Abstract
The molecular mechanism of the extrathymic generation of adaptive CD4+Foxp3+ regulatory T (iTreg) cells remains incompletely defined. We show that exposure of splenic CD4+CD25+Foxp3− cells to IL-2, but not other γc cytokines, resulted in Stat5 phosphorylation and induced Foxp3 expression in ~10% of the cells. Thus, IL-2/Stat5 signaling may be critical for Foxp3 induction in peripheral CD4+CD25+Foxp3− iTreg cell precursors. Herein, to further define the role of IL-2 in the formation of iTreg cell precursors as well as their subsequent Foxp3 expression, we designed a two-step iTreg cell differentiation model. During the initial “conditioning” step, CD4+CD25−Foxp3− naïve T cells were activated by TCR stimulation. Inhibition of IL-2 signaling via Jak3-Stat5 was required during this step to generate CD4+CD25+Foxp3− cells containing iTreg cell precursors. During the subsequent Foxp3-induction step driven by cytokines, IL-2 was the most potent cytokine to induce Foxp3 expression in these iTreg cell precursors. This two-step method generated a large number of iTreg cells with relatively stable expression of Foxp3, which were able to prevent CD4+CD45RBhigh cell-mediated colitis in Rag1−/− mice. Taken together, while initial inhibition of IL-2 signaling upon T cell priming generates iTreg cell precursors, subsequent activation of IL-2 signaling in these precursors induces the expression of Foxp3. These findings advance the understanding of iTreg cell differentiation, and may facilitate the therapeutic use of iTreg cells in immune disorders.
INTRODUCTION
CD4+CD25+ regulatory T (Treg) cells are able to suppress various immune responses against self and foreign antigens. The transcription factor Foxp3 is predominantly expressed in CD4+CD25+ Treg cells, and plays a central role in establishing and maintaining the Treg cell lineage. Deficiency of a functional Foxp3 gene in both humans and mice leads to ablation of Treg cells, severe autoimmunity, and early mortality (1, 2). Therefore, elucidating the factors that control Foxp3 expression will advance our understanding of Treg cell biology and its therapeutic application for immune diseases.
IL-2 has long been considered a major T-cell growth factor optimizing immune responses, as signaling through its high affinity IL-2R (consisting of the IL-2Rα [CD25], IL-2Rβ, and common gamma chain [γc] subunits) is essential for the expansion of recently activated effector T (Teff) cells (3). Therefore, it was somewhat unexpected that mice deficient in IL-2, IL-2Rα, or IL-2Rβ developed autoimmune diseases, often with lethal consequences (4, 5). Further studies revealed that Treg cells constantly express high affinity IL-2R. Indeed, since a constant supply of IL-2 is critical for Treg cell homeostasis, the lethal autoimmunity was eventually associated with an IL-2 signaling defect in Treg cells (3, 6). Thus, IL-2 proved to be an essential cytokine not only in Teff-mediated immunity but also in Treg-maintained immune tolerance (3, 7). Studies by our group and others have further shown that Treg cells exert their suppressive function on Teff cell responses at least partially through creating and modulating an IL-2-deprived environment (8, 9).
Foxp3-expressing Treg cells are either derived from the thymus as natural Treg (nTreg) cells or generated de novo from peripheral mature CD4+Foxp3− T cells in response to TCR stimulations as adaptive, or inducible, Treg (iTreg) cells (10). The low frequency of CD4+CD25+Foxp3+ cells in the thymus of IL-2Rβ−/− mice led to the assumption that IL-2 signaling is also critical for the thymic nTreg cell generation (6). Moreover, mice deficient in both IL-2 and IL-15 (also binding to IL-2Rβ) exhibit marked deficiency in thymic nTreg cells (11). Recently, a “two-step model” of thymic nTreg cell generation suggested that TCR/ligand interactions result in elevated CD25 (IL-2Rα) expression on Foxp3− CD4 single positive (CD4SP) thymocytes, followed by an IL-2-directed and TCR-independent step that subsequently induces Foxp3 expression in these CD25+Foxp3− CD4SP nTreg cell precursors (12, 13). Some other γc-dependent cytokines (IL-7 and IL-15) less effectively induced Foxp3 expression in nTreg cell precursors (14).
The essential polarizing cytokines involved in the differentiation of iTreg cells appear to be TGF-β and IL-2. In vitro activation of CD4+CD25−Foxp3− T cells in the presence of exogenous TGF-β results in a substantial percentage of Foxp3-expressing iTreg cells (15). Importantly, IL-2 was required for TGF-β-mediated iTreg cell differentiation, as addition of IL-2 neutralizing Ab in cultures or using IL-2-deficient T cells abrogated iTreg cell generation induced by exogenous TGF-β. In addition, only IL-2, but not other γc cytokines, was able to restore TGF-β-mediated Foxp3 expression in IL-2-deficient T cells (16, 17). Thus, IL-2 plays an essential and non-redundant role in TGF-β-mediated iTreg cell generation.
We have recently shown that in the absence of exogenous TGF-β and IL-2, TCR stimulation of neonatal T cells converted them into stable Treg cells (18). This led us to re-investigate the role of TGF-β and IL-2 in generating adult iTreg cells. In the current study, we first demonstrate that addition of IL-2 alone induces Stat5 phosphorylation and Foxp3 expression in ex vivo isolated peripheral CD4+CD25+Foxp3− iTreg cell precursors. Next, we show in a two-step model (including a TCR-directed “conditioning” stage and a cytokine-driven Foxp3-induction stage) that IL-2 plays a dynamic dual role in the differentiation of iTreg cells. At the initial conditioning stage upon TCR stimulation, inhibition of IL-2 signaling promotes the generation of iTreg cell precursors. Subsequently, IL-2 alone added at the Foxp3 induction phase induces Foxp3 expression in these iTreg cell precursors. This two-step process of iTreg cell differentiation does not require exogenous TGF-β, although direct blocking of TGF-β signals impaired such iTreg cell generation. The iTreg cells generated by this method exhibit relatively stable expression of Foxp3 and exert potent suppressive function both in vitro and in vivo.
MATERIALS AND METHODS
Mice
C57BL/6 (B6), B6.129P2-Il2tm1Hor/J (IL-2−/−), B6.129S7-Rag1tm1Mom/J (Rag1−/−), B6.Cg-Foxp3tm2Tch/J (Foxp3/GFP), and B6.Cg-Tg(Cd4-TGFBR2)16Flv/J (dnTGFBRII) mice were purchased from the Jackson laboratory (Bar Harbor, ME). B6.SJL-Ptprca/BoyAiTac (B6.SJL) congenic mice were obtained from Taconic Farms, Inc. (Hudson, NY). Animals were maintained at the University of Toledo specific pathogen-free animal facility according to institutional guidelines.
Antibodies, cytokines and Reagents
Fluorescence conjugated antibodies were purchased from BD Biosciences and eBioscience. Purified anti-CD3 and anti-CD28 mAbs, BD™ Phosflow Lyse/Fix Buffer I, BD™ Phosflow Perm/Wash Buffer I, and Annexin V-FITC apoptosis detection kit were purchased from BD Biosciences. Functional grade anti-IL-2 mAbs (clone S4B6 or JES6), anti-CD25 (PC61) mAb, and anti-Foxp3-PE and intracellular staining kit were purchased from eBioscience. CFSE was obtained from Molecular Probes. TGF-β neutralizing antibodies (9016 or 1D11), murine recombinant TSLP, and IL-7 were purchased from R&D Systems. Other murine recombinant cytokines were purchased from Peprotech. An inhibitor of TGF-β superfamily type I activin receptor-like kinase receptors (SB-431542) was purchased from Sigma-Aldrich. Jak3 inhibitor (CP690550) was purchased from Axon Biochemicals BV, whereas the Stat5 inhibitor [N′-((4-Oxo-4H-chromen-3-yl)methylene)nicotinohydrazide] and the Stat3 inhibitor (WP1066) were obtained from EMD Bioscience. Abs specific for Smad3 (C67H9) and phospho-Smad3 (C25A9) were purchased from Cell Signaling Technology. Anti-β-actin and lumin B mAbs were purchased from BD Biosciences and Santa Cruz Biotechnology, respectively.
