Abstract
We have examined mechanisms underlying the formation of pathologic Th17 cells using a transgenic mouse model in which autoreactive CD4+ T cells recognize influenza virus hemagglutinin (HA) as a ubiquitously expressed self-antigen, and induce inflammatory arthritis. The lymph nodes of arthritic mice contain elevated numbers of inflammatory monocytes (iMO) with an enhanced capacity to promote CD4+ Th17 cell differentiation, and a regional inflammatory response develops in the paw-draining lymph nodes by an IL-17-dependent mechanism. The activation of these Th17-trophic iMO precedes arthritis development, and occurs in the context of an autoreactive CD4+ Th1 cell response. Adoptive transfer of HA-specific CD4+ T cells into non-arthritic mice expressing HA as a self-antigen similarly led to the formation of Th1 cells and of iMO that could support Th17 cell formation, and notably, the accumulation of these iMO in the lymph nodes was blocked by IFN-γ neutralization. These studies show that autoreactive CD4+ Th1 cells directed to a systemically distributed self-antigen can promote the development of a regional Th17 cell inflammatory response by driving the recruitment of Th17-trophic iMO to the lymph nodes.
Introduction
Th17 cells are a subset of effector CD4+ T cells that can play an important role in maintaining effective anti-microbial immunity at mucosal surfaces such as the gut, but are also important participants in a variety of autoimmune diseases, including inflammatory arthritis and multiple sclerosis (1, 2). The processes that drive Th17 cell formation are not fully understood, particularly in the setting of autoimmune disease, where Th17 cell induction is pathological and presumably reflects a dysregulation of processes that are normally protective to the host.
Both in vitro and in vivo studies have shown that cytokines such as TGFβ 1, IL-6, and IL-23 can play important roles in promoting the differentiation of naïve CD4+ T cells into Th17 cells (3–5). More recently, an important role for gut residing bacteria in Th17 cell formation in vivo has become apparent, such that colonization of mice with particular commensal microbes (such as segmented filamentous bacterium) can profoundly influence the magnitude of Th17 cell responses that can be induced (6). Notably, these effects on Th17 cell formation were found to influence the development of autoimmune arthritis in K/BxN mice, indicating that commensal bacteria can play a role in promoting the formation of pathologic autoreactive Th17 cells in vivo (7). However, it is clear that additional factors must contribute to autoimmune disease development, in addition to those received from commensal bacteria, since most strains of mice carrying these bacteria do not develop autoimmunity. In particular, these findings do not explain why many autoimmune diseases show strong genetic linkages with MHC class II (MHCII) alleles, which imply an important role for CD4+ T cell recognition of self-peptides in the disease process (8). Indeed, mechanisms by which CD4+ T cell recognition of self-peptides might participate in and/or promote the formation of pathologic Th17 cells in autoimmune settings remain poorly understood.
We have examined this question in a mouse model of inflammatory arthritis, in which autoreactive CD4+ T cells responding to a self-peptide expressed by APCs induce arthritis by an IL-17 dependent mechanism (9). We show that autoimmune disease is initiated by a systemic autoreactive CD4+ Th1 response, which drives the formation of Th17-trophic inflammatory monocytes (iMO) that mobilize to the lymph nodes (LN). These studies provide a basis by which autoreactive CD4+ T cells responding to a systemically distributed antigen can promote a regional IL-17-mediated inflammatory response.
Materials and Methods
Mice
TS1, HACII, and TS1xHACII mice were previously described (10, 11). HACII.Cα−/− mice were generated by crossing HACII mice with TCR.Cα−/− mice that were bred to homozygosity for the H-2d haplotype and backcrossed onto the BALB/c background for at least 7 generations (12). TS1.CD45.1+/− congenic mice were generated by breeding TS1 mice with CD45.1+/+ mice on the BALB/c background that were purchased from Jackson Labs. Mice were housed under specific pathogen-free conditions at The Wistar Institute Animal Facility. All experiments were performed according to protocols approved by The Wistar Institutional Animal Care and Use Committee.
Assessment of arthritis, anti-IL-17 treatment, and antibiotic treatment
Mice were assessed weekly for the number of arthritic limbs beginning at 4 wk of age and continuing for at least 12 wk. For anti-IL-17 treatment, TS1xHACII mice received 3 i.p. injections per wk of 150 µg of an IL-17A neutralizing antibody (M210; provided by Amgen) or of an isotype control antibody (2A3; BioXcell) beginning at 4 wk of age.
Antibodies and flow cytometry
Single cell suspensions from spleens or lymph node were stained with the Live/Dead Fixable Aqua Dead Cell stain kit from Invitrogen (except when sorting) and then with fluorochrome labeled antibodies purchased from eBioscience or BD unless stated otherwise. The following clones were used in all experiments. Analysis and sorting of APCs: F4/80 (BM8), PDCA-1 (eBio927), CD19 (1D3), CD11b (M1/70), CD11c (N418), Ly6G (1A8), Ly6C (HK1.4), MHCII (M5/114.15.2), I-Ad (AMS-32.1), B220 (RA3-6B2), CD115 (AF598), CD49b (DX5), CCR2 (R&D, 475301). Analysis and sorting of T cells: CD4 (RM4-5), CD25 (PC61.6), IL-17 (eBio17B7), IFN-γ (XMG1.2), CD45.1 (A20), CD8 (53–6.7). Biotinylated 6.5 was prepared in-house and was detected with APC (BD) or Qdot655 (Invitrogen) conjugated streptavidin.
Cell sorting
For flow cytometric analysis and sorting of APC subsets, spleens and LN were minced and digested with 400 U/ml collagenase D (Roche) at 37 °C prior to use. Spleen and/or LN cells were sorted with a MoFlow (Dako Cytomation) or FACSAria (BD) cell sorter into the following subsets: inflammatory monocytes (B220−CD11c−Ly6G−CD11b+Ly6Chigh), conventional dendritic cells (B220−Ly6G−Ly6C−CD11b+/−CD11chigh), and B cells (CD11b−CD11c−Ly6C−B220high). For co-culture experiments hemagglutinin (HA)-specific CD4+CD25−6.5+ cells were sorted from the pooled spleens and LN of TS1.CD45.1+/− or TS1.Thy1.1+/− mice.
