Abstract
Introduction
Operant conditioning can gradually change the human soleus H-reflex. The protocol conditions the reflex near M-wave threshold. This study examined its impact on the reflexes at other stimulus strengths.
Methods
H-reflex recruitment curves were obtained before and after a 24-session exposure to an up-conditioning (HRup) or down-conditioning (HRdown) protocol and were compared.
Results
In both HRup and HRdown subjects, conditioning affected the entire H-reflex recruitment curve. In 5 of 6 HRup and 3 of 6 HRdown subjects, conditioning elevated (HRup) or depressed (HRdown), respectively, the entire curve. In the other HRup subject or the other 3 HRdown subjects, the curve was shifted to the left or to the right, respectively.
Discussion
H-reflex conditioning does not simply change the H-reflex to a stimulus of particular strength; it also changes the H-reflexes to stimuli of different strengths. Thus, it is likely to affect many actions in which this pathway participates.
Keywords: plasticity, motor learning, memory, rehabilitation, spinal cord
INTRODUCTION
The spinal cord changes throughout life. Activity-dependent plasticity in spinal cord neurons and synapses is prominent during development, contributes to skill acquisition and maintenance throughout later life, and occurs in response to trauma and disease1–5. Protocols that operantly condition spinal reflexes (such as the spinal stretch reflex and its electrical analog the H-reflex) provide powerful models for studying the mechanisms and impact of this plasticity4–6,3. Furthermore, because these reflex pathways serve in complex motor skills such as locomotion7–12, conditioning protocols may provide a new therapeutic approach to restoring function after spinal cord injuries or in other disorders13.
Long-term spinal reflex changes might be expected to have long-term effects on reflex recruitment curves (such as the H-reflex/M-wave relationship)14 comparable to the short-term changes in recruitment that occur with switching from one motor task to another (i.e., task-dependent modulation), with movement-induced reflex conditioning15, or over the different phases of locomotion (i.e., phase-dependent modulation)16–18. However, aside from Carp et al.19 and Dragert and Zehr14, studies of long-term reflex plasticity have typically focused on change in the reflex at a single point in the recruitment curve [e.g., at the maximum H-reflex (Hmax)20–22]. Information on the effects of reflex change on the entire recruitment curve (i.e., on the reflexes obtained with other stimulus amplitudes) could help to clarify the mechanisms and the functional impact of the reflex change.
In a recent study23, we showed that the human soleus H-reflex can be changed with an operant conditioning protocol. H-reflex increase (HRup subjects) or decrease (HRdown subjects) occurred while background EMG, M-wave, and subject posture remained unchanged. While the conditioning protocol focused on changing the H-reflex associated with an M-wave of a specific size, we also obtained H-reflex/M-wave recruitment curves in all sessions. These curves allow us to assess the impact of up- or down-conditioning on the H-reflexes elicited as stimulus strength varies across a broad range. This paper reports the results of this assessment. It provides a more comprehensive picture of the impact of the conditioning protocol on the functioning of the pathway that produces the H-reflex and thereby illuminates the implications of H-reflex conditioning for other behaviors to which the pathway contributes.
MATERIALS AND METHODS
Operant conditioning of the soleus H-reflex
The operant conditioning protocol for the human soleus H-reflex has been described in detail previously23 and is summarized here. Briefly, the protocol comprised 6 baseline sessions and 24 conditioning sessions spread over 10 weeks (i.e., 3/wk). Each subject's sessions always occurred at the same time of day to control for diurnal variations in H-reflex size24–27.
To elicit the H-reflex, the tibial nerve was stimulated in the popliteal fossa using surface self-adhesive Ag-AgCl electrodes (2.2×2.2 cm for the cathode and 2.2×3.5 cm for the anode, VerMed, Inc., Bellows Falls, VT) and a Grass S48 stimulator (with CCU1 constant current unit and SIU5 stimulus isolation unit; AstroMed, Inc., West Warwick, RI). The stimulating electrode pair was placed so as to minimize the H-reflex threshold and to avoid stimulation of other nerves. Soleus EMG was recorded with another pair of these electrodes placed longitudinally just below the gastrocnemii with their centers 3 cm apart. To monitor antagonist EMG, additional electrodes were placed over the belly of the tibialis anterior (TA) muscle. EMG activity was amplified, band-pass filtered (3–3,000 Hz), sampled at 5,000 Hz, and stored. To avoid session-to-session variability in electrode locations, their positions were initially mapped in relation to landmarks on the skin (e.g., scars or moles), and this mapping was used to place the electrodes in every session.