Cell preparation, cultures, and Flow Cytometry
Single cell suspensions were obtained from thymus and spleens of Foxp3/GFP, wild type B6, IL-2−/−, B6.SJL, or dnTGFBRII mice. To determine the existence of Treg cell precursors in thymus and spleen, CD4+CD8−CD25+Foxp3/GFP− thymocytes, CD4+CD8−CD25−Foxp3/GFP− thymocytes, CD4+CD25+Foxp3/GFP− splenocytes, and CD4+CD25−Foxp3/GFP− splenocytes were obtained from Foxp3/GFP mice by cell sorting, and were cultured with 10 U/ml IL-2 or 10 ng/ml other cytokines (IL-4, IL-7, or IL-15 as indicated in the text) for 3 days. The percentage of GFP+ T cells in the cultures was analyzed by flow cytometry. The phospho-Stat5 expression was determined by flow cytometry using anti-Stat5 (pY694)-PE according to the BD™ phosphoflow protocol.
For generating peripheral iTreg cells by the two-step method, 5 × 104 CD4+CD25−Foxp3/GFP− or CD4+CD25− splenocytes from different mice were cultured in 96 well flat bottom plates, and were stimulated with 4 µg/ml plate-bound anti-CD3 mAb and 2 µg/ml soluble anti-CD28 mAb for 3 days. During this TCR-directed conditioning phase, 10 µg/ml anti-IL-2 mAb, 10 µg/ml anti-CD25 mAb, 5 µg/ml anti-TGF-β mAb, 1 µM TGF-β inhibitor, 50 nM JAK3 inhibitor, 150 µM STAT3 inhibitor, 50 µM STAT5 inhibitor, or 2 ng/ml TGF-β was added into the cultures, respectively. The cells were then washed and cultured again, this time in the presence of 10 U/ml IL-2 or 10 ng/ml other cytokines for 3 days (the Foxp3-induction phase). Additional cultures were performed with various concentrations of plate bound anti-CD3 mAb, soluble anti-CD28, TGF-β, anti-TGF-β mAb, or TGF-β inhibitor as indicated in the text.
Some CD4+CD25− splenocytes were CFSE labeled prior to cultivation (8). Foxp3 expression was determined by Foxp3/GFP expression or by intracellular Foxp3 staining. CFSE dilution and the expression of CD25, CD69, GITR, CTLA4, Helios, Bcl-2, phospho-Stat5, and Annexin V were analyzed by a FACSCalibur flow cytometer (Becton Dickinson).
ELISA
CD4+CD25− B6 splenocytes stimulated with 4 µg/ml plate-bound anti-CD3 mAb and 2 µg/ml soluble anti-CD28 mAb were cultured in the presence or absence of anti-IL-2 mAb (JES6, 10 µg/ml), JAK3 inhibitor (CP690550, 50 nM) or STAT5 inhibitor (50 µM). After 3-day activation, the levels of TGF-β and IL-2 in culture supernatants were assessed by ELISA, using commercial kits following the manufacturer’s instructions (R&D Systems, Minneapolis, MN).
Western Blot analysis
CD4+CD25− B6 splenocytes stimulated by 4 µg/ml plate-bound anti-CD3 mAb and 2 µg/ml soluble anti-CD28 mAb were cultured in the presence or absence of anti-IL-2 mAb (JES6, 10 µg/ml) or TGF-β (2 ng/ml). Nuclear and cytoplasmic extracts were prepared from 3-day cultured cells using a commercial lysis buffer (NE-PER Nuclear and Cytoplasmic Extraction Reagents, Thermo Fisher Scientific, IL). Protein concentration was determined by Bradford assay using the Coomassie Protein Assay Reagent (Pierce, IL) and the Bio-Rad spectrophotometer. Equal amounts of protein (30 µg) were loaded in each lane and separated by SDS-polyacrylamide gel (10%) electrophoresis. Fractionated proteins were blotted onto a nitrocellulose membrane (Bio-Rad, CA). Blots were probed with anti-Smad3 (C67H9) Rabbit mAb, followed by horseradish peroxidase-labeled goat anti-rabbit IgG (Santa Cruz Biotechnology), and visualized by SuperSignal West Pico Chemiluminescent Substrate (Pierce) and the fluorchem 890 (Alpha Innotech, CA) detection system. To detect the phospho-Smad3 expression, the probed membrane was stripped (2% SDS, 62.5 mM Tris-HCl, 100 mM 2-ME, and pH adjusted to 6.5) and re-probed with Phospho-Smad3 (Ser423/425) (C25A9) (Cell Signaling Technology) followed by horseradish peroxidase-labeled goat anti-rabbit IgG (Bio-Rad), and visualized as above. The β-actin and lumin B served as internal loading controls for cytoplasmic and nuclear proteins, respectively.
Treg cell re-stimulation and in vitro suppression assay
CD4+CD25−Foxp3/GFP− cells were stimulated by anti-CD3/anti-CD28 mAbs for 3 days in the presence of 2 ng/ml TGF-β and 10 U/ml IL-2 (TGF-β-iTreg group), 10 µg/ml S4B6 anti-IL-2 mAb (Anti-IL-2-iTreg group), or 50 nM CP690550 (CP-iTreg group). Cultured cells were washed and rested with 10 U/ml IL-2 for another 3 days prior to sorting out Foxp3/GFP+ cells. Ex vivo sorted CD4+Foxp3/GFP+ splenocytes served as the nTreg cell group. These sorted Treg cells were re-cultured in 96 well flat bottom plates and stimulated by 4 µg/ml plate-bound anti-CD3 mAb and 2 µg/ml soluble anti-CD28 mAb for 3 days, followed by analysis of the Foxp3/GFP expression by flow cytometry.
For the in vitro suppression assay, 5 × 104/well B6.SJL CD4+CD25− T cells were CFSE labeled as previously described (8), and were used as responder cells. Suppressors were sorted CP-iTreg or nTreg cells, and were seeded with B6.SJL CD4+CD25− T cells in various ratios. Cells were stimulated for 3 days by 1.5 × 105/well CD3− syngeneic splenocytes and 0.5 µg/ml soluble anti-CD3 mAb. The proliferation of responder cells was determined by CFSE dilution in CD45.1+ T cells measured by flow cytometry.
Induction and prevention of colitis
Single cell suspensions were obtained from spleen and mesenteric lymph node (MLN) of B6 mice, and were labeled with anti-CD45RB-FITC, anti-CD25-PE and anti-CD4-PE-Cy5. CD4+CD45RBhigh T cells and CD4+CD45RBlowCD25+ nTreg cells were sorted out with a FACSAria cell sorter (Becton Dickinson, San Jose, CA). Foxp3/GFP+ CP-iTreg cells were sorted from the 6-day cultures with CP690550 conditioning and IL-2 resting. 4 × 105 CD4+CD45RBhigh T cells were suspended in 0.2 ml PBS and were injected i.v. into each RAG1−/− mouse. Some RAG1−/− mice were also injected with 4 × 105 CD4+CD45RBlowCD25+ nTreg cells or CP-iTreg cells. Mice were monitored weekly for body weight, and were euthanized 8 wks after T cell transfer. Cecum, proximal colon, mid-colon, and distal colon were removed and fixed in 10% buffered formalin, paraffin-embedded, sectioned, and stained with H&E. Inflammation was scored in a blinded fashion using a previously described scoring system (19).
Statistical analysis
Statistical analysis was performed using an unpaired, 2-tailed, Student’s t test to calculate p values. Calculated p values < 0.05 were considered statistically significant.
