Abstract
Population differences in age-related diseases and cancer could stem from differences in diet. To characterize DNA strand-breaking activities in selected foods/beverages, flavorings, and some of their constituent chemicals, we used p53R cells, a cellular assay sensitive to such breaks. Substances testing positive included reference chemicals: quinacrine (peak response, 51X) and etoposide (33X); flavonoids: EGCG (19X), curcumin (12X), apigenin (9X), and quercetin (7X); beverages: chamomile (11X), green (21X), and black tea (26X) and coffee (3 to 29X); and liquid smoke (4 to 28X). Damage occurred at dietary concentrations: etoposide near 5 μg/ml produced responses similar to a 1:1000 dilution of liquid smoke, a 1:20 dilution of coffee, and a 1:5 dilution of tea. Pyrogallol-related chemicals and tannins are present in dietary sources and individually produced strong activity: pyrogallol (30X), 3-methoxycatechol (25X), gallic acid (21X), and 1,2,4-benzenetriol (21X). From structure-activity relationships, high activities depended on specific orientations of hydroxyls on the benzene ring. Responses accompanied cellular signals characteristic of DNA breaks such as H2AX phosphorylation. Breaks were also directly detected by comet assay. Cellular toxicological effects of foods and flavorings could guide epidemiologic and experimental studies of potential disease risks from DNA strand-breaking chemicals in diets.
Keywords: DNA damage, p53, liquid smoke, tea, coffee, pyrogallol
1. Introduction
Health risks attributable to diet have been evaluated by both epidemiology and biological methods. Epidemiology, however, has poor resolution at the chemical level. For example, although screening of foods and individual chemicals for nucleotide mutagens is efficient using the Ames assay, screening for clastogens is inefficient and has employed a variety of cell- and animal-based assays.
Among Ames-negative foods, major dietary risks include the enhanced cancer risk from the consumption of high-tannin foods (Kirby, 1960; Morton, 1992). The subcutaneous administration of the tannin fractions from several plants (including tea) produced tumors at the injection site in rats (Kapadia et al., 1976). Hot teas and their constituents were linked to elevated risks of cancer (Morton, 1987). Processed meat intake may increase the risk of colorectal cancer by 20–50% (Chan et al., 2011; Santarelli et al., 2008). Salt, liquid smoke, nitrites and nitrates are widely used in meat processing. Liquid smoke caused a many-fold increase in DNA single-strand breaks (measured as DNA eluted in alkaline conditions from a filter) in rat gastric mucosa in vivo when given orally (Ohshima et al., 1989b) and induced mutations in human lymphocytes in vitro (Braun et al., 1987). An increased number of altered pyloric glands, a neoplasia precursor, were produced in rats on a diet containing 5% liquid smoke after a single intragastric administration of N-methyl-N’-nitroso-N-nitrosoguanidine (Shichino et al., 1992). The results of the Ames test, however, were equivocal (Braun et al., 1987; Putnam et al., 1999). The genotoxic potential of liquid smoke and many other food substances thus remains largely unexamined due to the limitations of the commonly used tests.
Efficient biological assays of DNA damage could aid epidemiologic studies of foods and flavorings. We used a well characterized p53-based luciferase reporter human cell line (Cunningham et al., 2004; Gallmeier et al., 2005; Sohn et al., 2002) and confirmatory assays to investigate the genotoxic properties of selected foods and flavorings.
2. Materials and Methods
2.1. Cell Lines and Cell Culture
p53R cells were created in our laboratory (Cunningham et al., 2004; Gallmeier et al., 2005; Sohn et al., 2002). p53R, HeLa (ATCC), and AAV-293 (Stratagene) cells were grown in DMEM with 10% (v/v) FBS, 1% (v/v) penicillin/streptomycin, and 20 mM HEPES. CHO AA8-Luc Tet-Off (Clontech) cells were supplemented with 100 μg/ml G418.
2.2. Substances Tested
Chemicals were from Sigma-Aldrich except as noted otherwise.
Positive controls
Quinacrine and etoposide served as positive controls for the p53R assay. Quinacrine, an intercalator and potential topoisomerase inhibitor inducing DNA strand breaks (Snyder and Arnone, 2002; Wang et al., 2005), strongly activates p53 in the p53R assay (Cunningham et al., 2004; Sohn et al., 2002). Etoposide is a potent topoisomerase inhibitor causing strand breakage and cytotoxicity, also strongly activating p53 in the assay (Sohn et al., 2002). Trichostatin A (TSA) was used to cause non-specific gene expression in CHO AA8-Luc Tet-Off cells (Cunningham et al., 2004). Hydrogen peroxide served as a positive control for the neutral comet assay.
Reference chemicals
A variety of reference chemicals were tested in the p53R assay. Among the chemotherapeutics, aminopterin and methotrexate are inhibitors of dihydrofolate reductase, fluorouracil (5-FU) forms a nucleotide analog inhibitor of thymidylate synthase, and vincristine sulfate disrupts microtubules. Cyclosporine is an immunosuppressant. Caffeine is known to inhibit ATM and ATR kinases (Sarkaria et al., 1999) and impair DNA-damage-mediated p53 induction (Kastan et al., 1991). Serine and retinoic acid were negative controls.
Foods
Among the tested items was celery extract, containing apigenin (Harnly et al., 2006). Apigenin, as with many flavonoids, is a known p53 activator (Cunningham et al., 2004; Sohn et al., 2002). Also tested was a distillate of a peat smoke condensate (an Islay Scotch, Laphroaig); several teas: chamomile tea (Wegmans) known to contain apigenin, green tea (Twinings), black tea (Twinings Irish Breakfast), and Lapsang Souchong tea (Twinings) containing pine smoke condensates; coffees: regular and decaffeinated (purchased prepared), smokeless tobacco (Skoal Long Cut); and a non-nutritive food additive: a nitrosamines mix (Supelco).
Condiments/flavorings
Tested substances included Tabasco pepper sauce (McIlhenny), tamari soy sauce (Kikkoman), fish sauce (Polar), oyster sauce (Polar), kim chee (Jo San), black bean sauce (Kikkoman), soybean paste (Awase Miso), roaster seaweed sushinori (Nagai’s), Japanese wasabi powder (Asian Gourmet), hickory smoke powder (Colorado Spice Co.), “smoke essence” powdered flavoring (Seasoning House), smoked paprikas (McCormick, Chiquilin), orange bitters (Angostura), aromatic bitters (Angostura, Peychauds), and 15 brands of liquid smoke (Wright’s Hickory, Cedar House Hickory, Colgin Hickory, Colgin Mesquite, Haddon House Hickory, Long Horn Grill Mesquite, Lazy Kettles Hickory, Stubb’s Hickory, Stubb’s Mesquite, Heart-Loc Hickory, Figaro Mesquite, Regal Foods Texas Style, PS Seasoning and Spices Charsol Supreme, PS Seasoning and Spices Mesquite, and LEM Hickory).
Individual Flavonoids and Other Selected Food Constituents
Individual flavonoids and other selected food constituents reported in teas, coffee, and foods (Harnly et al., 2006; Ramos, 2008) were tested: apigenin (in chamomile tea), epigallocatechin-3-gallate (EGCG) (in green tea), quercetin (in black tea), genistin, genestein, glutamic acid, sulforaphane, cyanidin chloride, hesperetin, resveratrol, curcumin, p-coumaric acid, (+) α-tocopherol, and apiin (Fluka).
Individual Components of Liquid Smoke
Individual components of liquid smoke, reported multiply in literature (Fiddler et al., 1970; Fiddler et al., 1966; Guillen et al., 1995; Hruza et al., 1974; Issenber. P et al., 1971; Kim et al., 1974; Knowles et al., 1975; Lustre and Issenber. P, 1969; Meier, 2008; Ohshima et al., 1989a; Sternitzke et al., 1992), were tested as isolated chemicals. These included pyrogallol, 3-methoxy catechol, benzopyrene, acetaldehyde (supplied in ethanol), hydroquinone, p-benzoquinone, 2-methoxy-4-propyl phenol, 2-methoxy-4-vinyl phenol, eugenol, furfuryl alcohol, furfural, 5-methyl furfural, m-cresol, o-cresol, p-cresol, phenol, guaiacol, 2-methoxy-4-methyl phenol, 3, 5-dimethyl phenol, syringol (2, 6-dimethyl phenol), 2, 6-dimethoxy phenol, 4-methyl-2, 6-dimethoxy phenol, maltol, 3-methyl catechol, pyrocatechol, acetovanillone, 1,2-dimethoxy benzene, vanillin, 2-cyclopentenone, and hydroxyacetone.
Compounds Resembling Pyrogallol
To evaluate structure-activity relationships (SARs), pyrogallol-like compounds were tested: 1,2,4-benzenetriol, gallic acid, 2,3,4-trihydroxybenzoic acid, gallacetophenone, tannic acid, 3,4,5-trihydroxybenzaldehyde, phloroglucinol, resorcinol, 3-methoxyphenol, 1,2,3-cyclohexanetriol (Tokyo Chemical Industry), 3,4,5-trihydroxybenzamide, 5-methyl benzene 1,2,3-triol, and 3,4,5-trihydroxymethylbenzoate. Cupric sulfate pentahydrate (J. T. Baker) was also tested because of the reported ability of cupric ions to enhance the effects of pyrogallol (Hayakawa et al., 1997; Khan and Hadi, 1998; Stich et al., 1981).
2.3. p53R Assay
Water or medium was used as the initial diluent for each substance. Dimethyl sulfoxide (DMSO) was generally used when the compound was not water-soluble. Culture medium was used for subsequent dilutions. Substances with a high salt content such as soy sauce, kim chee, and black bean sauce were tested over a dose range such that the final sodium concentration was maintained around or below 150 mM. For each treatment, cells were plated in triplicate and treated the following day with the chemical for 18 hours. A luciferase assay was performed (Promega Steady-Glo) using a PerkinElmer Microbeta Trilux plate reader. The mean for each triplet was plotted for each concentration tested.