In a few preliminary sessions prior to the first baseline session, the soleus and TA background EMG ranges were set to match the levels found during natural standing, and the target soleus M-wave size was set to the size of the M-wave that was just above threshold. In each H-reflex trial of each subsequent session, the soleus H-reflex was elicited while the subject maintained a natural standing posture and constant levels of soleus and TA background EMG. For all the H-reflex trials throughout the study, M-wave size was kept constant.
In each baseline session, 225 control H-reflexes (as 3 blocks of 75 trials) were elicited. In each conditioning session, 20 control H-reflexes were elicited as in the baseline sessions, and then 225 conditioned H-reflexes (in 3 blocks of 75 trials) were elicited. The difference between control and conditioned H-reflex trials was that, in the conditioned H-reflex trials, the subject was asked to increase (HRup subjects) or decrease (HRdown subjects) the H-reflex and was given visual feedback after each stimulus to indicate whether the resulting H-reflex was larger (HRup subjects) or smaller (HRdown subjects) than a criterion value. Thus, in control H-reflex trials the H-reflex was simply elicited, while in conditioned H-reflex trials the subject was encouraged to increase or decrease H-reflex size, and the H-reflex was immediately followed by feedback indicating whether it satisfied the size criterion. In the conditioning session, the size criterion for the first block of 75 conditioned H-reflex trials was based on the immediately preceding block of 20 control H-reflex trials, and the criterion values for the second and third blocks of conditioned H-reflex trials were based on the immediately preceding block of 75 conditioned H-reflex trials. The criterion was selected so that, if H-reflex sizes for the new block were similar to those for the previous block, 50–60% of the trials would be successful28. For each block, the subject earned a modest extra monetary reward when the success rate exceeded 50%. (See23 for full details.) In these conditioned trials, subjects were encouraged to develop a strategy for changing H-reflex size in the correct direction without changing posture or background EMG activity. Subject reports of their conditioning strategies are listed in Table 2 of Thompson et al.23.
As described fully in Thompson et al.23, over the course of the conditioning sessions the H-reflex elicited in both the initial 20 control trials and the 225 conditioning trials usually changed, increasing in HRup subjects and decreasing in HRdown subjects. The change in the control H-reflex reflected long-term plasticity in the reflex, while the greater change in the conditioned H-reflex reflected both long-term plasticity and within-session task-dependent adaptation (see Thompson et al.23 for full discussion).
Measurement of H-reflex/M-wave recruitment curve
At the beginning of each session, prior to the control and conditioning H-reflex trials described above, a full H-reflex/M-wave recruitment curve was obtained while the subject stood and maintained a defined level of EMG activity (typically 20–23 μV, corresponding to 10–20% of maximum voluntary contraction). Stimulus intensity was increased in increments of 1.2–2.5 mA from soleus H-reflex threshold to an intensity just above that needed to elicit the maximum M-wave (Mmax)29,30. Four EMG responses were averaged to measure the H-reflex and M-wave at each intensity.
Subjects
This study analyzes the H-reflex/M-wave recruitment curves from those subjects of the Thompson et al. (2009a) study in which the control H-reflex, as well as the conditioned H-reflex, changed in the correct direction (i.e., increased in HRup subjects and decreased in HRdown subjects). Thus, it focused on those subjects with clear evidence of long-term plasticity in the H-reflex pathway. This group comprised 12 adults (ages 21–55, 6 women and 6 men) with no known neurological disease or injury. The study was approved by the New York State Department of Health Institutional Review Board, and each person provided informed consent.
Data analysis
Using the recruitment data of the 6 baseline sessions and the last 6 conditioning sessions, H-reflex size was plotted as a function of the size of the accompanying M-wave. For the statistical analysis of the recruitment data, the Kolmogorov-Smirnov test was used, since it is sensitive to differences in both location and shape of sample distributions. To estimate Hmax and the corresponding M-wave size for the recruitment curves from the 6 baseline and last 6 conditioning sessions, second-order polynomial curves31 were fitted to the H-reflex sizes elicited by stimulus levels ranging from the level that elicited a threshold M-wave, through the level that elicited Hmax, to the level at which H-reflex size had fallen back to at least the 1/3 Hmax. Depending on the subject, this latter stimulus evoked an M-wave of 30–60% Mmax. These curves provided good fits to the data (average r2 = 0.61 for HRup and 0.62 for HRdown subjects). The ascending limb of the H-reflex recruitment curve has been described as sigmoidal, and a sigmoidal curve generally provides a good fit when the Hmax value is already calculated and available31–33. However, in this study, the H-reflex and M-wave measurements from 6 sessions were pooled to generate the H-reflex/M-wave recruitment curve and Hmax, and the corresponding M-wave size had to be estimated. Thus, a polynomial curve, which fits H-reflex/M-wave recruitment data as well as a sigmoidal curve fits32, was used.