RESULTS
IL-2 induces Stat5 phosphorylation and Foxp3 expression in peripheral CD4+CD25+Foxp3− iTreg cell precursors
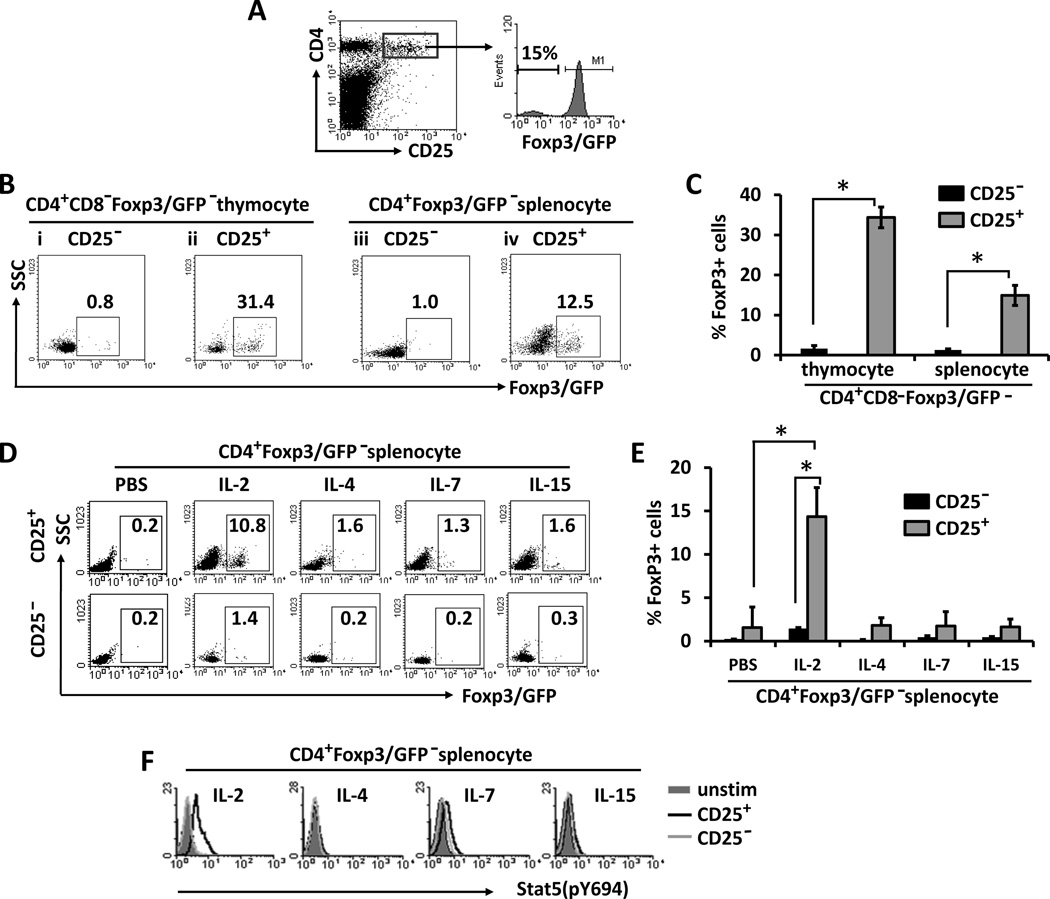
The discovery of nTreg cell precursors within CD25+Foxp3− CD4SP thymocytes and peripheral iTreg cell precursors within CD4+CD25+Foxp3− cells greatly advanced our understanding of Treg cell development (12, 20). We confirmed these findings in Foxp3/GFP reporter mice, as about 15% of CD4+CD25+ splenocytes were Foxp3/GFP− cells (Fig. 1A). Ex vivo exposure of purified CD4+CD25+Foxp3/GFP− thymocytes or splenocytes to 10 U/ml IL-2 alone in 3 day cultures resulted in conversion of more than 30% (Fig. 1B, ii and Fig. 1C) and 10% (Fig. 1B, iv and Fig. 1C) of cultured cells into Foxp3/GFP+ cells, respectively. In contrast, only up to 1% of CD4+CD25−Foxp3/GFP− thymocytes or splenocytes up-regulated Foxp3 expression in response to IL-2 (Fig. 1B, i and iii, and Fig. 1C). Therefore, the CD25+Foxp3− population, among both CD4SP thymocytes and CD4+ splenocytes, was always enriched for Treg cell precursors, which readily expressed Foxp3 upon exposure to IL-2.
Figure 1. Peripheral CD4+CD25+Foxp3− cells contain iTreg cell precursors, which express Foxp3 in response to IL-2.
(A) Splenocytes from Foxp3/GFP reporter mice were analyzed by flow cytometry. Cells are gated on CD4+CD25+ subset (dot plot; left panel), and the % Foxp3/GFP− within this subset is shown (histogram; right panel). (B and C) Sorted CD25− or CD25+ CD4+CD8−Foxp3/GFP− thymocytes, or CD25− or CD25+ CD4+Foxp3/GFP− splenocytes were cultured with 10 U/ml IL-2. Dot plots and the bar graph show the percentage of Foxp3/GFP+ cells in the day 3 cultures (* indicates p<0.05). (D–F) Sorted CD25− or CD25+ CD4+Foxp3/GFP− splenocytes were stimulated by 10 U/ml IL-2 or 10 ng/ml of the indicated γc-cytokines for 3 days. In D and E, Dot plots and the bar graph show the percentages of Foxp3/GFP+ cells in the day 3 cultures (* indicates p<0.05). In F, overlay histograms show the flow cytometric analysis of phospho-Stat5 expression within CD25− (gray line) or CD25+ (black line) CD4+Foxp3/GFP− splenocytes. CD4+Foxp3/GFP− splenocytes cultured without cytokine stimulation served as a control (unstim; shaded histogram). Data are representative of three independent experiments.
Other γc-dependent cytokines, including IL-4, IL-7, and IL-15, were much less effective than IL-2 in inducing Foxp3 expression in the cultures of CD4+CD25+Foxp3− splenocytes (Fig. 1D, upper panels and Fig. 1E). A putative explanation may be that IL-2 can potently induce Stat5 activation in CD4+CD25+Foxp3− splenocytes as shown by flow cytometric analysis of phosphorylated Stat5 (Stat5pY694) (Fig. 1F), while none of the remaining tested γc cytokines were as effective as IL-2 in activating Stat5 in CD4+CD25+Foxp3− splenocytes (Fig. 1F). All tested γc cytokines including IL-2 were ineffective at inducing Foxp3 expression (Fig. 1D, lower panels and Fig. 1E) and Stat5 phosphorylation (Fig. 1F) in CD4+CD25−Foxp3− splenocytes. Taken together, these results show that IL-2, but not other γc cytokines, effectively induces Stat5 activation and Foxp3 expression in CD4+CD25+Foxp3− iTreg cell precursors.
IL-2 deprivation upon TCR stimulation generates iTreg cell precursors
To further reveal the mechanism of iTreg cell differentiation, we established a two-step method to delineate the conditions for the formation of iTreg cell precursors (Fig. 2A). In the initial “conditioning” step, CD4+CD25−Foxp3/GFP− splenocytes isolated from Foxp3/GFP mice were stimulated with plate-bound anti-CD3 mAb and soluble anti-CD28 mAb, and exposed to various cytokines, mAbs, and inhibitors. Three days later, cells were washed and rested with IL-2 (10 U/ml) for an additional 3 days; this served as the “Foxp3-induction” step determining the presence of putative iTreg cell precursors after conditioning (Fig. 2A).
Figure 2. IL-2 restrains the formation of iTreg cell precursors.