In vitro co-cultures
FACS-purified APCs and HA-specific T cells were combined at T cell:APC ratios ranging from ~1:2 (LN APCs) to 1:10 (spleen APCs), and cultured at ~1.5×106 total cells/ml in cell culture media supplemented with 50 ng/ml IL-23 (R&D). After 7 d PMA, ionomycin, and brefeldin A were added to the cultures and IL-17 and IFN-γ production were assessed 5 h later by intracellular cytokine staining and flow cytometry.
Serum cytokine analysis
Serum was collected from whole blood and inflammatory cytokine expression was determined by multiplex ELISA using the Luminex platform (Millipore) at the University of Pennsylvania Human Immunology Core.
Adoptive transfer
CD4+ T cells were purified from the spleens and LN of TS1 mice by MACS-depletion of unwanted cells with antibodies against B220, I-Ad, CD11b, F4/80, and CD8. 106 cells/mouse were adoptively transferred into HACII.Cα−/− recipients by retro-orbital injection and recipient mice were sacrificed 7 d later for analysis. In some experiments recipients were injected i.p. with 0.5 mg of anti-IFN-γ (XMG1.2; BioXcell) or 0.5 mg of isotype control antibody (HRPN; BioXcell) on d 0 and d 3. For analysis of in vivo proliferation CD4+ T cells purified as described above were labeled with 5µM CFSE (Sigma-Aldrich) prior to adoptive transfer into BALB/c or HACII recipients, which were sacrificed 3 d later for analysis.
Statistical analysis
All statistical analyses were performed using Graphpad Prism software package. Unpaired, two-tailed t-tests were used for data analysis and the generation of P values.
Results
Arthritis in TS1xHACII mice is accompanied by a regional Th17 response to a systemic self-antigen
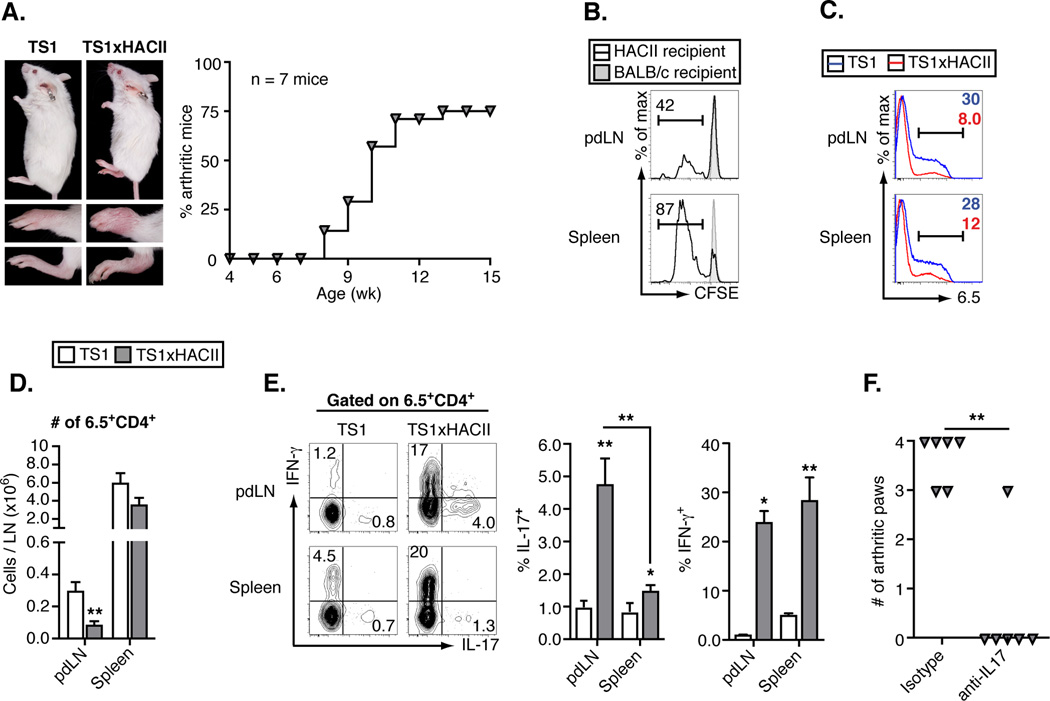
TS1 mice express a transgenic I-Ed-restricted TCR that recognizes the S1 determinant of influenza PR8 HA, and that can be detected with the clonotypic mAb 6.5 (10). HACII mice express HA as a neo-self antigen under the control of a MHCII promoter, and we previously reported that the majority of TS1xHACII mice (generated by mating TS1 mice with HACII mice) spontaneously develop inflammatory arthritis as they reach adulthood (Fig. 1A) (11). Arthritis depends on co-expression of the TS1 and HACII transgenes (since neither TS1 nor HACII mice develops the disease), and is driven by CD4+ T cells without a requirement for B cells, since TS1xHACII mice that congenitally lack B cells also develop arthritis (11). It is noteworthy that TS1xHACII mice develop arthritis as a prominent autoimmune manifestation, since the targeting of HA to MHCII+ cells results in its expression as a self-antigen by APCs throughout the body. Indeed, CD4+ T cells from TS1 mice underwent extensive division in both the spleens and LN following transfer into HACII mice (Fig. 1B), and HA-specific 6.5+CD4+ T cells are extensively deleted in TS1xHACII mice (Fig. 1, C and D), indicating that HA is a systemically expressed self-antigen in TS1xHACII mice.
Figure 1.
Arthritis in TS1xHACII mice is accompanied by a regional Th17 response to a systemic self-antigen. (A) Photographs of age and sex-matched TS1 and arthritic TS1xHACII mice, lower panels show front and rear paws. Graph shows arthritis incidence in a cohort of TS1xHACII mice. (B) CFSE dilution by CD4+ T cells from TS1 mice 3 d post-transfer into HACII or BALB/c recipients. (C) Histograms showing 6.5 staining by CD4+ T cells in pdLN and spleens of TS1 and arthritic TS1xHACII mice. (D) Numbers of 6.5+CD4+ T cells in pdLN and spleens of TS1 and arthritic TS1xHACII mice. (E) Ex vivo production of IFN-γ and IL-17 by 6.5+CD4+ T cells from pdLN and spleens of TS1 and arthritic TS1xHACII mice. (F) Arthritis incidence in 15 week old TS1xHACII mice given weekly injections of anti-IL-17 beginning at 4 wk of age. All data are means ± SEM of ≥ 5 independent determinations. *p<0.05; **p<0.01.
