Abstract
IL-2 signals during the primary response to infection are essential in shaping CD8+ T cell fate decisions. How CD8+ T cells integrate IL-2 signals in the development of functional memory is not well understood. Because IL-2 induces potent activation of the STAT5 transcription factor, we tested the role of STAT5 in CD8+ memory T cell differentiation and function using a model system in which STAT5 activity is inducibly abrogated upon CD8+ T cell activation. We report that STAT5 activity is broadly important for the expansion and effector function of all effector CTL subsets. After pathogen clearance, STAT5 was required for the survival of effector phenotype memory CTLs during the contraction phase. However, despite its role in supporting full primary CD8+ T cell expansion, and unlike IL-2, STAT5 activity is not required for the development of memory CD8+ T cells capable of robust secondary expansion upon rechallenge. Our findings highlight differential requirements for survival signals between primary and secondary effector CTL and demonstrate that IL-2-dependent programming of memory CD8+ T cells capable of secondary expansion and secondary effector differentiation is largely STAT5 independent.
Keywords: T cells, cell differentiation, memory, viral infection
Introduction
Environmental signals, including those from cytokines, play an essential role in effector and memory CD8+ T cell differentiation (1). The common gamma chain (γc)3 family cytokine IL-2 has been shown to drive CD8+ T cell effector and memory differentiation(2–5). We and others have shown a role for IL-2 during the primary immune response in driving differentiation of effector CTL (2–4), as well programming of memory CD8+ T cells capable of robust recall responses(3, 5).
How CD8+ T cells integrate signals from cytokines to determine fate decisions is not fully understood. Specifically, how do cytokines, such as IL-2, induce signaling pathways that result in long-lived memory CD8+ cells capable of protective recall responses? Several transcription factors, including T-box expressed in T cells (TBX21/T-bet) (6–11), Eomesodermin (Eomes) (6, 8, 12, 13), B-lymphocyte induced maturation protein (PRMD1/Blimp-1) (14–18), and B cell lymphoma 6 protein (Bcl-6) (14, 19, 20), have been shown to have crucial roles in CD8+ T cell effector differentiation and effector versus memory fate decisions. In the case of T-bet (6, 8–11) and Blimp-1 (14, 16–18), increased activity promotes a more differentiated terminal effector state in CD8+ T cells. On the other hand, Eomes (6, 8, 12, 13) and Bcl-6 (14, 19, 20) are associated with establishment of long-lived memory CD8+ T cell populations. In the case of effector CD4+ T cell differentiation, connections between cytokines and transcriptional programs are relatively well described and in several lineages involve STAT proteins (21). In contrast, the molecular connection between environmental signals such as cytokines received by CD8+ T cells during an immune response and the resulting transcriptional outcome driving differentiation towards terminal effector versus memory CD8+ T cell fate is less clear. The induction of transcription factors such as T-bet and Blimp-1 by cytokines appears to play a key role in this process (9, 22, 23).
We have shown a crucial role for the cytokine IL-2 in the differentiation of effector and memory CD8+ T cells. IL-2 signals during the primary response to infection are critical in promoting the differentiation of effector and effector memory CTL (3). Moreover, IL-2 signals received by CTL during the primary response program memory CD8+ T cells capable of robust secondary responses (3, 5) and secondary effector differentiation (3). We sought to determine the signaling pathways induced by IL-2 during a primary immune response that are involved in CD8+ T cell fate decisions and memory CD8+ T cell programming. The JAK3/STAT5, PI3K/Akt, and MAPK signaling pathways are potently activated by IL-2 (24). Of these pathways, PI3K/Akt and MAPK are activated by a variety of other cytokine receptors, TCR ligation and costimulatory signals (25–28) during T cell activation. Other γc cytokines in addition to IL-2, such as IL-7 and IL-15, also activate JAK3/STAT5, but the timing, magnitude and context of this signaling differ due to cytokine availability and differential regulation of the specific receptor subunits (29). IL-2 potently activates STAT5 during the differentiation of effector CTL. While IL-15 and IL-7 can also activate STAT5 during the effector CTL response, they play a dominant role in the maintenance of memory T cells after antigen clearance (29, 30). Moreover, IL-15 and IL-7 signals are not required for the programming of memory CD8+ T cells (3, 31, 32) as is the case with IL-2 (3, 5). IL-2 is an important catalyst for robust STAT5 activation during primary CTL responses, and we hypothesized that IL-2-mediated activation of STAT5 was a key step in the differentiation of highly functional CD8+ memory T cells.
Two isoforms of STAT5 exist, STAT5a and STAT5b, which have redundant functions in T cell homeostasis (33, 34). Mice with deletion of both stat5a/b fail to develop T cells (35, 36), demonstrating that STAT5 is essential during thymic selection. Recent reports examined the role of STAT5 in the survival of effector and memory CD8+ T cells after pathogen clearance (37, 38). In the absence of STAT5, effector CD8+ T cells show reduced accumulation at the peak of the primary response to infection, possibly due to their inability to induce Bcl-2 via STAT5 in response to IL-7 and IL-15 (38). Forced expression of a constitutively active form of STAT5 increased the numbers of effector CTL at the peak of the primary response to LCMV and augmented survival of all CD8+ T cell subsets through contraction and memory (37). These studies support a role for STAT5 signaling in effector and memory CD8+ T cell survival, mainly in the context of IL-15 and IL-7 signaling. However, high IL-2 levels potently drive STAT5 activation during the primary CTL response, and to date no studies have examined the role of STAT5 in differentially promoting effector and memory CTL subsets or in the programming of secondary CTL responses, two functions that have been described for IL-2 (3, 5). Here, we employ a unique experimental system in which STAT5 deletion is selectively targeted to activated CTL in an otherwise normal immune environment.
To determine the contribution of STAT5 to CD8+ effector and memory T cell differentiation, we employed a bone marrow chimera mouse model in which stat5a/stat5b are inducibly deleted upon CTL activation. We found that STAT5 was broadly important for all effector CD8+ T cell subsets during the primary response to acute infection, as well as the survival of effector phenotype CTL during contraction. After contraction, STAT5-independent memory CD8+ T cell populations were readily detectable, but whereas STAT5 was required for robust expansion and survival of primary effector CTL, STAT5-deficient memory CD8+ T cells mounted robust recall responses comparable to wild-type levels and underwent effective secondary effector CTL differentiation. Our findings highlight a differential requirement for survival signals mediated by STAT5 during primary and secondary CD8+ T cell responses. Moreover, our data suggest that IL-2 driven differentiation and programming of memory CD8+ T cells with robust recall potential is largely STAT5 independent.
Materials and Methods
Mice and Infections
4–6 week old B6.PL-Thy1a/CyJ (B6.PL, Thy1.1+) and C57BL/6 (B6, Thy1.2+) mice were purchased from Jackson Laboratories (Bar Harbor, ME). GzB-Cre (39), Rosa26-fl/stop/fl-YFP (RosaYFP) (40) (Jackson Laboratories), and stat5fl/fl(41)(supplied by L. Henighausen, NIDDK) mouse colonies were maintained at the University of Utah (Salt Lake City, UT). All experiments were performed with the approval of the IACUC committee at the University of Utah. Lymphocytic choriomeningitis virus (LCMV) strains Armstrong 53b and clone 13 were grown in BHK cells and titered in Vero cells as described (42). Primary infection was at a dose of 2 × 105 PFU i.p. For secondary challenges, mice were given 2 × 106 PFU LCMV-Cl.13 i.v. or 2 × 105 PFU LCMV-Arm i.p. Recombinant Listeria monocytogenes expressing the LCMV GP33–41 peptide (Lm-gp33) was propagated in BHI broth and agar plates as previously described (43–45). Prior to infection, bacteria were grown to log phase and concentration determined by measuring the O.D. at 600 nm (O.D. of 1 = 1 × 109 CFU/ml). For secondary challenges, mice were injected intravenously (i.v.) with 1 × 104 colony forming units (CFU).
