Abstract
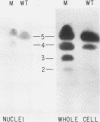
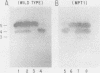
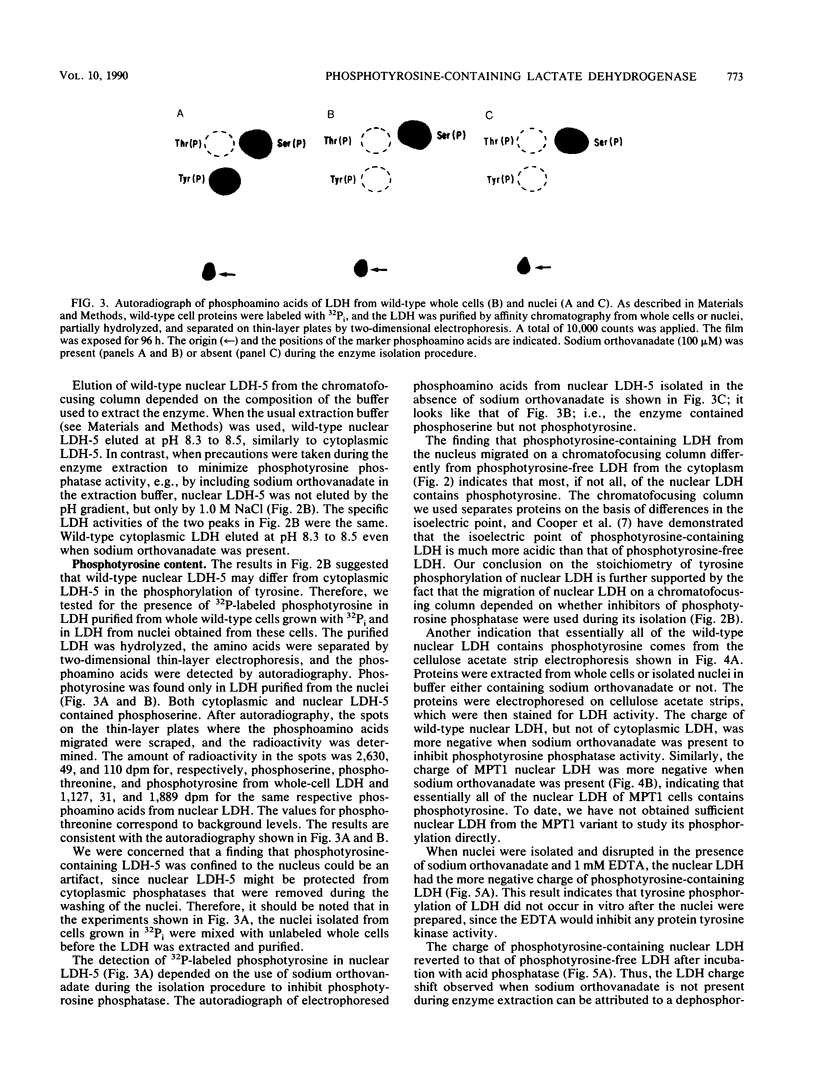
There are five lactate dehydrogenase (LDH) isoenzymes, composed of various combinations of two types of subunits. LDH-5, which contains only the LDH A subunit, is known to be present in both the cytoplasm and the nucleus, to act as a single-stranded DNA-binding protein possibly functioning in transcription and/or replication, and to undergo phosphorylation of tyrosine 238 in approximately 1% of the enzyme after cell transformation by certain tumor viruses. We have characterized LDH from wild-type PC12 pheochromocytoma cells and from a PC12 variant (MPT1) that exhibits altered lactate metabolism and altered expression of multiple genes. Wild-type and MPT1 cells contain different proportions of LDH isoenzymes, with LDH-5 being more predominant in wild-type cells than in the variant. A small fraction of LDH from PC12 cells contains phosphotyrosine. Approximately 99% of the total LDH activity is located in the cytoplasm, but all of the phosphotyrosine-containing LDH is located in the nucleus. Furthermore, essentially all of the nuclear LDH contains phosphotyrosine. These results suggest that tyrosine phosphorylation can affect its role in the nucleus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitler C. M., Zhang M. B., Howard B. D. PC12 variants deficient in catecholamine transport. J Neurochem. 1986 Oct;47(4):1286–1293. doi: 10.1111/j.1471-4159.1986.tb00752.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brew K., Vanaman T. C., Hill R. L. The role of alpha-lactalbumin and the A protein in lactose synthetase: a unique mechanism for the control of a biological reaction. Proc Natl Acad Sci U S A. 1968 Feb;59(2):491–497. doi: 10.1073/pnas.59.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo A., Biocca S., Corvaja N., Calissano P. Nuclear localization of a lactic dehydrogenase with single-stranded DNA-binding properties. Exp Cell Res. 1985 Nov;161(1):130–140. doi: 10.1016/0014-4827(85)90497-5. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Esch F. S., Taylor S. S., Hunter T. Phosphorylation sites in enolase and lactate dehydrogenase utilized by tyrosine protein kinases in vivo and in vitro. J Biol Chem. 1984 Jun 25;259(12):7835–7841. [PubMed] [Google Scholar]
- Cooper J. A., Reiss N. A., Schwartz R. J., Hunter T. Three glycolytic enzymes are phosphorylated at tyrosine in cells transformed by Rous sarcoma virus. Nature. 1983 Mar 17;302(5905):218–223. doi: 10.1038/302218a0. [DOI] [PubMed] [Google Scholar]
- Denton T., Howard B. D. A dopaminergic cell line variant resistant to the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. J Neurochem. 1987 Aug;49(2):622–630. doi: 10.1111/j.1471-4159.1987.tb02909.x. [DOI] [PubMed] [Google Scholar]
- Everse J., Kaplan N. O. Lactate dehydrogenases: structure and function. Adv Enzymol Relat Areas Mol Biol. 1973;37:61–133. doi: 10.1002/9780470122822.ch2. [DOI] [PubMed] [Google Scholar]
