This study reveals that the F-box protein COI1, which regulates jasmonate-mediated plant development and defense, is strictly regulated by a dynamic balance of SCFCOI1-mediated stabilization and 26S proteasome–mediated degradation and maintained at a protein level essential for proper biological functions in plants.
Abstract
Jasmonate regulates critical aspects of plant development and defense. The F-box protein CORONATINE INSENSITIVE1 (COI1) functions as a jasmonate receptor and forms Skp1/Cullin1/F-box protein COI1 (SCFCOI1) complexes with Arabidopsis thaliana Cullin1 and Arabidopsis Skp1-like1 (ASK1) to recruit its substrate jasmonate ZIM-domain proteins for ubiquitination and degradation. Here, we reveal a mechanism regulating COI1 protein levels in Arabidopsis. Genetic and biochemical analysis and in vitro degradation assays demonstrated that the COI1 protein was initially stabilized by interacting with ASK1 and further secured by assembly into SCFCOI1 complexes, suggesting a function for SCFCOI1 in the stabilization of COI1 in Arabidopsis. Furthermore, we show that dissociated COI1 is degraded through the 26S proteasome pathway, and we identified the 297th Lys residue as an active ubiquitination site in COI1. Our data suggest that the COI1 protein is strictly regulated by a dynamic balance of SCFCOI1-mediated stabilization and 26S proteasome–mediated degradation and thus maintained at a protein level essential for proper biological functions in Arabidopsis development and defense responses.
INTRODUCTION
In all eukaryotes, the ubiquitin/26S proteasome pathway plays a key role in regulating diverse aspects of developmental and physiological responses by selectively removing intracellular proteins (Moon et al., 2004; Smalle and Vierstra, 2004; Ho et al., 2008). These intracellular proteins are recognized and modified by the covalent attachment of polymeric ubiquitin chains via an ATP-dependent reaction cascade involving the sequential and coordinated action of three enzyme families, including the E1 ubiquitin-activating enzymes, the E2 ubiquitin-conjugating enzymes, and the E3 ubiquitin ligases (Hershko and Ciechanover, 1998). The best characterized class of E3 ubiquitin ligases is the SCF (SKP1/CUL1/F-box protein) E3 ligases, which contain core constant subunits, including Cullin1 (CUL1) and SKP1, and one variable F-box protein (Moon et al., 2004). SKP1 serves as a bridge between CUL1 and the F-box protein (Zheng et al., 2002). The N-terminal F-box motif of the F-box protein is responsible for interaction with SKP1, while its C-terminal WD40 motif or Leucine-rich repeat region binds targeted substrates to provide reaction specificity (Hua and Vierstra, 2011).
In Arabidopsis thaliana, there are ∼700 F-box proteins (Xu et al., 2009). These F-box proteins, which form distinct SCF complexes with Arabidopsis SKP-like proteins (ASK) and Arabidopsis CUL1, specifically recognize substrate proteins and dynamically regulate their abundance to modulate a diverse range of biological processes (Hellmann and Estelle, 2002; Stone and Callis, 2007). During the past two decades, tremendous progress has been achieved in understanding how F-box proteins control the abundance of short-lived regulatory proteins that mediate key plant functions, including hormonal signaling, light signaling, circadian clock control, organ development, self-incompatibility, cell cycle, and defense responses (Gray et al., 2001; del Pozo et al., 2002; Stirnberg et al., 2002; Más et al., 2003; Potuschak et al., 2003; Sasaki et al., 2003; Qiao et al., 2004; Imaizumi et al., 2005; Lechner et al., 2006; Santner and Estelle, 2010).
Recent studies have shown that regulating the abundance of F-box proteins themselves is essential to exert their proper biological functions in Arabidopsis. For example, the F-box protein ZEITLUPE (ZTL) functions as a circadian photoreceptor and is critical to the maintenance of circadian rhythmicity (Somers et al., 2004). Rhythmic changes in ZTL protein levels are necessary to sustain a normal circadian period in Arabidopsis (Kim et al., 2003). Further studies revealed that the GIGANTEA (GI) protein interacts with ZTL in a blue light–dependent manner (Kim et al., 2007), to stabilize the ZTL protein, which mediates the degradation of TIMING OF CAB EXPRESSION1 (TOC1), a central transcriptional repressor of core circadian genes (Más et al., 2003). The F-box proteins EBF1/2 modulate ethylene responses via regulating the abundance of their substrate protein, the EIN3/EIL1 transcription factor (Guo and Ecker, 2003; Potuschak et al., 2003). A recent study revealed that abundance of the EBF1/2 proteins adjusts in response to ethylene, which is vital for regulating EIN3/EIL1 degradation (An et al., 2010).
