Abstract
Objective
Posttraumatic epilepsy is prevalent, often difficult to manage, and currently cannot be prevented. While cooling is broadly neuroprotective, cooling-induced prevention of chronic spontaneous recurrent seizures has never been demonstrated. We examined the effect of mild passive focal cooling of the perilesional neocortex on the development of neocortical epileptic seizures after head injury in the rat.
Methods
Rostral parasagittal fluid percussion injury in rats reliably induces a perilesional, neocortical epileptic focus within weeks after injury. Epileptic seizures were assessed by 5-electrode video-electrocorticography (ECoG) 2–16 weeks post-injury. Focal cooling was induced with ECoG headsets engineered for calibrated passive heat dissipation. Pathophysiology was assessed by GFAP immunostaining, cortical sclerosis, gene expression of inflammatory cytokines IL-1α and IL-1β, and ECoG spectral analysis. All animals were formally randomized to treatment groups and data were analyzed blind.
Results
Cooling by 0.5–2°C inhibited the onset of epileptic seizures in a dose dependent fashion. The treatment induced no additional pathology or inflammation, and normalized the power spectrum of stage N2 sleep. Cooling by 2°C for 5.5 weeks beginning 3 days after injury virtually abolished ictal activity. This effect persisted through the end of the study, over ten weeks after cessation of cooling. Rare remaining seizures were shorter than in controls.
Interpretation
These findings demonstrate potent and persistent prevention and modification of epileptic seizures after head injury with a cooling protocol that is neuroprotective, compatible with the care of head-injury patients, and conveniently implemented. The required cooling can be delivered passively without Peltier cells or electrical power.
Keywords: trauma, epileptogenesis, pharmacoresistance, prophylaxis, hypothermia
INTRODUCTION
Post-traumatic epilepsy (PTE) complicates up to 75% of traumatic brain injuries (TBIs) depending on injury type, severity, the presence of risk factors such as acute seizures, and the duration of follow-up1–3. It is the leading cause of acquired epilepsy in young adults, and tends to be difficult to manage4,5. The mechanisms of posttraumatic epileptogenesis are unknown and there is currently no clinical intervention to cure PTE, prevent it, or even limit its severity6,7.
Indirect evidence suggests that brain cooling should be investigated for PTE prophylaxis. Hypothermia is neuroprotective after a variety of brain insults8–11, and suppresses seizures12. In animals, focal cooling inhibits electrically-induced kindling13, and head cooling acutely after head injury chronically elevates the threshold for chemically induced seizures14.
However, chronic spontaneous recurrent seizures (CSRSs) are the hallmark of epilepsy, and it is unknown whether a well tolerated magnitude of cooling, applied at a clinically feasible interval after brain injury, can prevent their development. The rostral parasagittal fluid percussion injury (rpFPI) model of PTE is ideal to investigate the issue. rpFPI is mechanically identical to human contusive closed head injury, reproduces key histopathological and pathophysiological sequelae15, and reliably results, within weeks after injury, in a high incidence of PTE with frequent focal seizures16–19. Before progressing to dual pathology months after injury, rpFPI-induced PTE first appears as frontal-lobe neocortical focal seizures, resembling frontal-lobe seizures common in human PTE18–22. In addition, this model is well characterized with respect to rat-to-rat and measurement-to-measurement variability in seizure frequency23, and statistical power analyses for the detection of CSRS prevention are available to optimize preclinical studies24.
We used formal randomization and blind analyses to examine the effects of continuous mild focal cooling on CSRSs and brain pathophysiology. We found that CSRSs could be potently and persistently prevented after rpFPI by a time-limited cooling of the perilesional neocortex by just 2°C. This small amount of cooling was achieved by passive heat dissipation, without Peltier cells or electrical power. This treatment is practical and compatible with the acute treatment of head injury patients.
METHODS
All procedures were approved by the University of Washington Institutional Animal Care and Use Committee. Experiments were designed randomized and analyzed by personnel kept blind to treatment and time point. The experimental methods and the design, construction and heat dissipation properties of the passive cooling headsets are described in detail in Supplementary Methods.
Briefly, Sprague-Dawley male rats (32–26 days old) were mechanically ventilated under halothane anesthesia, and a 3 mm diameter craniotomy was centered 2 mm posterior to Bregma, 3 mm from the midline. Animals were disconnected from the ventilator, administered an 8 ms pressure pulse (3.4 or 3.7 atm) through the FPI device, and ventilation resumed after a uniform 10s interval of posttraumatic apnea. Acute mortality after rpFPI was 9%. Epidural electrodes and exchangeable cooling or mock-cooling elements were incorporated into acrylic headsets that were securely anchored to the skull. Five epidural electrodes were implanted using procedures designed to avoid damage to the underlying neocortex. The entire assembly including cooling element, electrodes and connector pedestal was encased in dental acrylic and adhered to the skull. Heat dissipating properties of the headset were evaluated by temperature measurements and digital thermography.
Video-electrocorticographic (ECoG) monitoring was performed during and up to 2.5 months after termination of cooling. Each ECoG recording lasted 1 day, to fully sample the circadian cycle. Based on statistical power analyses (reference 24; Supplementary Fig. 3), recordings were acquired once or twice a week. All ECoG was manually analyzed by personnel kept blind to treatment and to time post-injury. Seizures had durations ranging from 1s to 4.5 min and were characterized by epileptiform ECoG patterns starting with visible trains of 150–250ms-long spikes, and with simultaneous stereotyped ictal behavioral changes according to a behavioral scale previously described18. Seizure frequencies and times spent seizing were determined for each rat for each week of recording. These data were logarithmically transformed prior to secondary analysis to maximize statistical power to detect treatment effects23,24. In one experiment, headsets were actively re-warmed to body temperature and video-ECoG recordings performed for 6–7 hours to examine whether CSRSs rebounded quickly.
Pathology was assessed in coronal sections taken from beneath the craniotomy near Bregma -2mm. Sections were immunostained for GFAP identically and together, using standard methods. Measurements of cortical thickness or GFAP optical density were performed blind with the aid of ImageJ software. Expression of IL-1α and IL-1β were measured blind by RT-PCR. Animals were transcardially perfused to flush the blood from the brain, and 5mm biopsy punches were rapidly collected from the perilesional and homologous contralateral cortices. RNA was extracted (RIN 9.9–10) with RNeasy extraction kit (Qiagen Inc., Valencia, CA). Gene expression assays were run using the 96.96 Gene Expression Dynamic Array™ IFC (Fluidigm, South San Francisco, CA) according to the manufacturer’s instructions. All gene expression levels were measured in triplicate and quantitated normalized to the geometric mean of the GAPDH, SDHA and HPRT1 housekeeping genes25.