2.4. Special Procedures for Liquid Smoke
To study the effect of desiccation on the ability of liquid smoke to evoke a p53 response, liquid smoke was vacuum-dried. To investigate the effect of filtration, liquid smoke was centrifuged at 500 × g for 5 min and the supernatant passed through a 0.2 μM filter. To determine whether charcoal adsorption could eliminate the p53-activating property, liquid smoke was treated as described (Carter, 1978). Briefly, 420 mg of activated charcoal (Sigma-Aldrich) was added to 3 ml of undiluted or 1:10 diluted liquid smoke. The mixture was vortex-mixed 10 times for 1 s intervals, shaken overnight, and centrifuged for 20 min at 3000 g. The supernatant was removed, centrifuged again for 20 min at 3000 × g, and the supernatant passed through a 0.2 μM filter. To adsorb liquid smoke with protein, liquid smoke (manufacturer’s stock) diluted in 5% milk (from dry skim milk) was used to treat cells. To explore the effect of chloroform extraction, equal volumes of liquid smoke and chloroform were mixed by shaking vigorously and vortexing for 30 s each. The mixture was centrifuged at 3000 g for 1 min. The aqueous and chloroform fractions were each vacuum-dried. The aqueous residue was reconstituted in water, and the chloroform residue, in DMSO. To examine the effect of various heat-treatments on the p53-activating property, liquid smoke, pyrogallol solution, or 3-methoxycatechol solution was moderately heated at 80°C for 24 h, boiled at 100°C for 1 h, or slow-cooked at 225°F for 8 h on a heat block. The manufacturer’s stock of liquid smoke, pyrogallol solution, or 3-methoxycatechol solution was baked in a conventional oven at 350°F for 1 h to simulate standard baking conditions. The tacky residue from cooking was restored to original volume by adding water.
2.5. Mixing Experiments
To investigate the behavior of complex mixtures in the p53R assay, several mixtures were prepared. The 1x mixture of the top eight most active chemical constituents was defined as containing 30 μg/ml pyrogallol, 7.5 μg/ml 3-methoxycatechol, 10 μg/ml 1,2,4-benzenetriol, 10 μg/ml gallic acid, 20 μg/ml 2,3,4-trihydroxybenzoic acid, 20 μg/ml gallacetophenone, 50 μg/ml hydroquinone, and 50 μg/ml p-benzoquinone; it was tested at 0.01x through 5x in the p53R assay. The 1x mixture of the top four most active chemicals was defined to contain 30 μg/ml pyrogallol, 7.5 μg/ml 3-methoxycatechol, 10 μg/ml 1,2,4-benzenetriol, and 10 μg/ml gallic acid; it was tested at 0.01x through 10x. The 1x mixture of pyrogallol and p-benzoquinone was defined to contain 30 μg/ml pyrogallol and 50 μg/ml p-benzoquinone at 1x; it was tested at 0.02x through 10x. The 1x mixture of Wright’s Hickory and Figaro Mesquite was defined to contain 0.001x Wright’s Hickory and 0.005x Figaro Mesquite at 1x; it was tested at 0.02x through 10x.
2.6. Immunoblot
Cells were lysed by rocking in a detergent (50 mM Tris-HCl, 150 mM NaCl, 1mM EDTA, and 1% v/v Triton X-100, and a protease inhibitor cocktail (Roche)) for 1 h at 4°C. The cell lysate was clarified by centrifugation. The protein concentration was determined by the DC protein assay (Bio-Rad). After adding a denaturant (2% m/v sodium dodecyl sulfate (SDS), 10% v/v glycerol, 0.002% m/v bromophenol blue, 2 mM EDTA, 50 mM Tris pH 6.8, and 1% v/v β-mercaptoethanol), samples were boiled and resolved on a 4–12% Bis-Tris gel (NuPAGE Invitrogen). After transfer to a polyvinylidene fluoride (PVDF) membrane (Pierce), blots were incubated with primary antibodies: p53 (Santa Cruz), p21 (Cell Signaling), γ-H2AX (Millipore), and GAPDH (Santa Cruz), followed by horseradish peroxidase (HRP)-conjugated anti-mouse IgG secondary antibodies (Santa Cruz). Membranes were developed with the Immobilon substrate (Millipore), and signals recorded on film.
2.7. Neutral Comet Assay
p53R cells were treated with 0.0005x Wright’s Hickory liquid smoke or 15 μg/ml pyrogallol for 30 min at 37°C. As a positive control, cells were also treated with 0.03% hydrogen peroxide for 30 min at 4°C. Cells were detached using 0.05% trypsin, diluted to 100,000 cells/ml, mixed with molten (37°C) 0.75% low melting agarose (1:10, v/v) and immediately layered onto pre-treated slides (Trevigen). Gels were incubated at 4°C in the dark for 30 min to adhere to the slides. Cells were then lysed in pre-chilled lysis buffer (2.5 M sodium chloride, 100 mM EDTA, 10 mM Tris, 1% m/v sodium lauroyl sarcosinate, and 1% v/v Triton X-100) overnight at 4°C. After a 15-min wash step in neutral TBE buffer, electrophoresis was performed in TBE at 23 V for 15 min at 4°C. Slides were then washed in water for 5 min, submerged in 70% ethanol for 5 min, and air-dried overnight. The slides were stained with SYBR Green (Molecular Probes) and imaged using a fluorescent microscope with a 10x objective and a Nikon Digital Eclipse DXM 1200 camera. The CometScore software was used to morphometrically integrate the tail moment of at least 45 randomly selected comets from each gel.
3. Results
The cytomegalovirus (CMV) promoter in CHO AA8-Luc Tet-Off cells is constitutively active, thus decrements reflected in the luciferase assay on these cells served as a read-out of toxicity due to chemical exposure. The p53 response (elevating the assay value) was superimposed on the toxicity of any given chemical exposure in the p53R assay. The typical dose-response relationship thus had a peak at a threshold value, beyond which cytotoxicity dominated. Positive controls for p53 activation are summarized in Table 1.
Table 1.
Reference Chemicals
| Compounda | Maximal response (fold increase) b | Concentration at maximal response |
|---|---|---|
| DNA intercalator/topo. inh. | ||
| Quinacrine | 51 | 5 μM (2 μg/ml) |
| Topoisomerase inhibitor | ||
| Etoposide | 33 | 10 μM (5.9 μg/ml) |
| Antimetabolite | ||
| Aminopterin | 6.4 | 320 μM (140 μg/ml) |
| 5-fluorouracil | 4.8 | 100 μM (13 μg/ml) |
| Antimicrotubule agent | ||
| Vincristine sulfate | 3.0 | 7.7 μM (7.1 μg/ml) |
| Metabolite | ||
| Retinoic acid | 1.9 | 100 μM (30 μg/ml) |
| Amino acid | ||
| Serine | 1.3 | 3.4 mM (360 μg/ml) |
| ATM/ATR inhibitor | ||
| Caffeine | 1.1 | 3.6 mM (17 mg/ml) |
| Antimetabolite | ||
| Methotrexate | 1.1 | 20 nM (9.1 ng/ml) |
| Immunosuppressant | ||
| Cyclosporin | 1.0 | 20 nM (24.1 ng/ml) |
Compounds were initially dissolved in DMSO except caffeine and serine, which were dissolved in culture medium. Quinacrine has multiple functions attributed.
Relative luciferase activity of p53R cells relative to untreated cells, tested over a wide dose range.
Among selected foods, flavorings, and constituents, the p53 activation assay gave strongly positive findings (Tables 2, 3, and 4). These included teas: chamomile tea (11X), green tea (21X), black tea (26X), and Lapsang Souchong tea (20X); coffees: regular (3 to 29X) and decaffeinated (8 to 23X); condiments/flavorings: all 15 brands of liquid smoke (4 to 28X, with a tendency for hickory products to test higher than mesquite.) and hickory smoke powder from Colorado Spice Co. having the aroma of liquid smoke (12X); celery extract (5X); and some flavonoids: apigenin (9X), EGCG (19X), quercetin (7X), and curcumin (12X). Black tea produced a strong p53 response irrespective of the method of extraction (boiling in water for 10 min versus extracting from a tea bag in hot water for 5 min). We tested different preparations of coffee from a local vendor to reflect conventional drinking practices and obtained different results from different brews. The values ranged from 3 to 29X for regular coffee and from 8 to 23X for decaffeinated coffee. We also found evidence for an aging effect: the activity diminished 10 to 15-fold with coffee storage over a one-week period. DNA-damaging activity was often seen at concentrations consumed dietarily; a 1:1000 dilution of liquid smoke, a 1:20 dilution of coffee, or a 1:5 dilution of brewed black tea produced responses similar to etoposide near 5 μg/ml.
Table 2.
Foods, flavorings
| Substancea | Maximal response (fold increase) | Concentration at maximal response |
|---|---|---|
| Food/Beverage | ||
| Coffee | ||
| Regular | 29 | 0.05xb |
| Decaffeinated | 23 | 0.05xb |
| Teas | ||
| Chamomile tea | 11 | 1xc |
| Green tea | 21 | 0.2xc |
| Black tea | 26 | 0.2xc |
| Lapsang Souchong tea | 20 | 0.02xc |
| Smokeless tobacco | ||
| Boiling water 10 min | 3.7 | 0.5xd |
| 37°C culture medium 1h | 1.8 | 0.5xe |
| Celery extract | 5.2 | 0.7xc |
| Islay Scotch: Laphroaig | 1.0 | 0.1xf |
| Condiment/flavoring | ||
| Fish sauce | 5.9 | 0.02xf |
| Oyster sauce | 4.6 | 0.05xf |
| Soy sauce | 1.1 | 0.005xf |
| Black bean sauce | 1.1 | 0.001xf |
| Tabasco sauce | 1.0 | 0.002xf |
| Soybean paste | 2.2 | 0.005xf |
| Roasted seaweed | 2.4 | 0.1x c |
| Kim Chee | 1.0 | 0.002xf |
| Wasabi powder | 2.4 | 200 μg/ml |
| Hickory smoke powder (Colorado Spice Co.) | 12 | 500 μg/ml |
| Smoke “essence” (Seasoning House) | 1.9 | 5 mg/ml |
| Smoked Paprika | ||
| McCormick | 1.0 | 200 μg/ml |
| Chiquilin | 1.1 | 1 μg/ml |
| Bitters | ||
| Aromatic | ||
| Angostura | 1.4 | 1 mg/ml |
| Peychaud’s | 1.2 | 100 ng/ml |
| Orange | ||
| Angostura | 1.3 | 1xg |
Substances were initially extracted or diluted in water, except for chamomile flowers (extracted in methanol), smoke essence and paprika (extracted in DMSO), and aromatic bitters (after desiccation, the tacky residue was weighed and reconstituted in water).
16 ounces of coffee beans were brewed in 180 ounces of water. The solution was vacuum-dried until around 90% of water evaporated. The resulting concentrated stock solution was arbitrarily designated 10x. The peak response varied between samples, ranging from 3 to 29X for regular and 8 to 23X for decaffeinated coffee.
