Phytochrome B (phyB) is the main light receptor responsible for the shade avoidance response in mature plants. Like other phytochromes (phys), it alternates between the Pfr form and the Pr form. Upon activation by red light (causing the switch to the Pfr conformation), phys interact with various signaling partners and translocate from the cytoplasm to the nucleus, where they are found in structures called photobodies (reviewed in Chen and Chory, 2011). Phytochromes are evolutionarily related to light-regulated His kinases, and the role of phosphorylation in phy signaling has been a matter of much debate. Two phosphatases interact with phyB, suggesting that phosphorylation might be play a role in its activity (Ryu et al., 2005; Phee et al., 2008). New work from Medzihradszky et al. (pages 535–544) shows that phosphorylation of Arabidopsis thaliana phyB increases its rate of reversion from the active Pfr form to the inactive Pr form.
Medzihradszky et al. began by testing whether phyB is indeed phosphorylated in vivo. They found that a phyB-TAP fusion protein purified from transgenic Arabidopsis seedlings was phosphorylated in the N-terminal domain, including on Ser-86. They then tested the function of phosphorylation in phyB signaling by mutating Ser-86 to Ala (representing the unphosphorylated state) or Asp (mimicking phosphorylation) and expressing the mutant proteins as fusions in a phyB mutant background. The phyBSer86Asp line showed decreased phyB signaling, especially at low fluences of R light, in terms of hypocotyl growth inhibition, cotyledon expansion, and shade avoidance. By contrast, the phyBSer86Ala line appeared to have enhanced phyB signaling activity.
In fact, the mutations altered virtually every facet of phyB function. Compared with the wild type, lower fluences of red light were able to induce nuclear accumulation and photobody formation of phyBSer86Ala, whereas higher fluences were necessary for phyBSer86Asp (see figure). The phyBSer86Asp mutation also led to reduced interaction with PHYTOCHROME INTERACTING FACTOR3 under nonsaturating light conditions in yeast two-hybrid assays.
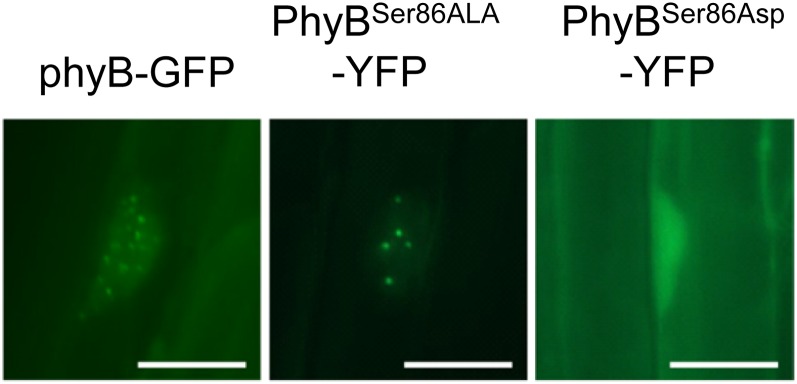
Photobody formation in the nucleus requires higher intensity light for phyBSer86Asp-YFP and lower for phyBSer86Ala-YFP. Dark-grown phyB-9 seedlings expressing the indicated proteins were treated with 2 μmol m−2 s−1 red light for 6 h. Bars = 10 μm. (Reprinted from Medzihradszky et al. [2013], Figure 3B.)
In an attempt to explain these myriad effects, the authors examined dark reversion, in which the Pfr form becomes inactive by reverting back to the Pr form. Importantly, they found that the phyBSer86Asp mutant reverted to the Pr form faster and to a greater extent than did the wild type, whereas phyBSer86Ala reverted more slowly than the wild type. These changes in dark reversion rate can explain all of the phenotypes caused by the Ser-86 mutations and shed light on the molecular mechanisms of phyB signaling. This thorough study from Medzihradszky et al. convincingly demonstrates that phosphorylation has a role in phyB function and that Ser-86 phosphorylation, in particular, is linked with phyB dark reversion.
References
- Chen M., Chory J. (2011). Phytochrome signaling mechanisms and the control of plant development. Trends Cell Biol. 21: 664–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzihradszky M., et al. (2013). Phosphorylation of phytochrome B inhibits light-induced signaling via accelerated dark reversion in Arabidopsis. Plant Cell 25: 535–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phee B.K., Kim J.I., Shin D.H., Yoo J., Park K.J., Han Y.J., Kwon Y.K., Cho M.H., Jeon J.S., Bhoo S.H., Hahn T.R. (2008). A novel protein phosphatase indirectly regulates phytochrome-interacting factor 3 via phytochrome. Biochem. J. 415: 247–255 [DOI] [PubMed] [Google Scholar]
- Ryu J.S., et al. (2005). Phytochrome-specific type 5 phosphatase controls light signal flux by enhancing phytochrome stability and affinity for a signal transducer. Cell 120: 395–406 [DOI] [PubMed] [Google Scholar]


