in 96 patients and unobtainable in 25 patients. Ulnar CMAPs were initially recordable in 115 patients and absent in 6. The mean AT CMAP amplitude was 1.95 mV with a standard deviation of 1.65 mV. The uncorrected Pearson correlation matrix for the 121 patients is shown in Table 2. There was a significant inverse correlation between AT CMAP amplitude and 3 individual variables: (1) the CMTNS (−0.36, p = 0.0001); (2) the leg strength (−0.39, p < 0.0001); and (3) the arm strength (−0.34, p = 0.0002). The sensory signs did not correlate well with the AT CMAP amplitudes.
Table 2.
Pearson Correlation Matrix for Anterior Tibialis CMAP Amplitudes in Patients with CMT1A (n = 121)
| Variable | Uncorrected Pearson Correlation Coefficient with AT CMAP Amplitude | p-Value |
|---|---|---|
| CMTNS | −0.3569 | 0.0001 |
| Vibration | −0.0319 | 0.7373 |
| Pinprick | −0.0834 | 0.3799 |
| Leg strength | −0.3889 | < 0.0001 |
| Arm Strength | −0.3400 | 0.0002 |
| Ulnar CMAP amplitude | 0.5495 | < 0.0001 |
Abbreviations: AT = Anterior Tibialis; CMAP = compound muscle action potential; CMTNS = Charcot Marie Tooth Neuropathy Score
We examined these associations more closely by utilizing the generalized estimating equations (GEE) model; this showed a significant direct correlation between AT CMAP amplitude and ulnar CMAP amplitude in the cross-sectional group (0.39, p < 0.001).
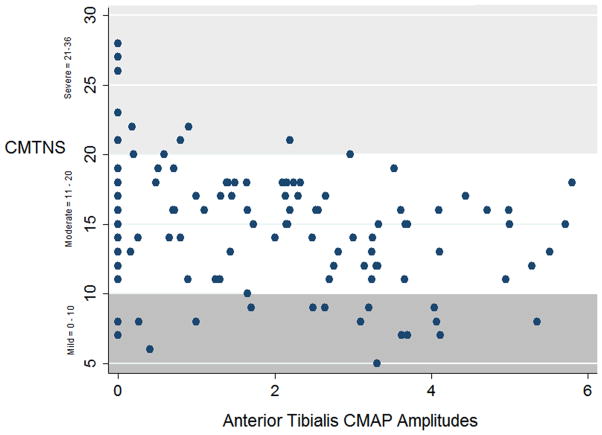
A scatter plot was created to visually characterize the association between AT CMAP amplitude and CMTNS score in the cross-sectional sample of 121 patients (see Figure 1). This showed that most patients with mild to moderate disability (CMTNS scores between 0–20), and even a few with the most severe disability (scores greater than 21) had recordable AT CMAPs. Of note, 21% of all patients with CMT1A had unrecordable AT CMAPs, and 5 out of 27 (18.5%) patients AT CMAPs during the course of their longitudinal evaluations, suggesting that AT CMAP amplitude could be followed longitudinally for most patients. There was a low “floor effect,” and therefore further worsening of CMAP amplitude could be detected in most patients. Only 12% had normal AT CMAP amplitudes, suggesting a low ceiling effect, since most patients had detectable abnormalities in the measurement.
Figure 1.
Scatter plot of the CMTNS scores and AT CMAP amplitudes in 121 CMT1A patients.
Longitudinal Data
Twenty-seven patients were followed longitudinally for periods between 1 and 6 years (mean = 4 years, S.D. = 2). In these patients the AT CMAP amplitude decreased by an average of 0.14 mV/year (S.D. = 0.48). In contrast, ulnar CMAP amplitude decreased by an average of 0.1 mV/year (S.D. = 0.61). The GEE model showed a significant negative correlation of −0.17 (p < 0.001) between the CMTNS and AT CMAP amplitudes when looked at in isolation. However, when all standardized measures (CMTNS, pinprick, vibration, leg strength, arm strength and ulnar CMAPs) were included in the GEE model, leg strength was the only variable that showed a significant inverse correlation with AT CMAP amplitude of −0.81 (p = 0.008) (Table 3).