(A) The schematic shows the two-step model for generating iTreg cells. During the initial conditioning step, purified T cells were stimulated by anti-CD3/anti-CD28 mAbs, and were treated with the indicated cytokines or mAbs for 3 days. During the subsequent Foxp3-induction step, cells were washed and re-cultured with 10 U/ml IL-2 for an additional 3 days. The frequency of Foxp3+ cells in the day 6 cultures was determined by Foxp3/GFP+ expression or intracellular staining of Foxp3. (B–D) Purified CD4+CD25−Foxp3/GFP− cells were cultured as in A in the presence of IL-2 or the indicated mAb treatments during the conditioning step as indicated (* indicates p<0.05). (E and F) IL-2−/− CD4+CD25− T cells were cultured as in A, and various concentrations of IL-2 were added during conditioning (* indicates p<0.05 compared to 0 U/ml IL-2). (G and H) CD4+CD25− T cells from IL-2−/− and B6.SJL CD45.1 congeneic mice were either cultured alone (left panels), or co-cultured together (right panels). The cultures contained different numbers of B6.SJL cells as indicated, but did not receive cytokine or mAb treatments during conditioning. Anti-CD45.2-APC staining was used to distinguish IL-2−/− cells from B6.SJL cells in the co-cultures. All histograms and bar graphs show the frequencies of Foxp3-expressing cells in the day 6 cultures (* indicates p<0.05). Data are representative of three independent experiments.
To determine the role of IL-2 in generating iTreg cell precursors, we added exogenous IL-2 or anti-IL-2 neutralizing mAbs during the initial conditioning step. As shown in Figure 2B and 2D, few Foxp3/GFP+ cells could be detected in the 6 day culture of CD4+CD25−Foxp3/GFP− splenocytes, which were treated with or without exogenous IL-2 during conditioning. In contrast, neutralizing IL-2 by addition of an anti-IL-2 mAb (clone S4B6 or JES6) during an initial 3-day conditioning step followed by an IL-2-driven Foxp3 induction step resulted in a high frequency (40%) of Foxp3/GFP+ cells (Fig. 2C and 2D). The Foxp3/GFP expression was induced during the IL-2-driven Foxp3-induction step (days 4 to 6), as very few Foxp3/GFP+ cells could be detected after 3-day conditioning by neutralizing IL-2 (Supplemental Fig. 1). The results from these experiments suggest that an initial IL-2 deprivation upon TCR stimulation generates iTreg cell precursors, which then express Foxp3 upon subsequent exposure to IL-2.
To confirm the above finding, IL-2−/− CD4+CD25− T cells were labeled with CFSE, and cultured using the same two-step method. After 6 days of culture, the Foxp3 expression was assessed by intracellular staining. To exclude many IL-2−/− T cells that were not activated and which were not proliferating, the flow cytometric analysis of cultured cells was gated only on dividing cells based on the CFSE dye dilution. As shown in Figure 2E and 2F, about 35% of the divided IL-2−/− CD4+CD25− T cells became Foxp3 positive. Addition of increasing concentrations of exogenous IL-2 during the conditioning step prevented Foxp3 induction in IL-2−/− T cells (Fig. 2E and 2F). Thus, IL-2 deprivation during the TCR-directed conditioning step is critical for the generation of iTreg cell precursors.
Induction of Foxp3 expression in IL-2−/− CD4+CD25− T cells was prevented when they were co-cultured with B6.SJL (CD45.1 congenic mice) CD4+CD25− T cells that can produce IL-2 upon TCR stimulation. This inhibitory effect was dependent on the concentration of B6.SJL cells (Fig. 2G, upper panels and Fig. 2H). Moreover, a significant frequency of Foxp3-expressing cells was also detected within the B6.SJL population in the co-culture group with the smallest fraction of IL-2 producing B6.SJL cells (Fig. 2G, lower panels and Fig. 2H). Therefore, sufficient IL-2 production from T cells upon TCR stimulation inhibits the generation of iTreg cell precursors.
Blockade of IL-2 signaling upon TCR stimulation generates iTreg cell precursors which become Foxp3+ iTreg cells upon exposure to IL-2
The IL-2Rαβγ complex associates with the intracellular tyrosine kinases: Jak1, that binds to IL-2Rβ chain and Jak3 that binds to IL-2Rγ chain. Binding of IL-2 to high affinity IL-2R results in autophosphorylation and activation of Jak1 and Jak3. We utilized an anti-IL-2 mAb (clone S4B6 or JES6), an anti-CD25 (IL2Rα) mAb (clone PC61), or a Jak3 inhibitor (CP690550) to define the role of IL-2 signaling in the formation of iTreg cell precursors. CD4+CD25−Foxp3/GFP− splenocytes isolated from Foxp3/GFP mice were cultured using the two-step method described in Figure 2A. Addition of anti-IL-2 mAb, anti-CD25 mAb, or CP690550 during the conditioning step did not induce Foxp3-expressing cells in 3 day cultures (Supplemental Fig. 1). However, subsequent exposure of these cells to 10 U/ml IL-2 resulted in an increase in Foxp3/GFP+ cells to 30–50% in the 6 day cultures (Fig. 3A and 3C). Thus, initial blockade of IL-2 signaling by different methods upon TCR stimulation generates iTreg cell precursors, which in turn express Foxp3 upon IL-2 exposure.
Figure 3. IL-2 signaling negatively regulates the formation of iTreg cell precursors, but IL-2 is the most potent cytokine to induce subsequent Foxp3 expression.
Purified CD4+CD25−Foxp3/GFP− T cells were used in the two-step model of iTreg cell generation. (A–C) During the conditioning step, cells were activated by anti-CD3/anti-CD28 mAbs for 3 days, and were either untreated (control group) or treated with 10 µg/ml anti-IL-2 mAb, 10 µg/ml anti-CD25 mAb, 50 nM CP690550, 150 µM Stat3 inhibitor, or 50 µM Stat5 inhibitor, as indicated. During the subsequent Foxp3-induction step, cells were exposed to 10 U/ml IL-2 for an additional 3 days. Representative dot plots (in A and B) and the bar graph (in C) show the frequencies of Foxp3/GFP+ cells in the day 6 cultures (* indicates p<0.05 compared to control). (D) Cells were treated with CP690550 during conditioning, followed by exposure to 10 U/ml IL-2 or 10 ng/ml of the indicated cytokines during the Foxp3-induction step. The bar graph shows the numbers of Foxp3-expressing cells in the day 6 cultures (* indicates p<0.05 compared to PBS treatment). (E) During the 3-day conditioning step, cells were activated by the indicated concentrations of anti-CD3/anti-CD28 mAbs, and were either untreated (−) or treated (+) with 50 nM CP690550. Cells were then exposed to 10 U/ml IL-2 for an additional 3 days. The bar graphs show the frequencies of Foxp3/GFP+ cells in the day 3 (i) or day 6 (ii) cultures (* indicates p<0.05). Data are representative of at least three independent experiments.
During the TCR-directed conditioning step, anti-IL-2 mAb, anti-CD25 mAb, or CP690550 only marginally down-regulated the phosphorylation of Stat5Y694 (Supplemental Fig. 2A). Moreover, in contrast to 250 µM Stat5 inhibitor, 50 µM Stat5 inhibitor did not decrease the phosphorylation of Stat5Y694 (Supplemental Fig. 2B). However, addition of 50 µM Stat5 inhibitor (but not Stat3 inhibitor) during the conditioning step was sufficient to generate 30–50% Foxp3/GFP+ cells in the 6-day culture of CD4+CD25−Foxp3/GFP− cells (Fig. 3B and 3C). Further studies are required to define how Stat5 and other transcription factors influence the programming of iTreg cell precursors.
We also investigated the effects of cytokines on the second Foxp3-induction step (Fig. 3D). Following an initial 3-day conditioning step of CD4+CD25−Foxp3/GFP− cells with Jak3 inhibitor (CP690550), the culture was washed and supplemented with 12 different cytokines. As shown in Figure 3D, IL-2 alone was the most potent cytokine for inducing Foxp3, while IL-7 and IL-15 were much less effective and other cytokines were not effective; IL-4 inhibited Foxp3 expression.