2 g of substance was extracted in 50 ml of solvent by boiling for 10 minutes. The solution was filtered and vacuum-dried until around 90% of original volume evaporated. The resulting concentrated stock solution was arbitrarily designated 10x.
1 g of substance was extracted in 25 ml of water by boiling for 10 minutes. The solution was filtered and vacuum-dried until around 90% of original volume evaporated. The resulting concentrated stock solution was arbitrarily designated 10x.
1 g of substance was extracted in 25 ml of culture medium by incubating at 37°C for 1 hour. The solution was filtered and vacuum-dried until around 90% of original volume evaporated. The resulting concentrated stock solution was arbitrarily designated 10x.
Manufacturer’s product was considered 1x. Scotch was toxic above 10% (4% alcohol by volume), but was tested negative after dessication and reconstitution in water
Manufacturer’s product was vacuum-dried until all of water and ethanol evaporated to its glycerin-rich residue. The resulting concentrate was arbitrarily designated 100x.
Table 3.
Brands of liquid smoke
| Brand | Ingredientsa | Max. response (fold increase) | Concentration at maximal responseb |
|---|---|---|---|
| Wright’s Hickory | W Ls | 28 | 0.001x |
| Haddon House Hickory | W Ls P | 24 | 0.001x |
| Lazy Kettles Brand Hickory | Ls | 24 | 0.0005x |
| Colgin Hickory | W Ls V Ml C Sa | 21 | 0.003x |
| PS Seas. & Spices Charsol Supreme | L A | 20 | 0.0002x |
| Cedar House Hickory | W Ls Ms | 17 | 0.001x |
| Heart-Loc Hickory | W Ls Ml C V Su | 15 | 0.002x |
| Regal Foods Texas Style | W Ls P | 14 | 0.001x |
| LEM Hickory | Ls P | 10 | 0.0002x |
| Colgin Mesquite | W Ms V Ml C Sp | 9 | 0.01x |
| Stubb’s Hickory | W Ss Ls V Hf C Sp | 6 | 0.05x |
| Figaro Mesquite | W Ms V Ss Su C Sp | 4 | 0.005x |
| Long Horn Grill Mesquite | W Ms V Su H | 4 | 0.01x |
| PS Seasoning and Spices Mesquite | Ms P D | 4 | 0.001x |
| Stubb’s Mesquite | W Ss V Hf Ms C Sp | 4 | 0.002x |
Ingredients are given in order stated on the product label. W, water. Ls, liquid smoke or hickory smoke, or smoke flavor, or smoke concentrate or condensate. Ms, mesquite smoke or smoke flavor, smoke concentrate, or condensate. V, vinegar. Ss, soy sauce or soy protein. Ml, molasses. Su, sugar. Ho, honey. P, polysorbate 80. D, dimethylpolysiloxane. Hf, high-fructose corn syrup. Sp, spices or flavoring. C, coloring agents. Su, sulfating agents. Sa, salt. A, acetic acid.
Manufacturer’s product was considered 1x.
Table 4.
Chemical constituents of foods and flavorings
| Chemicala | Maximal response (fold increase) | Concentration at maximal responseb |
|---|---|---|
| Flavonoids, other | ||
| EGCG | 19 | 100 μM |
| Curcumin | 12 | 27 μM |
| Apigenin | 8.7 | 260 μM |
| Quercetin | 7.0 | 250 μM |
| Genestein | 4.1 | 100 μM |
| Genistin | 2.2 | 100 μM |
| Hesperetin | 1.8 | 100 μM |
| Sulforaphane | 1.6 | 10 μM |
| Resveratrol | 1.6 | 10 μM |
| Cyanidin chloride | 1.4 | 100 μM |
| Glutamic acid | 1.3 | 6.8 μM |
| (+) α-tocopherol | 1.1 | 100 μM |
| Apiin | 1.1 | 100 μM |
| Nitrosamines mix | 1.1 | 2 μg/ml each component |
| p-Coumaric acid | 1.0 | 1 μM |
| Reported in liquid smoke in multiple publications | ||
| Pyrogallol | 30 | 15 μg/ml |
| 3-methoxycatechol | 25 | 7.5 μg/ml |
| Hydroquinone | 3.2 | 50 μg/ml |
| p-benzoquinone | 3.1 | 50 μg/ml |
| Eugenol | 2.3 | 0.0002x |
| 2-methoxy-4-propyl phenol | 2.2 | 0.00005x |
| 2-methoxy-4-vinyl phenol | 2.2 | 0.00001x |
| Benzopyrene | 2.1 | 5 μg/ml |
| p-cresol | 1.9 | 100 μg/ml |
| 2-methoxy-4-methylphenol | 1.8 | 0.0001x |
| Acetaldehyde | 1.6 | 1 mM |
| 2,6-dimethylphenol | 1.5 | 50 μg/ml |
| 3,5-dimethylphenol | 1.5 | 50 μg/ml |
| 1,2-dimethoxybenzene | 1.3 | 0.0005x |
| 2-cyclopentenone | 1.3 | 0.00001x |
| o-cresol | 1.3 | 0.000001x |
| 4-methyl-2,6-dimethoxyphenol | 1.2 | 10 μg/ml |
| Furfural | 1.2 | 0.0000002x |
| Furfuryl alcohol | 1.2 | 0.00001x |
| Guaiacol | 1.2 | 200 μg/ml |
| Syringol | 1.2 | 100 ng/ml |
| 3-methylcatechol | 1.1 | 5 μg/ml |
| 5-methylfurfural | 1.1 | 0.0002x |
| Acetovanillone | 1.1 | 100 ng/ml |
| Maltol | 1.1 | 100 ng/ml |
| m-cresol | 1.1 | 0.0001x |
| Hydroxyacetone | 1.0 | 0.00001x |
| Phenol | 1.0 | 100 μg/ml |
| Pyrocatechol | 1.0 | 100 ng/ml |
| Vanillin | 1.0 | 10 μg/ml |
| Similar to pyrogallolc | ||
| 1,2,4-benzenetriol | 21 | 10 μg/ml |
| Gallic acid | 21 | 10 μg/ml |
| 2,3,4-trihydroxybenzoic acid | 7.3 | 20 μg/ml |
| Gallacetophenone | 3.8 | 20 μg/ml |
| 3,4,5-trihydroxybenzamide | 3.7 | 100 μg/ml |
| Tannic acid | 1.4 | 100 ng/ml |
| 3-methoxyphenol | 1.3 | 0.000001x |
| 3,4,5-trihydroxymethylbenzoate | 1.2 | 500 ng/ml |
| 1,2,3-cyclohexanetriol | 1.1 | 1 μg/ml |
| 5-methylbenzene 1,2,3-triol | 1.1 | 2 μg/ml |
| 3,4,5-trihydroxybenzaldehyde | 1.1 | 100 ng/ml |
| Phloroglucinol | 1.0 | 500 ng/ml |
| Resorcinol | 1.0 | 200 ng/ml |
Dry chemicals were initially dissolved in DMSO, except for glutamic acid, hydroquinone, p-benzoquinone, p-cresol, guiacol, and phenol (in water or culture medium), nitrosamines mix (in methanol), and acetaldehyde (supplied in ethanol).
Dilutions of a liquid chemical are given as fractions of the pure compound, which was considered 1x.
Not a reported constituent or not multiply reported.
We found weak to negative responses with smokeless tobacco boiled in water for 10 min (4X) or incubated at 37°C for 1 h in culture medium to possibly simulate oral mucosal wetting (1.8X). Some flavonoids and other constituents tested weak (numbers given) or less than 2X (values not given). These included genistin (2X), genestein (4X), glutamic acid, sulforaphane, cyanidin chloride, hesperetin, resveratrol, p-Coumaric acid, (+) α-tocopherol, apiin, nitrosamines mix, Islay Scotch (under various conditions: desiccated and re-dissolved in water as well as directly diluted in water or medium), soy sauce, kim chee, black bean sauce, orange and aromatic bitters, smoked paprika, and a “smoke essence” powdered flavoring not having the aroma of liquid smoke.
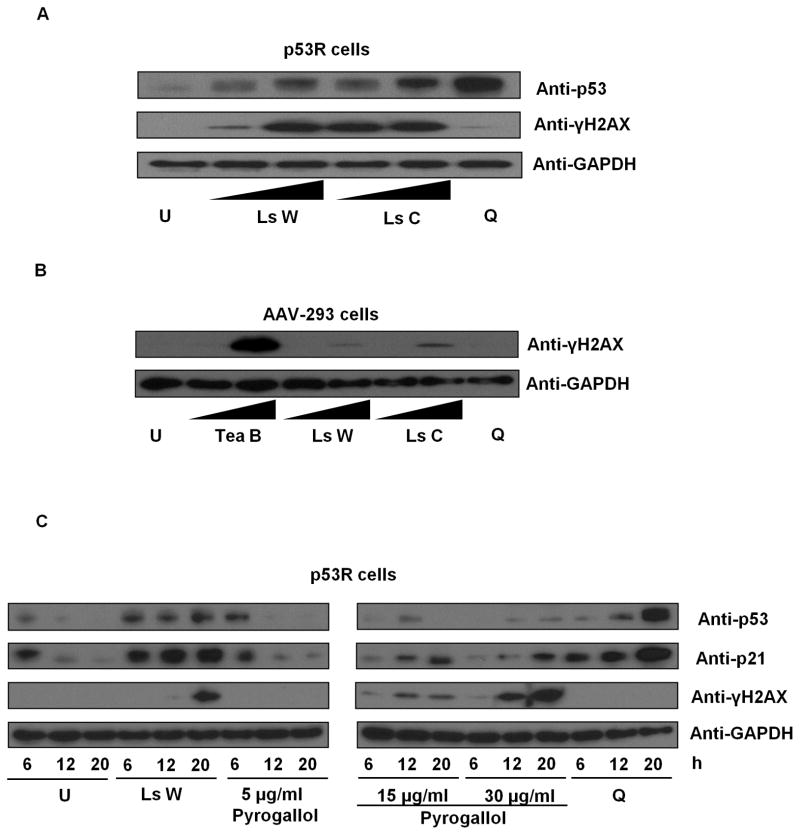
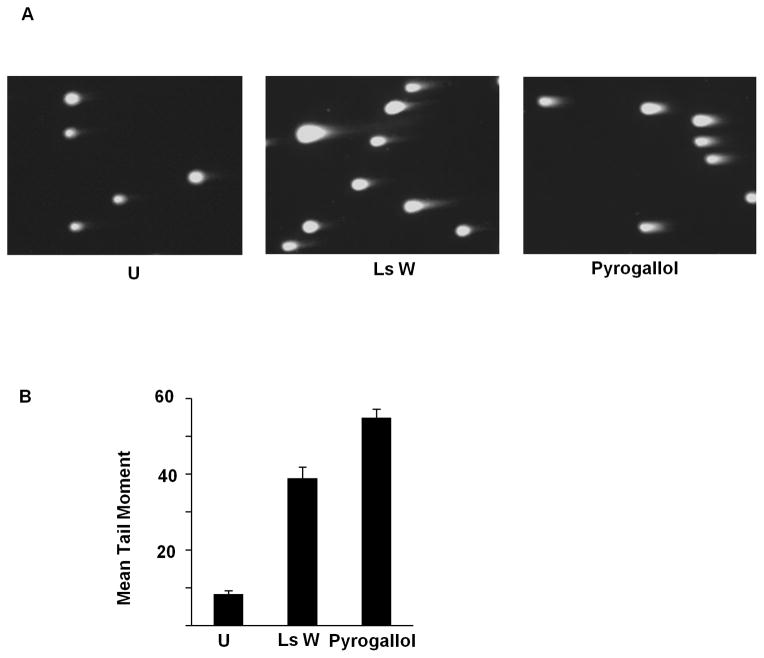
Due to surprisingly strong findings in teas and liquid smoke, further characterization was undertaken. We focused investigation on liquid smoke due to its known capacity to cause DNA damage in vivo, its expected paucity of p53-activating flavonoids, published lists of its constituents, and an expected analytic advantage from its extreme value in the p53R assay. The genotoxic activity in liquid smoke was confirmed using Wright’s Hickory and Cedar House Hickory by immunoblots detecting an increase in p53 protein level and γ-H2AX (Fig. 1A), indicating DNA double-strand breaks in p53R cells. H2AX phosphorylation was observed also in AAV-293 cells (Fig. 1B). Similar results were obtained after treatment with black tea (Fig. 1B). The p53 protein level was disregarded in AAV-293 cells, because the endogenous level is known to be high in these cells (Yin et al., 2011). With liquid smoke treatment, the level of γ-H2AX increased over time, as did p21, a transcriptional target of p53 (Fig. 1C). DNA double-strand breaks in p53R cells were also detected by the neutral comet assay (Fig. 2).