RESULTS
Comparison of the average H-reflex recruitment curves during the baseline sessions to those during the final conditioning sessions showed that the H-reflex elicited by the stimulus level used in the conditioning protocol had increased in all 6 HRup subjects and decreased in all 6 HRdown subjects. Thus, the curves were consistent with the effects of conditioning on the H-reflexes measured in the 20 control trials of each session.
H-reflex recruitment in HRup subjects
In 5 of the 6 HRup subjects, H-reflex size increased across a wide range of M-wave levels so that H-reflexes were larger across all or most of the recruitment curve. In 4 of these 5 subjects, the increase was significant (p < 0.05, Kolmogorov-Smirnov test). Consistent with this overall increase, Hmax (measured as the peak value of the fitted plot) increased to 129, 120, 110, 107, and 136% of its baseline value in the 5 subjects, respectively. At the same time, the recruitment curve did not move relative to M-wave size: in each of the 5 subjects, the size of the M-wave that accompanied Hmax changed by <1% of Mmax. In these HRup subjects, the recruitment curve did not shift horizontally or change in shape, rather the area under it increased. Figure 1A illustrates this change with the recruitment curves from 1 of these subjects. This figure also illustrates the broad recruitment curves found in these subjects.
Figure 1.
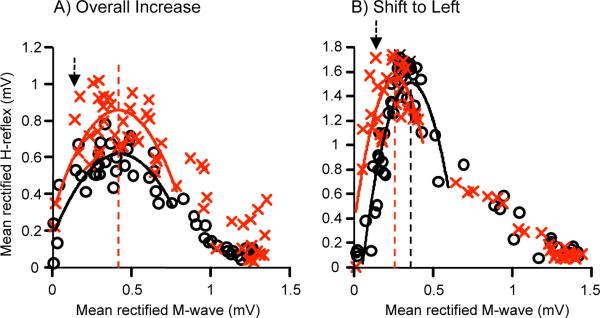
Average H-reflex recruitment curves for the 6 baseline sessions (o) and the last 6 conditioning sessions (x) of 2 HRup subjects. H-reflexes are plotted against the sizes of the accompanying M-waves. Second-order polynomial curves are fitted from the M-wave threshold to 50–70% Hmax of the down slope so that Hmax can be calculated for each curve (vertical lines). The arrow indicates the stimulus level used by the conditioning protocol (i.e., the M-wave size targeted by the protocol). In the subject in A (as in 5 of the 6 HRup subjects), the recruitment curve is broad, the entire curve is elevated by conditioning, and the stimulus level that produces Hmax does not change. In contrast, in the remaining HRup subject (B), the recruitment curve is narrow, the stimulus level that produces Hmax falls with conditioning, and the curve shifts to the left. In both subjects, the H-reflex produced by the stimulus level used in the conditioning protocol increases.
In contrast, in the remaining HRup subject, conditioning shifted the curve to the left. Hmax did not increase (i.e., final value 102% of baseline value), but the M-wave size associated with Hmax decreased by 5% of Mmax. Thus, while Hmax itself did not increase, the H-reflex sizes associated with stimuli just above M-wave threshold (including the stimulus used in the conditioning protocol) did increase. The leftward shift in the recruitment curve is readily apparent in Figure 1B. The curves are quite narrow compared to those in Figure 1A.
H-reflex recruitment in HRdown subjects
The 2 different patterns of change in the H-reflex recruitment curve seen with HRup conditioning were also evident with HRdown conditioning. In 3 of the 6 HRdown subjects, the H-reflex over the recruitment curve range was significantly decreased (p < 0.05, Kolmogrov-Smirnov test). In these HRdown subjects, the curves were broad, and H-reflex size decreased across a wide range of M-wave levels, so that H-reflexes were smaller across all or most of the recruitment curve. Consistent with this overall decrease, Hmax decreased to 67, 69, and 90% of its baseline value in the 3 subjects, respectively. At the same time, the recruitment curve did not move relative to M-wave size; in each subject, the size of the M-wave that accompanied Hmax changed by <1% of Mmax. In these HRdown subjects, the recruitment curve did not shift horizontally or change in shape; rather the area under it decreased. Figure 2A illustrates this change with the recruitment curves from 1 of these subjects.
Figure 2.