To examine how autoreactive 6.5+CD4+ T cells that evade deletion can promote arthritis development we first analyzed their representation and phenotype in the spleens and paw draining LN (pdLN) of arthritic TS1xHACII mice. There were increased frequencies of both IFN-γ- and IL-17-producing 6.5+CD4+ T cells (Th1 and Th17 cells, respectively) relative to TS1 mice (Fig. 1E). Notably, however, the percentage of 6.5+CD4+ T cells that were Th17 cells was significantly higher in the pdLN of arthritic TS1xHACII mice than in the spleens, while the percentages of 6.5+CD4+ T cells that were Th1 cells at these sites did not differ. Moreover, administration of an anti-IL-17A mAb led to a significant decrease in the numbers of arthritic paws that developed in TS1xHACII mice, consistent with other studies demonstrating a role for Th17 cells in inflammatory arthritis (Fig. 1F) (7, 9, 13). Together, these data show that TS1xHACII mice spontaneously develop an IL-17-dependent inflammatory arthritis that is accompanied by a regional increase in the frequency of HA-specific CD4+ Th17 cells in the pdLN.
Th17-trophic inflammatory monocytes accumulate in arthritic TS1xHACII mice
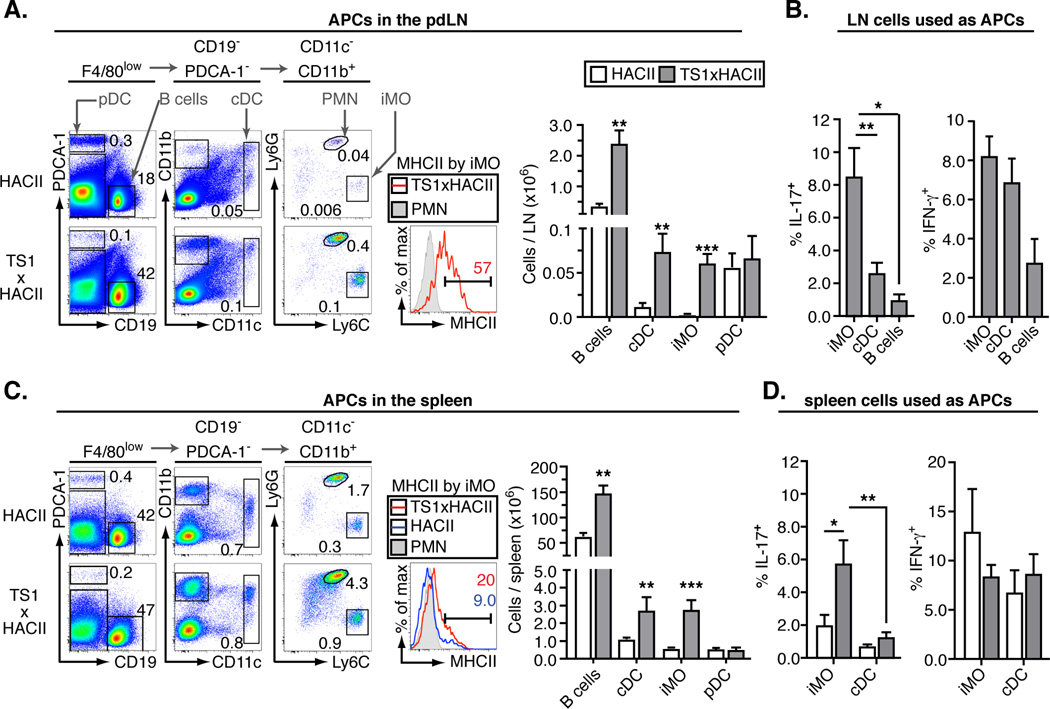
The HA self-antigen that is recognized by 6.5+CD4+ T cells in TS1xHACII mice is expressed by MHCII-expressing cells (including APCs), and we wanted to determine whether a particular APC type might be playing a prominent role in promoting the formation of autoreactive Th17 cells. We addressed this by first comparing the frequencies of various APC subsets in the pdLN of arthritic TS1xHACII mice with HACII mice. There were 6–8 fold increases in the numbers of CD19+ B cells and CD19−PDCA−CD11chigh conventional dendritic cells (cDC) in the pdLN of arthritic TS1xHACII mice relative to HACII mice. However, there was a much more dramatic increase in the number of CD19−PDCA-1−CD11c−CD11b+Ly6GlowLy6Chigh iMO (Fig. 2A); these cells were almost undetectable in the pdLN of HACII mice, and increased in frequency at least 30-fold in the pdLN of arthritic TS1xHACII mice. The iMO also expressed the markers CD115, F4/80, and CCR2 that are typical of monocytes, and lacked expression of the markers B220 and CD49b used to identify Ly6C+ plasmacytoid DCs and NK cells, respectively (Fig. S1) (14–16). CD19−PDCA-1−CD11c−CD11b+Ly6GhighLy6Cint polymorphonuclear (PMN) cells were also present in elevated numbers in arthritic mice, which is consistent with the ability of IL-17 to enhance the formation of these cells (17).
Figure 2.
Th17-trophic inflammatory monocytes accumulate in arthritic TS1xHACII mice. (A) Representative flow data showing APC subsets in the pdLN. The percent of total live cells falling within each gate is indicated. MHCII expression by iMO and the total numbers of each APC are shown in the accompanying panels. (B) Percentage of 6.5+CD4+ T cells that produced IL-17 or IFN-γ following 7 days of co-culture with APC subsets purified from the LN of arthritic TS1xHACII mice. (C) As in A, except for spleen. (D) As in B, except co-culture was with APC subsets purified from the spleens of HACII mice or arthritic TS1xHACII mice. All data are means ± SEM of ≥ 5 independent determinations. *p<0.05; **p<0.01; ***p<0.005.
