Abstract
Introduction
Nerve conduction studies provide information regarding the status of the peripheral nerve, but relationships with sensorimotor capacities that influence mobility have not been defined.
Methods
A secondary analysis was conducted of data from 41 older subjects (20 women, age 69.1 ± 8.3 years), 25 with diabetic neuropathy of varying severity, and 16 without diabetes or neuropathy. Measurements included routine fibular motor nerve conduction studies and laboratory-based determination of ankle inversion/eversion proprioceptive thresholds and ankle inversion/eversion motor function.
Results
Independent of age, fibular amplitude correlated robustly with ankle inversion/eversion proprioceptive thresholds (R2 = .591, p < .001), moderately with ankle inversion and eversion rates of torque generation (R2 = .216; p = .004 and R2 = .200; p = .006, respectively), and more strongly when fibular motor amplitude was normalized for body mass index (R2 = .350; p < .001 and R2 = .275; p = .001).
Discussion
Fibular motor amplitude was strongly associated with ankle sensorimotor capacities that influence lateral balance and recovery from perturbations during gait. The results suggest that nerve conduction study measures have potential for an expanded clinical role in evaluating mobility function in the population studied.
Keywords: Age, Balance, Diabetic Neuropathy, Muscle Strength, Proprioception
INTRODUCTION
Nerve conduction studies (NCS) have been used effectively to provide clinicians and researchers with information regarding the status of peripheral nerves. Despite being a physiologic study, NCS results correlate well with histologic and ultrasonographic findings.1,2 However, less research has been devoted to the ability of NCS to predict the sensorimotor capacities of a limb. The precise relationship between lower limb nerve conduction studies commonly used in clinical practice and lower limb sensorimotor function that influences mobility has not been defined clearly.
In general, prior work in the area suggests that compound muscle action potential (CMAP) amplitudes correlate with strength. For example, in 2 studies of patients with chronic inflammatory demyelinating polyradiculopathy, CMAP amplitude correlated with grip strength3 and overall muscle strength determined by dynamometry.4 Similarly, grip strength and CMAP amplitude were related in patients with Charcot-Marie-Tooth disease.5 Significant relationships between NCS and strength have also been found in focal peripheral neurologic disorders. Grip strength and CMAP amplitude correlated with one another in rats undergoing transection and repair of the median nerve,6 and strength of the first dorsal interosseous muscle was related to percentage of conduction block in subjects with ulnar neuropathy at the elbow.7 However, no studies have defined the relationship between a routine lower limb nerve conduction study and ankle sensory or motor function in humans.
It is well recognized that patients with diabetes mellitus experience a decrement in muscle strength. This decrement is more marked in the lower limbs,8 seems to affect those with symptomatic neuropathy most severely9 and is responsible for a diabetes-related decline in function with aging.10 This point is underscored by studies that confirm a markedly increased rate of injurious falls in older persons with neuropathy.11,12 Lateral falls are most likely to cause injury,13 and it is increasingly recognized that a decrement in lateral (or frontal plane) control leads to fall-related injury.14 As a result, the relationships between routine nerve conduction studies and ankle sensorimotor function relevant to frontal plane control are of clinical relevance. Therefore, the objective of this study was to identify relationships between NCS of the fibular nerve and ankle sensorimotor function relevant to frontal plane postural control in a secondary analysis of data from a group of subjects that included healthy older persons and older persons with a spectrum of distal symmetric polyneuropathy (DSPN) due to diabetes mellitus. It was hypothesized that increasing fibular motor amplitude (FMAmp) and conduction velocity (FMCV) would correlate with smaller (more precise) ankle proprioceptive thresholds (H1) and greater ankle strength (H2). The effect of age and ankle flexibility, both of which could potentially confound the relationships between NCS measures and ankle sensorimotor capacities, were also evaluated. It was hypothesized NCS measures would predict ankle sensorimotor capacities independent of the effect of age (H3a) and ankle flexibility (H3b).