- GOLDMAN R. D., KAPLAN N. O., HALL T. C. LACTIC DEHYDROGENASE IN HUMAN NEOPLASTIC TISSUES. Cancer Res. 1964 Apr;24:389–399. [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse F., Nasheuer H. P., Scholtissek S., Schomburg U. Lactate dehydrogenase and glyceraldehyde-phosphate dehydrogenase are single-stranded DNA-binding proteins that affect the DNA-polymerase-alpha-primase complex. Eur J Biochem. 1986 Nov 3;160(3):459–467. doi: 10.1111/j.1432-1033.1986.tb10062.x. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps M. P., Sefton B. M. Identification of multiple novel polypeptide substrates of the v-src, v-yes, v-fps, v-ros, and v-erb-B oncogenic tyrosine protein kinases utilizing antisera against phosphotyrosine. Oncogene. 1988 Apr;2(4):305–315. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee C. Y., Yuan J. H., Goldberg E. Lactate dehydrogenase isozymes from mouse. Methods Enzymol. 1982;89(Pt 500):351–358. doi: 10.1016/s0076-6879(82)89063-0. [DOI] [PubMed] [Google Scholar]
- PENNINGTON R. J. Biochemistry of dystrophic muscle. Mitochondrial succinate-tetrazolium reductase and adenosine triphosphatase. Biochem J. 1961 Sep;80:649–654. doi: 10.1042/bj0800649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel G. L., Thompson P. E. Immunoreactive helix-destabilizing protein localized in transcriptionally active regions of Drosophila polytene chromosomes. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6749–6753. doi: 10.1073/pnas.77.11.6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plimmer R. H. Esters of phosphoric acid: Phosphoryl hydroxyamino-acids. Biochem J. 1941 Apr;35(4):461–469. doi: 10.1042/bj0350461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharief F. S., Wilson S. H., Li S. S. Identification of the mouse low-salt-eluting single-stranded DNA-binding protein as a mammalian lactate dehydrogenase-A isoenzyme. Biochem J. 1986 Feb 1;233(3):913–916. doi: 10.1042/bj2330913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TATA J. R., ERNSTER L., LINDBERG O., ARRHENIUS E., PEDERSEN S., HEDMAN R. The action of thyroid hormones at the cell level. Biochem J. 1963 Mar;86:408–428. doi: 10.1042/bj0860408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taborsky G. Phosphoproteins. Adv Protein Chem. 1974;28:1–210. doi: 10.1016/s0065-3233(08)60230-2. [DOI] [PubMed] [Google Scholar]
- Tata J. R. Isolation of nuclei from liver and other tissues. Methods Enzymol. 1974;31:253–262. doi: 10.1016/0076-6879(74)31027-0. [DOI] [PubMed] [Google Scholar]
- Toth-Martinez B. L., Gál S., Kiss L. Chromatofocusing for separation of beta-lactamases. I. Microscale separation of RTEM- and chromosomally mediated beta-lactamases of Escherichia coli J6-2. J Chromatogr. 1983 Jun 24;262:373–378. doi: 10.1016/s0021-9673(01)88123-1. [DOI] [PubMed] [Google Scholar]
- Williams K. R., Reddigari S., Patel G. L. Identification of a nucleic acid helix-destabilizing protein from rat liver as lactate dehydrogenase-5. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5260–5264. doi: 10.1073/pnas.82.16.5260. [DOI] [PMC free article] [PubMed] [Google Scholar]