Table 3.
GEE Population-Averaged Model of Association Between AT CMAP Amplitude and Longitudinally Assessed Standardized Clinical Measures (n = 27)
| Variable | Correlation Coefficient with AT CMAP amplitude | Std err | p-Value | [95% CI] |
|---|---|---|---|---|
| CMTNS | −0.030 | 0.085 | 0.719 | −0.19 to 0.14 |
| Pinprick | −0.092 | 0.274 | 0.736 | −0.63 to 0.44 |
| Vibration | −0.076 | 0.231 | 0.741 | −0.53 to 0.38 |
| Arm Strength | 0.070 | 0.367 | 0.848 | −0.65 to 0.79 |
| Ulnar CMAP amplitude | 0.054 | 0.110 | 0.626 | −0.16 to 0.27 |
| Leg Strength | −0.806 | 0.304 | 0.008 | −1.40 to −0.21 |
Abbreviations: GEE= Generalized estimating equations; AT = Anterior Tibialis; CMAP = compound muscle action potential; CMTNS = Charcot Marie Tooth Neuropathy Score
DISCUSSION
CMT refers to a heterogeneous group of diseases caused by mutations in more than 50 genes. There is no cure for any form of CMT. The identification of disease-causing genes and investigations into the cell biology of how mutations in these genes cause neuropathy has made the development of rational therapy a possibility in CMT.5, 14 However, the lack of natural history data and concerns about the sensitivity of outcome measures used to measure natural history or treatment effects in clinical trials is currently limiting therapeutic advances for CMT. The CMTNS has been used as the primary outcome measure in natural history studies of CMT1A,7 CMT1X,15 and in multinational clinical trials of ascorbic acid treatment of CMT1A.10 CMAP amplitude of the ulnar nerve is a component of the CMTNS.7 However, despite the fact that the neuropathy in CMT is length-dependent, there are no physiologic measurements from the lower extremities in the CMTNS, largely because CMAPs are unobtainable from the EDB in most patients with CMT1A, the most common form of CMT.6 In this study we have found that an AT CMAP is present in approximately 80% of patients with CMT1A and that the amplitude decreases over time as the disease progresses. In addition, the rate of change over time of the AT CMAP amplitude is greater than the currently used electrophysiologic measure in the CMTNS, ulnar CMAP amplitude, in our study. Thus we believe that it can be used as an independent outcome measure or as a component of an outcome measure such as the CMTNS to quantify progression in patients with CMT1A and presumably other forms of CMT.
Physiological measurements, such as the AT CMAP, are particularly important to measure impairment or progression in CMT, because they are objective rather than subjective measurements and therefore are less subject to bias from the patient or examiner than measures taken from the history or neurological examination. The CMTNS is a validated measure of impairment7 and disease progression9 in CMT. However the first 3 components of the score are subjective opinions from patients concerning their leg and arm strength and loss of sensation in their legs. The next 4 components are motor and sensory findings on the neurological exam which depend on patient cooperation and examiner consistency to obtain reproducible results.7 The CMAP and SNAP amplitudes comprise the most objective part of the CMTNS. However, because SNAPs are severely reduced or absent in CMT1A, and EDB CMAPs are usually absent in most patients, fibular CMAP amplitudes have not been a part of the CMTNS despite the fact that fibular nerve-innervated muscles such as the AT are frequently impaired in CMT.16 The AT CMAP amplitude, either by itself or as part of the CMTNS, would provide an objective neurophysiological measurement from the lower extremity, which can be used to quantify patient impairment and progression in CMT.
There are several reasons to consider using the AT CMAP amplitude to replace other physiological measures in the CMTNS. The CMTNS was based on the Total Neuropathy Score (TNS), which was designed to measure impairment in length-dependent, predominantly sensory axonal neuropathies such as occur in diabetes or chemotherapy treatment.17 The CMTNS includes measurements of ulnar, median7 and, more recently, radial8 SNAP amplitudes as physiological measures of sensory loss. However, in our experience, these SNAPs are absent in most patients with CMT. As a result the inclusion of SNAPs acts as a ceiling effect in the CMTNS, as most patients receive the maximum possible, i.e. most severe, score on their entry visit and there is no potential to measure further impairment. Replacing these measurements with the AT CMAP would permit the elimination of these ceiling effects by a measurement from a lower extremity that would be subject to change over time.