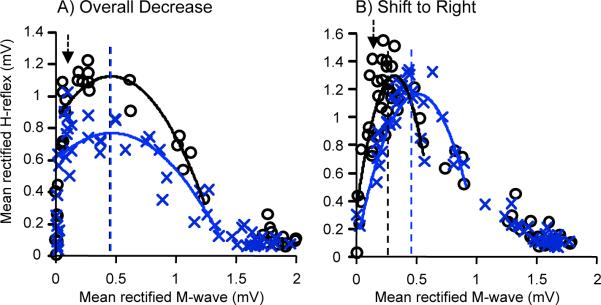
Average H-reflex recruitment curves for the 6 baseline sessions (o) and the last 6 conditioning sessions (x) of 2 HRdown subjects. H-reflexes are plotted against the sizes of the accompanying M-waves. Second-order polynomial curves are fitted from the M-wave threshold to 50–70% Hmax of the down slope so that Hmax can be calculated for each curve (vertical lines). The arrow indicates the stimulus level used by the conditioning protocol (i.e., the M-wave size targeted by the protocol). In the subject in A (as in 3 of the 6 HRdown subjects), the recruitment curve is broad, the curve is depressed by conditioning, and the stimulus level that produces Hmax does not change. In contrast, in the subject in B (as in the other 3 of the 6 HRdown subjects). the curve is narrow, the stimulus level that produces Hmax rises, and the curve shifts to the right. In both subjects, the H-reflex produced by the stimulus level used in the conditioning protocol decreases.
In contrast, in the other 3 HRdown subjects, the recruitment curves were narrow, and conditioning shifted the curve to the right. Hmax did not decrease (i.e., final values 99, 91, and 105% of baseline), but the M-wave size associated with Hmax increased by 12, 6, and 5%, respectively. Thus, while Hmax itself did not decrease, the H-reflex sizes associated with stimuli just above M-wave threshold (including the stimulus used in the conditioning protocol) did decrease. Figure 2B illustrates this rightward shift with the recruitment curves from 1 of these subjects. These curves are narrow compared to those in Figure 2A.
DISCUSSION
The results show that the effects of H-reflex conditioning were not limited to the H-reflexes elicited by a stimulus of the same strength as that used in the conditioning protocol. Conditioning affected the H-reflexes elicited across a broad range of stimulus strengths. Two different patterns of change were evident. In most subjects the recruitment curve was relatively broad, and it was simply elevated by up-conditioning or depressed by down-conditioning, without a change in the M-wave size at which Hmax was elicited. In the remaining subjects the curve was narrow, and conditioning did change the M-wave size at which Hmax was elicited, so that the curve was shifted to the left (by up-conditioning) or to the right (by down-conditioning). These changes in the recruitment curve cannot be explained as day-to-day variability, since each curve represents data from the 6 baseline sessions or the last 6 conditioning sessions.
These results raise at least 3 questions. First, why are there 2 different patterns of change in the recruitment curve? Second, how do the recruitment changes relate to the plasticity underlying H-reflex conditioning? Third, what are the possible functional and therapeutic implications of the results?
The factors shaping the recruitment curve changes
The impact of H-reflex conditioning on the recruitment curve (overall increase/decrease vs. right/left shift) appears to depend on the shape of the curve (i.e., broad vs. narrow), which differs across subjects. The shape of the curve is likely to reflect subject-specific relationships among stimulus strength, M-wave size, and H-reflex size.
In general, larger axons are more readily excited by external stimulation. Thus, since larger motoneurons have larger axons, the threshold M-wave is produced primarily by axons from the largest motoneurons. As stimulus strength increases, smaller and smaller motor axons join the M-wave, until Mmax is reached34. In contrast, the threshold H-reflex is produced mainly by axons from the smallest motoneurons, because they are more readily excited by primary afferent input. As stimulus strength increases, larger and larger motoneurons join the H-reflex. At some point, however, the stimulus rises to a point where the H-reflex stops increasing (i.e., Hmax), and then, with further stimulus increase, the H-reflex begins to decrease. The amplitude of Hmax and the stimulus level at which it occurs are determined by 2 factors. The first factor is the susceptibility of the motoneurons to excitation by primary afferent input. The second factor is the stimulus level at which the larger motoneurons that would be added to the H-reflex by further stimulus increase have already been added to the M-wave.
We suggest that the first factor, motoneuron susceptibility to primary afferent excitation, is the main determinant of Hmax in those subjects with broad recruitment curves. As the stimulus increases, the H-reflex increases until no more motoneurons are susceptible to primary afferent excitation, and it remains at this amplitude until continued stimulus increase eventually begins to recruit into the M-wave motoneurons that would otherwise participate in the H-reflex. In this case, H-reflex up- (or down-) conditioning simply increases (or decreases) Hmax and the entire curve, without significantly changing the stimulus strength (or M-wave size) associated with Hmax.
In contrast, we suggest that the second factor, the unavailability of motoneurons already recruited into the M-wave, is the main determinant of Hmax in the subjects with narrow recruitment curves. As the stimulus increases, more and more motoneurons join the H-reflex until no more are available, and then, as the stimulus rises further, motoneurons that had participated in the H-reflex are co-opted by the M-wave. In this case, H-reflex up- (or down-) conditioning decreases (or increases) the stimulus strength at which no more motoneurons are available to be recruited into the H-reflex, and thus shifts Hmax and the entire curve to the left (or right).