Statistical analyses were conducted using SPSS 10 (SPSS Inc., Chicago, IL). Unless indicated otherwise, black symbols indicate statistically significant differences, and open symbols denote measurements that do not differ significantly from their respective controls, which are always represented in gray. Statistical significance is set at p<0.05. All data are presented as mean±SEM.
RESULTS
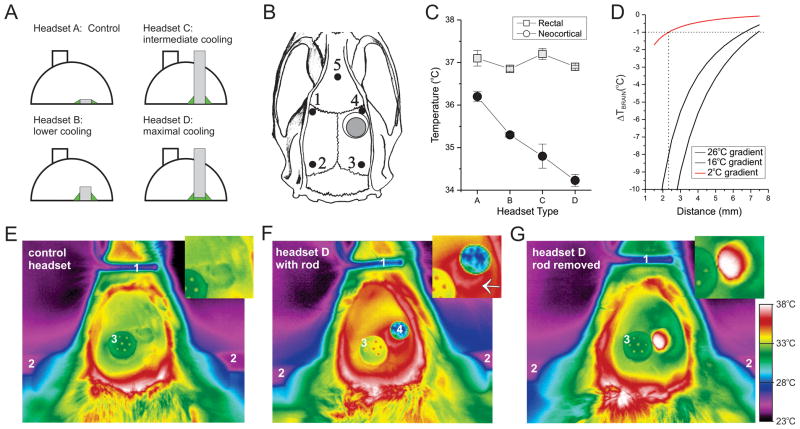
We designed different types of heat-dissipating ECoG headsets that provided graded passive cooling of the perilesional brain by up to 2°C (Fig. 1; Supplementary Figure 2). The metal elements in each headset type replace a 4mm diameter portion of the skull and enhance heat conduction from the brain to the headset (Fig. 1A). This results in warming of both the metal elements and the surrounding acrylic, increasing convective and radiant heat loss to the environment. Thermographic images show that D-type headsets were warmer than A-type control headsets (Fig. 1E–G). Headsets A–D dissipated heat (Supplementary Figure 4A–B) and cooled the neocortex (Fig. 1C) with the expected rank order efficacy without affecting systemic temperature. The temperature gradient induced by cooling the surface of the perilesional neocortex by 2°C was estimated in anesthetized rats by actively cooling the same cortical area to lower temperatures, measuring the induced temperatures at different distances, and then fitting the data and extrapolating based on the Fourier heat conduction law (Supplementary Figure 5). We found that 2°C cooling of the surface of the neocortex dissipates to <1°C within 2.3mm from the metal rod (Fig. 1D). These passive heat -dissipating ECoG headsets were used in 3 independent blind and randomized experiments.
Figure 1. Design and performance of passive focal-cooling headsets.
A) Headsets of dental acrylic incorporate a heat-dissipating piece (4mm diameter) of stainless steel placed in contact with the dura. In A-type control headsets, the metal element is a 250 μm thick disk. Steel rods, 1.5 mm (headset B) and 15 mm (headsets C and D) in length enhance the cooling capabilities of the headsets. The performance of D-type headsets is further enhanced by a thin layer of biocompatible silicone (green) that increases thermal contact with the brain. Metal elements are not to scale. Arrows indicate locations where the heat source is applied (red), and where temperature changes are measured (black) to estimate thermal conductance. B) Location of the 5 epidural electrodes (filled circles), the injury site (gray circle), and the metal element (hollow circle) in respect to the rat skull in all headsets. Note electrode 4 is perilesional. C) Focal neocortical cooling by different headset types in freely moving rpFPI rats. The rank order for brain cooling is the same as that for heat dissipation shown in Supplementary Figure 4B. Body temperature is unaffected by headset type. Statistical significance is color coded: black filled symbols indicate a statistically significant difference from the matched rectal temperature (gray symbols). D) Spatial extent of passive brain cooling by 2°C (red line) extrapolated from measurements of actively cooled rats (Supplementary Figure 5), and assuming that brain cooling dissipates as per the Fourier heat conduction law with the inverse of the distance as found for active cooling. E–G) Digital thermography (top view) of an acrylic control headset (E), a D-type headset (F), and a D-type headset minutes after removal of the cooling element (G). Insets in E–G are close-ups of the headsets above the injury sites. Note that the acrylic surface of the control headset is warmer than ambient temperature but cooler than the D-type headset. Brain heat is more efficiently dissipated to the acrylic and the environment by a D-type headset. F, inset) note the ring of warm acrylic at the base of the cooling rod (white arrow), demonstrating heat dissipation from the brain. G) Note the cooler acrylic headset surrounding the dura, which is at body temperature, after removal of the cooling element. Color scale applies to E–G.
Dose response of prolonged mild passive cooling on CSRSs
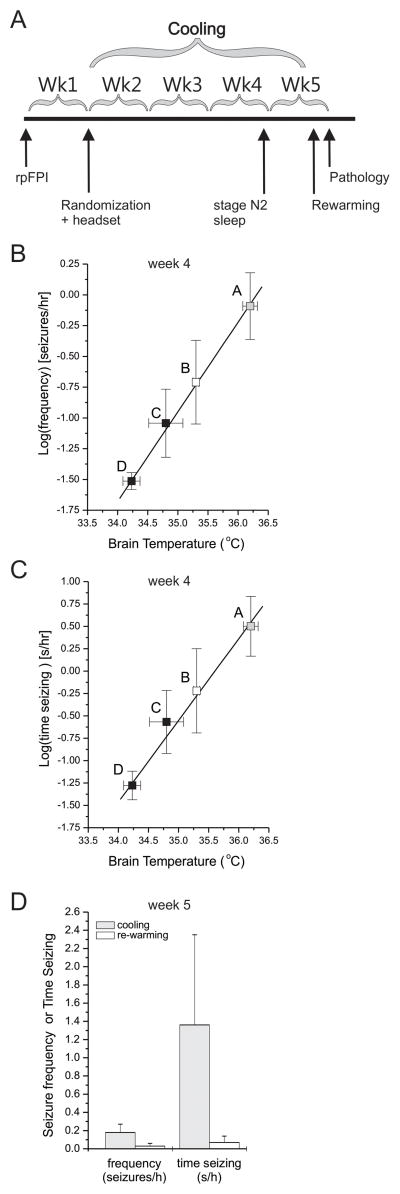
In the first experiment, the dose effect of passive focal cooling of the perilesional epileptic focus was examined with 39 rats randomized to treatment with the heat-dissipating headsets A, B, C or D from one to four weeks after 3.4atm rpFPI (Fig. 2A). One animal randomized to headset D was euthanized after a surgical error during implantation of the steel cooling rod, two animals died, and one lost the headset soon after randomization. The expected rank-order efficacy of cooling treatments (A<B<C<D) was evident by post-injury week 3 (Supplementary Figure 4C–D). At the end of the fourth week after rpFPI, rats treated with B-, C-, and D-type headsets had seizure frequencies lower by 48% (n=9; p=0.09), 81% (n=9; p=0.025) and 99% (n=8; p=0.001; Mann-Whitney), respectively, compared to controls (headset A; n=9). Times spent seizing were similarly decreased in rats with B- (p=0.10), C- (p=0.025) and D-type headsets (p=0.001; Mann-Whitney). The efficacy of the headsets in inhibiting the development of CSRSs paralleled both their thermal conductance and the magnitude of brain cooling they induced. The logarithms of seizure frequency and time spent seizing both varied linearly with perilesional brain cooling (Fig. 2B–C) and the headset thermal conductance (Supplementary Figure 4E–F).