MATERIALS AND METHODS
The subjects and methods for determining frontal plane ankle sensorimotor functions have been described previously.15
Subjects
Subjects were recruited from the University of Michigan Orthotics and Prosthetics Clinic, Endocrinology Clinic and the Older Americans Independence Center Human Subjects Core. The research protocol was approved by the University of Michigan Institutional Review Board, and all subjects provided written informed consent. Eligible subjects were between age 50 and 85 years, weighed not greater than 136 kilograms, had not fallen within the month prior to participation, and were free of central neurologic disease, vestibular disorders, symptomatic coronary artery disease, plantar skin sores or joint replacement within the previous year, symptomatic postural hypotension, severe musculoskeletal deformity (for example, amputation or Charcot changes), lower extremity or back pain that limited standing to less than 10 minutes, were able to walk 1 block or more and had greater than anti-gravity ankle strength (grade ≥ 3 by manual muscle testing). Subjects with DSPN had a history of type 2 diabetes mellitus confirmed by review of records and the ongoing use of oral hypoglycemic agents or insulin. The presence of a distal symmetric peripheral neuropathy (DSPN) was confirmed by: a) symptoms (subject reported altered sensation in the distal lower limbs); b) signs (Michigan Diabetes Neuropathy Score; MDNS) ≥ 10;16 c) bilaterally abnormal fibular motor NCS (recording over the extensor digitorum brevis, defined as amplitude < 2.0 mV, latency > 6.2 milliseconds, and/or conduction velocity < 41.0 m/s, using Nicolet Viking 4). Subjects without DSPN had no history of diabetes mellitus, no symptoms or signs of DSPN (MDNS < 10), and normal fibular NCS. Subjects were excluded if they reported a fall within 1 month of testing. As part of the initial examination, ankle range of motion was determined for dorsiflexion, inversion and eversion using standard goniometric techniques.17
Nerve Conduction Studies
Fibular motor NCS parameters served as independent variables. The limbs were warmed to 32.0 degree C as is standard, and temperatures were re-evaluated at the end of the studies and recorded. The recording electrode was placed over the extensor digitorum brevis muscle with the reference electrode distally over extensor tendon. The fibular nerve was stimulated 9 cm proximally and then, assuming a CMAP was obtained, just distal to the fibular head. Fibular CMAP amplitude (PMAmp) was measured from baseline to negative peak, and latencies and conduction velocities were determined in the usual fashion. Studies were all performed by the first author approximately 7 to 14 days prior to measurement of ankle sensorimotor functions.
Ankle Sensory Function
Frontal plane ankle proprioceptive threshold was determined while the subjects were standing, with the test foot in a 40 × 25 cm cradle which was rotated by an Aerotech 1000 servomotor equipped with an 8000-line rotary encoder. After an audible warning, a single ankle inversion or eversion rotation of 0.1 degree to 3 degrees was randomly presented at 5 degrees/s. The subject then tilted a joystick handle in the perceived direction of foot rotation. Four blocks of 25 trials (randomly, 10 eversion, 10 inversion, and 5 neutral, or dummy, trials) were presented interspersed with 2–5-min rest periods. The outcome measure was the ankle proprioceptive threshold, defined as the smallest rotational displacement of the ankle that a subject could reliably detect with 100% accuracy.
Ankle Motor Function
Ankle motor function was evaluated to determine maximal voluntary contraction (MVC) and rate of torque development (RTD). During MVC testing, subjects stood on a force platform touching the hand rails on both sides as needed. Subjects were then asked to lift one leg, shift their center of gravity as far lateral under their foot as they could, and lift their hands from the rails for 3 s. After a practice trial to confirm that the subject understood directions, the test was repeated 3 times for the lateral, and then likewise repeated for the medial margin of the foot. During testing of ankle frontal plane RTD, subjects stood on the test foot on a force plate (OR-6; Advanced Mechanical Technology, Inc.) and moved the center of ground support reaction from the lateral margin of the foot (by ankle inversion) to the medial margin (by ankle eversion) as quickly as possible, then again to the lateral margin, as described in prior work. Subjects were provided with real time visual feedback of the location of the center of ground reaction to inform their efforts. After a practice trial to confirm that the subject understood directions, 3 trials, each with 5 medial–lateral movements, were performed. Subjects were allowed to touch a horizontal railing to keep their balance. (Figure 1)
Figure 1.
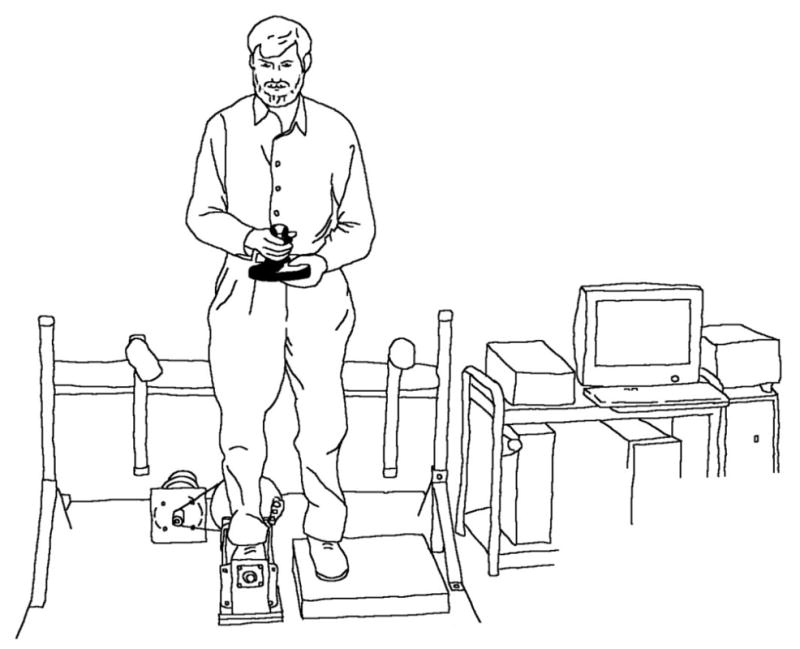
Device for measuring ankle inversion/eversion proprioceptive thresholds. Reproduced with permission from Son et al.37
Data Processing
Signals were amplified to volt levels before being acquired using a 12-bit analog-to-digital converter which sampled at 100 HZ. The MVC efforts at the ankle, as well as the maximal RTD, were normalized by individual body size, defined as body height multiplied by weight (units of N-m). Strength data were processed using a second-order least-squares polynomial fit (LabVIEW) to determine the peak value. The mean peak value obtained from the 3 trials for each test was used for the statistical analyses. To determine each proprioceptive threshold, the mean threshold perceived with 100% accuracy from the 4 blocks of 25 trials in each test direction was calculated. An overall measure of ankle proprioception was found from the sum of the inversion and eversion proprioception thresholds.