The plasticity responsible for H-reflex change
While the initial shape of the H-reflex recruitment curve affects the impact of H-reflex conditioning on the curve, the plasticity responsible for conditioning might also affect the changes in the curve. For example, if conditioning had its greatest effect on the participation of larger motoneurons in the H-reflex, it might be expected to change the H-reflexes elicited at higher stimulus levels. However, to date there is no evidence that the effect of up- or down-conditioning is concentrated on a particular portion of the motoneuron population. Rather, the effects of conditioning appear to be distributed across the population (e.g.,35,36). The recruitment curve changes reported here are consistent with this conclusion.
Furthermore, several kinds of spinal cord plasticity thought to account for H-reflex conditioning would be expected to affect the entire recruitment curve. Both the positive shift in motoneuron firing threshold that appears to account for down-conditioning36,37 and the change in polysynaptic afferent input that may contribute to up-conditioning38 would be likely to affect the H-reflexes elicited at a wide range of stimulus levels. Other possible mechanisms of reflex change, such as change in presynaptic inhibition at the Ia afferent synapse8,39,40, would probably also affect the entire recruitment curve. Changes in presynaptic inhibition of Ia afferents have been suggested as mechanisms of both short and long-term plasticity in the H-reflex pathway8,41,42,1,43,29,7. The H-reflex operant conditioning protocol produces both within-session task-dependent adaptation and long-term across-session change in H-reflex size23. The short-term, within-session change is likely to be presynaptic in origin, since the posture, background EMG, and M-wave size are kept the same throughout the session44,40. While it is possible that altered presynaptic inhibition also contributes to the long-term across-session change in H-reflex size, the long-term change appears to reflect changes in motoneuron properties, in terminals on the motoneuron, and/or in spinal interneurons (3,4 for review).
Functional and therapeutic implications
In each session, the soleus H-reflex recruitment curve was measured before the conditioning trials, while the subject simply maintained the background EMG levels and did not try to change the H-reflex. As discussed in detail in Thompson et al.23, the H-reflexes changes found in this control situation (i.e., when the subject is not trying to change the H-reflex) reflect long-term plasticity produced by the conditioning sessions, rather than short-term task-dependent adaptation. Our present results show that this plasticity affects H-reflexes across a wide range of stimulus levels. This finding is consistent with previous work that shows that the effects of H-reflex conditioning are still present in the stance and swing phases of locomotion and actually affect the participation of the soleus muscle in locomotion45. Taken together, this study and previous work show that H-reflex conditioning affects the overall functioning of the reflex pathway, including its participation in other motor actions such as locomotion.
This overall effect implies that appropriate H-reflex conditioning might be effective in ameliorating the functional disabilities associated with disorders such as spinal cord injury or stroke. Indeed, in rats with incomplete spinal cord injury, up-conditioning of the soleus H-reflex can strengthen the soleus locomotor burst and thereby restore symmetrical gait13. Initial studies in people with incomplete spinal cord injuries accompanied by spasticity suggest that down-conditioning of the soleus H-reflex may improve locomotion46. Because conditioning paradigms can focus on specific reflex pathways and can target reflexes other than the H-reflex (e.g., reciprocal inhibition47), they might be designed to address each individual's particular deficits. Reflex conditioning protocols might therefore provide a useful supplement to other therapeutic methods such as locomotor training48–51.
Conclusions
In summary, operant conditioning of the human soleus H-reflex changes all or most of the H-reflex recruitment curve. Depending on the individual, the change may be an overall increase (with up-conditioning) or decrease (with down-conditioning) in the curve or a shift in the curve to the left (with up-conditioning) or the right (with down-conditioning). These results are consistent with previous data that show that H-reflex conditioning affects the pathway's participation in other behaviors13,45; and they further support the possibility that conditioning protocols might provide a valuable new approach to reducing the motor deficits associated with spinal cord injuries and other neuromuscular disorders.
ACKNOWLEDGMENTS
We thank Dr. Gerwin Schalk for technical support, Dr. Dennis J. McFarland for advice in data analysis, and Drs. Jonathan S. Carp, Dennis J. McFarland, and Elizabeth Winter Wolpaw for comments on the manuscript. This work was supported in part by the New York State Spinal Cord Injury Research Trust (C023685(AKT)) and the National Institutes of Health (NS069551(AKT), HD36020(XYC), NS22189(JRW), and NS061823 (JRW&XYC)).