Figure 2. Dose-effect of passive focal cooling on focal neocortical epilepsy.
A) Schematic of the experimental protocol. One week after rpFPI rats are implanted with headsets A, B, C and D, providing graded cooling up to 2°C. B–C) The log-transformed seizure frequencies (B) and times spent seizing (C) measured 4 weeks after injury both varied as linear functions of the steady state temperatures (or inversely with the magnitude of cooling) measured in the perilesional neocortices of different rats implanted with the indicated headsets. Statistical significance shown is for seizure frequency or time seizing vs headset A. Statistical significance is color coded: black filled symbols indicate a statistically significant difference from controls (gray symbols). D) Seizures do not rebound acutely after termination of cooling in epileptic rats focally cooled with headsets C and D. Seizure frequencies and times spent seizing were measured in 7h recordings obtained at the same time of day on consecutive days during cooling and active rewarming to 36.5°C.
To determine whether seizures were directly controlled by the lower temperature and would rebound quickly upon termination of cooling, 5 epileptic rats that had been treated with C- (n=4) or D-type (n=1) headsets were recorded at week 5 post-injury on two consecutive days (Fig. 2D). Time-matched on-cooling reference data were obtained on the first day. On the second day, cooling rods were actively re-warmed to body temperature with an electrically insulated resistance and calibrated current flow. No animal exhibited an increase in seizure frequency or time spent seizing during the re-warming period, and mean seizure frequencies and times seizing were nominally lower during re-warming.
Pathophysiological examination after prolonged mild passive cooling
To determine whether the prolonged cooling treatment induced pathophysiological changes, we examined GFAP immunostaining, cortical thickness, and injury-induced changes in the power spectrum of stage N2 sleep after 3–4 weeks of cooling in the same animals used for the dose response study. In addition, a second experiment was conducted to examine the gene expression of inflammatory cytokines IL-1α and IL-1β after 3 weeks of cooling. Animals (n=21) were randomized to sham-injury or rpFPI with A-type headsets or rpFPI with D-type headsets in a 1:1:1 proportion.
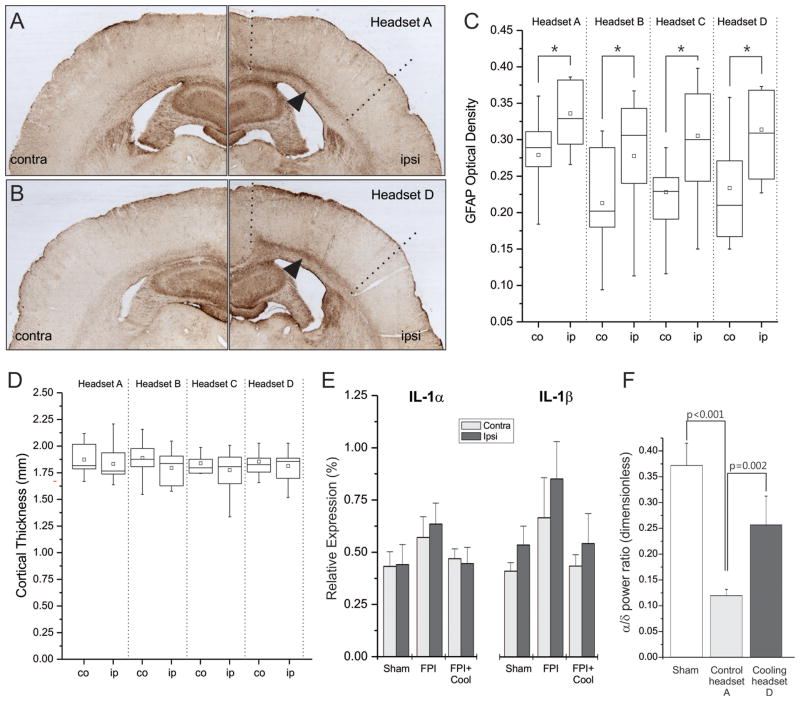
Densitometric analysis of GFAP-immunostained sections showed 4 weeks of cooling to have no effect on astroglial reactivity (Fig. 3A–C). Ipsilateral GFAP immunoreactivity was significantly elevated over contralateral levels in each treatment group, as expected for a lateral injury, but no differences were observed between cooled and untreated rpFPI rats (p=0.43, Kruskal-Wallace). Examination of neocortical thickness revealed no significant effects of cooling (Fig. 3D). No differences were observed either between ipsilateral and contralateral neocortices within each group, or between ipsilateral neocortices across groups. rpFPI significantly reduced the alpha/delta power ratio of stage N2 sleep (Headset A), compared to shams (p<0.001; Mann Whitney). Cooling Headset D significantly restored the alpha/delta power ratio compared to randomized headset-A controls (p=0.002, Mann-Whitney; Fig. 3F). This restorative effect was limited to the perilesional neocortex (Supplementary Figure 6), consistent with the induced temperature gradient (Fig. 1D).
Figure 3. Prolonged mild focal cooling does not induce pathophysiological changes.