Statistical Analyses
SPSS 19.0 for Windows was used for all analyses (SPSS, Inc., Chicago, Illinois). FMAmp and FMCV were independent variables, while age and ankle range of motion were considered as covariates. Dependent variables included ankle proprioceptive threshold (the sum of inversion and eversion thresholds), ankle inversion and eversion MVC, and ankle inversion and eversion RTD. The data were evaluated for normality via measures of skewness and kurtosis, and the presence of outliers by visual inspection; no data points were excluded. Descriptive statistics were calculated for all variables. Pearson product correlation coefficients were determined and used to identify relationships between the independent and dependent variables and between the covariates age and range of motion and the dependent variables. When the independent variable and a covariate both demonstrated significant relationships with a dependent variable, multiple regression was used to establish the independence and relative strength of those relationships.
RESULTS
Subjects
Ninety-one subjects were considered for study. Of these, 21 failed telephone screening, and 18 declined participation. Of the 52 remaining subjects, 3 had scheduling conflicts, and 5 failed the screen. Of those 44 remaining subjects, 1 was lost to follow-up, and 2 discontinued participation due to medical concerns. Therefore, 41 subjects were evaluated fully. Technical difficulties led to absence of data for 1 subject. The means and standard deviations of age, body mass index (BMI), and MDNS, together with the participant fibular NCS values, are shown in Table 1. Of the 25 subjects with diabetes, subject report of disease duration was 19 + 13.2 years, and duration of insulin use among the 13 subjects requiring it was 13.5 + 16.5 years. Subjects reported common symptoms ranging from numbness in the forefeet to numbness distal to the knees, and 11 subjects reported neuropathic pain or burning.
Table 1.
Demographic information and fibular nerve conduction study measures of the study subjects (Mean±SD)
| Parameter | Non-Diabetic Subjects
|
Diabetic Subjects
|
||||
|---|---|---|---|---|---|---|
| All n = 16 | Men n = 6 | Women n = 10 | All n = 25 | Men n = 15 | Women n = 10 | |
|
|
|
|||||
| Age (Yrs) | 67.8 ± 9.0 | 67.8 ± 11.0 | 67.8 ± 8.0 | 70.0 ± 8.2 | 71.5 ± 7.2 | 67.8 ± 9.4 |
| BMI* (Kg/m2) | 28.4 ± 7.2 | 26.2 ± 3.3 | 29.6 ± 8.7 | 32.4 ± 6.4 | 30.3 ± 5.4 | 35.7 ± 6.8 |
| MDNS (0 – 46) | 1.7 ± 3.8 | 2.5 ± 7.1 | 1.2 ± 1.5 | 13.6 ± 6.0 | 14.1 ± 6.5 | 12.7 ± 5.5 |
| FMCV** (m/s) | 44.9 ± 5.3 | 41.6 ± 6.6 | 46.5 ± 4.1 | 37.2 ± 5.6 | 37.2 ± 4.5 | 37.5 ± 7.6 |
| FMAmp (mV) | 4.7 ± 1.6 | 4.7 ± 2.1 | 4.7 ± 1.5 | 2.4 ± 2.1 | 2.0 ± 1.7 | 2.8 ± 2.6 |
| FMAmpBMI (mV/BMI) | 17.7 ± 8.4 | 18.7 ± 10.6 | 17.1 ± 7.7 | 7.5 ± 7.1 | 6.5 ± 5.4 | 8.8 ± 9.0 |
Mean Body Mass = 31.0 + 6.9 kg
Diabetic group vs. non-Diabetic group differences in FMCV, FMAmp and PMAmpBM were all significant (< .001).
H1: Fibular NCS Measures and Ankle Proprioceptive Thresholds
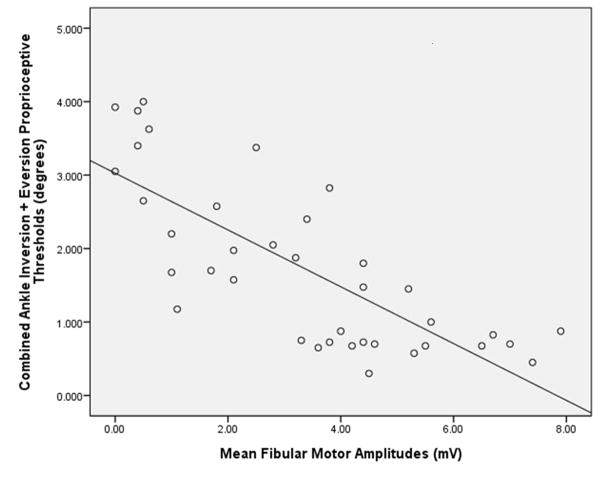
PNCV demonstrated a significant negative relationship with ankle proprioceptive thresholds (i.e., increased conduction velocity was associated with smaller, or more precise, proprioceptive thresholds) with the former explaining about 25% of the latter. (Table 2) FMAmp demonstrated a robust relationship with ankle proprioceptive thresholds, explaining almost 60% of its variability. (Figure 2)
Table 2.