Fig 1.
p53 and γ-H2AX protein levels after treatment with liquid smoke, black tea, and pyrogallol. (A) p53R cells were treated with Wright’s Hickory liquid smoke (Ls W) or Cedar House Hickory liquid smoke (Ls C) at 0.0005x and 0.001x for 18 h. Untreated cells (U) and 2 μg/ml quinacrine-treated cells (Q) served as a negative and a positive control for p53 induction respectively. Cellular p53 and γ-H2AX protein levels were evaluated by immunoblotting. (B) AAV-293 cells were treated with black tea (Tea B) at 0.05x and 0.2x or liquid smoke solutions (Ls W or Ls C) at 0.0005x and 0.001x for 18 h. Cellular γ-H2AX protein levels were assayed by immunoblotting. (C) p53R cells were treated with 0.001x Ls W, various concentrations of pyrogallol, or 2 μg/ml quinacrine for 6, 12, or 20 h. Cellular p53, p21, and γ-H2AX levels were assessed by immunoblotting.
Fig 2.
Neutral comet assay after treatment with liquid smoke and pyrogallol. The neutral comet assay was performed on p53R cells treated with 0.0005x Wright’s Hickory liquid smoke (Ls W) or 15 μg/ml pyrogallol. At least 45 cells were analyzed per sample and the mean tail moment of the cell population was calculated using CometScore. (A) Representative pictures of cells. (B) Mean tail moment for each treatment with error bars representing the standard error of the mean (SEM) for the cell population.
The p53-activating property in liquid smoke (Wright’s Hickory and Cedar House Hickory) was eliminated by standard baking conditions (350°F for 1 h). The p53-activating property in liquid smoke could not be eliminated, however, by different fractionation techniques including filtration, adsorption on charcoal or protein, chloroform extraction (the activity was retained in the aqueous fraction), desiccation (with or without alcohol), moderate heating (80°C for 24 hours), boiling (100°C for 1 hour), and slow cooking (225°F for 8 hours).
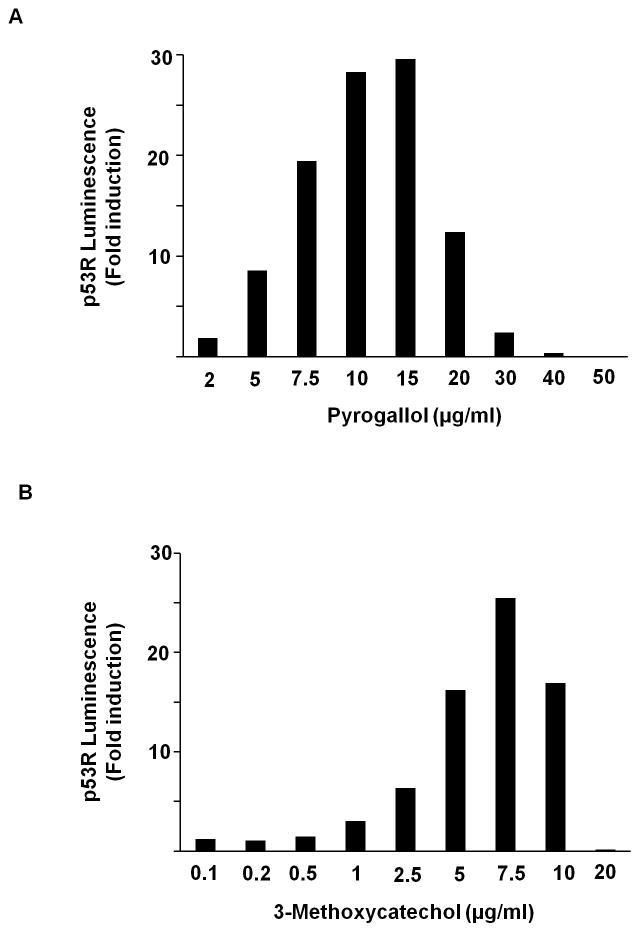
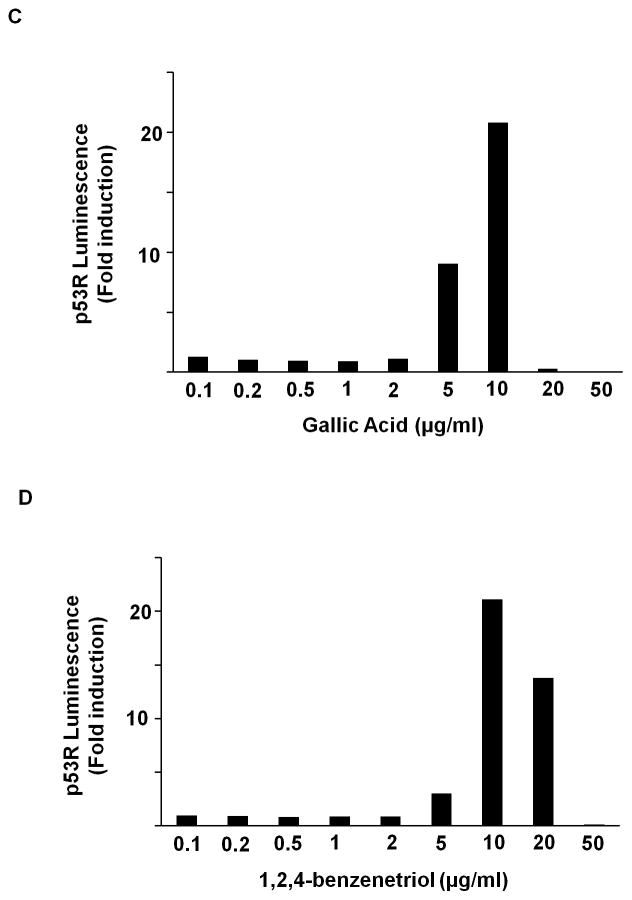
As we tested multiply reported individual components of liquid smoke in the p53R assay (Table 4), we encountered strong activity from pyrogallol (30X) and 3-methoxycatechol (25X) (Fig. 3). An additional 28 compounds known to constitute liquid smoke were negative (Table 4).
Fig 3.
p53 reporter activation after treatment with (A) pyrogallol, (B) 3-methoxycatechol, (C) gallic acid, and (D) 1,2,4-benzenetriol. p53R cells were plated in triplicate and treated with various doses of each compound for 18 h. p53 reporter activity was assessed in a luciferase assay. Luminescence was calculated relative to the untreated sample. The mean for each triplet was plotted for each concentration tested.
The possibility of pyrogallol being auto-luminescent was excluded by performing the luciferase assay in the absence of substrate and in the absence of cells. Concerns about non-specificity were addressed by treating CHO AA8-Luc Tet-Off cells with pyrogallol. Pyrogallol did not elevate luciferase gene expression in CHO AA8-Luc Tet-Off cells. The activity of pyrogallol was substantially reduced by standard baking conditions (350°F for 1 h), but was not eliminated by moderate heating (80°C for 24 h), boiling (100°C for 1 h), or slow cooking (225°F for 8 h). The neutral comet assay also confirmed the presence of DNA double-strand breaks after treatment of p53R cells with pyrogallol (Fig. 2). In contrast to pyrogallol, the strong activity of 3-methoxycatechol (25X) in the p53R assay was eliminated by moderate heating (80°C for 24 h), boiling (100°C for 1 h), slow cooking (225°F for 8 h) or standard baking (350°F for 1 h).
Because luciferase provides an integration of the p53 activity over time, and because p53 has oscillatory behavior (Geva-Zatorsky et al., 2006), we confirmed the p53 response to pyrogallol in p53R cells at 6, 12, and 20 h by immunoblotting for p53, p21, and γ-H2AX in a time-course experiment (Fig. 1C). Pyrogallol induced γ-H2AX in a dose- and time-dependent manner. 5 μg/ml pyrogallol did not alter p21, but higher doses of pyrogallol activated p21 expression in a time-dependent manner. The time-course of p53 stabilization was complex. 5 μg/ml pyrogallol gave a peak p53 protein response at 6 h. 15 μg/ml pyrogallol gave a peak response at 12 h in a manner characteristic of DNA-damaging agents. At 30 μg/ml, pyrogallol gave a muted response at the tested time-points. 2 μg/ml quinacrine produced a time-dependent p53 response which was not associated with γ-H2AX induction. This was not surprising because quinacrine is reported in some cells to activate p53 without causing significant genotoxicity (Gasparian et al., 2011; Wang et al., 2005).