GFAP-immunostained coronal sections obtained from injured rats treated with control headset A (A) or cooling headset D (B). In A–B, arrows indicate enhanced GFAP+ staining characteristic of the white-matter/layer VI region ipsilateral to the injury; dotted lines delimit the approximate ipsilateral regions of interest. C) Box plots summarize the blind densitometric analysis of GFAP immunostaining. Immunoreactivity was significantly greater ipsilateral to the injury in each treatment group but no treatment differences were detected. All asterisks indicate p<0.05. Hollow square=mean; Whiskers indicate max and min values. Contra=contralateral, ipsi=ipsilateral to the injury site. D) Box plots summarize the blind analysis of neocortical thickness showing no significant effects of cooling. No differences were observed either between ipsilateral and contralateral neocortices within each group, or between ipsilateral neocortices across groups. In C–D co=contralateral, ip=ipsilateral. E) Three weeks of treatment with cooling headset D do not increase inflammation of the perilesional and homologous contralateral neocortices, as demonstrated by nominally lower gene expression levels of inflammatory cytokines IL-1α and IL-1β compared to headset A controls and sham-injured animals. F) Three weeks of cooling restore the perilesional ECoG power spectrum of stage N2 sleep. Compared to sham injured animals, a reduction of the alpha/delta ratio is evident in mock-cooled (headset A) rpFPI animals. This is significantly increased in cooled (headset D) rpFPI animals relative to randomized headset A.
Gene expression levels of inflammatory cytokines IL-1α and IL-1β were nominally elevated 3.5 weeks after injury in perilesional and homologous contralateral neocortex of rpFPI animals (n=7) compared to age-matched controls (n=7). IL-1α and IL-1β gene expression in rpFPI animals treated with D-type headsets for 3 weeks was nominally lower than in headset A controls and nearly identical to the expression levels in shams (n=7; Fig. 3E).
Thus, we found no evidence for treatment-induced pathophysiological changes and, taken together, the data demonstrate that CSRS prevention is not due to damage to the neocortical epileptic focus.
CSRS prophylaxis by mild focal cooling after head injury
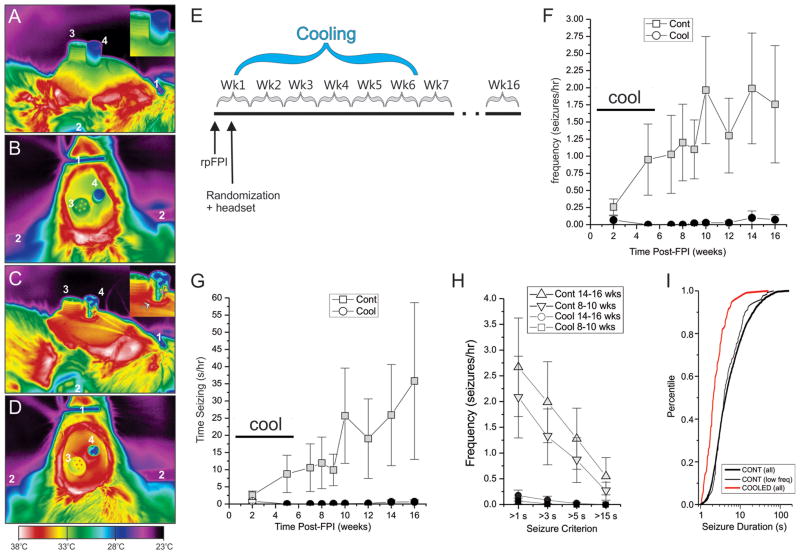
In the third experiment, twenty animals were randomized to mock-cooling or type-D cooling headsets after 3.7atm rpFPI. Cooling began 3 days after FPI and stopped 5.5 weeks later (Fig. 4E). Two animals lost their headsets at week 4 and 5 after injury, and were eliminated from the study. Animals were recorded on post-injury weeks 2 and 5, during cooling, and weeks 7, 8, 9, 10, 12, 14, 16 after termination of cooling. During cooling CSRSs were virtually abolished in treated animals, and this effect persisted for at least 10 weeks after cessation of cooling (Fig. 4F–H). The duration of the rare seizures recorded in the treated group (3.2±0.5s; n=116) in the weeks after cessation of cooling was significantly less than in controls (9.1±0.2s; n=3223; p<0.001; Mann-Whitney U). The cumulative frequency distribution of seizure duration in cooled rats was significantly left-shifted toward briefer seizures compared to controls (Fig. 4I; p<0.001, Kolmogorov-Smirnov). The shortening of seizures was confirmed with a Monte-Carlo method that assessed the likelihood that the seizures observed in the control and cooled groups could have been drawn from the same underlying distribution (Supplementary Figure 7). The aggregate effect of the treatment on seizure frequency and duration is a 99% less severe PTE syndrome, as indicated by time spent seizing.
Figure 4. Mild focal cooling prevents the development of CSRSs.
A–D) Thermal images of mock-cooling control headset (A, B) and a D-type cooling headset (C, D). Brain heat is more efficiently dissipated by a D-type headset with a steel rod than a control headset with an acrylic rod. A) Side view. Note the progressive cooling of the acrylic rod and headset with the distance from the brain. B) Top view. Note the cold top surface of the acrylic rod. C) Side view. Note the vertical artifact on the rod due to reflected light (gray arrow), and the ring of hot acrylic surrounding the base of the metal rod (white arrow). D) Top view. Note the ring of hot acrylic surrounding the steel rod, and the overall higher temperature of the whole headset compared to B. Throughout: 1= nose bar; 2= ear cone; 3= ECoG pedestal; 4=acrylic or steel rod. E) Schematic of the experimental protocol. Headsets for cooling and ECoG recordings were implanted 3 days post-injury. Cooling rods were replaced with acrylic rods 5 weeks later. F–G) seizure frequency (F) and time spent seizing (G) are virtually abolished in treated rats compared to controls both during and after treatment termination. Plots show data for seizures longer than 3s. H) Seizure-preventive effect of mild cooling shown for different duration-based seizure definitions in control and treated rats during weeks 8–10 and 14–16 post-injury. The effect is not sensitive to seizure definition. Statistical significance is color coded: black filled symbols indicate a statistically significant difference from the time-matched control (gray symbols). I) Cumulative frequency histograms of seizure durations actually observed in treated rats (COOLED all), all control rats (CONT all), and control rats with seizure frequencies comparable to those of cooled animals (CONT low freq). Cooling significantly left-shifts the distribution of seizure durations toward briefer seizures compared to both all controls and low seizure frequency controls (both p < 0.001, Kolmogorov-Smirnov).