Various concentrations of plate bound anti-CD3 mAb and soluble anti-CD28 mAb were also investigated in the two-step generation of iTreg cells. As shown in the Figure 3E, in the presence of CP690550 during the conditioning step, 4 µg/ml anti-CD3 mAb induced more iTreg cells than 0.5 µg/ml anti-CD3 mAb (Fig. 3E, ii). Thus, proper TCR stimulation is required to induce iTreg cells in this system. In the absence of CP690550 conditioning, none of the anti-CD3/CD28 mAb concentrations used was able to generate iTreg cells. Thus, conditioning of naïve T cells by TCR stimulation and IL-2 signaling blockade transforms them into iTreg cell precursors, which are robustly induced by IL-2 to become Foxp3 expressing iTreg cells.
Characterization of CD4+ T cells during iTreg cell generation
By CFSE labeling of CD4+CD25− cells prior to cultivation using the two-step method, we showed that a significant cell proliferation was retained in cultures from day 2 to day 6 (Fig. 4A) regardless of the conditioning treatment with 10 µg/ml anti-IL-2 mAb or 50 nM CP690550. Foxp3 expression in cells could not be significantly induced unless IL-2 was neutralized or IL-2 signaling was blocked during the initial conditioning step (Fig. 4A, 4B, and 4C). Addition of IL-2 into the conditioned cultures at day 3 then promptly induced Foxp3 expression within 12 hours, and further enhanced the frequency (Fig. 4A and 4B) and total number (Fig. 4C) of Foxp3-expressing cells in the following three days. Thus, these two events must happen in sequence for an efficient induction of precursors and a timely switch to iTreg cells. Moreover, a similar frequency of Foxp3+ cells was detected in each divided cell population (Supplemental Fig. 3A and 3B). It is possible that during the Foxp3 induction step, IL-2 up-regulates Foxp3 expression equally in each dividing cell population.
Figure 4. Characterizing the activation, proliferation, and survival of CD4+ T cells during the process of iTreg cell generation.
CD4+CD25− T cells were stimulated with anti-CD3/anti-CD28 mAbs for 3 days in the absence (control group) or presence of anti-IL-2 mAb or CP690550, followed by exposure to IL-2 for 3 days. Cells were analyzed on the indicated days after culture. (A–C) CD4+CD25− T cells were CFSE labeled prior to cell culture. On the indicated days, cells were intracellularly stained with anti-Foxp3-PE. In A and B, dot plots and the bar graph show the frequencies of Foxp3 expressing cells (* indicates p<0.05 compared to control at the same time point). In C, the line graph show the number of Foxp3+ cells in each well (* [anti-IL-2] or * [CP690550] indicates p<0.05 compared to control at the same time point). (D) Overlay histograms show the expression of CD25 and CD69 on the cultured cells. Shaded histograms represent un-cultured naïve CD4+CD25− T cells. (E) The overlay histograms show Bcl-2 expression in the cultured cells by intracellular staining with anti-Bcl-2-PE. Cells from the control group were also stained with isotype Ab-PE (Isotype Ab; shaded histogram). Data are representative of at least three independent experiments.
Significant cell proliferations were observed during iTreg cell generation (Fig. 4A), suggesting that this two-step process did not inhibit T-cell activation. Indeed, the evaluation of T-cell activation markers CD25 and CD69 after stimulation of CD4+CD25− wild type T cells showed that T-cell activation was not dramatically impaired by the conditioning treatment with 10 µg/ml anti-IL-2 mAb or 50 nM CP690550 (Fig. 4D). During the conditioning step, 10 µg/ml anti-IL-2 mAb was sufficient to neutralize IL-2 in the 3-day cultures. Nevertheless, blocking IL-2 signaling with 50 nM Jak3 inhibitor or 50 µM Stat5 inhibitor reduced (but did not completely inhibit) IL-2 production (Supplemental Fig. 3C).
We also investigated the possible pro-apoptotic effect produced in the conditioning step. As shown in supplemental Figure 4A and 4B, the conditioning treatment with anti-IL-2 mAb, anti-CD25 mAb, or Jak3 inhibitor (CP690550) at the indicated concentrations did not affect the proliferation of cells during iTreg cell generation, and it did not increase the frequency of apoptotic cells (annexin V positive) in the cultures. In fact, TCR stimulation enhanced expression of the anti-apoptotic molecule Bcl-2, and the conditioning treatment with 10 µg/ml anti-IL-2 mAb or 50 nM CP690550 did not inhibit Bcl-2 expression (Fig. 4E). These results demonstrate that in response to TCR stimulation, CD4+CD25− cells receiving diminished IL-2 signaling can be activated to proliferate, but their differentiation is directed into iTreg cell precursors. The described culture conditions do not diminish the survival of T cells despite the use of mAbs and inhibitors at the indicated concentrations.
Inhibition of TGF-β signaling upon TCR stimulation reduces iTreg cell differentiation
Addition of exogenous TGF-β during T cell activation potently up-regulates Foxp3 expression in T cells. We did not add exogenous TGF-β to the cultures during the process of iTreg cell generation, and the TGF-β concentration in the cultures was low (between 40–55 pg/ml) as shown in Figure 5A. Addition of 2 ng/ml exogenous TGF-β upon TCR stimulation increased the expression level of phosphorylated Smad3 both in the cytoplasmic fraction of T cells (Fig. 5B; left panel) and more predominantly in the nucleus (Fig. 5B; right panel). In contrast, neutralizing IL-2 in the absence of exogenous TGF-β did not up-regulate the expression of phosphorylated Smad3 in both locations (Fig. 5B). Thus, enhanced TGF-β signaling via activation of Smads is dispensable for the IL-2 deprivation-mediated generation of iTreg cell precursors.
Figure 5. Inhibiting TGF-β signaling decreases iTreg cell generation.
During the conditioning step, CD4+CD25− cells from wild-type or dnTGFBRII mice were treated without (control group) or with the indicated mAbs, inhibitors, or TGF-β. During the subsequent Foxp3-induction step, cells were exposed to IL-2. (A) The bar graph shows the concentration of TGF-β in the day 3 cultures of wild-type CD4+CD25− cells. (B) Western blots show the cytoplasmic (left panel) or nuclear (right panel) expression of Smad3 and phospho-Smad3 in the wild-type cells from day 3 cultures. (C–F) Dot plots and bar graphs show the frequencies of Foxp3-expressing cells in the day 6 cultures of wild-type cells (in C and D; * indicates p <0.05 compared to anti-IL-2 treated group) or dnTGFBRII CD4+CD25− cells (in E and F). (G) Anti-TGF-β mAb, TGF-β inhibitor (TGF-βi), or TGF-β was added into the cultures during the CP690550-conditioning step (left panel, i) or the later IL-2-exposing step (right panel, ii). The bar graphs show the frequencies of Foxp3-expressing cells in the day 6 cultures (* indicates p<0.05 compared to the gray bar). Data are representative of three independent experiments.