The iMO in the pdLN of arthritic TS1xHACII mice also expressed MHCII (along with endogenously synthesized HA-derived peptides synthesized under control of the MHCII promoter), indicating that they were capable of acting as APCs for 6.5+CD4+ T cells. We therefore examined their ability to promote the formation of IL-17-secreting 6.5+CD4+ T cells in vitro relative to other APC populations from the pdLN of arthritic TS1xHACII. Notably, iMO purified from the pdLN of arthritic TS1xHACII mice induced a significantly higher proportion of 6.5+CD4+ cells from TS1 mice to become IL-17 secretors than were induced by either B cells or cDC that had also been isolated from the pdLN (Fig. 2B). Inflammatory monocyte and cDC numbers were also elevated in the spleens of arthritic TS1xHACII mice, and although lower than was the case for iMO in the pdLN, the splenic iMO from arthritic TS1xHACII mice expressed higher levels of MHCII than iMO from the spleens of HACII mice (Fig. 2C). Splenic iMO from arthritic TS1xHACII mice again induced the formation of IL-17-secreting 6.5+CD4+ T cells more efficiently than was the case for cDC isolated from arthritic TS1xHACII mice, and they were also more efficient at inducing the formation of IL-17 secreting 6.5+CD4+ T cells than were either iMO or cDC obtained from the spleens of control HACII mice (Fig. 2D). Thus, the accumulation of IL-17-secreting 6.5+CD4+ T cells in the pdLN of arthritic TS1xHACII mice is associated with a sizable increase in the representation of an iMO population that is a more potent inducer of Th17 cells than is the case for other APC populations in the pdLN. Together, these data strongly suggesting that these “Th17-trophic” iMO are important in promoting the regional Th17 cell response that develops in the pdLN of arthritic TS1xHACII mice.
A systemic CD4+ Th1 cytokine response precedes arthritis in TS1xHACII mice
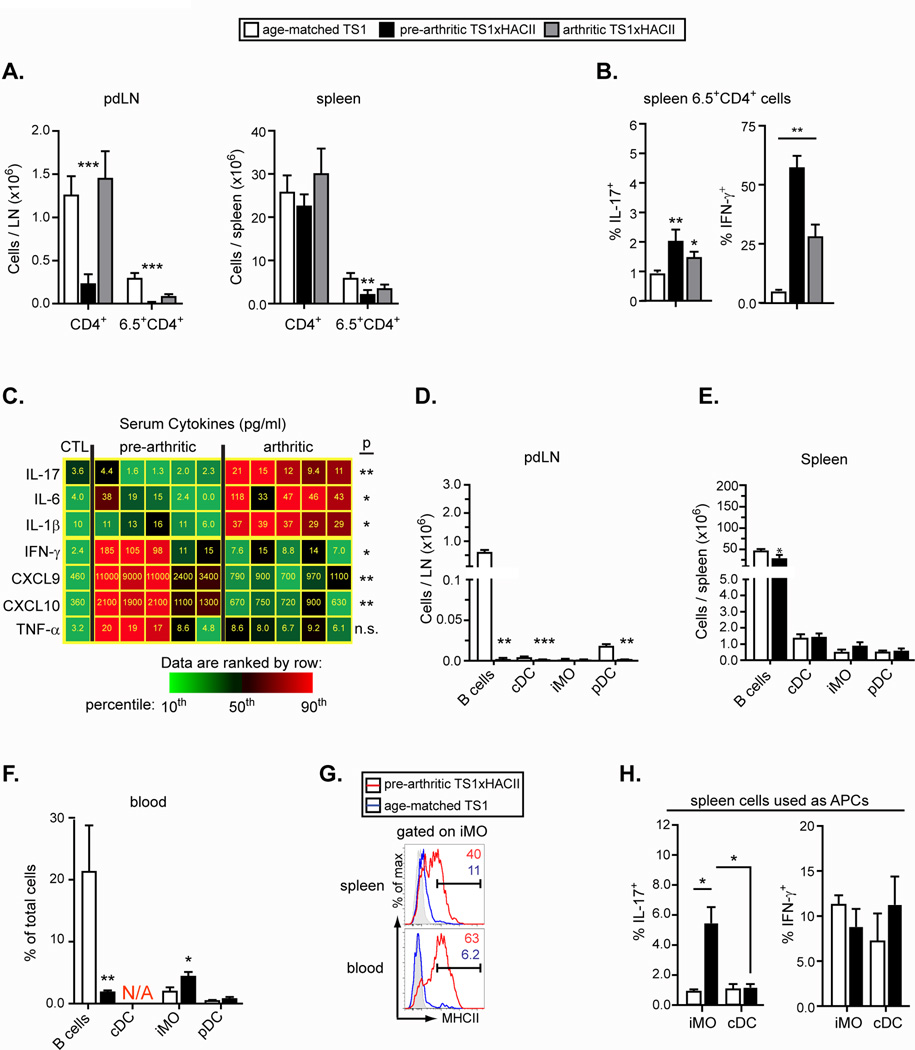
Arthritis arises spontaneously in adult TS1xHACII mice, and we were interested to evaluate processes occurring in younger mice that might play a predisposing role in arthritis development. The pdLN of 4–5 week old pre-arthritic TS1xHACII mice exhibited a marked hypocellularity, with total CD4+ T cells and 6.5+CD4+ T cells substantially underrepresented relative to both TS1 and arthritic TS1xHACII mice (Fig. 3A). The spleens of pre-arthritic TS1xHACII mice contained CD4+ and 6.5+CD4+ cells in numbers that were more comparable to the spleens of TS1 and arthritic TS1xHACII mice. Notably, more than 50% of 6.5+CD4+ splenocytes in pre-arthritic TS1xHACII mice were IFN-γ-secreting Th1 cells, compared to 25% and 5% of 6.5+CD4+ splenocytes in arthritic TS1xHACII and TS1 mice, respectively (Fig. 3B). Moreover, serum obtained from pre-arthritic TS1xHACII mice contained significantly higher levels of Th1-associated cytokines and chemokines (IFN-γ, CXCL9 and CXCL10) than serum from arthritic TS1xHACII mice; by contrast, the serum from arthritic TS1xHACII mice expressed significantly higher levels of Th17-associated cytokines (IL-17, IL-6 and IL-1β) than were found in serum form pre-arthritic TS1xHACII mice (Fig. 3C).
Figure 3.
A systemic CD4+ Th1 cytokine response precedes arthritis in TS1xHACII mice. (A) Number of CD4+ and 6.5+CD4+ T cells in the pdLN and spleens of TS1, pre-arthritic TS1xHACII, and arthritic TS1xHACII mice. (B) Percentage of 6.5+CD4+ splenocytes that produced IL-17 or IFN-γ (C) Heat map showing the concentrations of Th17 and Th1 cytokines in the serum of pre-arthritic and arthritic TS1xHACII mice. Average values from 7 control mice are shown in the leftmost column. The concentration of each cytokine is indicated within the tiles. P values refer to comparisons between pre-arthritic and arthritic TS1xHACII mice. (D–F) Number of APCs in the pdLN (D) and spleen (E), and percentages of cells in the blood (F) of 5 wk-old TS1xHACII and TS1 mice. N/A = equivalent subset not present. (G) MHCII expression by iMO in the spleens and blood of 5 wk-old TS1xHACII and TS1 mice. (H) Percentage of 6.5+CD4+ T cells from TS1 mice that produced IL-17 or IFN-γ following 7 d of co-culture with APC subsets purified from the spleens of 5 wk old TS1xHACII or HACII mice. All data are means ± SEM of N ≥ 5 independent determinations. *p<0.05; **p<0.01; ***p<0.005.