Irradiation chimeras
Host B6.PL (Thy1.1+) mice were given a dose of 900 rads using the analytical x-ray irradiator in the mouse vivarium at the University of Utah. The next day, mice received 5 × 106 bone marrow (BM) cells i.v. harvested from the femurs and tibias of Thy1.1+ or Thy1.2+ donor mice as indicated. BM cells were prepared by RBC lysis and depletion of CD3+ cells using biotinylated anti-CD3 antibody (eBioscience, San Diego, CA), anti-biotin magnetic beads (Miltenyi Biotec, Auburn, CA), and passage through magnetic column following manufacturer’s guidelines (Miltenyi Biotec). After 8–10 weeks, reconstitution within the CD8+ T cell compartment was determined using antibodies to the congenic markers Thy1.1 and Thy1.2.
Cell Suspensions and cell sorting
Splenocytes and lymph node cells were harvested at indicated time points post infection and placed in single cell suspension in RPMI 1640 with 10% FBS, L-glutamine, and penicillin/streptomycin prior to further analysis. Liver lymphocytes were harvested and subjected to collagenase digestion as previously described (46). Cell sorting of CD8+YFP+ cells was performed using a FACS Aria II cell sorter (BD Biosciences) at the University of Utah FACS Core Facility, followed by immediate adoptive transfer by i.v. injection into the indicated secondary hosts.
Flow cytometry and analysis
Antibodies (Abs) conjugated to fluorescent dyes were purchased from eBioscience (San Diego, CA), Biolegend (San Diego, CA), BD Biosciences (Mountain View, CA), or Santa Cruz Biotechnology (Santa Cruz, CA). Abs were specific for the following antigens: CD8, Thy1.1, Thy1.2, KLRG1, IL7Ra, CD62L, T-bet, Eomes, Bcl-6, GranzymeB, STAT5-pY694. For cell surface staining, single cell suspensions were incubated with Abs in FACS Buffer (PBS with 2% FBS and 0.02% sodium azide). For intracellular staining of T-bet and GranzymeB, cells were permeabilized and stained using the BD Cytofix/Cytoperm™ kit and manufacturer’s instructions (BD Biosciences). For intracellular staining of Eomes, cells were prefixed in 0.5% PFA to preserve the YFP signal, washed twice, then fixed using FoxP3 Fix/Perm Buffer kit and manufacturer’s instructions (eBioscience). To detect STAT5-pY694, the BD Phosflow™ kit and manufacturer’s protocol were followed (BD Biosciences). H-2Db/GP33–41 and H-2Db/NP396–404 tetramerswere prepared as previously described (47, 48). Multiparameter analysis of stained cells was performed using a FACSCanto II flow cytometer (BD Biosciences) and results were analyzed using FlowJo software (TreeStar, Ashland, OR).
Intracellular cytokine staining
Single cell suspensions in RPMI 1640 containing 10% FBS, L-glutamine and penicillin/streptomycin were incubated with 0.1 uM GP33–41 or NP396–404 peptides in the presence of GolgiPlug (BD Biosciences) for 4–5 hours. Cells were stained with cell surface Abs, fixed and permeablized using a kit (BD Biosciences) and stained with fluorescently labeled anti-cytokine Abs specific to IFN-γ, TNF-α, and IL-2 (eBioscience).
CTL assays
We used a CTL assay as previously described (6). EL-4 target cells were incubated with 0.1uM GP33–41 peptide for 2 hrs at 37°C. Cells were washed and then incubated with sorted STAT5 WT or STAT5 CKO P14 CTLs for 2 hrs at CTL to target ratios ranging from 3:1 to 0.1:1. The percentage of Annexin V+ target cells was determined by FACS using Annexin V-APC apoptosis detection kit (eBioscience). Specific lysis was determined by comparison of killing of target cells without peptide loading.
Real-time PCR
RNA was extracted from sorted YFP+CD8+ T cells using Trizol (Invitrogen, Carlsbad, CA). cDNA was prepared from the RNA and qRT-PCR was performed using Superscript™ III Platinum® Two-Step qRT-PCR Kit with SYBR® Green (Invitrogen, Carlsbad, CA) following the manufacturer’s guidelines. Primer sets used are as follows: EOMES: f-CCGCCCACTACAATGTTTTC, r-GAAATCTCCTGCCTCATCCA; T-bet: f-CCCACAAGCCATTACAGGAT, r-CCCTTGTTGTTGGTGAGCTT; Blimp-1: f- CGGGATGAACATCTACTTCTACACT, r-TTTCTTTCACGCTGTACTCTCTCTT; Bcl-6: f- CCGGCTCAATAATCTCGTGAA, r-GGTGCATGTAGAGTGGTGAGTGA; FasL: f- CATCACAACCACTCCCACTG, r-TACTGGGGTTGGCTATTTGC; Prf1: f- GCAGCTGAGAAGACCTATCAGGAC, r-TCTGAGCGCCTTTTTGAAGTC; Bcl-2: f- GTGGTGGAGGAACTCTTCAGGGATG, r-GGTCTTCAGAGACAGCCAGGAGAAATC; Bim: f-CGGATCGGAGACGAGTTCA, r-TTCAGCCTCGCGGTAATCA; mcl-1: f-AGAGCGCTGGAGACCCTG, r-CTATCTTATTAGATATGCCAGACC; Bcl-XL: f- GTAGTGAATGAACTCTTTCGGGATGG, r-ACCAGCCACAGTCATGCCCGTCAGG. Real-time PCR and analysis was performed using a Roche LightCycler® 480 (Roche, Indianapolis, IN).
Results
STAT5 is required for accumulation of primary CTL during acute infection
In order to study the role of STAT5 signals in CD8+ T cell effector and memory differentiation, we created a mouse bone marrow chimera model system in which stat5a and stat5b are deleted in activated CD8+ T cells, and the resulting STAT5-deficient CD8+ T cells are traceable by permanent expression of YFP (Fig 1A). Bone marrow was harvested from mice with the following genetic components: Cre recombinase driven by the GranzymeB promoter (39) (GzB-Cre), RosaYFP reporter construct (40), and the stat5a/b genes flanked by loxP sites (41) (stat5fl/fl), which we will subsequently refer to as “STAT5 conditional knockout (CKO)” bone marrow. The donor bone marrow was mixed 1:1 with wild-type B6.PL bone marrow (“WT B6”) and transplanted into irradiated B6.PL hosts. This set of chimeras will subsequently be referred to as STAT5 CKO chimeras. A second set of bone marrow chimeras was made in an identical fashion, except the donor bone marrow contained the stat5a/b genes without LoxP sites (stat5wt/wt) and will be referred to as STAT5 WT chimeras (Fig 1A). We distinguished between STAT5 (Thy1.2+) and B6.PL (Thy1.1+) donor-derived T cells over the course of infection based on differential expression of congenic Thy1 alleles. In this model system, activation of Cre activity upon CTL activation, driven by Granzyme B induction results in the deletion of LoxP-flanked stat5a/b as well as a LoxP-flanked stop codon preceding YFP under the control of the Rosa26 promoter. Because Granzyme B expression is an early event following CTL activation (39), permanent YFP expression serves as an effective surrogate marker for Cre-mediated gene deletion during CTL differentiation and expansion. Importantly, the mixed bone marrow chimera model system allows for the study of CTL-specific STAT5 activity without complications arising from differences in pathogen clearance.
FIGURE 1.