To test whether TGF-β signaling was required for the formation of iTreg cell precursors, CD4+CD25− T cells were stimulated with anti-CD3/anti-CD28 mAbs in the presence of conditioning treatment with 10 µg/ml anti-IL-2 mAb alone, or anti-IL-2 mAb plus either an anti-TGF-β mAb (9016 or 1D11; 5 µg/ml) or a TGF-β signaling inhibitor (SB 431542; 1 µM). Three days later, cells were washed and re-cultured with 10 U/ml IL-2. We found that neutralization of TGF-β or inhibition of TGF-β signaling resulted in a significant reduction in the frequency of Foxp3-expressing cells in the 6-day cultures when compared to the conditioning treatment with anti-IL-2 mAb alone (Fig. 5C and 5D). Furthermore, we assessed the iTreg cell differentiation using CD4+CD25− splenocytes isolated from dnTGFβRII mice, which express a dominant-negative form of the human TGF-β receptor II under the direction of the mouse CD4 promoter. Again, only 5–7% of Foxp3-expressing cells were present in the 6-day cultures that received conditioning treatment with anti-IL-2 mAb, anti-CD25 mAb, or Jak3 inhibitor followed by exposure to IL-2 (Fig. 5E and 5F). These results reveal that although significant TGF-β signaling via activation of Smads was not observed, the blockade of TGF-β signaling reduced IL-2 deprivation-mediated generation of iTreg cell precursors.
We further investigated whether the levels of TGF-β signaling affect the two-step generation of iTreg cells. CD4+CD25− T cells were stimulated with anti-CD3/anti-CD28 mAbs in the presence of conditioning treatment with 50 nM CP690550. Three days later, cells were washed and re-cultured with 10 U/ml IL-2. As shown in Figure 5Gi, addition of anti-TGF-β mAb or TGF-β inhibitor during the CP690550-conditioning step inhibited iTreg cell generation. By contrast, addition of exogenous TGF-β during the conditioning step dose-dependently increased the iTreg cell generation and could induce about 90% Foxp3+ cells in the day 6 cultures (Fig. 5G, i). However, addition of exogenous TGF-β or blockade of TGF-β signaling during the IL-2-exposing step only mildly modulated the iTreg cell generation (Fig. 5G, ii). Therefore, TGF-β signaling promotes iTreg cell generation at the early stage after TCR stimulation.
Two-step generated iTreg cells prevent experimental colitis
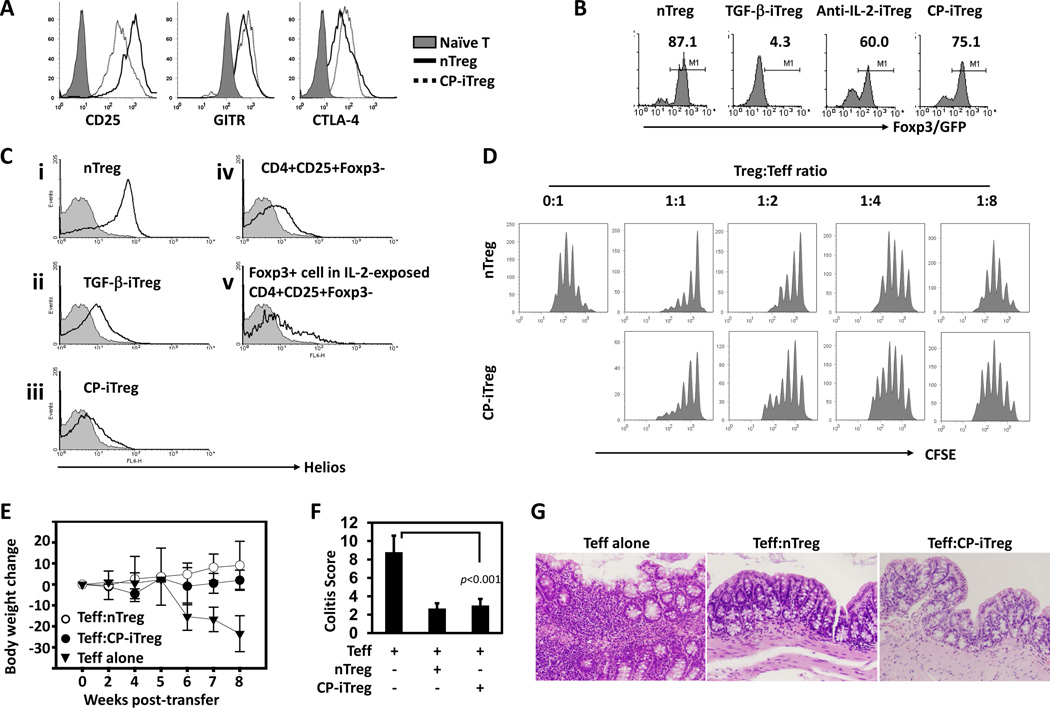
To characterize the phenotype and function of the CD4+Foxp3+ iTreg cells generated under IL-2 signal deprivation conditions in response to TCR stimulation, we examined additional membrane and intracellular markers, the stability of Foxp3 expression, and the suppressive function of these cells. Using our standard procedure, CD4+CD25−Foxp3/GFP− T cells were stimulated for 3 days with anti-CD3/anti-CD28 mAb in the presence of CP690550, followed by exposure to IL-2 for an additional 3 days. Flow cytometric analysis showed that these CP690550-conditioned Foxp3/GFP+ iTreg (CP-iTreg) cells in the 6-day cultures expressed high levels of CD25, GITR and CTLA4 on their surface, a cell surface phenotype that is similar to ex vivo sorted CD4+Foxp3/GFP+ nTreg cells (Fig. 6A).
Figure 6. iTreg cells exhibit relatively stable expression of Foxp3, and prevent experimental colitis.
(A–D) CD4+Foxp3/GFP+ nTreg cells were ex vivo sorted from Foxp3/GFP mice. TGF-β-iTreg cells were generated by TCR stimulation in the presence of exogenous TGF-β/IL-2. CP-iTreg or anti-IL-2-iTreg cells were generated using the two-step method when CP690550 or anti-IL-2 mAb was added during conditioning, respectively. In A, overlay histograms show the expression of CD25, GITR, and CTLA-4 on the indicated cell subsets. In B, Foxp3/GFP+ iTreg cells were sorted out from the day 6 cultures. Each Treg cell subset was re-stimulated with anti-CD3/anti-CD28 mAbs. Histograms show the frequencies of Foxp3/GFP+ cells 3 days after re-stimulation. In C, the indicated cell subsets (solid-line, unfilled histograms) were stained with anti-Helios-APC mAb, and Helios expression was compared to that of CD4+CD25− naïve T cells (gray shaded histograms). In D, CFSE-labeled SJL CD4+CD25− cells were stimulated by syngenic APCs plus soluble anti-CD3 mAb. Suppressors were sorted CP-iTreg or nTreg cells, and were seeded with SJL CD4+CD25− T cells in the indicated ratios. Histograms show the CFSE dilution in CD45.1+ cells. Data are representative of at least three independent experiments. (E–G) CD4+CD45RBhigh T cells and CD4+CD45RBlowCD25+ nTreg cells were ex vivo purified from wild-type mice. Foxp3/GFP+ CP-iTreg cells were sorted from the day 6 cultures with CP690550 conditioning. Rag1−/− mice were injected with 4 × 105 CD4+CD45RBhigh T cells alone (Teff alone group; n=5), or together with the same number of nTreg (Teff:nTreg group; n=3) or CP-iTreg cells (Teff:CP-iTreg group; n=5). The mice were monitored regularly for 8 weeks, and were then sacrificed to obtain colons for histological analysis. In E, the % body weight change of mice from each group is shown. In F, the bar graph shows the histology scores for Rag1−/− mice receiving CD4+CD45RBhigh T cells alone or together with nTreg or CP-iTreg cells. In G, representative hematoxylin and eosin stained colon sections from each group of Rag1−/− mice are shown.
When evaluated by previously described methods (18, 21), nTreg cells, but not CD4+Foxp3/GFP+ iTreg cells (generated with exogenous TGF-β; TGF-β-iTreg), stably maintained their Foxp3 expression upon repeated stimulation (Fig. 6B, left two panels). Interestingly, upon repeated TCR stimulation, 60–75% of the CD4+Foxp3/GFP+ iTreg cells obtained by our two-step culture conditions maintained their Foxp3 expression (Fig. 6B, right two panels, Anti-IL-2-iTreg and CP-iTreg). Nevertheless, CP-iTreg cells expressed lower levels of Helios than that of nTreg cells (Fig. 6C, i vs. iii). Helios expression was also low in TGF-β-iTreg cells (Fig. 6C, ii), ex vivo isolated CD4+CD25+Foxp3− splenocytes (Fig. 6C, iv), and Foxp3+ cells generated from the cultures of ex vivo isolated CD4+CD25+ Foxp3− splenocytes after IL-2 exposure (Fig. 6C, v).