The fibular nerve to the Anterior Tibialis muscle is a proximal innervation of the longest motor nerve in the leg, and thus provides an opportunity to study disease progression and neurophysiological impairment in the lower extremities in this slowly progressive disease. Since the CMTNS increases as a patient’s disease worsens and CMAP amplitudes typically decrease with age, we expected to see an inverse correlation between clinical impairment in patients with CMT1A and AT CMAP amplitude recorded from the fibular nerve at the AT. This study shows that there is a significant inverse relationship between AT CMAP amplitudes and leg strength, as seen in our longitudinal series. The weaker correlation between the amplitudes and the total CMTNS is probably due to leg strength being one of the components of the CMTNS. The correlation between AT and ulnar CMAP amplitudes may be due to the fact that patients with a severe neuropathy that has progressed to the arms often have severely affected legs as well. The scatter plot, which shows low ceiling and floor effects, suggest that AT CMAP amplitude could be a potentially useful trial outcome measure in the future.
CONCLUSION
We have demonstrated a significant inverse correlation between longitudinal changes in AT CMAP amplitudes and leg strength. This suggests that AT CMAP amplitude could be a sensitive outcome measure which is responsive to changes in the disease course over time. Future prospective studies with larger numbers of patients at several sites should determine the usefulness of AT CMAP amplitude as a potential trial outcome measure in some forms of CMT, particularly those that have symmetrical deficits.
Acknowledgments
This work was supported by grants from the National Institutes of Health [5U54NS065712-02 (MES); K23- NS072279-01 (SR)], the Muscular Dystrophy Association [MES], and the Charcot-Marie-Tooth Association [MES].
This work was presented as an abstract at the Charcot Marie Tooth Annual meeting following the Peripheral Nerve Society Meeting in Potomac, MD (June 25th–July 2nd, 2011)
ABBREVIATIONS
- AT
Anterior Tibialis
- CMAP
Compound Muscle Action Potential
- CMT1A
Charcot-Marie-Tooth Disease Type 1A
- CMTNS
Charcot-Marie-Tooth Neuropathy Score
- EDB
Extensor Digitorum Brevis
- NCS
Nerve Conduction Studies
- PMP22
Peripheral Myelin Protein 22 kD
- SNAP
Sensory Nerve Action Potential
References
- 1.Skre H. Genetic and clinical aspects of Charcot-Marie-Tooth’s disease. Clin Genet. 1974;6:98–118. doi: 10.1111/j.1399-0004.1974.tb00638.x. [DOI] [PubMed] [Google Scholar]
- 2.Thomas PK, Marques W, Jr, Davis MB, Sweeney MG, King RH, Bradley JL, et al. The phenotypic manifestations of chromosome 17p11.2 duplication. Brain. 1997;120:465–478. doi: 10.1093/brain/120.3.465. [DOI] [PubMed] [Google Scholar]
- 3.Lupski JR, de Oca-Luna RM, Slaugenhaupt S, Pentao L, Guzzeta V, Trask BJ, et al. DNA duplication associated with Charcot-Marie-Tooth disease type 1A. Cell. 1991;66:219–232. doi: 10.1016/0092-8674(91)90613-4. [DOI] [PubMed] [Google Scholar]
- 4.Raeymaekers P, Timmerman V, Nelis E, De Jonghe P, Hoogendijk JE, Baas F, et al. Duplication in chromosome 17p11.2 in Charcot-Marie-Tooth neuropathy type 1a (CMT 1a). The HMSN Collaborative Research Group. Neuromuscul Disord. 1991;1:93–97. doi: 10.1016/0960-8966(91)90055-w. [DOI] [PubMed] [Google Scholar]