When we examined the APC subsets present in pre-arthritic TS1xHACII mice we again found very low numbers of cells in the pdLN (Fig. 3D). By contrast, the spleens of pre-arthritic TS1xHACII mice contained similar numbers of APCs (including iMO) as were found in age-matched TS1 mice, and there was a significant increase in the frequency of circulating iMO in the blood of pre-arthritic TS1xHACII mice relative to their TS1 counterparts (Fig. 3, E and F). Notably, iMO in the spleens and blood of pre-arthritic TS1xHACII mice expressed MHCII at levels that were comparable to those found on iMO in the pdLN of arthritic TS1xHACII mice (compare Fig. 3G and Fig. 2A). Moreover, splenic iMO from pre-arthritic TS1xHACII mice were significantly better at inducing 6.5+CD4+ T cells from TS1 mice to become Th17 cells than was the case for either cDC from pre-arthritic TS1xHACII mice, or for cDC or iMO obtained from the spleens of age-matched HACII mice (Fig. 3H). Together, these data indicate that the autoreactive CD4+ T cell response in pre-arthritic TS1xHACII mice differs from that observed in arthritic TS1xHACII mice in that it is characterized by IFN-γ production, and occurs predominantly in the spleen rather than the pdLN. Notably, the formation of “Th17-trophic” iMO precedes the onset of an inflammatory response in the pdLN and arthritis.
Autoreactive CD4+ T cells promote activation of Th17-trophic inflammatory monocytes via IFN-γ production
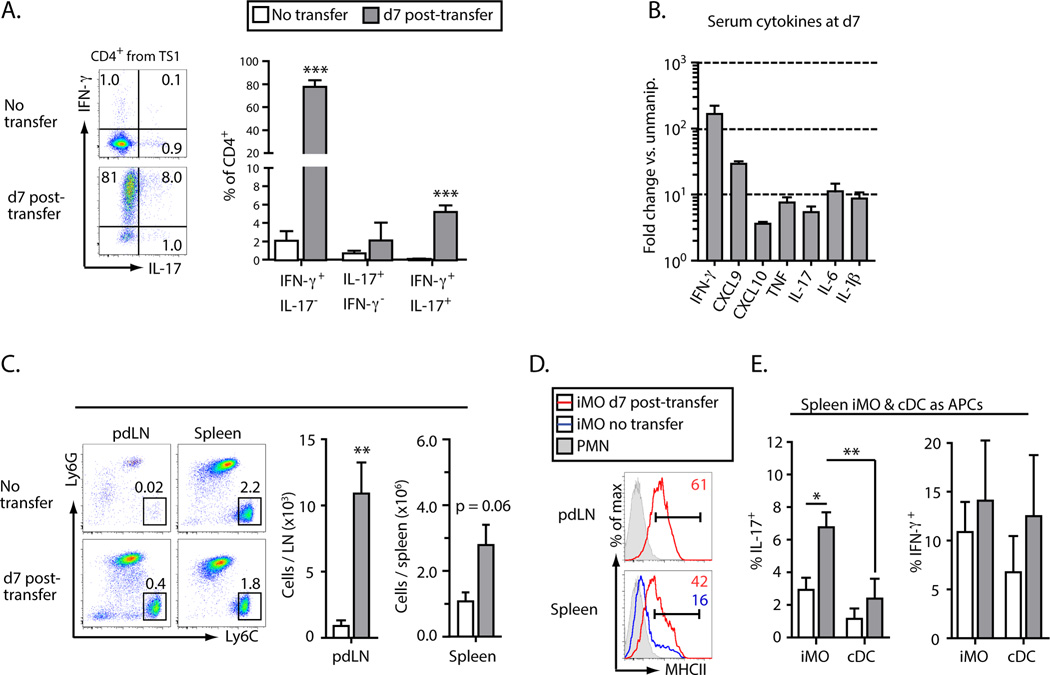
To examine whether the autoreactive CD4+ Th1 cells that arise in pre-arthritic TS1xHACII mice can play a direct role in the formation of Th17-trophioc iMO we used an adoptive transfer approach in which CD4+ T cells from TS1 mice were introduced into T cell-deficient mice that express the HACII self-antigen (HACII.Cα−/− mice). By 7 d post-transfer 80% of the donor CD4+ T cells in the spleens and LN of recipient mice were IFN-γ-secreting cells, with a smaller subset co-producing IL-17 or producing IL-17 alone (Fig. 4A). The serum of the recipient mice also contained elevated concentrations of pro-inflammatory cytokines relative to mice that had not received T cells, with IFN-γ showing the greatest increase (>100 fold), consistent with the high frequency of IFN-γ-secreting cells detected by intracellular cytokine staining (Fig. 4B). The introduction of HA-specific CD4+ T cells also led to increased numbers of MHCII+ iMO in the pdLN and spleens of recipient mice (Fig. 4, C and D), and as had been observed in intact TS1xHACII mice, these iMO were significantly more capable of inducing Th17 cell formation than were cDC from HACII.Cα−/− mice that had received CD4+ T cells, or iMO or cDC from unmanipulated HACII mice (Fig. 4E).
Figure 4.
Autoreactive T cells promote the activation of Th17-trophic inflammatory monocytes. CD4+ T cells from TS1 mice were transferred into HACII.Cα−/− mice (“recipient mice”). All data were collected at 7 d post-transfer. (A) Production of IFN-γ and IL-17 by CD4+ T cells from a TS1 mouse analyzed directly ex vivo or at 7 d post-transfer. (B) Fold increase in the indicated cytokines in the serum of recipient versus unmanipulated mice. (C) Representative flow data of iMO and PMN in the pdLN and spleens. The frequency of each subset as a percentage of total cells is indicated on each plot. The total number of iMO in the spleens and pdLN of recipient mice is shown in the accompanying chart. (D) Histograms showing MHCII expression by iMO in the spleens and pdLN of recipient mice. (E) Percentages of 6.5+CD4+ T cells that produced IL-17 or IFN-γ following 7 d of co-culture with APCs isolated from the spleens of unmanipulated HACII or recipient mice. All data are means ± SEM of N ≥ 3 independent determinations. *p<0.05; **p<0.01; ***p<0.005.