Abbreviations
- Hmax
maximum H-reflex
- HRup
H-reflex up-conditioning
- HRdown
H-reflex down-conditioning
- Mmax
maximum M-wave
- TA
Tibialis Anterior
REFERENCES
- 1.Stein RB, Yang JF, Belanger M, Pearson KG. Modification of reflexes in normal and abnormal movements. Prog Brain Res. 1993;97:189–196. doi: 10.1016/s0079-6123(08)62277-3. [DOI] [PubMed] [Google Scholar]
- 2.Zehr EP. Training-induced adaptive plasticity in human somatosensory reflex pathways. J Appl Physiol. 2006;101:1783–1794. doi: 10.1152/japplphysiol.00540.2006. [DOI] [PubMed] [Google Scholar]
- 3.Wolpaw JR. What can the spinal cord teach us about learning and memory? Neuroscientist. 2010;16:532–549. doi: 10.1177/1073858410368314. [DOI] [PubMed] [Google Scholar]
- 4.Wolpaw JR. Spinal cord plasticity in acquisition and maintenance of motor skills. Acta Physiol (Oxf) 2007;189:155–169. doi: 10.1111/j.1748-1716.2006.01656.x. [DOI] [PubMed] [Google Scholar]
- 5.Wolpaw JR, Tennissen AM. Activity-dependent spinal cord plasticity in health and disease. Ann Rev Neurosci. 2001;24:807–843. doi: 10.1146/annurev.neuro.24.1.807. [DOI] [PubMed] [Google Scholar]
- 6.Wolpaw JR. The complex structure of a simple memory. Trends Neurosci. 1997;20:588–594. doi: 10.1016/s0166-2236(97)01133-8. [DOI] [PubMed] [Google Scholar]
- 7.Zehr EP, Stein RB. What functions do reflexes serve during human locomotion? Prog Neurobiol. 1999;58:185–205. doi: 10.1016/s0301-0082(98)00081-1. [DOI] [PubMed] [Google Scholar]
- 8.Stein RB. Presynaptic inhibition in humans. Prog Neurobiol. 1995;47:533–544. doi: 10.1016/0301-0082(95)00036-4. [DOI] [PubMed] [Google Scholar]
- 9.Brooke JD, Cheng J, Collins DF, McIlroy WE, Misiaszek JE, Staines WR. Sensori-sensory afferent conditioning with leg movement: gain control in spinal reflex and ascending paths. Prog Neurobiol. 1997;51:393–421. doi: 10.1016/s0301-0082(96)00061-5. [DOI] [PubMed] [Google Scholar]
- 10.Yang JF, Stein RB. Phase-dependent reflex reversal in human leg muscles during walking. J Neurophysiol. 1990;63:1109–1117. doi: 10.1152/jn.1990.63.5.1109. [DOI] [PubMed] [Google Scholar]
- 11.Sinkjaer T, Andersen JB, Larsen B. Soleus stretch reflex modulation during gait in humans. J Neurophysiol. 1996;76:1112–1120. doi: 10.1152/jn.1996.76.2.1112. [DOI] [PubMed] [Google Scholar]
- 12.Lamont EV, Zehr EP. Task-specific modulation of cutaneous reflexes expressed at functionally relevant gait cycle phases during level and incline walking and stair climbing. Exp Brain Res. 2006;173:185–192. doi: 10.1007/s00221-006-0586-4. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, Chen XY, Jakeman LB, Chen L, Stokes BT, Wolpaw JR. Operant conditioning of H-reflex can correct a locomotor abnormality after spinal cord injury in rats. J Neurosci. 2006;26:12537–12543. doi: 10.1523/JNEUROSCI.2198-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dragert K, Zehr EP. Bilateral neuromuscular plasticity from unilateral training of the ankle dorsiflexors. Exp Brain Res. 2011;208:217–227. doi: 10.1007/s00221-010-2472-3. [DOI] [PubMed] [Google Scholar]
- 15.Mezzarane RA, Klimstra M, Lewis A, Hundza SR, Zehr EP. Interlimb coupling from the arms to legs is differentially specified for populations of motor units comprising the compound H-reflex during “reduced” human locomotion. Exp Brain Res. 2011;208:157–168. doi: 10.1007/s00221-010-2467-0. [DOI] [PubMed] [Google Scholar]
- 16.Kido A, Tanaka N, Stein RB. Spinal reciprocal inhibition in human locomotion. J Appl Physiol. 2004;96:1969–1977. doi: 10.1152/japplphysiol.01060.2003. [DOI] [PubMed] [Google Scholar]