To investigate the suppressive function of two-step generated iTreg cells, we cultured nTreg cells or CP-iTreg cells together with CFSE-labeled syngeneic CD4+CD25− T cells, which were stimulated by syngeneic APCs and soluble anti-CD3 mAb. We found that nTreg cells and CP-iTreg cells suppressed CD4+CD25− T cell proliferation in a similar fashion (Fig. 6D). Based on these experiments we concluded that IL-2 signal deprivation-conditioned CP-iTreg cells exhibit relatively stable expression of Foxp3 and exert suppressive function in vitro similar to nTreg cells, but remain different from nTreg cells in Helios expression.
To assess the in vivo suppressive function of CP-iTreg cells, we transferred sorted CD4+CD45RBhi T cells alone, or together with either CP-iTreg cells or nTreg cells into Rag1−/− mice. Host mice receiving CD4+CD45RBhi T cells alone dramatically lost body weight within 8 weeks after cell transfer, while host mice receiving CD4+CD45RBhi T cells together with either CP-iTreg cells or nTreg cells remained healthy without losing body weight (Fig. 6E). The average histology scores of nTreg or CP-iTreg cell-treated groups were similar and were indicative of a low degree of inflammation compared with that of mice that did not receive Treg cells (Fig. 6F and 6G). Thus, CP-iTreg cells are suppressive in vivo, and prevent experimental colitis in mice.
DISCUSSION
IL-2 is the principal T-cell growth factor normally produced by Teff cells in response to antigenic stimulation. Binding of IL-2 to high affinity IL-2R on Teff cells upon antigen stimulation positively regulates the magnitude and duration of Teff cell responses, and is even required for the generation of functional memory T cells (3, 22). Nevertheless, IL-2 also plays a critical role in maintaining tolerance by controlling Treg cell homeostasis and function (3, 7). In particular, IL-2 is the major growth factor for CD4+CD25+Foxp3+ Treg cells. The lethal autoimmune phenotype observed in mice deficient in IL-2, IL-2Rα, or IL-2Rβ is attributable to a Treg cell defect (4, 5). Moreover, an IL-2:anti-IL-2 mAb complex has been shown to dramatically expand Treg cells in vivo, and prevent experimental autoimmune encephalomyelitis as well as islet allograft rejection in mice by expanding Treg cells (23, 24). Low dose IL-2 treatment also reverses the onset of type 1 diabetes in NOD mice through enhancing CD25 and Foxp3 expression in pancreatic Treg cells, which may subsequently up-regulate Treg cell function (25). More profoundly, IL-2 is also involved in Treg cell generation, and induction of Foxp3 expression in CD25+Foxp3− CD4SP thymocytes and peripheral CD4+CD25+Foxp3− T cells (12, 20). Because Treg cell generation also requires TCR stimulation, we sought to understand how IL-2 signals during T-cell priming influence different outcomes (e.g. generating Teff cell vs. iTreg cell responses) in T cells.
In this study, we investigated the role of IL-2 signaling in iTreg cell generation using a two-step differentiation model, which includes an initial TCR-driven conditioning step generating iTreg cell precursors and a subsequent cytokine-driven step inducing Foxp3 expression in those precursors. In the TCR-driven conditioning step, inhibition of IL-2 signaling via Jak3-Stat5 conditions naïve CD4+CD25−Foxp3− T cells to become CD4+CD25+Foxp3− cells which contain iTreg cell precursors. In contrast, sufficient IL-2/Jak3/Stat5 signals during TCR activation abrogate the generation of iTreg cell precursors, reflecting the fact that quantitative differences in IL-2 signaling at this differentiation step led to distinct outcomes of either dominant Teff cell responses or iTreg cell responses. Currently, there is no specific marker for iTreg cell precursors which are recognized as a CD4+CD25+Foxp3− population, and their bona fide presence may be determined by the consequent expression of Foxp3 upon exposure to cytokines. Among 12 tested cytokines, IL-2 was the most potent cytokine to induce Foxp3 expression in iTreg cell precursors while IL-7 and IL-15 were less effective. These results may suggest that IL-2 deficiency protects naïve T cells from apoptosis by switching them to an iTreg precursor line, but their further development is dependent upon timely exposure to γc cytokines to induce Foxp3 expression.
Differentiation of naïve T cells into various Teff cell subsets requires TCR stimulation and distinct polarizing cytokines. For the iTreg cell differentiation, TGF-β appears to be the most potent polarizing cytokine (15, 26). In most in vitro studies, high amounts of exogenous TGF-β (at the ng/ml level) are needed to convert TCR-stimulated naïve CD4+ T cells into Foxp3-expressing iTreg cells (15, 18). Such high amounts of TGF-β may be rare under physiological conditions. Moreover, in the presence of exogenous TGF-β (at the ng/ml level), addition of IL-2 during the initial TCR stimulation does not inhibit iTreg generation and even facilitates the generation of high frequencies of iTreg cells (16, 17). Nevertheless, these TGF-β-iTregs promptly lose Foxp3 expression upon TCR re-stimulation (Fig. 6B). We demonstrated in our two-step process of iTreg cell differentiation that exogenous TGF-β is not required as the need for downstream Smad2/Smad3 signaling seemed to be minimal. However, a low level of TGF-β signaling is needed with yet undefined TGF-β origin, as neutralizing TGF-β or blocking TGF-βR signals decreased iTreg cell generation. Addition of exogenous TGF-β during the conditioning step dose-dependently increased the iTreg cell generation and could induce about 90% Foxp3+ cells in the day 6 cultures (Fig. 5G, i). Therefore, the two-step iTreg development is favored when environmental TGF-β levels are relatively high.
The transcription factor Stat5 is part of the downstream IL-2 signaling pathway. Activated Stat5 translocates to the nucleus and binds to the promoter region and an intronic regulatory DNA element within the Foxp3 locus (11, 27, 28), suggesting its direct role in transcriptional regulation of Foxp3. Indeed, Stat5−/− mice that survive for only 6 to 8 weeks generate very few Foxp3-expressing CD4 cells, a similar phenotype as Jak3−/− and Il2rg−/− mice (27). In addition, expressing a constitutively active Stat5 in mice leads to expansion of Treg cells, and restores Treg cell numbers even in the absence of IL-2 (11, 13). Thus, Stat5 is required for Treg cell development and homeostasis. The intriguing findings of this paper do not contradict these previous reports, as IL-2/Stat5 signaling is needed to induce Foxp3 expression in iTreg cell precursors. However, the striking observation of our work reveals that TCR stimulation under a low IL-2/Stat5 signaling condition (i.e. IL-2 deficient T cells, IL-2 neutralizing mAb, IL-2Rα blocking mAb, as well as Jak3 or Stat5 inhibition) generates iTreg cell precursors. Recently, analyzing mice with a deletion of the Stat5a/b amino termini or with a T cell-specific deletion of Stat5 showed that these mice have residual Stat5 function and thus have some Treg cells (11, 27), suggesting that a low Stat5 signaling threshold allows for the development of Treg cell precursors. Further studies should discriminate the delicate balance between the effects of Stat5 signaling on the generation of iTreg cell precursors versus the differentiation into Teff cells.