- 5.Niemann A, Berger P, Suter U. Pathomechanisms of mutant proteins in Charcot-Marie-Tooth disease. Neuromolecular Med. 2006;8:217–242. doi: 10.1385/nmm:8:1-2:217. [DOI] [PubMed] [Google Scholar]
- 6.Krajewski KM, Lewis RA, Fuerst DR, Turansky C, Hinderer SR, Garbern J, et al. Neurological dysfunction and axonal degeneration in Charcot-Marie-Tooth disease type 1A. Brain. 2000;123:1516–27. doi: 10.1093/brain/123.7.1516. [DOI] [PubMed] [Google Scholar]
- 7.Shy ME, Blake J, Krajewski K, Fuerst DR, Laura M, Hahn AF, et al. Reliability and validity of the CMT neuropathy score as a measure of disability. Neurology. 2005;64:1209–1214. doi: 10.1212/01.WNL.0000156517.00615.A3. [DOI] [PubMed] [Google Scholar]
- 8.Murphy SM, Herrmann DN, McDermott MP, Scherer SS, Shy ME, Reilly MM, et al. Reliability of the CMT neuropathy score (second version) in Charcot-Marie-Tooth disease. J Peripher Nerv Syst. 2011 Sep;16(3):191–198. doi: 10.1111/j.1529-8027.2011.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shy ME, Chen L, Swan ER, Taube R, Krajewski KM, Herrmann D, et al. Neuropathy progression in Charcot-Marie-Tooth disease type 1A. Neurology. 2008;70:378–383. doi: 10.1212/01.wnl.0000297553.36441.ce. [DOI] [PubMed] [Google Scholar]
- 10.Pareyson D, Reilly MM, Schenone A, Fabrizi GM, Cavallaro T, Santoro L, et al. CMT-TRIAAL; CMT-TRAUK groups. Ascorbic acid in Charcot-Marie-Tooth disease type 1A (CMT-TRIAAL and CMT-TRAUK): a double-blind randomised trial. Lancet Neurol. 2011 Apr;10(4):320–8. doi: 10.1016/S1474-4422(11)70025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallardo E, García A, Combarros O, Berciano J. Charcot-Marie-Tooth disease type 1A duplication: spectrum of clinical and magnetic resonance imaging features in leg and foot muscles. Brain. 2006;129(Pt 2):426–37. doi: 10.1093/brain/awh693. [DOI] [PubMed] [Google Scholar]
- 12.Chung KW, Suh BC, Shy ME, Cho SY, Yoo JH, Park SW, et al. Different clinical and magnetic resonance imaging features between Charcot-Marie-Tooth disease type 1A and 2A. Neuromuscul Disord. 2008 Aug;18(8):610–8. doi: 10.1016/j.nmd.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Preston DC, Shapiro BE. Clinical-Electrophysiologic Correlations. 2. Philadelphia: Elsevier/Butterworth Heinemann; 2005. Electromyography and Neuromuscular Disorders; pp. 148–149. [Google Scholar]
- 14.Reilly MM, Shy ME. Diagnosis and new treatments in genetic neuropathies. J Neurol Neurosurg Psychiatry. 2009;80:1304–1314. doi: 10.1136/jnnp.2008.158295. [DOI] [PubMed] [Google Scholar]
- 15.Shy ME, Siskind C, Swan ER, Krajewski KM, Doherty T, Fuerst DR, et al. CMT1X phenotypes represent loss of GJB1 gene function. Neurology. 2007;68:849–855. doi: 10.1212/01.wnl.0000256709.08271.4d. [DOI] [PubMed] [Google Scholar]
- 16.Harding AE, Thomas PK. The clinical features of hereditary motor and sensory neuropathy types I and II. Brain. 1980;103:259–280. doi: 10.1093/brain/103.2.259. [DOI] [PubMed] [Google Scholar]
- 17.Cornblath DR, Chaudhry V, Carter K, Lee D, Seysedadr M, Miernicki M, et al. Total neuropathy score: validation and reliability study. Neurology. 1999;53:1660–1664. doi: 10.1212/wnl.53.8.1660. [DOI] [PubMed] [Google Scholar]