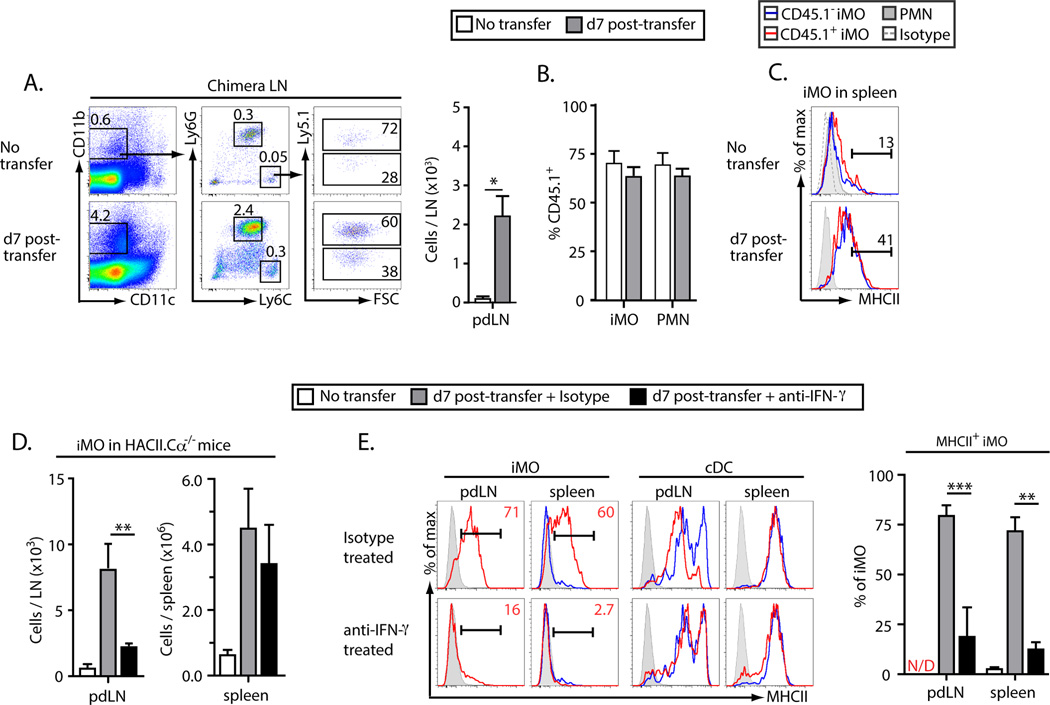
To identify signals that the CD4+ Th1 cells were providing to induce the formation of Th17-trophic iMO we first generated mixed bone-marrow chimeras in which 75 % of marrow-derived cells expressed the HACII transgene and were CD45.1+, and the remaining 25 % were CD45.1− and lacked expression of HACII. When CD4+ T cells from a TS1 mouse were transferred into these chimeras there was a significant increase in the number of iMO in their pdLN compared to control HA-chimeric mice that had not received T cells (Fig. 5A). However, the frequencies of HA+ to HA− iMO (based on Ly5.1 expression) in the LNs of recipient mice were no different than in chimeras that had been left unmanipulated as controls, and were similar to that of PMN, which were uniformly MHCII− and would therefore not be expected to interact with the transferred T cells in an antigen-specific manner (Fig. 5B). Moreover, MHCII was upregulated by iMO in the spleens of recipient mice independent of HA expression (Fig. 5C).
Figure 5.
IFN-γ promotes in vivo activation of Th17-trophic inflammatory monocytes. (A–C) Lethally irradiated BALB/c mice were reconstituted with mixed bone marrow from HACII.CD45.1+ (75%) and BALB/c.CD45.1− (25%) mice. Four weeks after reconstitution CD4+ T cells from a TS1 mouse were adoptively transferred into the chimeras and data were collected at 7 d post-transfer. (A) Representative flow data showing the accumulation of iMO in the pdLN of chimeric mice at d7 post-transfer. Gating for live CD19−PDCA-1− cells is shown. (B) The frequency of iMO and PMN that were CD45.1+ in the pdLN of chimeric mice at d7 post-transfer. (C) Representative histograms showing MHCII expression by iMO and PMN in the spleens of chimeric mice at d7 post-transfer. Flow data are representative of 3 independent experiments with at least one mouse per condition per experiment. (F–G) As in Figure 4 except mice were treated at d0 and d3 with an anti-IFN-γ neutralizing mAb. (F) Number of iMO in the pdLN of recipient mice treated with anti-IFN-γ or an isotype control mAb. (G) Histograms showing MHCII expression by iMO and cDC in the spleens and pdLN of recipient mice treated with anti-IFN-γ or an isotype control mAb. All numerical data are means ± SEM of at least 3 independent experiments.
We then repeated the approach of transferring CD4+ T cells into HACII.Cα−/− mice, and in this case treated the recipients either with an anti-IFN-γ mAb or an isotype control antibody. The number of iMO was significantly reduced in the pdLN of mice that received the anti-IFN-γ mAb (Fig. 5D). Moreover, there was little or no expression of MHCII on the iMO that were present in either the pdLN or the spleens of mice that were treated with anti-IFN-γ mAb, while MHCII expression by cDC in the spleens of recipient mice was unaffected by anti-IFN-γ treatment (Fig. 5E). Together, these data indicate that IFN-γ produced by CD4+ Th1 cells responding to a self-antigen can play a direct role in the activation of Th17-trophic iMO, and that CD4+ T cell recognition of self-antigen expressed by the iMO themselves is not required for this activation.