Relationships between fibular nerve conduction study measures and ankle sensorimotor capacities
| Conduction Velocity R/p value/R2 N = 35 |
Amplitude R/p value/R2 N = 40 |
Amplitude nBMI R/p value/R2 N = 40 |
|
|---|---|---|---|
| Ankle Inv/Ev Prop Th | −.493/.003/.243 | −.768/<.001/.591 | −.679/0.001/.461 |
| Ankle Inversion MVC | −.063/.736/.004 | .310/.070/.096 | .367/.030/.135 |
| Ankle Eversion MVC | .171/.350/.029 | .368/.025/.135 | .379/.021/.144 |
| Ankle Inversion RTD | .306/0.089/.094 | .465/.004/.216 | .592/<.001/.350 |
| Ankle Eversion RTD | .342/.055/.117 | .447/.006/.200 | .524/.001/.275 |
Inv/Ev Prop Th = Ankle Inversion/Eversion Proprioceptive Threshold; MVC = Maximum Voluntary Contraction; RTD = Rate of Torque Development; nBMI = normalized for BMI
Figure 2.
Scatterplot with a regression line depicting the relationship between fibular motor amplitudes (millivolts) and frontal plane ankle proprioceptive thresholds (degrees; R2 = 0.591)
H2: Fibular NCS Measures and Ankle Motor Function
FMCV demonstrated no significant relationship with ankle inversion or eversion MVC. FMAmp demonstrated statistically significant, but weak, relationships with these same motor functions. Similarly, FMCV showed significant but weak relationships with ankle inversion and eversion RTD. However FMAmp significantly correlated with ankle inversion and eversion RTD. When FMAmp was normalized for BMI (fibular amplitude/BMI × 100; FAmpBMI), the correlations became stronger and explained over a third of the ankle inversion and eversion RTD.
H3.a.: Ankle Sensorimotor Function and Age
There were no significant relationships between age and any of the ankle sensorimotor functions except for ankle inversion RTD. In that instance the 2 variables showed a significant negative correlation of 0.331 (p = .039). When age was introduced into a regression equation with FMAmp and FMAmpBMI, the significance of these measures remained (p = <.001 and .006, respectively) and that of age did not (p = .051 and .060, respectively).
H.3.b.: Ankle Sensorimotor Function and Ankle Range of Motion
Dorsiflexion range of motion demonstrated significant correlations with proprioceptive threshold (−.363/.018), inversion RTD (.435/.006) and eversion RTD (.331/.040). However, all became insignificant when entered into a regression model including FMAmp or FMAmpBMI. No significant relationships between any of the ankle sensorimotor function measures and ankle inversion or eversion range of motion were identified.
Post-Hoc Analysis: Considering Subjects With and Without DSPN Separately
Although the study was not designed or planned for the purpose of analyzing fibular NCS and ankle sensorimotor functions within the more narrow ranges of peripheral neurologic health offered by the subjects with and without, DSPN, such an analysis is of interest to clinicians. Therefore, a post-hoc analysis was performed to determine whether relationships between NCS and ankle sensorimotor function remained when subjects with and without diabetic DSPN were considered separately. Among subjects with DSPN (n = 25) ankle proprioceptive threshold and FMAmp were strongly correlated (−.660/.000). However, the relationships between ankle inversion RTD and FMAmp and FMAmpBMI were not significant (.205/.326 and .273/.186, respectively).
When subjects without DSPN (n = 16) were considered separately, there were negative relationships between ankle proprioceptive threshold and FMCV (r/p = −.737/.002) and FMAmp (−.494/.061). There were also significant relationships between ankle inversion RTD and FMAmp (0.630/.028), and FAmpBMI (0.674/.016).
Summary of Results
In summary, FMAmp correlated more strongly with ankle sensorimotor functions than did FMCV. FMAmp demonstrated a strong inverse relationship with frontal plane ankle proprioceptive threshold, predicting almost 60% of the proprioceptive threshold. The indirect relationship indicates that, as FMAmp increases, the proprioceptive threshold decreases or becomes more precise. This same relationship between FMAmp and ankle sensory function was present when the subjects with and without DSPN were considered separately. FMAmp also correlated significantly with ankle motor function, but the relationship was not as robust. More specifically, FMAmp predicted only 10 to 15% of ankle inversion or eversion MVC. However, the relationships between FMAmp and ankle RTD were stronger; the former predicted about 20% of the latter. When FMAmp was normalized for BMI it then explained approximately 1/3 of ankle inversion and eversion RTD.