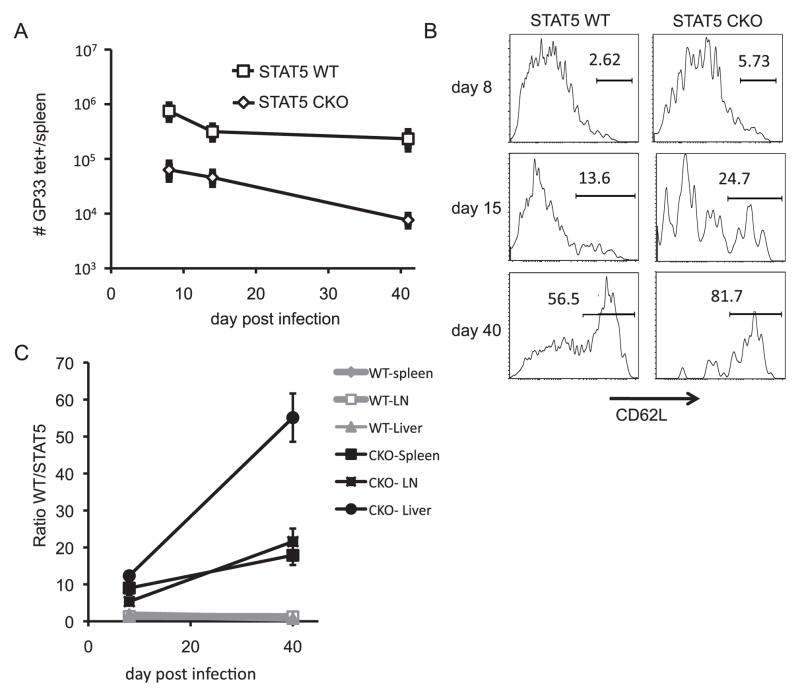
STAT5 is required for full primary expansion of CD8+ T cells. A, Schematic showing creation of STAT5 WT and STAT5 CKO bone marrow chimeras as a model system to study CD8+ T cell responses in the absence of STAT5, absent complications arising from differences in viral clearance. B, Representative flow plots showing Thy1.2+CD8+YFP+ populations in both STAT5 WT and STAT5 CKO chimeras prior to infection and at day 8 post LCMV-Arm infection. C, Representative flow plots show YFP expression by KLRG1hiTh1.2+CD8+ T cells as well as IL-7Rα and KLRG1 expression by CD8+Th1.2+YFP+ T cells in the spleen at day 8 post-infection. D, Splenocytes from STAT5 WT or STAT5 CKO chimeras harvested on day 8 or day 40 post LCMV infection were cultured with 500 U/mL mIL-2 for 20 minutes, then stained for phospho-STAT5. Representative histogram shows p-STAT5 levels in unstimulated controls (light gray shaded), CD8+YFP+ T cells from STAT5 WT chimeras (dark gray shaded), and CD8+YFP+ T cells from STAT5 CKO chimeras (black line). E, The ratio of “WT” (Thy1.1+) to “STAT5” (Thy1.2+) within the CD8+ T cell compartment are shown for both sets of chimeras prior to (day 0) and day 8 post LCMV infection. F, Total numbers of CD8+ T cells from “WT” and “STAT5” components on day 8 post LCMV infection in STAT5 WT and CKO chimeras. Error bars indicate the SEM (n=3–4 mice per time point). Results are representative of 4–5 independent experiments.
Prior to infection, small numbers of YFP+ cells could be detected among the STAT5 WT (~5%) and at much lower levels among the STAT5 KO (~0.5%) CD8+ T cells (Fig. 1B). These cells were largely CD44loCD122loand CD62Lhi, indicating that they did not arise through homeostatic proliferation and do not represent a pre-existing memory phenotype population (Supp. Fig. 1). Following infection with LCMV-Armstrong, CD8+ T cell expansion was evident in both STAT5 WT and STAT5 CKO chimeras, and both groups of chimeras cleared the infection as expected (data not shown). A clearly distinguishable CD8+YFP+ population was seen in both STAT5 WT and STAT5 CKO chimeras on day 8 post-infection (Fig 1B). When gated on STAT5 WT or STAT5 CKO donor CD8+ T cells (Thy1.2+), more than 90% of KLRG1-expressing effector CTL also expressed YFP, confirming that activation of YFP and expression of the YFP reporter was highly efficient (Fig. 1C). Additionally, YFP+CD8+ T cells from either WT or CKO donors populated the effector (KLRG1hiIL-7Rαlo) and memory precursor (KLRG1loIL-7Rαhi) pools with similar frequency (Fig. 1C).
We confirmed that STAT5 activity was absent from the CD8+YFP+ population within the STAT5 CKO chimeras at effector and memory time points. Using an anti-phospho-STAT5 antibody that efficiently stains phosphorylated STAT5 in mouse T cells (37, 38), we observed that STAT5 CKO YFP+ CTLs failed to phosphorylate STAT5 upon ex vivo exposure to IL-2, as compared STAT5 WT CTLs, as compared to unctimulated (Fig. 1C) and isotype (data not shown) controls. We also measured the deletion of stat5a/b by genomic PCR and the expression of STAT5a mRNA by RT-PCR. FACS-sorted STAT5 WT YFP+ CTL expressed ~15–20-fold higher levels of STAT5a mRNA, whereas genomic PCR revealed a ~15-fold decrease in stat5a/b genomic DNA, both numbers roughly consistent with the FACS sort purity (Supp. Fig. 2).
Following LCMV infection, we observed a defect in the overall expansion of CD8+YFP+ effector CTLs in the spleen in the absence of STAT5. While STAT5 WT chimeras displayed similar expansion of CD8+ T cells derived from either donor, STAT5 CKO chimeras displayed deficient expansion by CD8+ T cells derived from the STAT5 CKO donor (Fig. 1E). By day 8 post-infection, the CD8+ T cell population in the spleens of STAT5 CKO chimeras was almost entirely derived from the WT donor (Fig. 1F), indicating a decreased accumulation of STAT5 CKO CD8+ T cells at day 8 post infection compared to WT.
We examined wildtype and STAT5 CKO CD8+ T cells for differences in anti-apoptotic, pro-survival, or pro-apoptotic gene expression on day 8 post infection with LCMV and found no significant differences in expression of the pro-survival genes Bcl-2 and myeloid leukemia cell differentiation protein 1 (Mcl-1) and no difference in expression of the pro-apoptotic gene Bim (Table I). STAT5 WT CD8+ T cells showed a modest but significant increase in expression of the pro-survival molecule B cell lymphoma extra large (Bcl-xL) over STAT5 CKO CD8+ T cells (Table I).
Table I.
Gene expression in WT STAT5 CD8+ compared to CKO STAT5 CD8+ T cells on day 8 post-infection with LCMV. Significant fold differences (p<0.05, n=4 per group) are bolded.
| Gene | Fold expression in WT compared to CKO |
|---|---|
| Tbet | 1.57 (p=0.008) |
| Blimp-1 | 1.82 (p=0.021) |
| Perforin | 1.55 (p=0.010) |
| Bcl-6 | 0.23 (p=0.041) |
| EOMES | 1.42 (p=0.150) |
| FasL | 0.95 (p=0.859) |
| Bcl2 | 0.71 (p=0.365) |
| Bcl-XL | 1.77 (p=0.046) |
| Bim | 1.17 (p=0.642) |
| Mcl-1 | 0.88 (p=0.654) |
We next examined whether STAT5 deficiency similarly affected antigen-specific CD8+ T cells responding to acute infection by staining with MHC Class I tetramers for two immunodominant LCMV epitopes. While the frequency of H-2Db/GP33–41 and H-2Db/NP396–404 tetramer+YFP+ cells was similar in the STAT5 WT and STAT5 CKO donor CD8+ T cell populations (Fig. 2A), due to the overall loss in CD8+ T cells the total number of STAT5 CKO antigen specific CD8+ T cells was substantially lower than WT at the peak of the response in the spleen (Fig. 2B). Because measuring total numbers does not take into account the change in frequency of donor CD8+ T cells seen between day 0 and day 8 post infection (Fig. 1D), we normalized the CTL response in each chimera to the predicted response based on the ratio of donor WT to donor STAT5 WT or STAT5 CKO CD8+ T cells prior to infection. We found that the STAT5 CKO antigen specific CD8+ T cell response was approximately 4 fold lower than the wild-type response seen STAT5 WT CTLs (Fig. 2C). In all, the STAT5 WT YFP+ CTL response was similar in magnitude to the response of WT donor CTL in the same host, as well as the WT control CTL in the STAT5 CKO chimeras (data not shown)
FIGURE 2.