DISCUSSION
The principal finding of these blind and randomized studies is that mild focal cooling of the perilesional neocortex potently prevents CSRSs after head injury, both during and after treatment, without inducing pathology, inflammation or silencing neuronal activity. rpFPI induces a severe progressive PTE syndrome 16,17 characterized by a high frequency of focal CSRSs resistant to classic 23 and investigational 24 antiepileptic drugs. The magnitude of cooling required to prevent CSRSs was much smaller than anticipated: a clear dose-response relationship could be demonstrated with graded cooling by up to just 2°C. This was achieved with ECoG headsets engineered to provide graded passive heat dissipation. This is the first demonstration of non-pharmacological prophylaxis of CSRSs.
Passive mild focal cooling prevents CSRSs
Our initial efforts to prevent CSRSs in freely moving rats employed water-cooled ECoG headsets (Supplementary Figure 1). However, rpFPI rats implanted with these headsets, but never actively cooled, failed to develop the expected PTE. This suggested that the magnitude of cooling required to suppress the development of spontaneous seizures was much smaller than expected on the basis of previous studies of provoked seizures12,26, and that it could be achieved by passive heat dissipation. Thus, we designed and built a series of heat dissipating headsets to passively cool the perilesional neocortex with varying efficacy (Fig. 1; Supplementary Fig. 2).
Focal cooling by 2°C resulted in virtually complete prevention of CSRSs both during and after treatment (Fig. 4). The temperature gradient induced by these headsets dissipated to less than 1°C within 2.3 mm from the center of the cooling rod (Fig. 1D), confirming that the seizure onset zone is in the perilesional neocortex, as previously indicated by depth-electrode17 and grid18 recordings. This prophylactic effect persisted for the duration of the study, over 10 weeks after termination of cooling. While the untreated group presented marked progression in seizure frequency and time spent seizing consistent with previous observations17, the cooled group did not. At the end of the study, the treated group had much lower seizure frequency (Fig. 4F) and time spent seizing (Fig. 4G) than the control group did in the early weeks postinjury, suggesting that the prevention of CSRSs would persist long after the 4 months investigated.
Rare short remaining seizures
The residual seizures observed after treatment were rare, and they were shorter than in the control group (Figs. 4I, 5, Supplementary Figure 7). Their occurrence may reflect a cooling protocol that has not yet been optimized. The cooling protocol used in these initial studies may not adequately treat the margins of the seizure-onset zones in some rats. Indeed, the cooling gradient dropped off to less than 1°C within 2.5 mm from the center of the cooling piece, i.e. 0.5 mm from its edge (Fig. 1D), and we know that cooling of the perilesional cortex by just 1°C achieved sub-maximal effects in the dose response study (Fig. 2B–C). Thus, we predict that larger cooling gradients, obtained by increasing the area of the cooling surface and/or the magnitude of cooling applied, will result in complete prevention of seizures. Also, optimization of the cooling protocol was beyond the scope of our studies, and it is possible that earlier treatments applied for different durations may prove to be even more effective.
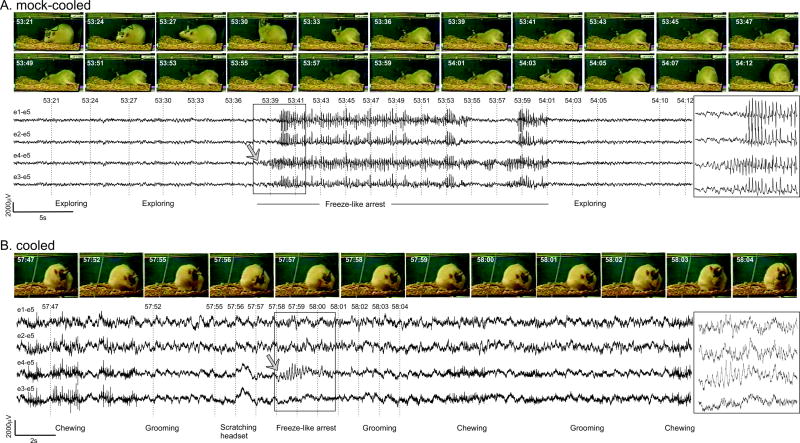
Figure 5. Video-ECoG of chronic spontaneous recurrent focal seizures in mock-cooled and cooled rpFPI animals.
Representative CSRSs observed 4 months postinjury in the prophylactic study of 5.5 weeks of mild focal cooling started 3 days postinjury (Fig. 4). A) A typical focal seizure in the mock-cooling group (control). Exploratory behavior is evident in frames captured prior to the onset of seizure. Epileptic ECoG is first detected by the perilesional electrode e4. Static posture is evident in 12 consecutive frames captured during the epileptic ECoG discharge, and movement resumes after termination of the seizure. Note the perilesional focal onset. B) One of the rare short seizures observed in the cooled group. Grooming behavior is evident in changes in the position of the animal’s head and paws in frames captured before onset of the seizure. Epileptic ECoG is detected only by the perilesional electrode (e4–e5). Ictal motion arrest is indicated by animal’s static posture in frames captured during the epileptic ECoG. Resumption of grooming is evident after termination of the discharge. Note the high frequency muscle artifact during chewing, the absence of artifact during headset scratching at 57:56, and the perilesional focal onset of the short seizure. Numbers shown to the left of ECoG traces indicate recording and reference electrodes as shown in Fig. 1B. Boxes at right show brief seizure on an expanded time scale. Gray arrows indicate ECoG seizure focal onset.
In any case, there is no evidence in our study that these rare short CSRSs represent a progressive disorder and can promote secondary epileptogenesis. The treated group did not show progression of epilepsy, and even more than 10 weeks after cooling termination displayed seizure frequency (Fig. 4F) and time spent seizing (Fig. 4G) comparable to those during the earliest weeks postinjury, and more than an order of magnitude lower than in controls. This is consistent with our previous report that kindling appears to depend on the frequency of neocortical seizures in FPI animals 19.