Previous studies suggested that cupric ions could augment the DNA-damaging effects of pyrogallol in vitro (Hayakawa et al., 1997; Stich et al., 1981). Copper is present in the chromatin, and chemical agents can mobilize endogenous copper to induce DNA fragmentation (Burkitt et al., 1996). We treated cells with 68 μM Cu2+ or 272 μM Cu2+ in the presence of increasing concentrations of pyrogallol. Cupric ions did not amplify the activity of pyrogallol here.
We explored the behavior of complex mixtures in the p53R assay. A mixture of the top eight most active components gave 27X activity, whereas a mixture of the top four components gave 18X activity. A mixture of Wright’s Hickory (strong activity) and Figaro Mesquite (weak activity) diluted the stronger activity as expected for additive interactions. A mixture of pyrogallol (strong activity) and p-benzoquinone (weak activity) also diluted the p53 response as expected. Thus, we did not find clear evidence for or against synergy (a greater-than-additive effect) among these compounds from this preliminary qualitative exploration. Additional quantitative explorations of mixed components might reveal interactions of interest.
In an effort to identify the essential structural groups (the pharmacophore) accounting for the DNA-damaging activity in pyrogallol and pyrogallol-related compounds, we screened a number of related compounds and observed the following activities in the p53R assay (Table 4 and Fig. 3): 1,2,4-benzenetriol (21X), gallic acid (21X), 2,3,4-trihydroxybenzoic acid (7.3X), gallacetophenone (3.8X), 3,4,5-trihydroxybenzamide (3.7X), tannic acid (1.4X), 3-methoxyphenol (1.3X), 3,4,5-trihydroxymethylbenzoate (1.2X), 1,2,3-cyclohexanetriol (1.1X), 3,4,5-trihydroxybenzaldehyde monohydrate (1.1X), 5-methylbenzene 1,2,3-triol (1.1X), phloroglucinol (1X),, and resorcinol (1X). We concluded that the three adjacent hydroxyl groups on a benzene ring were necessary but not sufficient for strong activity among this structural family, for additional substitution on the benzene ring could impair the activity.
4. Discussion
p53-based assays emerged with the discovery of the DNA sequence bound by p53 (Kern, 1991; Kern et al., 1991) and the discovery of p53 activation by DNA strand breaks (Kastan et al., 1991). There is a long history of using p53 for qualitative assays, but few authors have adapted p53-responsive assays as quantitative screening tools (Cunningham et al., 2004; Gasparian et al., 2011; Gurova et al., 2005; Sohn et al., 2002; Wang and El-Deiry, 2003). When testing dose-response relationships, chemicals inducing DNA strand breaks produce a peak reporter activity centered at one concentration. We assume that most of the measured effects follow the predictions of Loewe additivity (Berenbaum, 1978). Effects are truncated at higher concentrations when overtaken by cellular toxicity, which reduces the ability of the reporting cells to respond or survive in culture (Cunningham et al., 2004). This theory is an imprecise simplification, as shown by the non-linearity of some dose-response curves.
It is likely that, even when using isolated chemicals, multiple mechanisms of action and of toxicity are engaged during the exposure. Threshold effects can occur, as when compensating mechanisms are overwhelmed and other forms of cellular toxicity further impair any compensating mechanism. High amounts of nucleotide excision repair would cause interactions between DNA regions undergoing repair, leading to double-strand breaks in DNA, even when cells are exposed to chemicals that do not directly induce strand breaks. Based on the simplest assumptions, the peak p53R activity, measured in a given assay setting, is expected to be a value innate to a tested chemical, being independent of the concentration of the starting material. Active DNA-damaging chemicals, when tested in the presence of simple toxins having no particular DNA-damaging ability, could yield values lower than the peak activity of the same chemical tested alone, but would not be expected to yield a higher value.
When tested in the presence of other DNA-damaging agents, the resulting mixture’s peak activity could reflect at most the peak activity of the most potent DNA-damaging agent, in the absence of any synergistic interaction. Since different DNA-damaging agents are unlikely to have the same toxicity threshold, synergistic interactions are difficult to evaluate in a complex mixture of such chemical agents and for the sake of simplicity are not considered here. These assumptions, to the extent that they hold true, permit an analytic fractionation of a complex mixture to identify at least some of the compound(s) responsible for the peak activity. The responsible compound(s) should, when tested alone, have a peak activity at least as high as did the mixture. When the mixture has a very high peak activity, the number of suitable candidate compounds will be small. In a mixture containing multiple toxic substances, the compound(s) responsible for the peak activity should be present in relatively higher concentration(s). Otherwise, the mixture’s peak activity would be truncated prematurely in a dose-escalation experiment owing to the toxicity from these other, assay-negative constituents. The peak activity would be variable when tested in different settings, such as when using different measures of p53 activation (immunoblots of p53 protein, p53 mRNA transcripts, genes downstream of p53 in signal transduction of the DNA-damage response), different cell lines, and conditions preventing/stimulating drug uptake or preventing/augmenting cytotoxicity. To some extent, it is thus valuable to maintain the test situation constant when comparing the tested substances. The peak activity is expected to decrease when testing active chemicals diluted adequately with inactive chemicals.
Constituents of teas and coffees with relevant activities for a p53-based screen for clastogens include flavonoids, tannins, and pyrogallol/gallic acid subunits. Flavonoids are known to activate p53 in the p53R assay (Sohn et al., 2002). Many are reported to be topoisomerase inhibitors (Bandele and Osheroff, 2008; Birt et al., 2001; Lopez-Lazaro et al., 2011; Lopez-Lazaro et al., 2007a; Lopez-Lazaro et al., 2007b; Markovits et al., 1989; Strick et al., 2000). Tannins and pyrogallol/gallic acid subunits are present in substantial amounts in teas and coffees (Chaturvedula and Prakash, 2011; Hussain et al., 2008; Kanwal et al., 2009; Lang et al., 2006; Muller et al., 2006). Gallic acid and EGCG are topoisomerase inhibitors reportedly mediated by the pyrogallol moiety (Lopez-Lazaro et al., 2011). Pyrogallol-like compounds in acellular and cellular conditions are known to damage DNA by activated oxygen species (Hayakawa et al., 1997; Stich et al., 1981).
From our findings and using some allowance for imprecision, we presume that apigenin (≈9X) might fully explain the activity in chamomile tea (≈11X) and in celery extract (≈5X). Aqueous standardized chamomile extract contains approximately 1.2% apigenin (Srivastava and Gupta, 2007). Celery contains 1.3 mg of apigenin per 100 g of fresh weight (Harnly et al., 2006). The future use of quantitative methods would provide more certainty in these rough attributions. Further, EGCG (19X) could largely explain the activity in green tea (21X). However, quercetin (7X) could not fully explain the activity in black tea (26X). EGCG is present in green tea but not in black tea because during black tea production, the catechins are converted to theaflavins and thearubigins (Lorenz et al., 2009). Nevertheless, black tea contains large amounts of pyrogallol and gallic acid among other complex products (Hussain et al., 2008; Kanwal et al., 2009).
Health concerns about the carcinogenic potential of liquid smoke have not been comprehensively addressed; inconclusive to weak results were obtained from the Ames test (Braun et al., 1987; Putnam et al., 1999). We found, as with teas and coffee, strong activities causing cellular DNA-damage-responses after treatment with liquid smoke. Pyrogallol is a major constituent of liquid smoke (Issenber.P et al., 1971; Kim et al., 1974; Knowles et al., 1975; Meier, 2008; Ohshima et al., 1989a; Sternitzke et al., 1992). Pyrogallol and liquid smoke share an unusual strength of p53R activity, similar chemical stabilities upon heating, and other similar chemical properties.
Pyrogallol and flavonoid toxicities are closely related. Many flavonoids contain a catechol or pyrogallol moiety; EGCG contains both a gallic acid and a pyrogallol moiety. A structure-activity analysis of polyphenols found that flavonoids containing a pyrogallol moiety had the most cytotoxic activity, causing apoptosis (Mitsuhashi et al., 2008; Saeki et al., 2000). In our SAR study, pyrogallol-like compounds had high p53R activities dependent on specific orientations of the three hydroxyl groups on the benzene ring. They had generally reduced activity when further modified by side-groups, methylation, or polymerization. For example, pyrogallol (30X) was more potent than 3-methoxycatechol (25X) or gallic acid (21X), whereas tannic acid (1.4X) and syringol (1.2X) were both very weak. A similar trend was observed in an in vitro DNA-cleavage assay (Khan and Hadi, 1998). Likewise, the clastogenic activity of di- and tri-hydroxylated phenolics in CHO cells was also reduced by their methylation (Stich et al., 1981). Pyrogallol and tannin subunits thus suggest unifying structural features of the DNA-damaging activities in foods and flavorings. Pyrogallol and related structures are likely responsible for the strong activity in liquid smoke and perhaps some teas and coffee.
Our results are derived from a particular cell line, RKO colorectal cancer cells, the parental line for p53R cells. p53R assays reflect a chemical’s intracellular accumulation and metabolism, the cellular repair functions subsequent to a chemical exposure, and any influences of the culture medium. It is interesting to note that our measured p53R activities of individual compounds do not closely match the relative levels of DNA damage measured in earlier assays produced in vitro by similar chemical panels (Hayakawa et al., 1997) or produced in vivo using the CHO cell clastogenic assay measuring visible chromosomal aberrations (Stich et al., 1981). The p53R assay might be more sensitive than these earlier assays.
Pyrogallol is a trihydroxylated phenol, all hydroxyl groups being adjacent. It is obtained by the heating (pyrolysis) of gallic acid, which is a building block for some flavonoids (such as EGCG) and for tannins. Pyrogallol is highly water-soluble, but weakly soluble in chloroform, similar to the fractionation pattern observed for the p53R-activating property of liquid smoke. It boils at a much higher temperature (309°C) than water and would not enter conventional distillates even when other smoky flavorings would, as in the distillation of Scotch. Pyrogallol is toxic to most vital organs and known to generate free radicals in cells, among other biological interactions (NIEHS, 1998; NTP, 2012). It is ingested from smoked foods, smoke condensates in food, cigarette smoke, and some ground waters (Knowles et al., 1975; Ohshima et al., 1989a). Among many uses, it is a topical anti-psoriatic medication, a component of hair dyes, and used as a photographic developer (NTP, 2012). Pyrogallol is present in tea, coffee, cigarette smoke, bread crust, roasted malt, and cocoa powder (Hussain et al., 2008; Kanwal et al., 2009; Lang et al., 2006; NTP, 2012). Pyrogallol is efficiently formed during coffee roasting from carbohydrates and amino acids (the Maillard reaction) and from quinic acid moieties (Muller et al., 2006).