Antigen specific STAT5 CKO primary responses are reduced in number compared to wildtype. A, Representative flow plots show the frequency of antigen specific (H2Db-GP33–41 tetramer+) YFP+ cells within the STAT5 WT or STAT5 CKO CD8+ population in the spleen on day 8 post LCMV infection. B, The bar graph indicates the total numbers of STAT5 WT and STAT5 CKO CD8+YFP+ T cells on day 8 post infection that bind tetramers for two immunodominant LCMV-specific epitopes. C, Differences in absolute numbers seen on day 8 post-infection for antigen-specific CD8+ T cells were normalized to reflect changing ratios of WT B6 and STAT5 CD8+ counterparts seen between day 0 and day 8 post infection (Fig 1D). Graphs showing the % of the normalized response (where the donor B6 WT CD8+tetramer+ response is considered 100%) are displayed for two antigen-specific CD8+ T cell populations in a representative experiment. Error bars represent the SEM (n=3–4 per group). D, Confirmation of the absence of STAT5 activity within antigen specific YFP+CD8+ T cells from STAT5 CKO Chimeras. Splenocytes from STAT5 WT and CKO Chimeras were harvested on day 8 post LCMV infection, surface stained for CD8 and tetramers, cultured with 500U/mL IL-2 for 20 minutes, then stained for pSTAT5. Representative histogram shows pSTAT5 levels in unstimulated controls (gray shaded) and H2Db-GP33–41 tetramer+YFP+CD8+ T cells from either STAT5 WT (black line) or STAT5 CKO chimeras (gray line).
These differences were not due to inefficient deletion of STAT5, as antigen specific YFP+ STAT5 CKO CTLs displayed virtually no STAT5 phosphorylation at day 8 post-infection upon exposure to IL-2 (Fig. 2D). Similar results were found at day 5 post-infection (data not shown), the earliest time point at which we were able to detect tetramer-binding CTL in the spleen. While we cannot pinpoint in vivo STAT5 inactivation precisely, in vitro activation of CD8+ STAT5 CKO T cells resulted in undetectable STAT5 activity within 48 hours of activation (data not shown).
Therefore, we conclude that our model system results in rapid inhibition of STAT5 activity and that STAT5 is an important survival signal for CD8+ T cells undergoing expansion during primary immune challenge. We cannot rule out the possibility that STAT5 provides key survival and/or differentiation signals in the first hours after activation.
STAT5 influences CTL function but does not drive CTL differentiation
To explore whether STAT5 influenced effector CTL differentiation, we examined the localization, expression of effector molecules and function of effector CTLs in the absence of STAT5. Antigen-specific wild-type and STAT5 CKO CD8+ T cells were present at similar frequencies in the liver of LCMV infected mice on day 8 post infection (Fig. 3A), demonstrating that CTL form and traffic to peripheral sites of infection even in the absence of STAT5. Additionally, STAT5 CKO CD8+ T cells produced IFN-γ and TNF-α at similar frequencies and levels upon peptide restimulation of splenocytes (Fig 3B) and liver lymphocytes (data not shown). STAT5 CKO tetramer+ CD8+ T cells readily expressed similar levels of the cytolytic molecule GranzymeB (GzB) as STAT5 WT tetramer+ cells on day 8 post-infection (Fig 3C). In addition, STAT5 CKO CTL de-granulated normally upon peptide restimulation, as measured by the surface expression of CD107a (LAMP-1) following peptide restimulation (data not shown). STAT5 CKO CTL also expressed similar levels of Ki-67 compared to STAT5 WT CTL, suggesting that defects in accumulation were not due to poor cell division, but rather poor survival (data not shown).
FIGURE 3.
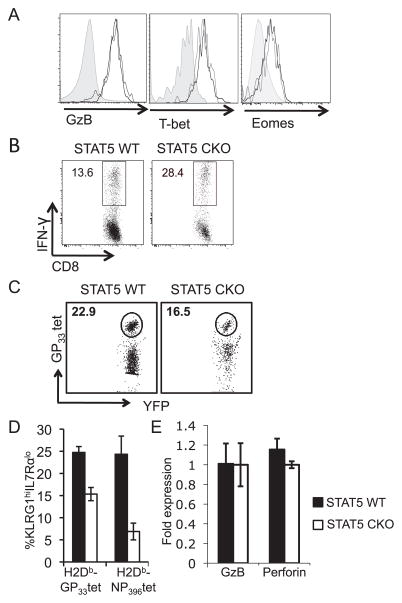
Effector CTL function is reduced in the absence of STAT5. A, Representative flow plots show the frequency of antigen specific (gp33 tetramer+) YFP+ cells within STAT5 WT or STAT5 CKO CD8+ T cell populations in the liver on day 8 post LCMV infection. B, Cytokine expression was determined by ex vivo GP33–41 peptide restimulation of splenocytes on day 8 post LCMV infection. Representative flow plots display IFN-γ and TNF-α-production gated on CD8+YFP+ T cells from STAT5 WT or STAT5 CKO chimeras. C, Intracellular expression of Granzyme B, T-bet, Eomes, and Bcl-6 on day 8 post-infection in STAT5 WT (black line) and STAT5 CKO (gray line) GP33–41 tetramer+ CD8+ T cells. Gray filled histograms display isotype control staining. D, Target cells loaded with GP33–41 peptide were incubated with STAT5 WT or STAT5 CKO YFP+ CTL FACS sorted from spleens at day 8 post-infection. CTL induced lysis was measured by staining with Annexin V after 2 hrs. Percent specific lysis was determined by comparison to Annexin V+ target cells that were not loaded with peptide. Error bars represent the SEM (n=3–4 per group). Results are representative of 3–4 independent experiments.
Modest differences in mRNA levels (Table I) were seen for two transcription factors, T-bet and Eomes, that are associated with effector CTL (9, 11) and effector to memory CD8+ T cell transition (8, 12), respectively. However, protein expression levels of T-bet and EOMES were not changed in the absence of STAT5 (Fig 3C). Transcriptional control of CD8+ T cell terminal differentiation versus memory formation is mediated by the inverse activity of Blimp-1 and Bcl-6 (14). We saw a reduced level of Blimp-1 mRNA and an increased level of Bcl-6 mRNA in CD8+ T cells lacking STAT5 compared to wildtype (Table I). Interestingly, STAT5 has been shown to inhibit TFH differentiation in effector CD4+ T cells through increasing Blimp-1 expression and ultimately negatively regulating Bcl-6, which drives TFH fate (49, 50). When we examined protein levels of Bcl-6, we did not see differences (Fig 3C), but it remains possible that STAT5 impacts Blimp-1/Bcl-6 activity at early time points after activation and thus may favor effector CTL differentiation in a manner similar to what is seen for CD4+ T cells.
To test effector function more directly, we compared target cell lysis of STAT5 WT and STAT5 CKO CD8+ T cells on day 8 post-infection. We found approximately a 2-fold decrease in killing on a per cell basis in the absence of STAT5 (Fig 3D). This could be potentially explained by the lower levels of Perforin (Prf1) mRNA expressed by STAT5 CKO CTL compared to wild-type CTL (Table I) and is consistent with a report that STAT5 binds the Prf1 promoter and drives Prf1 expression in CTL (4).