- 17.Capaday C, Stein RB. Difference in the amplitude of the human soleus H reflex during walking and running. J Physiol. 1987;392:513–522. doi: 10.1113/jphysiol.1987.sp016794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Capaday C, Stein RB. Amplitude modulation of the soleus H-reflex in the human during walking and standing. J Neurosci. 1986;6:1308–1313. doi: 10.1523/JNEUROSCI.06-05-01308.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carp JS, Tennissen AM, Chen XY, Wolpaw JR. H-reflex operant conditioning in mice. J Neurophysiol. 2006;96:1718–1727. doi: 10.1152/jn.00470.2006. [DOI] [PubMed] [Google Scholar]
- 20.Thompson AK, Estabrooks KL, Chong S, Stein RB. Spinal reflexes in ankle flexor and extensor muscles after chronic central nervous system lesions and functional electrical stimulation. Neurorehabil Neural Repair. 2009;23:133–142. doi: 10.1177/1545968308321067. [DOI] [PubMed] [Google Scholar]
- 21.Ozmerdivenli R, Bulut S, Urat T, Ayar A. The H- and T-reflex response parameters of long- and short-distance athletes. Physiol Res. 2002;51:395–400. [PubMed] [Google Scholar]
- 22.Nielsen J, Crone C, Hultborn H. H-reflexes are smaller in dancers from The Royal Danish Ballet than in well-trained athletes. Eur J Appl Physiol Occup Physiol. 1993;66:116–121. doi: 10.1007/BF01427051. [DOI] [PubMed] [Google Scholar]
- 23.Thompson AK, Chen XY, Wolpaw JR. Acquisition of a simple motor skill: task-dependent adaptation plus long-term change in the human soleus H-reflex. J Neurosci. 2009;29:5784–5792. doi: 10.1523/JNEUROSCI.4326-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carp JS, Tennissen AM, Chen XY, Wolpaw JR. Diurnal H-reflex variation in mice. Exp Brain Res. 2006;168:517–528. doi: 10.1007/s00221-005-0106-y. [DOI] [PubMed] [Google Scholar]
- 25.Wolpaw JR, Seegal RF. Diurnal rhythm in the spinal stretch reflex. Brain Res. 1982;244:365–369. doi: 10.1016/0006-8993(82)90099-3. [DOI] [PubMed] [Google Scholar]
- 26.Chen XY, Wolpaw JR. Circadian rhythm in rat H-reflex. Brain Res. 1994;648:167–170. [PubMed] [Google Scholar]
- 27.Lagerquist O, Zehr EP, Baldwin ER, Klakowicz PM, Collins DF. Diurnal changes in the amplitude of the Hoffmann reflex in the human soleus but not in the flexor carpi radialis muscle. Exp Brain Res. 2006;170:1–6. doi: 10.1007/s00221-005-0172-1. [DOI] [PubMed] [Google Scholar]
- 28.Chen XY, Wolpaw JR. Operant conditioning of H-reflex in freely moving rats. J Neurophysiol. 1995;73:411–415. doi: 10.1152/jn.1995.73.1.411. [DOI] [PubMed] [Google Scholar]
- 29.Zehr EP, Stein RB. Interaction of the Jendrassik maneuver with segmental presynaptic inhibition. Exp Brain Res. 1999;124:474–480. doi: 10.1007/s002210050643. [DOI] [PubMed] [Google Scholar]
- 30.Kido A, Tanaka N, Stein RB. Spinal excitation and inhibition decrease as humans age. Can J Physiol Pharmacol. 2004;82:238–248. doi: 10.1139/y04-017. [DOI] [PubMed] [Google Scholar]
- 31.Stein RB, Estabrooks KL, McGie S, Roth MJ, Jones KE. Quantifying the effects of voluntary contraction and inter-stimulus interval on the human soleus H-reflex. Exp Brain Res. 2007;182:309–319. doi: 10.1007/s00221-007-0989-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klimstra M, Zehr EP. A sigmoid function is the best fit for the ascending limb of the Hoffmann reflex recruitment curve. Exp Brain Res. 2008;186:93–105. doi: 10.1007/s00221-007-1207-6. [DOI] [PubMed] [Google Scholar]
- 33.Wilmink RJ, Slot PJ, Sinkjaer T. Modeling of the H-reflex facilitation during ramp and hold contractions. J Comput Neurosci. 1996;3:337–346. doi: 10.1007/BF00161092. [DOI] [PubMed] [Google Scholar]
- 34.Henneman E, Mendell LM. Handbook of Physiology: Section 1: The Nervous System Volume II, Part 1 &2: Motor Control. American Physiological Society; 1980. Functional organization of motoneuron pool and its inputs; pp. 423–507. [Google Scholar]