The F-box protein CORONATINE INSENSITIVE1 (COI1) (Xie et al., 1998) functions as a receptor for jasmonate (Katsir et al., 2008; Yan et al., 2009; Sheard et al., 2010), an important plant hormone essential for plant defense and development (Farmer and Ryan, 1992; Staswick et al., 1992; Howe et al., 1996; McConn et al., 1997; Cheong and Choi, 2003; Farmer et al., 2003; Wasternack, 2007). COI1 forms SCFCOI1 complexes with CUL1 and ASK1/2 (Xu et al., 2002) and recruits its substrates, the jasmonate ZIM-domain proteins (JAZs) (Chini et al., 2007; Thines et al., 2007; Yan et al., 2007), for ubiquitination and degradation via the 26S proteasome pathway. Degradation of JAZs activates their downstream transcription factors or complexes, including MYC2/3/4 (Cheng et al., 2011; Fernández-Calvo et al., 2011; Niu et al., 2011), MYB21/24 (Song et al., 2011), and WD-repeat/bHLH/MYB (Qi et al., 2011), to mediate various jasmonate responses. Although understanding the molecular basis for COI1 function in regulation of jasmonate responses has received significant attention, the mechanism by which cells regulate and maintain the required level of the F-box protein COI1 itself to properly execute its biological function in Arabidopsis remains unclear.
In this study, we revealed a mechanism regulating the abundance of COI1 in Arabidopsis. We demonstrate that the COI1 protein is stabilized by the integrity of the SCFCOI1 complex. Furthermore, we show that disassociated COI1 is degraded through the 26S proteasome pathway. Our data suggest that the COI1 protein is regulated by a dynamic balance of SCFCOI1-mediated stabilization and 26S proteasome–mediated degradation and maintained at a proper protein level essential for exerting its biological functions in Arabidopsis. It will be interesting to determine whether this mechanism is more widely used for regulation of other Arabidopsis F-box proteins by SCF complexes, in addition to their regulatory role in substrate degradation.
RESULTS
ASK1 Is Required to Maintain COI1 Stability
Previous studies have shown that the abundance of some F-box proteins is dynamically regulated by hormones in Arabidopsis (An et al., 2010; Liu and Stone, 2010; Jurado et al., 2008, 2010). To examine the effect of plant hormones on abundance of the F-box protein COI1, we treated Arabidopsis wild-type seedlings with various plant hormones in the presence of the protein synthesis inhibitor cycloheximide (CHX). Immunoblot analysis showed that treatments with plant hormones, including abscisic acid, 1-aminocyclopropane-1-carboxylic acid, 6-benzylaminopurine, gibberellin, salicylate, jasmonic acid, jasmonic acid methyl ester (MeJA), jasmonoyl-Ile, cis(+)-12-oxophytodienoic acid, and coronatine (a molecular mimic of jasmonoyl-Ile), had no obvious effect on COI1 abundance. The COI1 protein level was unchanged over 24 h in hormone-treated Arabidopsis seedlings in which protein synthesis was inhibited by CHX (Figure 1A). These results indicate that COI1 experiences a high degree of stability in Arabidopsis.
Figure 1.
ASK1 Regulates COI1 Stability.
(A) Ten-day-old Arabidopsis seedlings were treated with CHX (1 mM) for 2 h, followed by additional 24-h treatment with 20 μM cis(+)-12-oxophytodienoic acid (OPDA), (±)-jasmonic acid (JA), MeJA, (±)-jasmonoyl-Ile (JA-Ile), (±)-abscisic acid (ABA), 1-aminocyclopropane-1-carboxylic acid (ACC), 6-benzylaminopurine (6-BA), gibberellic acid GA3 (GA), indole-3-acetic acid (IAA), salicylic acid (SA), or coronatine (COR). Total protein extracted from the treated seedlings was separated by SDS-PAGE and immunoblotted with anti-COI1 antibody (α-COI1). The PVDF membrane was stained with Memstain to visualize the large subunit of ribulose-1,5-bisphosphate as a control for equal loading (Loading Control). CK1 and CK2 refer to the treatment with CHX for 0 h (CK1) and 26 h (CK2).
(B) Immunoblot analysis of COI1 expression in yeast cells. Yeast cells transfected with constructs pBridge-COI1 (COI1), pBridge-JAZ1-COI1 (COI1+JAZ1), and pBridge-ASK1-COI1 (COI1+ASK1) were lysed in detergent. The supernatants of whole-cell lysates were subjected to immunoblot analysis using COI1 antiserum (α-COI1). The PVDF membrane was stained with Memstain to serve as loading control.
(C) Leaves from 4-week-old Arabidopsis Landsberg erecta (the wild type [WT]) and ask1-1 (ask1) plants were harvested for protein extraction and subjected to immunoblot analysis using the COI1 antibody (α-COI1) or the ASK1 antibody (α-ASK1).