Discussion
Inflammatory arthritis is a prominent disease manifestation in a number of systemic autoimmune disorders including rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE). Since MHCII alleles display by far the strongest genetic linkages with diseases such as SLE and RA, it is likely that CD4+ T cell recognition of self-peptides drives these disease processes, but how autoreactive CD4+ T cells can cause both systemic and joint-targeted autoimmune manifestations remains poorly understood, at least partly because the antigens that are recognized by autoreactive CD4+ T cells in human inflammatory arthritis are mostly undefined (18, 19). In TS1xHACII mice, the target autoantigen recognized by autoreactive CD4+ T cells (i.e. HA) is expressed selectively by APCs, which is notable in light of findings indicating that peripheral blood lymphocytes from RA patients exhibit an autologous mixed lymphocyte reaction suggestive of an autoreactive CD4+ T cell response to APCs (and APC-derived peptides) (19, 20). Moreover, in both SLE and RA tissues other than the joints can also be affected by inflammatory processes. Indeed, these autoimmune diseases are typically classified as “systemic” because they involve multiple tissues and organ systems, and it is noteworthy in this regard that TS1xHACII mice develop inflammatory processes affecting the lungs and exhibit additional evidence of systemic immune activation resembling the processes that can occur in patients that develop autoimmune inflammatory arthritis (11). The studies here describe an autoimmune process in which an initial systemic Th1-dominated autoreactive CD4+ T cell response against an APC-derived peptide leads to the activation of iMO that are biased toward inducing Th17 cell responses. Since iMO have an intrinsic capacity to mobilize from sites such as the blood and the spleen to the LNs and tissues, their activation by autoreactive CD4+ T cells provides a basis by which an autoimmune response that is systemic in nature can exhibit regional manifestations
The reason that inflammatory arthritis is a prominent manifestation of the autoimmune response in TS1xHACII mice most likely reflects the sensitivity of the joints to the activities of IL-17 (21). We have shown that blockading IL-17A inhibits arthritis development in TS1xHACII mice, and IL-17 has been shown to promote joint pathology in other mouse models of inflammatory arthritis. In SKG mice (which harbor a mutation in the ZAP-70 gene that causes increased self-reactivity and autoreactive Th17 cell formation) synoviocytes produce CCL20 which attracts CCR6-bearing Th17 cells to the joints (22). In the K/BxN arthritis model IL-17-producing T cells can augment autoantibody-induced arthritis and were enriched in inflamed joints of arthritic mice (23). Increased frequencies of IL-17-secreting CD4+ T cells were similarly found to promote arthritis development in another mouse model, even when these T cells lacked specificity for joint antigens (24). Our data provide evidence that the mobilization of iMO by autoreactive CD4+ T cells can also contribute to the development of a regional autoimmune response in inflammatory arthritis, and resembles studies showing that the inflamed synovium and synovial fluid of patients with active RA contain activated monocytes that specifically promote Th17 responses (25). Increased iMO activity may also promote arthritis development through activation of CD4+ Th17 cells that react with joint-derived antigens, since recent studies in this system suggested that the CD4+ Th17 cells that cause joint inflammation include cells using non-clonotypic TCRs, suggesting the a process of “epitope-spreading” may contribute to the development of arthritis in TS1xHACII mice (9).
As is also the case in RA, TS1xHACII mice develop inflammatory arthritis as adults, and we have shown here that the autoimmune environment is distinct in young pre-arthritic and older arthritic TS1xHACII mice. Thus, the accumulation of autoreactive Th17 cells in the pdLN of arthritic TS1xHACII mice was accompanied by a shift in serum cytokines towards elevated expression of Th17-related cytokines, and away from the elevated levels of Th1-related cytokines found in younger mice. It was also notable that the LNs of pre-arthritic mice exhibited a marked hypocellularity relative to age-matched TS1 controls. This hypocellularity in the LNs, but not in the spleens, could be an indication that the inflammatory environment of young TS1xHACII mice interferes with the formation and/or activity of lymphoid tissue inducer cells, which play a crucial role in LN formation (26). Additional studies will be necessary to determine why LN formation is impaired in young TS1xHACII mice, but at this stage it is notable that impaired LN formation in young TS1xHACII mice may contribute to the delay in arthritis development until TS1xHACII mice become adults.
The biphasic cytokine response in TS1xHACII mice (Th1-dominated in pre-arthritic mice, Th17-dominated in arthritic mice) is also likely a reflection of an autoimmune environment in which various cytokines exert counter-regulatory effects. For example, IFN-γ can be a negative regulator of IL-17 production, and may tend to inhibit IL-17 production at early stages of the autoimmune response (27, 28). The decreased levels of IFN-γ in older mice may in turn be a consequence of increased IL-6 production (which was elevated in the serum of arthritic TS1xHACII mice), since IL-6 has been found to inhibit IFN-γ production and promote Th17 cell formation (4, 29). However, it is also possible that the IFN-γ-induced activation of iMO contributes to this effect, since monocytes secrete a number of inflammatory cytokines that support Th17 responses (including IL-6), and monocyte production of IL-6 is enhanced by IFN-γ (30, 31). Similarly, activated monocytes and other myeloid cells can produce IL-23 which is likely to play an important role in supporting Th17 cell formation, and IL-23 can itself directly and indirectly induce myeloid cell activation, as indicated by studies in which elevated levels of IL-23 were found to lead to arthritis development (32–34). In these respects then, the activation of iMO by autoreactive CD4+ Th1 cells during the initial phases of the autoimmune response may be viewed as both a consequence of, and a contributor to an evolving autoimmune environment.
Supplementary Material
Acknowledgments
Support: This work was supported by grants from the NIH (AI24541), NCI (P30 CA10815), Sibley Memorial Hospital, and the Commonwealth of Pennsylvania to AC. DMS was supported by NCI T32 CA09171.