DISCUSSION
In this study of a group of older persons with a spectrum of peripheral neurologic health and function, FMAmp demonstrated a strong relationship with frontal plane ankle proprioceptive thresholds, moderate relationships with frontal plane ankle RTD and statistically significant, but relatively weak, relationship with ankle MVC. These findings support the hypotheses formulated prior to the study. The relationship between FMAmp and ankle motor function was stronger when the former was normalized for BMI. FMCV was significantly and negatively correlated with ankle proprioceptive threshold, but the strength of the relationship was much weaker than between FMAmp and proprioceptive threshold. Contrary to the hypothesis, FMCV did not correlate with measures of ankle motor function, although there were trends with regard to ankle inversion/eversion RTD. Importantly, neither age nor ankle range of motion significantly influenced any of the ankle sensorimotor capacities when FMAmp or FMAmpBMI were taken into account. Lastly, the findings persisted to a large degree when the subjects with and without diabetic DSPN were considered separately.
The findings relating frontal plane proprioception at the ankle with FMAmp are novel and somewhat surprising, given that only motor nerve function was evaluated. The findings suggest a close relationship between the FMAmp and large fiber sensory function. Similar findings were noted in patients with chronic inflammatory demyelinating polyneuropathy in that significant correlations between sensory sum scores and summated CMAPs in the lower limbs were identified.18 In addition, FMAmp was just as effective at predicting ankle inversion motor function as it was ankle eversion motor function. This was found despite the former motion being produced by the tibial-innervated posterior tibialis muscle along with the fibular-innervated anterior tibialis muscle, while the latter is produced almost exclusively by the fibular-innervated fibularis longus. Taken together the data suggest that FMAmp is a representation of general ankle sensorimotor function in the population studied and is not solely a reflection of the function of the fibular motor nerve itself.
The findings relating strength and FMAmp are consistent with other work. For example Andersen and Mogensen found that decreased peak velocity of ankle dorsal and plantar flexors correlated with neuropathy severity in a group of subjects with longstanding diabetes mellitus.19 In other work, FMAmp showed significant negative correlations with nerve fiber density, a measure of collateral sprouting, which in turn showed negative correlations with ankle dorsiflexion peak torque.20 Additionally, a strong negative relationship between foot muscle volume and a neuropathy severity score (which included nerve conduction studies) was identified.21 Lastly, in a large study of older adults with and without diabetes mellitus, FMAmp significantly correlated with ankle dorsiflexion strength, and this relationship remained when only subjects without diabetes mellitus were included.22 Additionally, the presence of a DSPN without diabetes increased the risk of physical decline.23 Taken together, the data suggest a strong relationship between PMAmp and ankle motor function, and this appears to be true regardless of the presence of diabetes mellitus. In contrast to the other work, we evaluated ankle motor function in the frontal plane using a closed chain technique and normalized the strength for BMI. The fact that FMAmp remained strongly correlated with ankle strength despite between-study differences in measurement technique suggests the finding is robust in older persons with and without diabetes mellitus.
FMCV showed weaker relationships than FMAmp for all ankle sensorimotor capacities. Although we are not aware of any other work relating nerve conduction studies and proprioceptive thresholds, others have also noted that the relationship between ankle motor function and FMCV is weaker than that between ankle motor function and FMAmp.22,24,25 The findings are consistent with a decreased FMAmp being a more reliable indicator of axon loss, which is directly related to loss of strength, than demyelination which is less strongly related to strength loss.26 Other possible reasons for this finding include a positive relationship between FMAmp and calf muscle density in older men and women27 and an inverse relationship between FMAmp and nerve fiber density, a measure of collateral reinnervation in diabetic DSPN (20). Therefore FMAmp appears to be a marker for axon loss, leg muscle density and collateral reinnervation. All 3 of these factors would be expected to negatively impact strength and, in the case of the last, particularly influence rate of torque generation given the temporal desynchronization of muscle fiber depolarization in the setting of extensive collateral reinnervation.
The relationships between FMAmp and FMAmpBMI and ankle sensorimotor capacities appear to have been confounded by neither age nor range of motion. Although aging reduces rapid generation of ankle torque,28 the independent effect of FMAmp on ankle force generation is consistent with prior evidence that diminished peripheral neurologic function independently diminished lower limb physical function in older women.10 The relationship between ankle range of motion and sensorimotor function has not been explored thoroughly, however, the plausibility of a negative relationship between range of motion and ankle motor function is suggested by findings of a strong relationship between diabetes mellitus, reduced ankle range of motion, and degenerative change in tendons in older persons.29 Other factors associated with diabetes may have influenced the findings. For example, diabetes-associated neurocognitive factors may have diminished attention, which could have influenced the data, particularly the proprioceptive threshold protocol which requires concentration for an extended period.
It is notable that RTD, a more dynamic measure of motor function than MVC, correlated more strongly with PMAmp than did MVC. This finding is consistent with other work which suggests that Type II fast twitch fibers are preferentially lost in the setting of distal denervation and aging.30,31 Given the time critical nature of neuromuscular responses to loss of balance and perturbation during gait,28,32,33 the clinical relevance of the relationship between FMAmp and ankle motor function increases.