(D) RT-PCR analysis of the COI1 transcript levels (COI1) in the wild type and ask1-1 described in (C). ACTIN1 was used as an internal control.
(E) Real-time quantitative PCR analysis of COI1 transcript levels in the wild type and ask1-1 described in (C). Error bars represent sd (n = 6).
(F) Real-time quantitative PCR analysis of the VEGETATIVE STORAGE PROTEIN1 (VSP1) gene in 2-week-old seedlings of Landsberg erecta (WT) and ask1-1 (ask1) treated with or without MeJA at the indicated concentrations. Error bars represent sd (n = 6).
Stabilities of F-box proteins ZTL and Homolog of Slimb (HOS) are maintained by the ZTL-interacting protein GI and the HOS substrate IκBα, respectively (Li et al., 2004; Kim et al., 2007). To investigate the molecular basis for COI1 stability, we examined whether the COI1 protein was stabilized by COI1-interacting protein ASK1 or its substrate protein JAZ. When the COI1 gene was coexpressed with ASK1 in a yeast pBridge expression system, high levels of the COI1 protein were detected in the supernatant of lysed yeast cells. However, under equivalent growth conditions, the COI1 protein was hardly detected when COI1 was coexpressed with JAZ1 or expressed alone (Figure 1B). These results clearly indicate that ASK1, but not JAZ1, played an important role in COI1 stability in a heterologous system.
To verify the role for ASK1 in COI1 stability in planta, we investigated whether disruption of ASK1 results in reduced COI1 levels by comparing the COI1 level between the wild type and ask1-1, an Arabidopsis mutant with a Ds insertion in the ASK1 gene (Yang et al., 1999). As shown in Figure 1C, the COI1 protein level was severely reduced in ask1-1 compared with the wild type, whereas the COI1 mRNA level was equivalent in both genotypes, as revealed by RT-PCR (Figure 1D) and real-time quantitative PCR analysis (Figure 1E). Consistent with the reduction of COI1 protein in ask1-1, expression of VSP1, a typical jasmonate-responsive gene (Anderson, 1991; Staswick, 1994), was also reduced in ask1-1 (Figure 1F). These results demonstrate that ASK1 is required to maintain stability of the COI1 protein for eliciting jasmonate responses.
Mutation in CUL1 Results in a Reduction of COI1 Protein in Arabidopsis
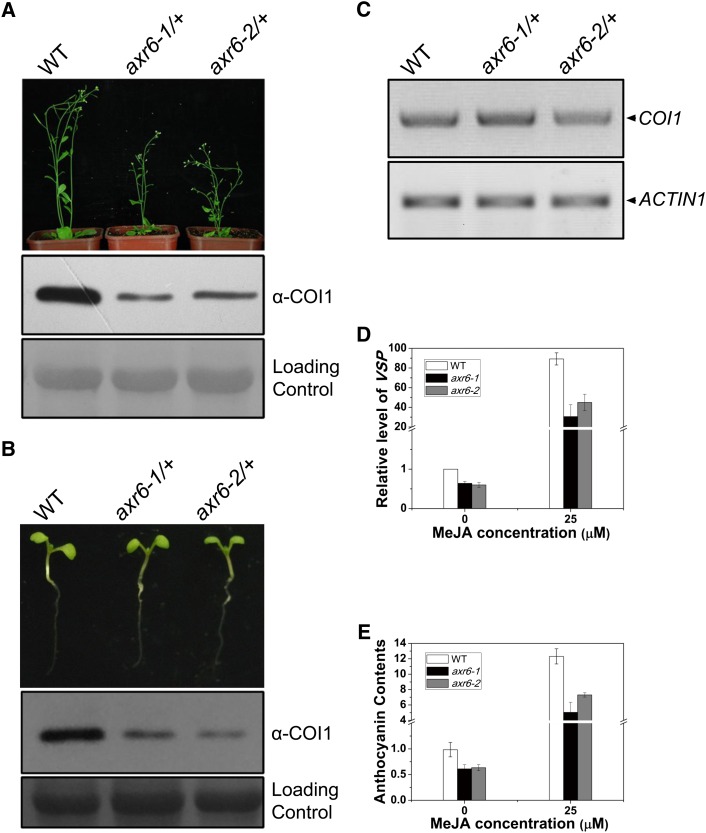
ASK1 interacts directly with COI1 (Xu et al., 2002) and stabilizes the COI1 protein (Figure 1). Here, we investigated whether the stability of COI1 is also controlled by another COI1-associated protein, CUL1, which forms an SCF complex with COI1 via ASK1 (Xu et al., 2002; Ren et al., 2005; Hua and Vierstra, 2011). As the axr6-1 and axr6-2 homozygous mutants with missense mutations of Phe-111 to Val and Ile residues, respectively, in CUL1 are seedling lethal (Hobbie et al., 2000; Hellmann et al., 2003), axr6-1/+ and axr6-2/+ heterozygous mutants were used for immunoblot analysis of COI1 protein.