Overall, our data suggests that on a per cell basis, STAT5 deficiency has a modestly adverse impact on CTL effector function. This is similar to the modest decrease in effector function seen in CTL that do not receive IL-2 signals (3), and it is likely that IL-2 driven acquisition of effector function is mediated at least to some degree by STAT5. However, taking into account that STAT5 does not affect peripheral localization of effector CTL and that STAT5 CKO CTL express Granzyme B, cytokines, transcription factors, and FasL equal to wildtype (Fig. 3, Table I), we conclude that STAT5 plays only a modest role in the development of primary effector CTL function.
CTL differentiation is heterogeneous, including effector and memory precursor CD8+ T cell subsets that form part of the overall effector CTL pool. These two populations can be distinguished based on cell surface expression of killer cell lectin-like receptor subfamily G member 1 (KLRG1) and IL7Rα (9). Others and we have shown that IL-2 signals drive differentiation of terminal effector phenotype CTL during the primary response (2, 3). To determine if IL-2 driven effector differentiation could be mediated by STAT5, we examined the differentiation of STAT5 CKO antigen specific CD8+ T cells based on IL7Ra and KLRG1 expression following LCMV infection. We found that the total numbers of STAT5 CKO IL7RαloKLRG1hi CD8+ T cells were decreased at the peak of the effector response and declined at nearly a 10-fold faster pace than STAT5 WT IL7RαloKLRG1hi CD8+ T cells between days 8 and 40 post-infection (Fig 4A,C). Whereas CD8+ T cells activated in the absence of IL-2 signals generate normal numbers of memory precursor CTL (3), STAT5 CKO memory precursor IL7RαhiKLRG1lo CD8+ T cells were also reduced at effector time points and declined rapidly between days 8 and 40 post-infection (Fig 4B,C). We conclude that in contrast to IL-2, STAT5 does not selectively drive differentiation of effector phenotype CTL and is instead important for the emergence of all CTL subsets through the peak of expansion. After clearance of pathogen, STAT5 signals are important for maintaining IL7RαloKLRG1hi phenotype CTL through contraction and into the memory phase
FIGURE 4.
STAT5 signals during primary expansion are broadly important for all CD8+ T cell subsets responding to acute infection. Graphs display the total number of KLRG1hiIL7Rαlo (terminal effector phenotype) (A) and KLRG1loIL7Rαhi (memory precursor/memory phenotype) (B) at the indicated time points post infection with LCMV-Arm for STAT5 WT and STAT5 CKO tetramer binding CD8+ T cells. Numbers within the plot indicate fold decline between day 8 and day 40. C, Representative flow plots display the frequency of CD8+ T cell subsets as determined by KLRG1 and IL7Ra expression at the indicated time points for STAT5 WT and STAT5 CKO tetramer-binding YFP+CD8+ T cells. Results are representative of 3–4 separate experiments. Error bars represent the SEM (n=3–4 per group).
STAT5 signals are important for maintenance of tissue-residing memory CTL after pathogen clearance
Memory CD8+ T cell populations were detectable in the spleen in the absence of STAT5 following LCMV infection, although at lower numbers than wildtype (Fig. 5A). Importantly, surviving memory cells did not express detectable STAT5 (Fig 6A), indicating that memory CTL can emerge in the absence of STAT5, albeit at lower levels. We did not examine memory CD8+ T cell survival and homeostasis beyond 6 weeks post infection due to the known role of IL-15 and IL-7, in long term memory T cell maintenance (30). At early memory time points, the absence of STAT5 resulted in rapid loss of CD62Llo effector memory CTL in the spleen (Fig 5B) and the preferential and specific decline of tissue-homing effector memory CTL in the liver (Fig. 5C). These findings are consistent with the role of IL-2 in effector memory CTL differentiation and suggest that IL-2-driven STAT5 activation is a key driver of effector memory CTL establishment (3, 5). After pathogen clearance, STAT5 signaling is particularly important for effector memory and tissue residing memory CD8+ T cell survival (Fig. 5B–C).
FIGURE 5.
STAT5 signals are important for establishment and maintenance of effector memory and tissue residing CD8+ T cells. A, Graphs display the total numbers of antigen specific STAT5 WT and STAT5 CKO YFP+CD8+ T cells in the spleen at the indicated time points post LCMV infection. B, Histograms show representative CD62L staining in STAT5 WT and CKO YFP+CD8+ T cells in the spleen at indicated time points. C, Ratio of H2Db-GP33–41 tetramer binding CD8+ T cells derived from WT B6 counterpart (Thy1.1+) compared to STAT5 counterpart YFP+CD8+ T cells (Thy1.2+) in the indicated tissues (spleen mesenteric lymph nodes, liver) at day 8 and day 40 post-infection. Results are representative of 3–4 independent experiments and error bars represent the SEM (n=3–4 per group).
FIGURE 6.
STAT5-deficient memory CD8+ T cells are capable of robust recall responses. A, Schematic showing adoptive transfer strategy for determining recall capacity of STAT5 CKO CD8+ T cells compared to WT. CD8+YFP+ memory T cells were FACS sorted from pooled spleens derived from either STAT5 WT or STAT5 CKO chimeras approximately 6 weeks post LCMV-Arm infection. Sorted CD8+YFP+ T cells were analyzed for the frequency of H2Db-GP33–41 and NP396- tetramer binding memory CD8+ T cells, and then adoptively transferred into naïve B6 hosts. The next day, the naïve B6 hosts were infected with LCMV clone 13. B, Representative flow plots show the frequencies of tetramer-binding YFP+CD8+ STAT5 WT and STAT5 CKO secondary responders in the spleens of B6 hosts on day 5 post-infection. C, Bar graphs display the fold expansion of antigen specific STAT5 WT and STAT5 CKO CD8+YFP+ cells on day 5 post-infection compared to numbers adoptively transferred prior to infection. Results are representative of 3–4 independent experiments and error bars represent the SEM (n=3–4 per group).
STAT5 CKO memory CTL have robust recall capacity
Because IL-2 signals during primary CTL activation are required for the formation of memory CTL capable of robust secondary responses (3, 5), we assessed the ability of STAT5 WT and STAT5 CKO memory CTL to respond to rechallenge. Prior to rechallenge, we verified that antigen specific memory CD8+YFP+ T cells from STAT5 CKO chimeras did not express detectable STAT5 (Fig. 6A). Direct rechallenge of memory phase bone marrow chimeras is difficult to interpret due to the inability to distinguish between recall responses by YFP+ memory CD8+ T cells and recruitment of YFP− memory or naïve CD8+ T cells that subsequently express YFP. Therefore, we used an adoptive transfer strategy to determine recall capacity in the absence of STAT5. STAT5 WT or STAT5 CKO CD8+YFP+ memory CTL were FACS-purified from the spleens of mixed chimeras 6 weeks after LCMV infection, analyzed for frequency of CD8+tetramer+ cells and transferred into naïve B6 hosts. Twenty-four hours after adoptive transfer, the naïve B6 hosts were infected with LCMV clone 13 to induce recall responses by YFP+ memory CD8+ T cells (Fig 6B). Similar rechallenges were done using LCMV Armstrong or recombinant Listeria monocytogenes expressing GP33–41 (Lm-gp33) as a secondary stimulus, with similar results (data not shown).