High quantities of pyrogallol and similar compounds exist in substances found by us to have high activities (liquid smoke, teas, and coffee) in the p53R assay. Pyrogallol is absorbed from oral ingestion and metabolized to 2-O-methylpyrogallol with 6% detected in urine as such (Bakke, 1970; NTP, 2012). It can be formed from gallic acid and related polyphenols by intestinal bacteria (Daykin et al., 2005; Meselhy et al., 1997; Schantz et al., 2010; Soni, 2012; Yoshida and Yamada, 1985). Brewed black tea was reported to contain 17 nM gallic acid (Kanwal et al., 2009). Brewed green tea contained 35 nM pyrogallol and 73 nM gallic acid (Kanwal et al., 2009). Brewed coffee contained 75 nM pyrogallic acid (Kanwal et al., 2009). Dried green tea leaves contained pyrogallol at the concentration of 133–418 mg/g and gallic acid at 1–13 mg/g (Hussain et al., 2008). In dried black tea leaves, the concentration of pyrogallol was 60–171 mg/g, and that of gallic acid, 7–17 mg/g (Hussain et al., 2008).
DNA-breakage by phenolic compounds, including trihydroxyphenols and flavonoids, is widely reported, although the literature emphasized in vitro studies of purified reaction components and the generation of reactive oxygen species (Kawanishi et al., 1989; Kim et al., 2008; Lee et al., 1995; Said Ahmad et al., 1992; Yamada et al., 1985). Pyrogallol is also reported to induce mutations in some strains of Salmonella (NTP, 2012), to induce chromosomal aberrations in CHO cells (Stich et al., 1981), cultured human lymphocytes (NIEHS, 1998), and bone marrow cells in vivo in mice (NIEHS, 1998), to induce micronuclei and sister chromatid exchanges in V79 cells (Glatt et al., 1989), to induce mutations in L5178Y mouse lymphoma cells (NIEHS, 1998), and to increase the frequency of micronucleated polychromatic erythrocytes in mice (Gocke et al., 1981). Its clastogenic potential is thought to follow an order wherein trihydroxylated phenols are more active than dihydroxylated, dihydroxylated more than monohydroxylated phenols (Stich et al., 1981). Pyrogallol does not require a metal ion catalyst to produce ·OH radicals in vitro. It is a more potent generator of hydrogen peroxide in vitro than other polyphenols (pyrogallol > 1,2,4-benzenetriol > hydroquinone > catechol) (Lee and Lin, 1994; NTP, 2012). Pyrogallol-like compounds are reported to stimulate neoplasia and other hyperproliferative lesions. 3-methyoxycatechol in the drinking water induced little gastric hyperplasia, but greatly enhanced hyperplasia and papilloma formation when sodium nitrite was co-administered with 3-methyoxycatechol (Hirose et al., 1990). 3-methyoxycatechol also promoted esophageal carcinogenesis and fore-stomach carcinogenesis, the latter being augmented with sodium nitrite co-administration (Hirose et al., 1993). Pyrogallol and related structures thus may be a major DNA-damaging entity in the diet and upon smoke inhalation. Cancer and other diseases of aging are often seen as a product of our modern industrial age (Dunn, 2012). Dietary changes have included higher consumption of such beverages as tea and coffee, resulting in enhanced exposure to pyrogallol-like clastogenic substances. Speculatively, population differences in any resulting disease risks could reflect differences in diet, gut bacterial flora, and bowel transit time. Specific areas of interest, such as the possible protective effects of individual foods such as green tea, are not addressed directly by our study. The properties detected by p53R cells were more widely distributed among foods and do not take into account any genoprotective effects or tissue differences. Our acute exposure studies also do not directly address the clinical situation of chronic exposure nor of exposures in non-gastrointestinal tissues, where blood-borne chemicals would be at low concentrations.
Our findings have practical implications. Future studies should assess whether pyrogallol and related compounds have DNA-damaging properties in the concentrations and forms typically encountered by humans and investigate the physiology used by the gastrointestinal mucosa and other cells to limit DNA damage from pyrogallol-like activities. One wonders whether there are molecular signatures of exposure in tissues, especially in terms of mutation fixation and any effect on human tumorigenesis. It is feasible to survey whether other major food substances contain similar DNA-damaging activities. For example, one could examine naturally smoked foods for similar DNA-damaging activity. It will be helpful to determine whether the variation in p53R assay results among otherwise similar foods (such as different brands) can be explained using quantitative analysis of the candidate chemicals implicated here. If the DNA-damaging activities of liquid smoke were thought to be deleterious, it might be possible to replace liquid smoke with other, safer, smoky substances. One might identify constituents of liquid smoke missed in earlier studies due to choice of solvents. It might also be advisable to measure the intake of pyrogallol-like substances as well as their levels in urine and serum samples in epidemiologic studies. Methods have been developed to monitor ingested exposure by measuring the amount of pyrogallol or its conjugates in urine and serum samples, and have found application as a diagnostic marker for oak toxicosis in cattle (Bakke, 1970; NTP, 2012; Tor et al., 1996).
Together with our earlier studies (Cunningham et al., 2004; Gallmeier et al., 2005; Sohn et al., 2002), especially our use of the p53R assay quantitatively on tens of thousands of compounds (Sohn et al., 2002), this report presents future directions for genotoxicity screening and further establishes the utility and informativeness of biological assays as a screening method for environmental exposures. The ToxCast Program of the U.S. Environmental Protection Agency also uses cell lines incorporating multiple potential signals. This strategy was employed semi-quantitatively and qualitatively on about three hundred compounds (Judson et al., 2010; Knight et al., 2009) and is currently being used to analyze compounds to prioritize them for further testing under the Endocrine Disruption Screening Program (Mahadevan et al., 2011). Future cellular toxicological screens of foods and flavorings could be augmented with chemical characterization, fractionation, and testing of constituent chemicals to define the major sources of DNA strand-breaking activity in food and flavoring. Pyrogallol-related compounds are likely to dominate the assay results in foods and flavorings containing them, with flavonoids providing widespread but less intense activities. Such a knowledge base may prove useful in guiding studies of epidemiologic risks of age-related diseases and cancer that would address DNA strand-breaking chemicals in diets.
Highlights.
We used a cellular biological (p53R) assay to screen for DNA-damaging activity in selected foods and flavorings.
Potent DNA-damaging activity was seen in tea, coffee, and liquid smoke at concentrations consumed dietarily.
DNA double-strand breaks were confirmed by the comet assay and immunoblots reporting H2AX phosphorylation.
Pyrogallol-like substances are likely to be a major source of genotoxic activity in food and flavoring.
These findings have implications for evaluation of dietary risks in epidemiologic studies and in designing safer diets.
Acknowledgments
This work was supported by the National Institutes of Health grant CA62924 and the Everett and Marjorie Kovler Professorship in Pancreas Cancer Research. We appreciated ongoing discussions with Drs. James Harnly and Pei Chen of the United States Department of Agriculture Agricultural Research Service and with Dr. Surojit Sur of our institution.
Abbreviations
- γ-H2AX
phosphorylated histone protein H2AX
- 5-FU
fluorouracil
- ATM
ataxia telangiectasia mutated
- ATR
ataxia telangiectasia and Rad3-related protein
- CHO
Chinese hamster ovary
- CMV
cytomegalovirus
- DMEM
Dulbecco’s modified Eagle’s medium
- DMSO
dimethyl sulfoxide
- EGCG
epigallocatechin-3-gallate
- FBS
fetal bovine serum
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- PVDF
polyvinylidene fluoride
- SAR
structure-activity relationship
- SDS
sodium dodecyl sulfate
- SEM
standard error of the mean
- TBE
Tris/Borate/EDTA
- TSA
trichostatin A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bakke OM. O-methylation of simple phenols in the rat. Acta Pharmacol Toxicol (Copenh) 1970;28:28–38. doi: 10.1111/j.1600-0773.1969.tb00526.x. [DOI] [PubMed] [Google Scholar]
- Bandele OJ, Osheroff N. (−)-Epigallocatechin gallate, a major constituent of green tea, poisons human type II topoisomerases. Chem Res Toxicol. 2008;21:936–943. doi: 10.1021/tx700434v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenbaum MC. A method for testing for synergy with any number of agents. J Infect Dis. 1978;137:122–130. doi: 10.1093/infdis/137.2.122. [DOI] [PubMed] [Google Scholar]
- Birt DF, Hendrich S, Wang W. Dietary agents in cancer prevention: flavonoids and isoflavonoids. Pharmacol Ther. 2001;90:157–177. doi: 10.1016/s0163-7258(01)00137-1. [DOI] [PubMed] [Google Scholar]
- Braun AG, Busby WF, Jr, Jackman J, Halpin PA, Thilly WG. Commercial hickory-smoke flavouring is a human lymphoblast mutagen but does not induce lung adenomas in newborn mice. Food Chem Toxicol. 1987;25:331–335. doi: 10.1016/0278-6915(87)90131-1. [DOI] [PubMed] [Google Scholar]