- 35.Carp JS, Chen XY, Sheikh H, Wolpaw JR. Operant conditioning of rat H-reflex affects motoneuron axonal conduction velocity. Exp Brain Res. 2001;136:269–273. doi: 10.1007/s002210000608. [DOI] [PubMed] [Google Scholar]
- 36.Carp JS, Wolpaw JR. Motoneuron plasticity underlying operantly conditioned decrease in primate H-reflex. J Neurophysiol. 1994;72:431–442. doi: 10.1152/jn.1994.72.1.431. [DOI] [PubMed] [Google Scholar]
- 37.Halter JA, Carp JS, Wolpaw JR. Operantly conditioned motoneuron plasticity: possible role of sodium channels. J Neurophysiol. 1995;73:867–871. doi: 10.1152/jn.1995.73.2.867. [DOI] [PubMed] [Google Scholar]
- 38.Carp JS, Wolpaw JR. Motoneuron properties after operantly conditioned increase in primate H-reflex. J Neurophysiol. 1995;73:1365–1373. doi: 10.1152/jn.1995.73.4.1365. [DOI] [PubMed] [Google Scholar]
- 39.Capaday C, Stein RB. The effects of postsynaptic inhibition on the monosynaptic reflex of the cat at different levels of motoneuron pool activity. Exp Brain Res. 1989;77:577–584. doi: 10.1007/BF00249610. [DOI] [PubMed] [Google Scholar]
- 40.Capaday C, Stein RB. A method for simulating the reflex output of a motoneuron pool. J Neurosci Methods. 1987;21:91–104. doi: 10.1016/0165-0270(87)90107-5. [DOI] [PubMed] [Google Scholar]
- 41.Morita H, Petersen N, Christensen LO, Sinkjaer T, Nielsen J. Sensitivity of H-reflexes and stretch reflexes to presynaptic inhibition in humans. J Neurophysiol. 1998;80:610–620. doi: 10.1152/jn.1998.80.2.610. [DOI] [PubMed] [Google Scholar]
- 42.Nielsen J, Petersen N, Crone C. Changes in transmission across synapses of Ia afferents in spastic patients. Brain. 1995;118:995–1004. doi: 10.1093/brain/118.4.995. [DOI] [PubMed] [Google Scholar]
- 43.Yang JF, Fung J, Edamura M, Blunt R, Stein RB, Barbeau H. H-reflex modulation during walking in spastic paretic subjects. Can J Neurol Sci. 1991;18:443–452. doi: 10.1017/s0317167100032133. [DOI] [PubMed] [Google Scholar]
- 44.Stein RB, Capaday C. The modulation of human reflexes during functional motor tasks. Trends Neurosci. 1988;11:328–332. doi: 10.1016/0166-2236(88)90097-5. [DOI] [PubMed] [Google Scholar]
- 45.Chen Y, Chen XY, Jakeman LB, Schalk G, Stokes BT, Wolpaw JR. The interaction of a new motor skill and an old one: H-reflex conditioning and locomotion in rats. J Neurosci. 2005;25:6898–6906. doi: 10.1523/JNEUROSCI.1684-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thompson AK, Wolpaw JR, Abel BM, Lichtman SW, DeFrancesco E, Pomerantz F. Operant down-conditioning of the soleus H-reflex appears to improve gait in people with incomplete spinal cord injury. Washington, D. C.: 2011. p Program No. 917.912. [Google Scholar]
- 47.Chen XY, Chen L, Chen Y, Wolpaw JR. Operant conditioning of reciprocal inhibition in rat soleus muscle. J Neurophysiol. 2006;96:2144–2150. doi: 10.1152/jn.00253.2006. [DOI] [PubMed] [Google Scholar]
- 48.Maegele M, Muller S, Wernig A, Edgerton VR, Harkema SJ. Recruitment of spinal motor pools during voluntary movements versus stepping after human spinal cord injury. J Neurotrauma. 2002;19:1217–1229. doi: 10.1089/08977150260338010. [DOI] [PubMed] [Google Scholar]
- 49.Wernig A, Nanassy A, Muller S. Laufband (LB) therapy in spinal cord lesioned persons. Prog Brain Res. 2000;128:89–97. doi: 10.1016/S0079-6123(00)28009-6. [DOI] [PubMed] [Google Scholar]
- 50.Harkema SJ, Hurley SL, Patel UK, Requejo PS, Dobkin BH, Edgerton VR. Human lumbosacral spinal cord interprets loading during stepping. J Neurophysiol. 1997;77:797–811. doi: 10.1152/jn.1997.77.2.797. [DOI] [PubMed] [Google Scholar]
- 51.Edgerton VR, Courtine G, Gerasimenko YP, Lavrov I, Ichiyama RM, Fong AJ, Cai LL, Otoshi CK, Tillakaratne NJ, Burdick JW, Roy RR. Training locomotor networks. Brain Res Rev. 2008;57:241–254. doi: 10.1016/j.brainresrev.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