Antigen-specific STAT5 WT and STAT5 CKO YFP+CD8+ T cells were clearly visible in the spleens of the infected hosts at day 5 post rechallenge (Fig 6C). In contrast to the primary response, STAT5 CKO memory CD8+ T cells expanded at levels similar to wild-type when rechallenged (Fig. 6C–D). This is in striking contrast to the defective accumulation upon rechallenge of memory CD8+ T cells generated in the absence of IL-2 (3, 5). As in the primary response, STAT5 CKO secondary CTLs expressed normal levels of Granzyme B (Fig. 7A), effector cytokines (Fig. 7B) and differentiation-associated transcription factors T-bet and Eomes (Fig. 7A). When rechallenged with Lm-gp33, STAT5 CKO secondary CTLs trafficked to the liver normally (Fig. 7C). There was a modest decrease in formation of secondary KLRG1hiIL7Ralo phenotype CD8+ T cells in the absence of STAT5 (Fig 7D), but STAT5 CKO secondary CTLs expressed normal levels of Perforin mRNA (Fig. 7E), in contrast to the primary CTL response. Additionally, STAT5 CKO secondary effector CTL de-granulated normally following restimulation, as measured by surface CD107a expression (data not shown). Our results indicate that STAT5 has little effect on secondary effector CTL differentiation. We conclude that IL-2-driven programming of memory CD8+ T cells capable of robust secondary expansion is largely STAT5 independent.
FIGURE 7.
STAT5 is not required for secondary effector CTL differentiation. A, Intracellular staining of STAT5 WT (black line) and STAT5 CKO (gray line) CD8+ T cells for GzB, T-bet, and Eomes on day 5 post rechallenge (experiment performed as described in Fig 6A). Gray shaded histograms are isotype controls. B, Intracellular cytokine staining for IFN-γ was performed after ex vivo restimulation on day 5 post-rechallenge. Splenocytes were restimulated with GP33–41 and NP396- peptides in the presence of Brefeldin A. Staining of IFN-γ is shown in CD8+YFP+ T cells. C, Representative flow plots show the presence of secondary CTL in the liver of Lm-gp33 rechallenged mice on day 5 post infection. D, Bar graphs show the frequency of secondary KLRG1hiIL7Ralo phenotype CD8+ T cells within YFP+ populations on day 5 post-rechallenge. E, Bar graph indicates the fold difference in mRNA expression at day 5 post-rechallenge between sorted STAT5 WT and STAT 5 CKO CD8+YFP+ T cells. Error bars are SEM (n=3–4 per group).
Discussion
Our results demonstrate a broad role for STAT5 signaling in the primary response to infection in promoting the accumulation of effector CTL, and a more selective role for STAT5 in the survival of effector phenotype and establishment and survival of tissue residing memory CD8+ T cells after pathogen clearance. Despite its role in promoting robust primary effector CTL responses, we make two novel observations. First, STAT5 is not required for IL-2 driven memory differentiation, reflecting the potential participation of other IL-2-dependent signaling pathways in this process. Second, the requirement for STAT5 activity in the primary and secondary CTL response is different, with secondary CTL differentiating and expanding normally in the absence of STAT5. A study using direct infection of mice with conditional stat5 deletion driven by a Type I IFN responsive promoter showed that cell division of wildtype and STAT5-deficient CD8+ T cells on day 8 post-infection was the same (38). Our observations of poor primary expansion in the absence of STAT5 also likely reflect poor survival, and we propose that the survival of secondary effector CTL is controlled by distinct mechanisms. Additionally, the absence of IL-2 signaling during the in vivo response to acute infection impacts survival while having little effect on cell division (5).
Consistent with reduced survival in the absence of STAT5, we observed reduced expression of the pro-survival molecule Bcl-xL in STAT5 CKO CD8+ T cells at day 8, similar to its expression in the absence of IL-2 signals (3). It is known that STAT proteins, including STAT5, can induce Bcl-xL in several cell types (51). While it is possible that IL-2 induced STAT5 could promote survival of CD8+ T cells during the primary response through induction of Bcl-xL, T cells lacking Bcl-xL (all isoforms) were able to mount normal responses to Listeria monocytogenes infection (52). Thus, the functional impact of differences in Bcl-xL mRNA levels during primary CTL expansion in the absence of STAT5 is not clear.
We find that STAT5 signals are broadly important for all subsets of effector CTL cell during primary expansion, suggesting that STAT5 does not drive CD8+ T cell fate decisions during the primary response. This is in contrast to the specific role of IL-2 in preferentially promoting the differentiation of effector phenotype CTL (3). Another γc family member, IL-21, is able to activate STAT5, though to a lesser degree than IL-2 (53), and has recently been identified as being important in CD8+ T cell differentiation in the context of chronic (54–56) and acute infection (57). Additional STAT5 activation during the primary response is likely stimulated by IL-7 and IL-15, and we have found that the combined absence of IL-2 and IL-15 enhances the defect in terminal effector CTL differentiation during the primary response (3). Our results suggest that STAT5 may have graded effects on CTL differentiation. While some loss of STAT5 activation induced by IL-2 could influence effector phenotype CTL formation, STAT5 activation induced by other cytokines may be sufficient for the differentiation of memory precursor CTL. Complete loss of STAT5, however, results in a defect in both terminal effector phenotype and memory precursor CTL.
IL-15 selectively promotes survival of effector phenotype CTL after viral clearance. Although IL-15 induces STAT5 signaling, we observed a switch from a broad requirement for STAT5 during expansion to a more selective requirement for its activity in the maintenance of effector phenotype and long lived effector memory CD8+ T cells during contraction and into the memory phase. This could be due to different levels of STAT5 activation induced by IL-15 and IL-7 (58). IL-7 also efficiently activates STAT5, and it has roles in both the generation and survival of memory CTL (32). Alternatively, another report has shown that effector phenotype CD8+ T cells are more dependent on STAT5 signals for survival during contraction (37, 38). Collectively, these reports and ours emphasize that for different CD8+ T cells subsets, there appears to be differential usage of and access to common signaling cascades induced by common gamma chain cytokines at distinct stages of differentiation.
Importantly, we observed the emergence of a STAT5-deficient memory CD8+ T cell population 6 weeks after infection, albeit at much lower levels than what was seen for wildtype. This brings to question the survival signals that are used in the absence of STAT5 and to what degree these signals are used under normal conditions. A recent report suggests that STAT5-independent survival signals could be utilized in the formation of memory CD8+ T cell populations (58), but the nature of those signals remain unknown. Another recent study showed that memory precursor CTL were able to activate PI3K/Akt in an IL-15-dependent manner more efficiently than effector phenotype CTL (37). However, hyper-activation of this pathway was detrimental to long-term survival of memory CD8+ T cells.
Despite being a key mediator of IL-2 signals, we found that STAT5 was dispensable for the ability of memory CD8+ T cells to mount robust secondary responses and undergo secondary effector CTL differentiation. This highlights a different requirement for STAT5 during primary and secondary CD8+ T cell responses, as STAT5-deficient primary responses were significantly reduced compared to wildtype. Moreover, this is in striking contrast as to what is seen for memory CD8+ T cells generated in the absence of IL-2 signals, which fail to mount successful recall responses and fail to differentiate into secondary effector CTL (3, 5). Thus, the mechanism by which IL-2 signals during the primary response program memory CD8+ T cells is independent of STAT5 and remains to be defined.
Alternative candidate pathways induced by IL-2 that could playa role in memory CTL differentiation include STAT3 and PI3K/Akt. STAT3 signals are also induced by IL-2 (53), and a role for STAT3 in persistence of memory precursor CTL in mice (57) and CD8+ TCM formation in humans (59) has been suggested. Like STAT5, STAT3 is activated by a variety of other cytokines, such as IL-21 and IL-6 (53, 60), and it is unclear what proportion of STAT3 activity during the primary CTL response is IL-2-dependent. The phenotype of STAT3-deficient and IL-2Rα-deficient CD8+ T cells do not align, as IL-2 is important for terminal effector CTL (3), whereas STAT3 appears to be important for memory precursor phenotype CD8+ T cells (57).