- Burkitt MJ, Milne L, Nicotera P, Orrenius S. 1,10-Phenanthroline stimulates internucleosomal DNA fragmentation in isolated rat-liver nuclei by promoting the redox activity of endogenous copper ions. Biochem J. 1996;313 (Pt 1):163–169. doi: 10.1042/bj3130163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P. Preparation of ligand-free human serum for radioimmunoassay by adsorption on activated charcoal. Clin Chem. 1978;24:362–364. [PubMed] [Google Scholar]
- Chan DS, Lau R, Aune D, Vieira R, Greenwood DC, Kampman E, Norat T. Red and processed meat and colorectal cancer incidence: meta-analysis of prospective studies. PLoS One. 2011;6:e20456. doi: 10.1371/journal.pone.0020456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedula VSP, Prakash I. The aroma, taste, color and bioactive constituents of tea. Journal of Medicinal Plants Research. 2011;5:2110–2124. [Google Scholar]
- Cunningham SC, Ryu B, Sohn TA, Kern SE. Nonspecific enhancement of gene expression by compounds identified in high-throughput cell-based screening. Biotechniques. 2004;37:120–122. doi: 10.2144/04371DD01. [DOI] [PubMed] [Google Scholar]
- Daykin CA, Van Duynhoven JP, Groenewegen A, Dachtler M, Van Amelsvoort JM, Mulder TP. Nuclear magnetic resonance spectroscopic based studies of the metabolism of black tea polyphenols in humans. J Agric Food Chem. 2005;53:1428–1434. doi: 10.1021/jf048439o. [DOI] [PubMed] [Google Scholar]
- Dunn B. Cancer: Solving an age-old problem. Nature. 2012;483:S2–6. doi: 10.1038/483S2a. [DOI] [PubMed] [Google Scholar]
- Fiddler W, Doerr RC, Wasserma Ae. Composition of an Ether-Soluble Fraction of a Liquid Smoke Solution. Journal of Agricultural and Food Chemistry. 1970;18:310. [Google Scholar]
- Fiddler W, Doerr RC, Wasserma Ae, Salay JM. Composition of Hickory Sawdust Smoke. Furans and Phenols. Journal of Agricultural and Food Chemistry. 1966;14:659. [Google Scholar]
- Gallmeier E, Winter JM, Cunningham SC, Kahn SR, Kern SE. Novel genotoxicity assays identify norethindrone to activate p53 and phosphorylate H2AX. Carcinogenesis. 2005;26:1811–1820. doi: 10.1093/carcin/bgi132. [DOI] [PubMed] [Google Scholar]
- Gasparian AV, Burkhart CA, Purmal AA, Brodsky L, Pal M, Saranadasa M, Bosykh DA, Commane M, Guryanova OA, Pal S, Safina A, Sviridov S, Koman IE, Veith J, Komar AA, Gudkov AV, Gurova KV. Curaxins: anticancer compounds that simultaneously suppress NF-kappaB and activate p53 by targeting FACT. Sci Transl Med. 2011;3:95ra74. doi: 10.1126/scitranslmed.3002530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geva-Zatorsky N, Rosenfeld N, Itzkovitz S, Milo R, Sigal A, Dekel E, Yarnitzky T, Liron Y, Polak P, Lahav G, Alon U. Oscillations and variability in the p53 system. Mol Syst Biol. 2006;2:2006 0033. doi: 10.1038/msb4100068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatt H, Padykula R, Berchtold GA, Ludewig G, Platt KL, Klein J, Oesch F. Multiple activation pathways of benzene leading to products with varying genotoxic characteristics. Environ Health Perspect. 1989;82:81–89. doi: 10.1289/ehp.898281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gocke E, King MT, Eckhardt K, Wild D. Mutagenicity of cosmetics ingredients licensed by the European Communities. Mutat Res. 1981;90:91–109. doi: 10.1016/0165-1218(81)90072-0. [DOI] [PubMed] [Google Scholar]
- Guillen MD, Manzanos MJ, Zabala L. Study of a Commercial Liquid Smoke Flavoring by Means of Gas-Chromatography Mass-Spectrometry and Fourier-Transform Infrared-Spectroscopy. Journal of Agricultural and Food Chemistry. 1995;43:463–468. [Google Scholar]
- Gurova KV, Hill JE, Guo C, Prokvolit A, Burdelya LG, Samoylova E, Khodyakova AV, Ganapathi R, Ganapathi M, Tararova ND, Bosykh D, Lvovskiy D, Webb TR, Stark GR, Gudkov AV. Small molecules that reactivate p53 in renal cell carcinoma reveal a NF-kappaB-dependent mechanism of p53 suppression in tumors. Proc Natl Acad Sci U S A. 2005;102:17448–17453. doi: 10.1073/pnas.0508888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnly JM, Doherty RF, Beecher GR, Holden JM, Haytowitz DB, Bhagwat S, Gebhardt S. Flavonoid content of U.S. fruits, vegetables, and nuts. J Agric Food Chem. 2006;54:9966–9977. doi: 10.1021/jf061478a. [DOI] [PubMed] [Google Scholar]
- Hayakawa F, Kimura T, Maeda T, Fujita M, Sohmiya H, Fujii M, Ando T. DNA cleavage reaction and linoleic acid peroxidation induced by tea catechins in the presence of cupric ion. Biochim Biophys Acta. 1997;1336:123–131. doi: 10.1016/s0304-4165(97)00019-6. [DOI] [PubMed] [Google Scholar]
- Hirose M, Fukushima S, Hasegawa R, Kato T, Tanaka H, Ito N. Effects of sodium nitrite and catechol or 3-methoxycatechol in combination on rat stomach epithelium. Jpn J Cancer Res. 1990;81:857–861. doi: 10.1111/j.1349-7006.1990.tb02657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose M, Tanaka H, Takahashi S, Futakuchi M, Fukushima S, Ito N. Effects of sodium nitrite and catechol, 3-methoxycatechol, or butylated hydroxyanisole in combination in a rat multiorgan carcinogenesis model. Cancer Res. 1993;53:32–37. [PubMed] [Google Scholar]
- Hruza DE, Praag MV, Heinsohn H. Isolation and Identification of Components of Tar of Hickory Wood Smoke. Journal of Agricultural and Food Chemistry. 1974;22:123–126. [Google Scholar]
- Hussain I, Khan H, Saleem M, Marwat GA, Iqbal Y. Investigation of organic constituents of commercial tea brands and fresh tea leaves by high-performance liquid chromatography. Journal of the Chemical Society of Pakistan. 2008;30:571–576. [Google Scholar]
- Issenber P, Kornreic, Lustre AO. Recovery of Phenolic Wood Smoke Components from Smoked Foods and Model Systems. Journal of Food Science. 1971;36:107. [Google Scholar]
- Judson RS, Houck KA, Kavlock RJ, Knudsen TB, Martin MT, Mortensen HM, Reif DM, Rotroff DM, Shah I, Richard AM, Dix DJ. In vitro screening of environmental chemicals for targeted testing prioritization: the ToxCast project. Environ Health Perspect. 2010;118:485–492. doi: 10.1289/ehp.0901392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwal S, Fu XH, Su XG. Highly sensitive flow-injection chemiluminescence determination of pyrogallol compounds. Spectrochimica Acta Part a-Molecular and Biomolecular Spectroscopy. 2009;74:1046–1049. doi: 10.1016/j.saa.2009.08.047. [DOI] [PubMed] [Google Scholar]
- Kapadia GJ, Paul BD, Chung EB, Ghosh B, Pradhan SN. Carcinogenicity of Camellia sinensis (tea) and some tannin-containing folk medicinal herbs administered subcutaneously in rats. J Natl Cancer Inst. 1976;57:207–209. doi: 10.1093/jnci/57.1.207. [DOI] [PubMed] [Google Scholar]
- Kastan MB, Onyekwere O, Sidransky D, Vogelstein B, Craig RW. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991;51:6304–6311. [PubMed] [Google Scholar]
- Kawanishi S, Inoue S, Kawanishi M. Human DNA damage induced by 1,2,4-benzenetriol, a benzene metabolite. Cancer Res. 1989;49:164–168. [PubMed] [Google Scholar]
- Kern SE. Consensus DNA sequence bound by p53. The FASEB Conference; Saxtons River, VT. 1991. [Google Scholar]
- Kern SE, Kinzler KW, Bruskin A, Jarosz D, Friedman P, Prives C, Vogelstein B. Identification of p53 as a sequence-specific DNA-binding protein. Science. 1991;252:1708–1711. doi: 10.1126/science.2047879. [DOI] [PubMed] [Google Scholar]
- Khan NS, Hadi SM. Structural features of tannic acid important for DNA degradation in the presence of Cu(II) Mutagenesis. 1998;13:271–274. doi: 10.1093/mutage/13.3.271. [DOI] [PubMed] [Google Scholar]
- Kim K, Kurata T, Fujimaki M. Studies on Smoke Flavor .2. Identification of Flavor Constituents in Carbonyl, Noncarbonyl Neutral and Basic Fractions of Aqueous Smoke Condensates. Agricultural and Biological Chemistry. 1974;38:53–63. [Google Scholar]
- Kim SW, Han YW, Lee ST, Jeong HJ, Kim SH, Kim IH, Lee SO, Kim DG, Kim SZ, Park WH. A superoxide anion generator, pyrogallol, inhibits the growth of HeLa cells via cell cycle arrest and apoptosis. Mol Carcinog. 2008;47:114–125. doi: 10.1002/mc.20369. [DOI] [PubMed] [Google Scholar]
- Kirby KS. Induction of tumours by tannin extracts. Br J Cancer. 1960;14:147–150. doi: 10.1038/bjc.1960.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight AW, Little S, Houck K, Dix D, Judson R, Richard A, McCarroll N, Akerman G, Yang C, Birrell L, Walmsley RM. Evaluation of high-throughput genotoxicity assays used in profiling the US EPA ToxCast chemicals. Regul Toxicol Pharmacol. 2009;55:188–199. doi: 10.1016/j.yrtph.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Knowles ME, Gilbert J, MacWeeny DJ. Phenols in smoked, cured meats: nitrosation of phenols in liquid smokes and in smoked bacon. J Sci Food Agric. 1975;26:267–276. doi: 10.1002/jsfa.2740260306. [DOI] [PubMed] [Google Scholar]
- Lang R, Mueller C, Hofmann T. Development of a stable isotope dilution analysis with liquid chromatography-tandem mass spectrometry detection for the quantitative analysis of di- and trihydroxybenzenes in foods and model systems. J Agric Food Chem. 2006;54:5755–5762. doi: 10.1021/jf061118n. [DOI] [PubMed] [Google Scholar]
- Lee SF, Liang YC, Lin JK. Inhibition of 1,2,4-benzenetriol-generated active oxygen species and induction of phase II enzymes by green tea polyphenols. Chem Biol Interact. 1995;98:283–301. doi: 10.1016/0009-2797(95)03652-0. [DOI] [PubMed] [Google Scholar]