These short seizures were brief enough to be disregarded in most preclinical studies, which typically arbitrarily exclude seizures lasting less than 5–15s. However, we have characterized the electroclinical semiology of rpFPI-PTE based on the clinical practice of requiring chronic, recurrent, spontaneous epileptiform ECoG with simultaneous stereotyped behavioral changes independently from the duration of the spells27,28. As in humans, rpFPI-PTE includes clinical seizures ranging 1 second to several minutes18,28. Importantly, seizures of all durations responded to cooling, whose effect did not depend on duration-based seizure definitions (Fig. 4H). While further refinements of the cooling protocol may achieve the complete prevention of even the shortest seizures (<3s), these could be subclinical and go unnoticed in humans depending on the location of the focus18,19. For perspective, if a patient with intractable complex partial seizures only has non-disabling simple partial seizures after epilepsy surgery, this would be categorized as an Engel Class I outcome.
Properties of CSRS prophylaxis by mild focal cooling
Our studies show that it is cooling, and not some other feature of the treatment, that accounts for the observed prevention and shortening of CSRSs after FPI: 1) seizure prevention did not depend on the contact between dura and the metal; headset C and D differed only in a thin layer of silicon separating the metal rod from the dura (Figs. 1A, 2B–C); 2) the logarithm of seizure frequency and time seizing were linearly depended on both the thermal conductance of the headsets (Supplementary Figure 4E–F), and on the steady state neocortical cooling they induced (Fig. 2B–C), and 3) these dose dependent effects were demonstrated in the absence of treatment-induced pathophysiological changes (Fig. 3), excluding the possibility that the focus was silenced by treatment-induced cortical damage.
It is unlikely that a direct inhibitory effect of cooling on neuronal/synaptic activity is the predominant mechanism accounting for the observed inhibition of CSRSs during treatment because: 1) the effect of mild cooling appears to be time dependent, inducing a progressive decrease in CSRSs during treatment (Supplementary Fig. 4C–D); 2) acute re-warming of the headsets to body temperature for several hours did not result in seizure rebound (Fig. 2D), and 3) the alpha/delta spectral power ratio during stage N2 sleep was increased by cooling (Fig. 3F). Since this ratio was decreased after rpFPI (Supplementary Figure 6), consistent with the FPI-induced depression in neuronal and synaptic excitability29,30, its increase represents an increase in, and a normalization of cortico-thalamic neuronal/synaptic activity.
CSRSs did not return for months after termination of cooling indicating that: 1) the bulk of epileptogenesis occurs within the first 6 weeks after injury, during which the treatment was delivered, and 2) the development of CSRSs is not irreversibly determined in the first 3 days post-injury, allowing an ample window for intervention. The cooling treatment predominantly affected seizure frequency, but the rare residual seizures are also significantly shorter than those in untreated rpFPI controls. This effect on seizure duration is independent from that on frequency because it was observed even when comparing the treated group to control animals with comparable seizure frequency (Fig. 4I and Supplementary Fig. 7). Further work is needed to identify the mechanisms mediating CSRS prevention induced by mild cooling. Hypothermia alters many cellular and systemic pathophysiological processes10,11,31 that may contribute in a complex fashion to neuroprotection and the observed persistent CSRSs prevention. In particular, recent studies linking epileptogenesis to neurodegeneration32 suggest that cooling-induced moderation of inflammatory and apoptotic processes10,11,31,33,34 may inhibit epileptogenesis by sparing critical neuronal populations. Thus, our findings suggest that head-injury induced epileptogenesis may also be affected by pharmacological anti-inflammatory treatments as recently hypothesized 48.
Translational potential of mild cooling for human TBI
While a number of pharmacological agents have failed to demonstrate prevention of CSRSs in clinical trials6, there is preclinical evidence that epileptogenesis may be modulated pharmacologically using the α2-adrenergic antagonist atipamezole35. This class of drugs, however, may further elevate intracranial pressure in severe head injury patients with impaired cerebrovascular autoregulation36. In addition, atipamezole is proepileptic35, and may exacerbate acute seizures after head injury. Therefore this class of drugs needs scrutiny before use in head injury patients.
In contrast, mild cooling is safe and beneficial in both humans and animals after severe head injury8,11,37–39. Numerous studies have shown hypothermic treatments to provide neuroprotection and improve functional and cognitive recovery after experimental brain injuries 11,39, 46,47. In addition, single center clinical trials of relatively prolonged (>48 hr) periods of mild (34–35°C) systemic hypothermia after TBI have demonstrated decreased mortality, reduced posttraumatic edema and intracranial pressure and improved functional recovery37,40–42. Tokutomi et al.38 argued that cooling by 1.5°C to 2°C is optimal for TBI treatment. Finally, two trials have demonstrated that selective cooling of the TBI brain to 33°C–35°C results in improved outcome compared to normothermia and/or comparable systemic hypothermia42,43. In addition, cooling controls seizures12, making hypothermic treatments particularly attractive for the acute and sub-acute management of head injury patients.
Accordingly, we found that continuous 2°C cooling for 5 weeks resulted in no increase in astroglial reactivity (Fig. 3C), a sensitive indicator of neuropathology44, and neocortical sclerosis, as measured by cortical thinning (Fig. 3D). The gene expression of inflammatory cytokines IL-1α and IL-1β, sensitive and non-specific markers of neuroinflammation45, were nominally lower in the perilesional neocortices of cooled rats compared to rpFPI controls (Fig. 3E). In addition, we found that the injury-related changes in spectral features of the ECoG were partially normalized in the cooled perilesional neocortex (Fig. 3F), indicating a localized restoration of normal neuronal/synaptic function.
A limitation of preclinical prophylactic studies is that they must be restricted in time, and ours lasted 4 months. One can never exclude that a delayed form of epileptogenesis might occur after a sufficiently long time after the observation period. Ultimately the indefinite persistence of seizure prevention –and its clinical value- can only be demonstrated with a trial in humans and with clinical experience. This clinical trial should be conducted after further preclinical optimization of the magnitude of cooling and of the delay/duration of treatment after injury. Indeed, the lack of neuropathology after intermitted cooling by 17–27°C for a month26 suggests the magnitude of focal brain cooling could be safely increased. In addition, the normalization of IL-1α and IL-1β levels after just 3 weeks of cooling (Fig. 3E) suggests that cooling for less than 5 weeks may be sufficient.