A more likely candidate is IL-2-dependent PI3K/Akt activation. While this signaling pathway is induced in CD8+ T cells by a variety of sources, the additive effects of multiple signals, including IL-2, in the induction of PI3K/Akt activation for memory CD8+ T cell differentiation could be important. Moreover, several recent reports have suggested that the kinase mammalian target of rapamycin (mTOR), which is downstream of PI3K/Akt activation, is a key regulator of effector and memory CTL fate decisions and survival (61–63). Therefore, a likely future hypothesis is that IL-2 mediates its effects on the programming of secondary effector CTL responses at least in part through PI3K/Akt activation.
Supplementary Material
Acknowledgments
We acknowledge the technical assistance of J. Cassiano, as well as D.C. Jay and C. Kim for thoughtful discussion and critical review of the manuscript.
Footnotes
Research reported in this publication was supported by the National Institute Of Allergy And Infectious Diseases of the National Institutes of Health under Award Number R01AI080830. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations used in this manuscript: common gamma chain, γc; T-box expressed in T cells , TBX21/T-bet; Eomesodermin, Eomes; B lymphocyte induced maturation protein, Blimp-1; B cell lymphoma 6 protein (Bcl-6); Protein Kinase B, PKB, also known as Akt; C57BL/6, B6; lymphocytic choriomeningitis virus, LCMV; Listeria monocytogenes expressing recombinant LCMV GP33-41 peptide, Lm-gp33; antibodies, Abs; GranzymeB, GzB; B cell lymphoma extra large, Bcl-xL; Perforin, Prf1; killer cell lectin-like receptor subfamily G member 1, KLRG1
References
- 1.Cox MA, Harrington LE, Zajac AJ. Cytokines and the inception of CD8 T cell responses. Trends Immunol. 2011;32:180–186. doi: 10.1016/j.it.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalia V, Sarkar S, Subramaniam S, Haining WN, Smith KA, Ahmed R. Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity. 2010;32:91–103. doi: 10.1016/j.immuni.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell DM, Ravkov EV, Williams MA. Distinct roles for IL-2 and IL-15 in the differentiation and survival of CD8+ effector and memory T cells. J Immunol. 2010;184:6719–6730. doi: 10.4049/jimmunol.0904089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pipkin ME, Sacks JA, Cruz-Guilloty F, Lichtenheld MG, Bevan MJ, Rao A. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 2010;32:79–90. doi: 10.1016/j.immuni.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cruz-Guilloty F, Pipkin ME, Djuretic IM, Levanon D, Lotem J, Lichtenheld MG, Groner Y, Rao A. Runx3 and T-box proteins cooperate to establish the transcriptional program of effector CTLs. J Exp Med. 2009;206:51–59. doi: 10.1084/jem.20081242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Intlekofer AM, Takemoto N, Kao C, Banerjee A, Schambach F, Northrop JK, Shen H, Wherry EJ, Reiner SL. Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells. J Exp Med. 2007;204:2015–2021. doi: 10.1084/jem.20070841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, Gapin L, Ryan K, Russ AP, Lindsten T, Orange JS, Goldrath AW, Ahmed R, Reiner SL. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 9.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sullivan BM, Juedes A, Szabo SJ, von Herrath M, Glimcher LH. Antigen-driven effector CD8 T cell function regulated by T-bet. Proc Natl Acad Sci U S A. 2003;100:15818–15823. doi: 10.1073/pnas.2636938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeo CJ, Fearon DT. T-bet-mediated differentiation of the activated CD8+ T cell. Eur J Immunol. 2011;41:60–66. doi: 10.1002/eji.201040873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banerjee A, Gordon SM, Intlekofer AM, Paley MA, Mooney EC, Lindsten T, Wherry EJ, Reiner SL. Cutting edge: The transcription factor eomesodermin enables CD8+ T cells to compete for the memory cell niche. J Immunol. 2010;185:4988–4992. doi: 10.4049/jimmunol.1002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, Banica M, DiCioccio CB, Gross DA, Mao CA, Shen H, Cereb N, Yang SY, Lindsten T, Rossant J, Hunter CA, Reiner SL. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 14.Crotty S, Johnston RJ, Schoenberger SP. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nat Immunol. 2010;11:114–120. doi: 10.1038/ni.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ji Y, Pos Z, Rao M, Klebanoff CA, Yu Z, Sukumar M, Reger RN, Palmer DC, Borman ZA, Muranski P, Wang E, Schrump DS, Marincola FM, Restifo NP, Gattinoni L. Repression of the DNA-binding inhibitor Id3 by Blimp-1 limits the formation of memory CD8+ T cells. Nat Immunol. 2011;12:1230–1237. doi: 10.1038/ni.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kallies A, Xin A, Belz GT, Nutt SL. Blimp-1 transcription factor is required for the differentiation of effector CD8(+) T cells and memory responses. Immunity. 2009;31:283–295. doi: 10.1016/j.immuni.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 17.Rutishauser RL, Martins GA, Kalachikov S, Chandele A, Parish IA, Meffre E, Jacob J, Calame K, Kaech SM. Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31:296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin H, Blackburn SD, Intlekofer AM, Kao C, Angelosanto JM, Reiner SL, Wherry EJ. A role for the transcriptional repressor Blimp-1 in CD8(+) T cell exhaustion during chronic viral infection. Immunity. 2009;31:309–320. doi: 10.1016/j.immuni.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ichii H, Sakamoto A, Hatano M, Okada S, Toyama H, Taki S, Arima M, Kuroda Y, Tokuhisa T. Role for Bcl-6 in the generation and maintenance of memory CD8+ T cells. Nat Immunol. 2002;3:558–563. doi: 10.1038/ni802. [DOI] [PubMed] [Google Scholar]
- 20.Ichii H, Sakamoto A, Kuroda Y, Tokuhisa T. Bcl6 acts as an amplifier for the generation and proliferative capacity of central memory CD8+ T cells. J Immunol. 2004;173:883–891. doi: 10.4049/jimmunol.173.2.883. [DOI] [PubMed] [Google Scholar]
- 21.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gong D, Malek TR. Cytokine-dependent Blimp-1 expression in activated T cells inhibits IL-2 production. J Immunol. 2007;178:242–252. doi: 10.4049/jimmunol.178.1.242. [DOI] [PubMed] [Google Scholar]
- 23.Takemoto N, Intlekofer AM, Northrup JT, Wherry EJ, Reiner SL. Cutting Edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J Immunol. 2006;177:7515–7519. doi: 10.4049/jimmunol.177.11.7515. [DOI] [PubMed] [Google Scholar]
- 24.Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–479. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- 25.Kane LP, Weiss A. The PI-3 kinase/Akt pathway and T cell activation: pleiotropic pathways downstream of PIP3. Immunol Rev. 2003;192:7–20. doi: 10.1034/j.1600-065x.2003.00008.x. [DOI] [PubMed] [Google Scholar]
- 26.Okkenhaug K, Bilancio A, Farjot G, Priddle H, Sancho S, Peskett E, Pearce W, Meek SE, Salpekar A, Waterfield MD, Smith AJ, Vanhaesebroeck B. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science. 2002;297:1031–1034. doi: 10.1126/science.1073560. [DOI] [PubMed] [Google Scholar]
- 27.Song J, Lei FT, Xiong X, Haque R. Intracellular signals of T cell costimulation. Cell Mol Immunol. 2008;5:239–247. doi: 10.1038/cmi.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ashwell JD. The many paths to p38 mitogen-activated protein kinase activation in the immune system. Nat Rev Immunol. 2006;6:532–540. doi: 10.1038/nri1865. [DOI] [PubMed] [Google Scholar]