The findings have potential for clinical application. By their nature, clinical estimates of lower limb proprioceptive function are subjective. The objective nature of the fibular nerve conduction study allows a greater degree of certainty regarding proprioceptive function than is likely possible using other routinely available clinical techniques. This is particularly true given the strength of the association between FMAmp and proprioceptive thresholds. Although less strong, the relationship between PAmp and ankle inversion/eversion RTD, particularly when normalized for BMI, is still significant. Given the time critical nature of effective neuromuscular responses to loss of balance, frontal plane ankle RTD must be robust to reliably perform single limb stance and tolerate perturbations during gait without sacrificing gait speed or efficiency.28,32,33,34 Given this, and the clear association between reduced lower limb proprioception and falls,35 FMAmp appears to have potential for an expanded clinical role, extending beyond the provision of information regarding the presence or absence of DSPN and the health of the specific fibular nerve tested. If so, then decreased FMAmp may be an objective marker of patients with mild balance impairment, a group for whom a simple home exercise program can be helpful.36
This study has a number of limitations. Although the sample size is relatively large given the highly quantitative and technical nature of the sensorimotor measurements, the subjects studied may not accurately reflect the population of older persons in general, resulting in sampling error. The measurement of ankle motor function in a closed chain fashion (i.e., with the subject standing) is a more physiologic method given the role of the ankle muscles during the stance phase of the gait cycle; however, the technique may include some force from frontal plane hip muscles, and full muscle activation could not be confirmed. If so, it would be anticipated that the ability of FMAmp to predict ankle motor function would be diminished. In addition, the investigators were not blinded to subject status, and the study coordinator was often present for NCS and, one to two weeks later, determination of ankle sensorimotor function. Bias could have been introduced in this manner, although this is not likely, given that the results of the quantitative sensorimotor measurements were not available until data analyses were done well after the testing was completed. Another limitation is the decision to study lower limb capacities involved in frontal plane control in contrast to the typically studied muscles of sagittal plane control (e.g., knee extensors, ankle dorsi/plantar flexors). As a result, we cannot report on the relationship between nerve conduction studies and ankle dorsi/plantar flexion proprioceptive thresholds or motor function, and the importance of rapid ankle plantar flexor force generation has been demonstrated in the recovery from an anterior perturbation.32,37 Only the fibular nerve was studied, as the data represent a secondary analysis from related research. It is possible that similar or stronger relationships with other NCS measures such as tibial motor and sural sensory NCS exist.
In summary, the data suggest a strong negative relationship between FMAmp and ankle inversion/eversion proprioceptive thresholds, and moderate positive relationships between FMAmp and ankle inversion/eversion RTD, particularly when FMAmp is normalized for BMI. These findings appear to be independent of age, ankle range of motion and the presence of diabetic neuropathy. Future work should confirm these findings and evaluate the utility of other routine lower limb NCS in predicting quantified sensorimotor function. If the results reported in this study are confirmed in future studies, then older patients with low FMAmp responses can be assumed to have impairments in frontal plane ankle sensorimotor function that impacts lateral balance. Recent research suggests that strengthening frontal plane muscles at the hip (adductor/abductor) may allow compensation for such distal impairments.15
Acknowledgments
Support provided by National Institutes of Health (RO1 AG026569-01), PHS grants (P30AG024824), and the Swiss National Foundation (IZK)Z3_133925).
The authors also acknowledge James A. Albers, M.D., PhD for expert opinion and manuscript review.
Abbreviations used
- CMAP
Compound Muscle Action Potential
- DSPN
Distal Symmetric Peripheral Neuropathy
- MVC
Maximum Voluntary Contraction
- NCS
Nerve Conduction Studies
- FMAmp
Fibular Motor Amplitude
- FMAmpBMI
Fibular Motor Amplitude normalized for BMI
- FMCV
Fibular Motor Conduction Velocity
- RTD
Rate of Torque Development
Footnotes
A portion of the material within the manuscript was presented at the Association of Academic Physiatrists meeting March 1, 2012 in Las Vegas, Nevada.