T cells produce IL-2 in response to TCR stimulation. We now show that this natural process not only optimizes Teff cell function, but also restrains the formation of iTreg cell precursors. This finding implies that iTreg cell differentiation in vivo is limited under immunogenic antigen stimulation. Indeed, iTreg cell generation is generally identified under certain defined conditions, such as suboptimal antigen presentation, chronic low dose exposure to antigens, and in unique environments such as gut-associated lymphoid tissues (10, 29). In particular, Kretschmer et al. have shown that the conversion of truly naive CD4 T cells into iTreg cells in vivo was achieved by minute antigen doses with suboptimal dendritic cell activation (30). More strikingly, in the same host, adoptively transferred IL-2−/− T cells converted into iTreg cells two- to three-fold more efficiently than did IL-2-competent T cells (30). Our study likely provides an insight into the mechanism of their finding, as low IL-2/Stat5 signaling in IL-2−/− T cells during the TCR-directed conditioning step induces the formation of iTreg cell precursors, which may subsequently receive paracrine IL-2 from IL-2-competent T cells for Foxp3 induction. We thus speculate that when T cells receive suboptimal or chronic antigen stimulation in the absence of robust IL-2 signaling some of these cells develop into Treg cell precursors that then remain in the environment where, over time, sufficient levels of environmental IL-2 for Foxp3 induction become available. This two-step iTreg generation still requires low levels of TGF-β and should be favored in unique environments where TGF-β levels are relatively high, such as gut-associated lymphoid tissues (10, 29). Compared to iTreg generation by TCR-stimulation in the presence of high amounts of exogenous TGF-β (at the ng/ml level) and IL-2, our model may mimic and provide insights into the biological conditions favorable for iTreg generation as high amounts of IL-2 and TGF-β (at the ng/ml level) are not often present at the same time physiologically.
In summary, IL-2/Stat5 signaling during TCR stimulation negatively regulates the formation of iTreg cell precursors, but positively regulates the subsequent Foxp3 expression in these precursors. The iTreg cells generated by our two-step method showed relatively stable Foxp3 expression upon TCR re-stimulation (in comparison to exogenous TGF-β-induced iTreg cells), and prevented CD4+CD45RBhigh cell-mediated colitis in Rag1−/− mice. Importantly, the number of generated iTreg cells in the 6 day culture was five-fold more than that of the initial number of cultured naïve T cells. These aspects of our two-step generated iTreg cells may facilitate the clinical application of iTreg cells as a cell therapy for immune disorders.
Supplementary Material
Abbreviations used in this article
- CD4SP
CD4 single positive
- CP-iTreg
CP690550-conditioned Foxp3/GFP+ iTreg
- iTreg
adaptive Treg
- nTreg
natural Treg
- Teff
effector T
- Treg
regulatory T
Footnotes
This work was supported by National Institutes of Health Grant HL69723 & P30 DK079638, American Heart Association Grant 11SDG7690000, and the University of Toledo Biomedical Research Innovation Award.
Reference List
- 1.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat. Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 2.Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nat. Immunol. 2007;8:457–462. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- 3.Malek TR. The biology of interleukin-2. Annu. Rev. Immunol. 2008;26:453–479. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- 4.Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki H, Kundig TM, Furlonger C, Wakeham A, Timms E, Matsuyama T, Schmits R, Simard JJ, Ohashi PS, Griesser H, Taniguchi T, Paige CJ, Mak TW. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta. Science. 1995;268:1472–1476. doi: 10.1126/science.7770771. [DOI] [PubMed] [Google Scholar]
- 6.Malek TR, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rbeta-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 2002;17:167–178. doi: 10.1016/s1074-7613(02)00367-9. [DOI] [PubMed] [Google Scholar]
- 7.Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat. Rev. Immunol. 2012;12:180–190. doi: 10.1038/nri3156. [DOI] [PubMed] [Google Scholar]
- 8.Wang G, Khattar M, Guo Z, Miyahara Y, Linkes SP, Sun Z, He X, Stepkowski SM, Chen W. IL-2-deprivation and TGF-beta are two non-redundant suppressor mechanisms of CD4+CD25+ regulatory T cell which jointly restrain CD4+CD25− cell activation. Immunol. Lett. 2010;132:61–68. doi: 10.1016/j.imlet.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat. Immunol. 2007;8:1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 10.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T Cells: Mechanisms of Differentiation and Function. Annu. Rev. Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J. Immunol. 2007;178:280–290. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- 12.Lio CW, Hsieh CS. A two-step process for thymic regulatory T cell development. Immunity. 2008;28:100–111. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burchill MA, Yang J, Vang KB, Moon JJ, Chu HH, Lio CW, Vegoe AL, Hsieh CS, Jenkins MK, Farrar MA. Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity. 2008;28:112–121. doi: 10.1016/j.immuni.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vang KB, Yang J, Mahmud SA, Burchill MA, Vegoe AL, Farrar MA. IL-2, -7, and -15, but not thymic stromal lymphopoeitin, redundantly govern CD4+Foxp3+ regulatory T cell development. J. Immunol. 2008;181:3285–3290. doi: 10.4049/jimmunol.181.5.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-beta to convert naive CD4+CD25− cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J. Immunol. 2007;178:2018–2027. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- 17.Davidson TS, DiPaolo RJ, Andersson J, Shevach EM. Cutting Edge: IL-2 is essential for TGF-beta-mediated induction of Foxp3+ T regulatory cells. J. Immunol. 2007;178:4022–4026. doi: 10.4049/jimmunol.178.7.4022. [DOI] [PubMed] [Google Scholar]
- 18.Wang G, Miyahara Y, Guo Z, Khattar M, Stepkowski SM, Chen W. "Default" generation of neonatal regulatory T cells. J. Immunol. 2010;185:71–78. doi: 10.4049/jimmunol.0903806. [DOI] [PubMed] [Google Scholar]
- 19.Izcue A, Hue S, Buonocore S, Arancibia-Carcamo CV, Ahern PP, Iwakura Y, Maloy KJ, Powrie F. Interleukin-23 restrains regulatory T cell activity to drive T cell-dependent colitis. Immunity. 2008;28:559–570. doi: 10.1016/j.immuni.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schallenberg S, Tsai PY, Riewaldt J, Kretschmer K. Identification of an immediate Foxp3(−) precursor to Foxp3(+) regulatory T cells in peripheral lymphoid organs of nonmanipulated mice. J. Exp. Med. 2010;207:1393–1407. doi: 10.1084/jem.20100045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polansky JK, Kretschmer K, Freyer J, Floess S, Garbe A, Baron U, Olek S, Hamann A, von BH, Huehn J. DNA methylation controls Foxp3 gene expression. Eur. J. Immunol. 2008;38:1654–1663. doi: 10.1002/eji.200838105. [DOI] [PubMed] [Google Scholar]
- 22.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Webster KE, Walters S, Kohler RE, Mrkvan T, Boyman O, Surh CD, Grey ST, Sprent J. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J. Exp. Med. 2009;206:751–760. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 25.Grinberg-Bleyer Y, Baeyens A, You S, Elhage R, Fourcade G, Gregoire S, Cagnard N, Carpentier W, Tang Q, Bluestone J, Chatenoud L, Klatzmann D, Salomon BL, Piaggio E. IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. J. Exp. Med. 2010;207:1871–1878. doi: 10.1084/jem.20100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Selvaraj RK, Geiger TL. A kinetic and dynamic analysis of Foxp3 induced in T cells by TGF-beta. J. Immunol. 2007;178:7667–7677. doi: 10.4049/jimmunol.178.12.7667. [DOI] [PubMed] [Google Scholar]
- 27.Yao Z, Kanno Y, Kerenyi M, Stephens G, Durant L, Watford WT, Laurence A, Robinson GW, Shevach EM, Moriggl R, Hennighausen L, Wu C, O'Shea JJ. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood. 2007;109:4368–4375. doi: 10.1182/blood-2006-11-055756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;30:616–625. doi: 10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30:626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von BH. Inducing and expanding regulatory T cell populations by foreign antigen. Nat. Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.