- 29.Schluns KS, Lefrancois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3:269–279. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- 30.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Haring JS, Jing X, Bollenbacher-Reilley J, Xue HH, Leonard WJ, Harty JT. Constitutive expression of IL-7 receptor alpha does not support increased expansion or prevent contraction of antigen-specific CD4 or CD8 T cells following Listeria monocytogenes infection. J Immunol. 2008;180:2855–2862. doi: 10.4049/jimmunol.180.5.2855. [DOI] [PubMed] [Google Scholar]
- 32.Hand TW, Morre M, Kaech SM. Expression of IL-7 receptor alpha is necessary but not sufficient for the formation of memory CD8 T cells during viral infection. Proc Natl Acad Sci U S A. 2007;104:11730–11735. doi: 10.1073/pnas.0705007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imada K, Bloom ET, Nakajima H, Horvath-Arcidiacono JA, Udy GB, Davey HW, Leonard WJ. Stat5b is essential for natural killer cell-mediated proliferation and cytolytic activity. J Exp Med. 1998;188:2067–2074. doi: 10.1084/jem.188.11.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakajima H, Liu XW, Wynshaw-Boris A, Rosenthal LA, Imada K, Finbloom DS, Hennighausen L, Leonard WJ. An indirect effect of Stat5a in IL-2-induced proliferation: a critical role for Stat5a in IL-2-mediated IL-2 receptor alpha chain induction. Immunity. 1997;7:691–701. doi: 10.1016/s1074-7613(00)80389-1. [DOI] [PubMed] [Google Scholar]
- 35.Yao Z, Cui Y, Watford WT, Bream JH, Yamaoka K, Hissong BD, Li D, Durum SK, Jiang Q, Bhandoola A, Hennighausen L, O’Shea JJ. Stat5a/b are essential for normal lymphoid development and differentiation. Proc Natl Acad Sci U S A. 2006;103:1000–1005. doi: 10.1073/pnas.0507350103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seki Y, Yang J, Okamoto M, Tanaka S, Goitsuka R, Farrar MA, Kubo M. IL-7/STAT5 cytokine signaling pathway is essential but insufficient for maintenance of naive CD4 T cell survival in peripheral lymphoid organs. J Immunol. 2007;178:262–270. doi: 10.4049/jimmunol.178.1.262. [DOI] [PubMed] [Google Scholar]
- 37.Hand TW, Cui W, Jung YW, Sefik E, Joshi NS, Chandele A, Liu Y, Kaech SM. Differential effects of STAT5 and PI3K/AKT signaling on effector and memory CD8 T-cell survival. Proc Natl Acad Sci U S A. 2010;107:16601–16606. doi: 10.1073/pnas.1003457107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tripathi P, Kurtulus S, Wojciechowski S, Sholl A, Hoebe K, Morris SC, Finkelman FD, Grimes HL, Hildeman DA. STAT5 is critical to maintain effector CD8+ T cell responses. J Immunol. 2010;185:2116–2124. doi: 10.4049/jimmunol.1000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacob J, Baltimore D. Modelling T-cell memory by genetic marking of memory T cells in vivo. Nature. 1999;399:593–597. doi: 10.1038/21208. [DOI] [PubMed] [Google Scholar]
- 40.Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC developmental biology. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cui Y, Riedlinger G, Miyoshi K, Tang W, Li C, Deng CX, Robinson GW, Hennighausen L. Inactivation of Stat5 in mouse mammary epithelium during pregnancy reveals distinct functions in cell proliferation, survival, and differentiation. Mol Cell Biol. 2004;24:8037–8047. doi: 10.1128/MCB.24.18.8037-8047.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984;160:521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen H, Slifka MK, Matloubian M, Jensen ER, Ahmed R, Miller JF. Recombinant Listeria monocytogenes as a live vaccine vehicle for the induction of protective anti-viral cell-mediated immunity. Proc Natl Acad Sci U S A. 1995;92:3987–3991. doi: 10.1073/pnas.92.9.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slifka MK, Shen H, Matloubian M, Jensen ER, Miller JF, Ahmed R. Antiviral cytotoxic T-cell memory by vaccination with recombinant Listeria monocytogenes. Journal of virology. 1996;70:2902–2910. doi: 10.1128/jvi.70.5.2902-2910.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams MA, Ravkov EV, Bevan MJ. Rapid culling of the CD4+ T cell repertoire in the transition from effector to memory. Immunity. 2008;28:533–545. doi: 10.1016/j.immuni.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams MA, Bevan MJ. Shortening the infectious period does not alter expansion of CD8 T cells but diminishes their capacity to differentiate into memory cells. J Immunol. 2004;173:6694–6702. doi: 10.4049/jimmunol.173.11.6694. [DOI] [PubMed] [Google Scholar]
- 47.Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. doi: 10.1126/science.274.5284.94. [DOI] [PubMed] [Google Scholar]
- 48.Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 49.Johnston RJ, Choi YS, Diamond JA, Yang JA, Crotty S. STAT5 is a potent negative regulator of TFH cell differentiation. J Exp Med. 2012;209:243–250. doi: 10.1084/jem.20111174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nurieva RI, Podd A, Chen Y, Alekseev AM, Yu M, Qi X, Huang H, Wen R, Wang J, Li HS, Watowich SS, Qi H, Dong C, Wang D. STAT5 Protein Negatively Regulates T Follicular Helper (Tfh) Cell Generation and Function. J Biol Chem. 2012;287:11234–11239. doi: 10.1074/jbc.M111.324046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grad JM, Zeng XR, Boise LH. Regulation of Bcl-xL: a little bit of this and a little bit of STAT. Curr Opin Oncol. 2000;12:543–549. doi: 10.1097/00001622-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 52.Zhang N, He YW. The antiapoptotic protein Bcl-xL is dispensable for the development of effector and memory T lymphocytes. J Immunol. 2005;174:6967–6973. doi: 10.4049/jimmunol.174.11.6967. [DOI] [PubMed] [Google Scholar]
- 53.Kovanen PE, Leonard WJ. Cytokines and immunodeficiency diseases: critical roles of the gamma(c)-dependent cytokines interleukins 2, 4, 7, 9, 15, and 21, and their signaling pathways. Immunol Rev. 2004;202:67–83. doi: 10.1111/j.0105-2896.2004.00203.x. [DOI] [PubMed] [Google Scholar]
- 54.Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324:1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Frohlich A, Kisielow J, Schmitz I, Freigang S, Shamshiev AT, Weber J, Marsland BJ, Oxenius A, Kopf M. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 2009;324:1576–1580. doi: 10.1126/science.1172815. [DOI] [PubMed] [Google Scholar]
- 56.Yi JS, Du M, Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009;324:1572–1576. doi: 10.1126/science.1175194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cui W, Liu Y, Weinstein JS, Craft J, Kaech SM. An interleukin-21-interleukin-10-STAT3 pathway is critical for functional maturation of memory CD8+ T cells. Immunity. 2011;35:792–805. doi: 10.1016/j.immuni.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kemp RA, Pearson CF, Cornish GH, Seddon BP. Evidence of STAT5-dependent and -independent routes to CD8 memory formation and a preferential role for IL-7 over IL-15 in STAT5 activation. Immunol Cell Biol. 2010;88:213–219. doi: 10.1038/icb.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Siegel AM, Heimall J, Freeman AF, Hsu AP, Brittain E, Brenchley JM, Douek DC, Fahle GH, Cohen JI, Holland SM, Milner JD. A critical role for STAT3 transcription factor signaling in the development and maintenance of human T cell memory. Immunity. 2011;35:806–818. doi: 10.1016/j.immuni.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O’Sullivan LA, Liongue C, Lewis RS, Stephenson SE, Ward AC. Cytokine receptor signaling through the Jak-Stat-Socs pathway in disease. Molecular immunology. 2007;44:2497–2506. doi: 10.1016/j.molimm.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 61.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 2010;32:67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.