- Lee SF, Lin JK. Generation of Hydrogen Peroxide, Superoxide Anion and the Hydroxyl Free Radical from Polyphenols and Active Benzene Metabolites: Their Possible Role in Mutagenesis. J Biomed Sci. 1994;1:125–130. doi: 10.1007/BF02257986. [DOI] [PubMed] [Google Scholar]
- Lopez-Lazaro M, Calderon-Montano JM, Burgos-Moron E, Austin CA. Green tea constituents (−)-epigallocatechin-3-gallate (EGCG) and gallic acid induce topoisomerase I- and topoisomerase II-DNA complexes in cells mediated by pyrogallol-induced hydrogen peroxide. Mutagenesis. 2011;26:489–498. doi: 10.1093/mutage/ger006. [DOI] [PubMed] [Google Scholar]
- Lopez-Lazaro M, Willmore E, Austin CA. Cells lacking DNA topoisomerase II beta are resistant to genistein. J Nat Prod. 2007a;70:763–767. doi: 10.1021/np060609z. [DOI] [PubMed] [Google Scholar]
- Lopez-Lazaro M, Willmore E, Jobson A, Gilroy KL, Curtis H, Padget K, Austin CA. Curcumin induces high levels of topoisomerase I- and II-DNA complexes in K562 leukemia cells. J Nat Prod. 2007b;70:1884–1888. doi: 10.1021/np070332i. [DOI] [PubMed] [Google Scholar]
- Lorenz M, Urban J, Engelhardt U, Baumann G, Stangl K, Stangl V. Green and black tea are equally potent stimuli of NO production and vasodilation: new insights into tea ingredients involved. Basic Res Cardiol. 2009;104:100–110. doi: 10.1007/s00395-008-0759-3. [DOI] [PubMed] [Google Scholar]
- Lustre AO, Issenber P. Volatile Components of Hardwood Sawdust Smoke - Components of Phenolic Fraction. Journal of Agricultural and Food Chemistry. 1969;17:1387. [Google Scholar]
- Mahadevan B, Snyder RD, Waters MD, Benz RD, Kemper RA, Tice RR, Richard AM. Genetic toxicology in the 21st century: reflections and future directions. Environ Mol Mutagen. 2011;52:339–354. doi: 10.1002/em.20653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovits J, Linassier C, Fosse P, Couprie J, Pierre J, Jacquemin-Sablon A, Saucier JM, Le Pecq JB, Larsen AK. Inhibitory effects of the tyrosine kinase inhibitor genistein on mammalian DNA topoisomerase II. Cancer Res. 1989;49:5111–5117. [PubMed] [Google Scholar]
- Meier D. Additives: Smoke Flavorings. In: LMLaT, Nollet F, editors. Handbook of Processed Meats and Poultry Analysis. CRC Press; 2008. pp. 109–128. [Google Scholar]
- Meselhy MR, Nakamura N, Hattori M. Biotransformation of (−)-epicatechin 3-O-gallate by human intestinal bacteria. Chem Pharm Bull (Tokyo) 1997;45:888–893. doi: 10.1248/cpb.45.888. [DOI] [PubMed] [Google Scholar]
- Mitsuhashi S, Saito A, Nakajima N, Shima H, Ubukata M. Pyrogallol structure in polyphenols is involved in apoptosis-induction on HEK293T and K562 cells. Molecules. 2008;13:2998–3006. doi: 10.3390/molecules13122998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton JF. Tannin and oesophageal cancer. Lancet. 1987;2:327–328. doi: 10.1016/s0140-6736(87)90908-1. [DOI] [PubMed] [Google Scholar]
- Morton JF. Widespread Tannin Intake Via Stimulants and Masticatories, Especially Guarana, Kola Nut, Betel Vine, and Accessories. Plant Polyphenols : Synthesis, Properties, Significance. 1992;59:739–765. doi: 10.1007/978-1-4615-3476-1_45. [DOI] [PubMed] [Google Scholar]
- Muller C, Lang R, Hofmann T. Quantitative precursor studies on di- and trihydroxybenzene formation during coffee roasting using “in bean” model experiments and stable isotope dilution analysis. J Agric Food Chem. 2006;54:10086–10091. doi: 10.1021/jf062727y. [DOI] [PubMed] [Google Scholar]
- NIEHS. Pyrogallol: Review of Toxicological Literature. National Institute of Environmental Health Sciences, National Institutes of Health; 1998. [Google Scholar]
- NTP. NTP Technical Report on the Toxicology and Carcinogenesis Studies of Pyrogallol in F344/N Rats and B6C3F1/N Mice. National Toxicology Program, National Institues of Health; 2012. [Google Scholar]
- Ohshima H, Friesen M, Malaveille C, Brouet I, Hautefeuille A, Bartsch H. Formation of direct-acting genotoxic substances in nitrosated smoked fish and meat products: identification of simple phenolic precursors and phenyldiazonium ions as reactive products. Food Chem Toxicol. 1989a;27:193–203. doi: 10.1016/0278-6915(89)90069-0. [DOI] [PubMed] [Google Scholar]
- Ohshima H, Furihata C, Matsushima T, Bartsch H. Evidence of potential tumour-initiating and tumour-promoting activities of hickory smoke condensate when given alone or with nitrite to rats. Food Chem Toxicol. 1989b;27:511–516. doi: 10.1016/0278-6915(89)90046-x. [DOI] [PubMed] [Google Scholar]
- Putnam KP, Bombick DW, Avalos JT, Doolittle DJ. Comparison of the cytotoxic and mutagenic potential of liquid smoke food flavourings, cigarette smoke condensate and wood smoke condensate. Food Chem Toxicol. 1999;37:1113–1118. doi: 10.1016/s0278-6915(99)00104-0. [DOI] [PubMed] [Google Scholar]
- Ramos S. Cancer chemoprevention and chemotherapy: dietary polyphenols and signalling pathways. Mol Nutr Food Res. 2008;52:507–526. doi: 10.1002/mnfr.200700326. [DOI] [PubMed] [Google Scholar]
- Saeki K, Hayakawa S, Isemura M, Miyase T. Importance of a pyrogallol-type structure in catechin compounds for apoptosis-inducing activity. Phytochemistry. 2000;53:391–394. doi: 10.1016/s0031-9422(99)00513-0. [DOI] [PubMed] [Google Scholar]
- Said Ahmad M, Fazal F, Rahman A, Hadi SM, Parish JH. Activities of flavonoids for the cleavage of DNA in the presence of Cu(II): correlation with generation of active oxygen species. Carcinogenesis. 1992;13:605–608. doi: 10.1093/carcin/13.4.605. [DOI] [PubMed] [Google Scholar]
- Santarelli RL, Pierre F, Corpet DE. Processed meat and colorectal cancer: a review of epidemiologic and experimental evidence. Nutr Cancer. 2008;60:131–144. doi: 10.1080/01635580701684872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkaria JN, Busby EC, Tibbetts RS, Roos P, Taya Y, Karnitz LM, Abraham RT. Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 1999;59:4375–4382. [PubMed] [Google Scholar]
- Schantz M, Erk T, Richling E. Metabolism of green tea catechins by the human small intestine. Biotechnol J. 2010;5:1050–1059. doi: 10.1002/biot.201000214. [DOI] [PubMed] [Google Scholar]
- Shichino Y, Tatematsu M, Ohshima H, Bartsch H, Furihata C, Ito N. Effects of hickory-smoke condensate on development of pepsinogen 1-altered pyloric glands in rats. Food Chem Toxicol. 1992;30:859–864. doi: 10.1016/0278-6915(92)90051-l. [DOI] [PubMed] [Google Scholar]
- Snyder RD, Arnone MR. Putative identification of functional interactions between DNA intercalating agents and topoisomerase II using the V79 in vitro micronucleus assay. Mutat Res. 2002;503:21–35. doi: 10.1016/s0027-5107(02)00028-3. [DOI] [PubMed] [Google Scholar]
- Sohn TA, Bansal R, Su GH, Murphy KM, Kern SE. High-throughput measurement of the Tp53 response to anticancer drugs and random compounds using a stably integrated Tp53-responsive luciferase reporter. Carcinogenesis. 2002;23:949–957. doi: 10.1093/carcin/23.6.949. [DOI] [PubMed] [Google Scholar]
- Soni M, Sharma KP, John PJ. Characterization of Pyrogallol Production from Gallic Acid by Enterobacter spp. Journal of Microbiology and Biotechnology Research. 2012;2:327–336. [Google Scholar]
- Srivastava JK, Gupta S. Antiproliferative and apoptotic effects of chamomile extract in various human cancer cells. J Agric Food Chem. 2007;55:9470–9478. doi: 10.1021/jf071953k. [DOI] [PubMed] [Google Scholar]
- Sternitzke A, Legrum W, Netter KJ. Effects of phenolic smoke condensates and their components on hepatic drug metabolizing systems. Food Chem Toxicol. 1992;30:771–781. doi: 10.1016/0278-6915(92)90079-z. [DOI] [PubMed] [Google Scholar]
- Stich HF, Rosin MP, Wu CH, Powrie WD. The action of transition metals on the genotoxicity of simple phenols, phenolic acids and cinnamic acids. Cancer Lett. 1981;14:251–260. doi: 10.1016/0304-3835(81)90151-8. [DOI] [PubMed] [Google Scholar]
- Strick R, Strissel PL, Borgers S, Smith SL, Rowley JD. Dietary bioflavonoids induce cleavage in the MLL gene and may contribute to infant leukemia. Proc Natl Acad Sci U S A. 2000;97:4790–4795. doi: 10.1073/pnas.070061297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tor ER, Francis TM, Holstege DM, Galey FD. GC/MS determination of pyrogallol and gallic acid in biological matrices as diagnostic indicators of oak exposure. Journal of Agricultural and Food Chemistry. 1996;44:1275–1279. [Google Scholar]
- Wang W, El-Deiry WS. Bioluminescent molecular imaging of endogenous and exogenous p53-mediated transcription in vitro and in vivo using an HCT116 human colon carcinoma xenograft model. Cancer Biol Ther. 2003;2:196–202. doi: 10.4161/cbt.2.2.347. [DOI] [PubMed] [Google Scholar]
- Wang W, Ho WC, Dicker DT, MacKinnon C, Winkler JD, Marmorstein R, El-Deiry WS. Acridine derivatives activate p53 and induce tumor cell death through Bax. Cancer Biol Ther. 2005;4:893–898. doi: 10.4161/cbt.4.8.2134. [DOI] [PubMed] [Google Scholar]
- Yamada K, Shirahata S, Murakami H, Nishiyama K, Shinohara K, Omura H. DNA Breakage by Phenyl Compounds. Agricultural and Biological Chemistry. 1985;49:1423–1428. [Google Scholar]
- Yin J, Zhang YM, Yang L, Li HD, Xue ZF. Effective inhibition of specific gene by adeno-associated virus (AAV)-mediated expression of small interfering RNA. African Journal of Biotechnology. 2011;10:2844–2849. [Google Scholar]
- Yoshida H, Yamada H. Microbial-Production of Pyrogallol through Decarboxylation of Gallic Acid. Agricultural and Biological Chemistry. 1985;49:659–663. [Google Scholar]