Abbreviations used in this article
- HA
hemagglutinin
- iMO
inflammatory monocyte
- MHCII
class II MHC
- LN
lymph node
- pdLN
paw draining LN
- cDC
conventional dendritic cell
- PMN
polymorphonuclear cell
- RA
rheumatoid arthritis
- SLE
systemic lupus erythematosus
References
- 1.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu. Rev. Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 2.Peck A, Mellins ED. Precarious balance: Th17 cells in host defense. Infect. Immun. 2010;78:32–38. doi: 10.1128/IAI.00929-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 4.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 5.McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O’Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat. Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, Littman DR, Benoist C, Mathis D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nepom GT, Byers P, Seyfried C, Healey LA, Wilske KR, Stage D, Nepom BS. HLA genes associated with rheumatoid arthritis. Identification of susceptibility alleles using specific oligonucleotide probes. Arthritis Rheum. 1989;32:15–21. doi: 10.1002/anr.1780320104. [DOI] [PubMed] [Google Scholar]
- 9.Oh S, Aitken M, Simons DM, Basehoar A, Garcia V, Kropf E, Caton AJ. Requirement for diverse TCR specificities determines regulatory T cell activity in a mouse model of autoimmune arthritis. J. Immunol. 2012;188:4171–4180. doi: 10.4049/jimmunol.1103598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirberg j, Baron A, Jakob S, Rolink A, Karjalainen K, von Boehmer H. Thymic selection of CD8+ single positive cells with a class II major histocompatibility complex-restricted receptor. J. Exp. Med. 1994;180:25–34. doi: 10.1084/jem.180.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rankin AL, Reed AJ, Oh S, Cozzo PC, Guay HM, Larkin J, iii, Panarey L, Aitken MK, Koeberlein B, Lipsky PE, Tomaszewski JE, Naji A, Caton AJ. CD4+ T Cells Recognizing a Single Self-Peptide Expressed by APCs Induce Spontaneous Autoimmune Arthritis. J. Immunol. 2008;180:833–841. doi: 10.4049/jimmunol.180.2.833. [DOI] [PubMed] [Google Scholar]
- 12.Mombaerts P, Clarke AR, Rudnicki MA, Iacomini J, Itohara S, Lafaille JJ, Wang L, Ichikawa Y, Jaenisch R, Hooper ML, Tonegawa S. Mutations in T-cell antigen receptor genes alpha and beta block thymocyte development at different stages. Nature. 1992;360:225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- 13.Hirota k, Hashimoto M, Yoshitomi H, Tanaka S, Nomura T, Yamaguchi T, Iwakura Y, Sakaguchi N, Sakaguchi S. T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis. J. Exp. Med. 2007;204:41–47. doi: 10.1084/jem.20062259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 15.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat. Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 16.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 17.Schwarzenberger P, La Russa V, Miller A, Ye P, Huang W, Zieske A, Nelson S, Bagby GJ, Stoltz D, Mynatt RL, Spriggs M, Kolls JK. IL-17 stimulates granulopoiesis in mice: use of an alternate, novel gene therapy-derived method for in vivo evaluation of cytokines. J. Immunol. 1998;161:6383–6389. [PubMed] [Google Scholar]
- 18.Buckner JH, Nepom GT. Genetics of rheumatoid arthritis: is there a scientific explanation for the human leukocyte antigen association? Curr. Opin. Rheumatol. 2002;14:254–259. doi: 10.1097/00002281-200205000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Goronzy JJ, Weyand CM. Developments in the scientific understanding of rheumatoid arthritis. Arthritis Res. Ther. 2009;11:249. doi: 10.1186/ar2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas R, Lipsky PE. Could endogenous self-peptides presented by dendritic cells initiate rheumatoid arthritis? Immunol. Today. 1996;17:559–564. doi: 10.1016/s0167-5699(96)20030-1. [DOI] [PubMed] [Google Scholar]
- 21.LubbertsE E, Koenders MI, van den Berg WB. The role of T-cell interleukin-17 in conducting destructive arthritis: lessons from animal models. Arthritis Res. Ther. 2005;7:29–37. doi: 10.1186/ar1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, Yamaguchi T, Nomura T, Ito H, Nakamura T, Sakaguchi N, Sakaguchi S. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J. Exp. Med. 2007;204:2803–2812. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobs JP, Wu H-J, Benoist C, Mathis D. IL-17-producing T cells can augment autoantibody-induced arthritis. Proc. Natl. Acad. Sci. U.S.A. 2009;106:21789–21794. doi: 10.1073/pnas.0912152106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murakami M, Okuyama Y, Ogura H, Asano S, Arima Y, Tsuruoka M, Harada M, Kanamoto M, Sawa Y, Iwakura Y, Takatsu K, Kamimura D, Hirano T. Local microbleeding facilitates IL-6- and IL17-dependent arthritis in the absence of tissue antigen recognition by activated T cells. J. Exp. Med. 2011;208:103–114. doi: 10.1084/jem.20100900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans HG, Gullick NJ, Kelly S, Pitzalis C, Lord GM, Kirkham BW, Taams LS. In vivo activated monocytes from the site of inflammation in humans specifically promote Th17 responses. Proc. Natl. Acad. Sci. U.S.A. 2009;106:6232–6237. doi: 10.1073/pnas.0808144106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu. Rev. Immunol. 2012;30:647–675. doi: 10.1146/annurev-immunol-020711-075053. [DOI] [PubMed] [Google Scholar]
- 27.Chu CQ, Swart D, Alcorn D, Tocker J, Elkon KB. Interferon-gamma regulates susceptibility to collagen-induced arthritis through suppression of interleukin-17. Arthritis Rheum. 2007;56:1145–1151. doi: 10.1002/art.22453. [DOI] [PubMed] [Google Scholar]
- 28.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2006;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diehl S, Anguita J, Hoffmeyer A, Zapton T, Ihle JN, Fikrig E, Rincon M. Inhibition of Th1 differentiation by IL-6 is mediated by SOCS1. Immunity. 2000;13:805–815. doi: 10.1016/s1074-7613(00)00078-9. [DOI] [PubMed] [Google Scholar]
- 30.Danis VA, Millington M, Hyland VJ, Grennan D. Cytokine production by normal human monocytes: inter-subject variation and relationship to an IL-1 receptor antagonist (IL-1Ra) gene polymorphism. Clin. Exp. Immunol. 1995;99:303–310. doi: 10.1111/j.1365-2249.1995.tb05549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biondillo DE, Konicek SA, Iwamoto GK. Interferon-gamma regulation of interleukin 6 in monocytic cells. Am. J. Physiol. 1994;267:L564–L568. doi: 10.1152/ajplung.1994.267.5.L564. [DOI] [PubMed] [Google Scholar]
- 32.McGeachy MJ, Cua DJ. The link between IL-23 and Th17 cell-mediated immune pathologies. Semin. Immunol. 2007;19:372–376. doi: 10.1016/j.smim.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 33.Sherlock JP, Joyce-Shaikh B, Turner SP, Chao C-C, Sathe M, Grein J, Gorman DM, Bowman EP, McClanahan TK, Yearley JJH, Eberl G, Buckley CD, Kastelein RA, Pierce RH, Laface DM, Cua DJ. IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+CD4-CD8- entheseal resident T cells. Nature Med. 2012;18:1069–1076. doi: 10.1038/nm.2817. [DOI] [PubMed] [Google Scholar]
- 34.Adamopoulos IE, Tessmer M, Chao C-C, Adda S, Gorman D, Petro M, Chou C-C, Pierce RH, Yao W, Lane NE, Laface D, Bowman EP. IL-23 is critical for induction of arthritis, osteoclast formation, and maintenance of bone mass. J. Immunol. 2011;187:951–959. doi: 10.4049/jimmunol.1003986. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.