Conclusions
Focal 2°C cooling dramatically diminished the development of CSRS after head injury, caused no additional pathology and partially restored one indicator of brain function. This small magnitude of cooling was achieved by passive heat dissipation, without electrical power, circuitry or moving parts, indicating that a practical implementation of cooling-based therapies may be easier than previously anticipated. Thus, it has promise as a practical epilepsy prophylaxis after human TBI, and further investigation is warranted to define the full potential of this novel therapeutic modality.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health [NS053928 to RD, NS042936 to SMR, and EB007362 to FD], CURE [5154001.01 to RD; 5154001.05 to MDS; both made possible by a cooperative agreement with the U.S. Army Medical Research & Materiel Command], and by the University of Washington. We acknowledge the assistance of the University of Washington Center for Ecogenetics & Environmental Health [P30ES07033] and of the Center on Human Development and Disability [P30HD02274]. DRV current affiliation: University of California at Los Angeles. SMR current affiliation: Mercy Clinic Child Neurology, St. Louis MO.
References
- 1.Agrawal A, Timothy J, Pandit L, Manju M. Post-traumatic epilepsy: an overview. Clin Neurol Neurosurg. 2006;108:433–439. doi: 10.1016/j.clineuro.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Frey LC. Epidemiology of posttraumatic epilepsy: a critical review. Epilepsia. 2003;44(Suppl 10):11–7. doi: 10.1046/j.1528-1157.44.s10.4.x. [DOI] [PubMed] [Google Scholar]
- 3.Eftekhar B, Sahraian MA, Nouralishahi B, et al. Prognostic factors in the persistence of posttraumatic epilepsy after penetrating head injuries sustained in war. J Neurosurg. 2009;110(2):319–26. doi: 10.3171/2008.4.17519. [DOI] [PubMed] [Google Scholar]
- 4.Semah F, Picot MC, Adam C, et al. Is the underlying cause of epilepsy a major prognostic factor for recurrence? Neurology. 1998;51:1256–1262. doi: 10.1212/wnl.51.5.1256. [DOI] [PubMed] [Google Scholar]
- 5.Lu Y, Yu W, Wang X. Efficacy of topiramate in adult patients with symptomatic epilepsy: an open-label, long-term, retrospective observation. CNS Drugs. 2009;23:351–9. doi: 10.2165/00023210-200923040-00006. [DOI] [PubMed] [Google Scholar]
- 6.Temkin NR. Preventing and treating posttraumatic seizures: the human experience. Epilepsia. 2009;50(Suppl 2):10–3. doi: 10.1111/j.1528-1167.2008.02005.x. [DOI] [PubMed] [Google Scholar]
- 7.Lowenstein DH. Epilepsy after head injury: an overview. Epilepsia. 2009;50 (Suppl 2):4–9. doi: 10.1111/j.1528-1167.2008.02004.x. [DOI] [PubMed] [Google Scholar]
- 8.Linares G, Mayer SA. Hypothermia for the treatment of ischemic and hemorrhagic stroke. Crit Care Med. 2009;37(7 Suppl):S243–9. doi: 10.1097/CCM.0b013e3181aa5de1. [DOI] [PubMed] [Google Scholar]
- 9.Peterson K, Carson S, Carney N. Hypothermia treatment for traumatic brain injury: a systematic review and meta-analysis. J Neurotrauma. 2008;25:62–71. doi: 10.1089/neu.2007.0424. [DOI] [PubMed] [Google Scholar]
- 10.Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med. 2009;37(7 Suppl):S186–202. doi: 10.1097/CCM.0b013e3181aa5241. [DOI] [PubMed] [Google Scholar]
- 11.Sahuquillo J, Vilalta A. Cooling the injured brain: how does moderate hypothermia influence the pathophysiology of traumatic brain injury. Curr Pharm Des. 2007;13:2310–22. doi: 10.2174/138161207781368756. [DOI] [PubMed] [Google Scholar]
- 12.Rothman SM. The therapeutic potential of focal cooling for neocortical epilepsy. Neurotherapeutics. 2009;6:251–7. doi: 10.1016/j.nurt.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burton J, Peebles G, Binder D, et al. Transcortical cooling inhibits hippocampal-kindled seizures in the rat. Epilepsia. 2005;46:1881–1887. doi: 10.1111/j.1528-1167.2005.00299.x. [DOI] [PubMed] [Google Scholar]
- 14.Atkins CM, Truettner JS, Lotocki G, et al. Post-traumatic seizure susceptibility is attenuated by hypothermia therapy. Eur J Neurosci. 2010;32:1912–20. doi: 10.1111/j.1460-9568.2010.07467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson HJ, Lifshitz J, Marklund N, et al. Lateral fluid percussion brain injury: a 15-year review and evaluation. J Neurotrauma. 2005;22:42–75. doi: 10.1089/neu.2005.22.42. [DOI] [PubMed] [Google Scholar]
- 16.D’Ambrosio R, Fairbanks JP, Fender JS, et al. Posttraumatic epilepsy following fluid percussion injury in the rat. Brain. 2004;127:304–14. doi: 10.1093/brain/awh038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D’Ambrosio R, Fender JS, Fairbanks JP, et al. Progression from frontal-parietal to mesial-temporal epilepsy after fluid percussion injury in the rat. Brain. 2005;128:174–188. doi: 10.1093/brain/awh337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Ambrosio R, Hakimian S, Stewart T, et al. Functional definition of seizure provides new insight into post-traumatic epileptogenesis. Brain. 2009;132:2805–21. doi: 10.1093/brain/awp217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curia G, Levitt M, Fender JS, et al. Impact of Injury Location and Severity on Posttraumatic Epilepsy in the Rat: Role of Frontal Neocortex. Cereb Cortex. 2010;21:1574–1592. doi: 10.1093/cercor/bhq218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williamson PD, Spencer DD, Spencer SS, et al. Complex partial seizures of frontal lobe origin. Ann Neurol. 1985;18:497–504. doi: 10.1002/ana.410180413. [DOI] [PubMed] [Google Scholar]
- 21.Diaz-Arrastia R, Agostini MA, Frol AB, et al. Neurophysiologic and neuroradiologic features of intractable epilepsy after traumatic brain injury in adults. Arch Neurol. 2000;57:1611–6. doi: 10.1001/archneur.57.11.1611. [DOI] [PubMed] [Google Scholar]
- 22.Bancaud J, Talairach J. Clinical semiology of frontal lobe seizures. In: Chauvel P, Delgado-Escueta AV, Halgren E, Bancaud J, editors. Advances in Neurology. Vol. 57. New York: Raven Press; 1992. pp. 3–58. [PubMed] [Google Scholar]