References
- 1.van Veen BK, Schellens RL, Stegeman DF, Schoonhoven R, Gabreels-Festen AA. Conduction velocity distributions compared to fiber size distributions in normal human sural nerve. Muscle & Nerve. 1995;18(10):1121–7. doi: 10.1002/mus.880181008. [DOI] [PubMed] [Google Scholar]
- 2.Bayrak AO, Bayrak IK, Turker H, Elmali M, Nural MS. Ultrasonography in patients with ulnar neuropathy at the elbow: comparison of cross-sectional area and swelling ratio with electrophysiological severity. Muscle & Nerve. 2010;41(5):661–6. doi: 10.1002/mus.21563. [DOI] [PubMed] [Google Scholar]
- 3.Bril V, Banach M, Dalakas MC, Deng C, Donofrio P, Hanna K, Hurtung HP, Hughes RAC, Katzberg HL, Norman M, Ingemar SJ, Van Doorn PA. Electrophysiologic correlations with clinical outcomes in CIDP. Muscle & Nerve. 2010;42(4):492–7. doi: 10.1002/mus.21733. [DOI] [PubMed] [Google Scholar]
- 4.Harbo T, Andersen H, Jakobsen J. Length-dependent weakness and electrophysiological signs of secondary axonal loss in chronic inflammatory demyelinating polyradiculoneuropathy. Muscle & Nerve. 2008;38(2):1036–45. doi: 10.1002/mus.21000. [DOI] [PubMed] [Google Scholar]
- 5.Videler AJ, van Dijk JP, Beelen A, deVisser M, Nollet F, vanSchaik IN. Motor axon loss is associated with hand dysfunction in Charcot-Marie Tooth disease 1a. Neurology. 2008;71(16):1254–60. doi: 10.1212/01.wnl.0000327643.05073.eb. [DOI] [PubMed] [Google Scholar]
- 6.Wang H, Sorenson EJ, Spinner RJ, Windebank AJ. Electrophysiologic findings and grip strength after nerve injuries in the rat forelimb. Muscle & Nerve. 2008;38(4):1254–65. doi: 10.1002/mus.20971. [DOI] [PubMed] [Google Scholar]
- 7.Allen MD, Doherty TJ. Assessing weakness in patients with ulnar neuropathy: comparison between a custom hand muscle dynamometer and a pinch dynamometer. Am J Phys Med Rehabil. 2011;90:923–9. doi: 10.1097/PHM.0b013e31822415b6. [DOI] [PubMed] [Google Scholar]
- 8.Andersen H, Nielsen S, Mogensen CE, Jakobsen J. Muscle strength in type 2 diabetes. Diabetes. 2004;53(6):1543–8. doi: 10.2337/diabetes.53.6.1543. [DOI] [PubMed] [Google Scholar]
- 9.Andreassen CS, Jakobsen J, Andersen H. Muscle weakness: a progressive late complication in diabetic distal symmetric polyneuropathy. Diabetes. 2006;55(3):806–12. doi: 10.2337/diabetes.55.03.06.db05-1237. [DOI] [PubMed] [Google Scholar]
- 10.Resnick HE, Stansberry KB, Harris TB, Tirivedi M, Smith K, Morgan P, Vinik AI. Diabetes, peripheral neuopathy, and old age disability. Muscle & Nerve. 2002;25(1):43–50. doi: 10.1002/mus.1217. [DOI] [PubMed] [Google Scholar]
- 11.Richardson JK, Ching C, Hurvitz EA. The relationship between electromyographically documented peripheral neuropathy and falls. J Amer Geriatr Soc. 1992;40(10):1008–1012. doi: 10.1111/j.1532-5415.1992.tb04477.x. [DOI] [PubMed] [Google Scholar]
- 12.Cavanagh PR, Derr JA, Ulbrecht JS, Maser RE, Orchard TJ. Problems with gait and posture in neuropathic patients with insulin-dependent diabetes mellitus. Diabetes Med. 1992;9:469–474. doi: 10.1111/j.1464-5491.1992.tb01819.x. [DOI] [PubMed] [Google Scholar]
- 13.Greenspan SL, Meyers ER, Maitland LA, Resnick NJ, Hayes WC. Fall severity and bone mineral density as risk factors for hip fracture in ambulatory elderly. JAMA. 1994;271(2):128–33. [PubMed] [Google Scholar]
- 14.Hilliard MJ, Martinez KM, Janssen I, Edwards B, Mille ML, Zhang Y, et al. Lateral balance factors predict future falls in community-living older adults. Arch Phys Med Rehabil. 2008;89:1708–13. doi: 10.1016/j.apmr.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allet L, Kim H, Ashton-Miller JA, DeMott T, Richardson JK. Frontal plane hip and ankle sensorimotor function, not age, predicts unipedal stance time. Muscle & Nerve. 2012;45:578–85. doi: 10.1002/mus.22325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17(11):1281–1289. doi: 10.2337/diacare.17.11.1281. [DOI] [PubMed] [Google Scholar]
- 17.Delbridge L, Perry P, Marr S, Arnold N, Yue DK, Turtle JR, Reeve TS. Limited joint mobility in the diabetic foot: relationship to neuropathic ulceration. Diabet Med. 1988;5:333–7. doi: 10.1111/j.1464-5491.1988.tb01000.x. [DOI] [PubMed] [Google Scholar]
- 18.Rajabally YA, Narasimhan M. Characteristics and correlates of sensory function in chronic inflammatory demyelinating polyneuropathy. J Neurol Sci. 2010;297:11–4. doi: 10.1016/j.jns.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Andersen H, Mogensen PH. Disordered mobility of large joints in association with neuropathy in patients with long-standing insulin-dependent diabetes. Diabetic Med. 1997;14(3):221–227. doi: 10.1002/(SICI)1096-9136(199703)14:3<221::AID-DIA338>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 20.Andersen H, Stalberg E, Gjerstad MA, Jakobsen J. Association of muscle strength and electrophysiological measures of reinnervation in diabetic neuropathy. Muscle & Nerve. 1998;21:1647–54. doi: 10.1002/(sici)1097-4598(199812)21:12<1647::aid-mus4>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 21.Andersen H, Gjerstad MD, Jakobsen J. Atrophy of foot muscles; a measure of diabetic neuropathy. Diabetes Care. 2004;27:2382–5. doi: 10.2337/diacare.27.10.2382. [DOI] [PubMed] [Google Scholar]