- 23.Eastman CL, Verley DR, Fender JS, et al. ECoG studies of valproate, carbamazepine and halothane in frontal-lobe epilepsy induced by head injury in the rat. Exp Neurol. 2010;224:369–88. doi: 10.1016/j.expneurol.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eastman CL, Verley DR, Fender JS, et al. Antiepileptic and antiepileptogenic performance of carisbamate after head injury in the rat: blind and randomized studies. J Pharmacol Exp Ther. 2011;336:779–90. doi: 10.1124/jpet.110.175133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vandesompele J, De Preter K, Pattyn F, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002 Jun 18;3(7):RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujii M, Inoue T, Nomura S, et al. Cooling of the epileptic focus suppresses seizures with minimal influence on neurologic functions. Epilepsia. 2012;53:485–493. doi: 10.1111/j.1528-1167.2011.03388.x. [DOI] [PubMed] [Google Scholar]
- 27.Leppik IE. Seizures. In: Leppik IE, editor. Contemporary diagnosis and management of the patient with epilepsy. Handbooks In Health Care. Pennsylvania: Newtown; 2006. pp. 9–20. [Google Scholar]
- 28.D’Ambrosio R, Miller JW. What is an epileptic seizure? Unifying definitions in clinical practice and animal research to develop novel treatments. Epilepsy Curr. 2010;10:61–6. doi: 10.1111/j.1535-7511.2010.01358.x. Comment in Epilepsy Curr 2010; 10(4):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D’Ambrosio R, Maris DO, Grady MS, et al. Selective loss of hippocampal long-term potentiation, but not depression, following fluid percussion injury. Brain Res. 1998;786:64–79. doi: 10.1016/s0006-8993(97)01412-1. [DOI] [PubMed] [Google Scholar]
- 30.Cohen AS, Pfister BJ, Schwarzbach E, et al. Injury-induced alterations in CNS electrophysiology. Prog Brain Res. 2007;161:143–69. doi: 10.1016/S0079-6123(06)61010-8. [DOI] [PubMed] [Google Scholar]
- 31.González-Ibarra FP, Varon J, López-Meza EG. Therapeutic hypothermia: critical review of the molecular mechanisms of action. Front Neurol. 2011;2:4. doi: 10.3389/fneur.2011.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Norwood BA, Bumanglag AV, Osculati F, et al. Classic hippocampal sclerosis and hippocampal-onset epilepsy produced by a single “cryptic” episode of focal hippocampal excitation in awake rats. J Comp Neurol. 2010;518:3381–407. doi: 10.1002/cne.22406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi J, Koh S. Role of brain inflammation in epileptogenesis. Yonsei Med J. 2008;49:1–18. doi: 10.3349/ymj.2008.49.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol. 2011;7:31–40. doi: 10.1038/nrneurol.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pitkänen A, Narkilahti S, Bezvenyuk Z, et al. Atipamezole, an alpha(2)-adrenoceptor antagonist, has disease modifying effects on epileptogenesis in rats. Epilepsy Res. 2004;61:119–40. doi: 10.1016/j.eplepsyres.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 36.Stover JF, Steiger P, Stocker R. Controversial Issues Concerning Norepinephrine and Intensive Care Following Severe Traumatic Brain Injury. Eur J Trauma. 2006;32:10–27. [Google Scholar]
- 37.Tokutomi T, Miyagi T, Takeuchi Y, et al. Effect of 35 degrees C hypothermia on intracranial pressure and clinical outcome in patients with severe traumatic brain injury. J Trauma. 2009;66:166–73. doi: 10.1097/TA.0b013e318157dbec. [DOI] [PubMed] [Google Scholar]
- 38.Tokutomi T, Morimoto K, Miyagi T, et al. Optimal temperature for the management of severe traumatic brain injury: effect of hypothermia on intracranial pressure, systemic and intracranial hemodynamics, and metabolism. Neurosurgery. 2003;52:102–11. [PubMed] [Google Scholar]
- 39.Dietrich WD, Bramlett HM. The evidence for hypothermia as a neuroprotectant in traumatic brain injury. Neurotherapeutics. 2010;7:43–50. doi: 10.1016/j.nurt.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fox JL, Vu EN, Doyle-Waters M, et al. Prophylactic hypothermia for traumatic brain injury: a quantitative systematic review. CJEM. 2010;12:355–64. doi: 10.1017/s1481803500012471. [DOI] [PubMed] [Google Scholar]
- 41.Tolias CM, Bullock MR. Critical appraisal of neuroprotection trials in head injury: what have we learned? NeuroRx. 2004;1:71–9. doi: 10.1602/neurorx.1.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qiu W, Zhang Y, Sheng H, et al. Effects of therapeutic mild hypothermia on patients with severe traumatic brain injury after craniotomy. J Crit Care. 2007;22:229–35. doi: 10.1016/j.jcrc.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 43.Liu WG, Qiu WS, Zhang Y, et al. Effects of selective brain cooling in patients with severe traumatic brain injury: a preliminary study. J Int Med Res. 2006;34:58–64. doi: 10.1177/147323000603400107. [DOI] [PubMed] [Google Scholar]
- 44.Eng LF, Ghirnikar RS. GFAP and astrogliosis. Brain Pathol. 1994;4:229–37. doi: 10.1111/j.1750-3639.1994.tb00838.x. [DOI] [PubMed] [Google Scholar]
- 45.Basu A, Krady JK, Levison SW. Interleukin-1: a master regulator of neuroinflammation. J Neurosci Res. 2004;78:151–6. doi: 10.1002/jnr.20266. [DOI] [PubMed] [Google Scholar]
- 46.Bramlett HM, Green EJ, Dietrich WD, Busto R, Globus MY, Ginsberg MD. Posttraumatic brain hypothermia provides protection from sensorimotor and cognitive behavioral deficits. J Neurotrauma. 1995;12:289–98. doi: 10.1089/neu.1995.12.289. [DOI] [PubMed] [Google Scholar]
- 47.Doll H, Truebel H, Kipfmueller F, Schaefer U, Neugebauer EA, Wirth S, Maegele M. Pharyngeal selective brain cooling improves neurofunctional and neurocognitive outcome after fluid percussion brain injury in rats. J Neurotrauma. 2009;26:235–42. doi: 10.1089/neu.2008.0741. [DOI] [PubMed] [Google Scholar]
- 48.Vezzani A, Friedman A, Dingledine RJ. The role of inflammation in epileptogenesis. Neuropharmacology. 2012 Apr 13; doi: 10.1016/j.neuropharm.2012.04.004. [Epub ahead of print] http://dx.doi.org/10.1016/j.neuropharm.2012.04.004. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.