- 22.Strotmeyer ES, deRekeneire N, Schwartz AV, Resnick HE, Goodpaster BH, Faulkner KA, Shorr RI, Vinik AI, Harris TB, Newman AB. Sensory and motor peripheral nerve function and lower-extremity quadriceps strength: the health, aging and body composition study. J Am Geriatr Soc. 2009;57:2004–10. doi: 10.1111/j.1532-5415.2009.02487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inzitari M, DiCarlo A, Baldereschi M, et al. Risk and predictors of motor performance decline in a normally functioning population-based sample of elder subjects: the Italian longitudinal study on aging. J Am Geriatr Soc. 2006;54:318–24. doi: 10.1111/j.1532-5415.2005.00584.x. [DOI] [PubMed] [Google Scholar]
- 24.Strotmeyer ES, de Rekeneire N, Schwartz AV, Faulkner KA, Resnick HE, Goodpaster BH, Shorr RI, Vinik AI, Harris TB, Newman AB. The relationship of reduced peripheral nerve function and diabetes with physical performance in older white and black adults. Diabetes Care. 2008;32(9):1767–72. doi: 10.2337/dc08-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDermott MM, Guralnik JM, Albay M, Bandinelli S, Miniati B, Ferrucci L. Impairments of muscles and neres associated with peripheral arterial disease and their relationship with lower imbremity functioning: the InCHIANTI study. J Am Geriatr Soc. 2004;52:405–10. doi: 10.1111/j.1532-5415.2004.52113.x. [DOI] [PubMed] [Google Scholar]
- 26.Arezzo JC, Zotova E. Electrophysiologic measures of diabetic neuropathy: mechanism and meaning. In Rev Neurogiol. 2002;50:229–55. doi: 10.1016/s0074-7742(02)50079-9. [DOI] [PubMed] [Google Scholar]
- 27.Lauretani F, Bandinelli S, Bartali B, Di Iorio A, Giacomini V, Corsi AM, guralnik JM, Ferrucci L. Axonal degeneration affects muscle density in older men and women. Neurobiol Aging. 2006;27:1145–54. doi: 10.1016/j.neurobiolaging.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thelen DG, Schultz AB, Alexander NB, Ashton-Miller JA. Effects of age on rapid ankle torque development. J Gerontol; Med Sci. 1996;51A(5):M226–32. doi: 10.1093/gerona/51a.5.m226. [DOI] [PubMed] [Google Scholar]
- 29.Abate M, Schiavone C, Pelotti P, Salini V. Limited joint mobility in elderly subjects with type II diabetes mellitus. Arch Gerontol Geriatr. 2011;53(2):135–40. doi: 10.1016/j.archger.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Bishop DL, Milton RL. The effects of denervation location on fiber type mix in self-reinnervated mouse soleus muscles. Experiment Neurol. 1997;147:151–8. doi: 10.1006/exnr.1997.6605. [DOI] [PubMed] [Google Scholar]
- 31.Andersen JL. Muscle fiber type adaptation in the elderly human muscle. Scand J Med Sci Sports. 2003;13:40–7. doi: 10.1034/j.1600-0838.2003.00299.x. [DOI] [PubMed] [Google Scholar]
- 32.Robinovitch SN, Heller B, Lui A, Cortez J. Effect of strength and speed of torque development on balance recovery with the ankle strategy. J Neurophysiol. 2002;88:613–20. doi: 10.1152/jn.2002.88.2.613. [DOI] [PubMed] [Google Scholar]
- 33.Gutierrez EM, Helber MD, Dealva D, Ashton-Miller JA, Richardson JK. Mild diabetic neuropathy affects ankle motor function. Clin Biomech. 2001;16(6):522–28. doi: 10.1016/s0268-0033(01)00034-1. [DOI] [PubMed] [Google Scholar]
- 34.Allet L, Kim H, Ashton-Miller JA, Richardson JK. Which lower limb sensory and motor functions are required for functional gait on uneven surfaces in older persons with diabetic neuropathy? doi: 10.1016/j.pmrj.2012.05.002. submitted PM&R Journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lord, Menz HB, Tiedemann A. A physiological profile approach to falls risk assessment and prevention. Physical Therapy. 2003 Mar;83(3):237–52. [PubMed] [Google Scholar]
- 36.Yang XJ, Hill K, Moore K, Williams S, Dowson L, Borschmann K, Simpson JA, Dharmage SC. Effectiveness of a targeted exercise intervention in reversing older people’s mild balance dysfunction: a randomized controlled trial. Phys Ther. 2012;92(1):24–37. doi: 10.2522/ptj.20100289. [DOI] [PubMed] [Google Scholar]
- 37.Pijnappels M, Bobbert MF, van Dieen JH. Contribution of the support limb in control of angular momentum after tripping. J Clin Biomech. 2004;37:1811–18. doi: 10.1016/j.jbiomech.2004.02.038. [DOI] [PubMed] [Google Scholar]