Compared with the wild type, COI1 protein levels were clearly reduced in axr6-1/+ and axr6-2/+ adult plants (Figure 2A), whereas the COI1 mRNA levels were unchanged (Figure 2C). Similar results were obtained for 10-d-old seedlings, which exhibited normal morphological phenotypes (Figure 2B), and minimized potential effect of the severe morphological defects on COI1 stability in axr6-1/+ and axr6-2/+ adult plants (Figure 2A). Consistent with reduction of the COI1 protein, jasmonate induction of VSP1 expression and anthocyanin accumulation were also reduced in axr6-1/+ and axr6-2/+ compared with the wild type (Figures 2D and 2E). These results suggest a role for CUL1 in the regulation of COI1 stability.
Figure 2.
Mutations in CUL1 Affect COI1 Stability.
(A) and (B) Proteins were extracted from adult plants (A) and seedlings (B) of Arabidopsis Columbia (the wild type [WT]), axr6-1/AXR6 (axr6-1/+), and axr6-2/AXR6 (axr6-2/+) heterozygous mutant plants and subjected to immunoblot using the COI1 antiserum (α-COI1). Morphological phenotypes of 5-week-old adult plants (top panel in [A]) and 7-d-old seedlings (top panel in [B]) are also shown.
(C) RT-PCR analysis of the COI1 transcript levels (COI1) in the Arabidopsis plants, as indicated.
(D) Real-time quantitative PCR analysis of the VSP1 (VSP) transcripts in the wild type, axr6-1/+, and axr6-2/+ treated with MeJA (25 μM) or solvent. Error bars represent sd (n = 6).
(E) Anthocyanin accumulation in the wild type, axr6-1/+, and axr6-2/+ treated with MeJA (25 μM) or solvent. The o.d. (535 nm–650 nm)/g fresh weight was regarded as the Y value. Error bars represent sd (n = 6).
[See online article for color version of this figure.]
Missense mutations of Phe-111 to Val and Ile residues in CUL1 do not reduce the CUL1 level (Hellmann et al., 2003) but compromise the ability of CUL1 to interact with ASK1 and attenuate assembly of the ASK1-COI1 into SCF complexes (Ren et al., 2005). Attenuation of the COI1-ASK1 assembly into SCFCOI1 by the CUL1 mutations of Phe-111 to Val and Ile residues causes decreased COI1 protein levels in axr6-1/+ and axr6-2/+ (Figures 2A and 2B), suggesting that integrity of SCFCOI1 is essential for the COI1 stability in Arabidopsis.
The COI1 Protein Is Regulated at the Posttranslational Level in Arabidopsis
We next generated transgenic lines constitutively overexpressing COI1 to test whether high and stable COI1 transcript levels would affect COI1 protein abundance in Arabidopsis. The COI1 gene, tagged with hemagglutinin (HA) at its C terminus and Flag at its N terminus, was driven by the cauliflower mosaic virus (CaMV) 35S promoter and introduced into the coi1-1 mutant.
The transgenic line, referred as to coi1:HiCOI1 (coi1-1 highly expressing COI1), had an ∼10-fold increase in COI1 transcripts as determined by RT-PCR and real-time quantitative PCR analysis (Figure 3A). Immunoblot analysis showed that coi1:HiCOI1 retained the normal COI1 protein level, similar to that in the wild type (Figure 3B), suggesting that the high level of COI1 transcripts was unable to further elevate COI1 protein levels in coi1:HiCOI1. Consistent with the COI1 level, the coi1:HiCOI1 plants exhibited full complementation with restoration of normal jasmonate responses, as indicated by jasmonate-mediated root inhibition (Figure 3C), anthocyanin accumulation (Figure 3F), and plant fertility (stamen length, anther dehiscence, pollen viability, and seed production; Figures 3D and 3E). Similar observations were obtained when other independent transgenic lines were analyzed. These data suggest that overexpression of COI1 transcript level is unable to further increase the COI1 protein abundance in Arabidopsis, indicating that the COI1 protein level was regulated at a posttranslational level.
Figure 3.
The Significantly Increased COI1 Transcripts Were Unable to Elevate the COI1 Protein Level.
(A) RT-PCR (top panel) and real-time quantitative PCR (bottom panel) of the COI1 transcript level in the leaves of 4-week-old Arabidopsis Columbia (the wild type [WT]), coi1-1 (coi1), and coi1:HiCOI1 plants. Error bars represent sd (n = 4).
(B) Immunoblot analysis of the COI1 protein level in the plants described in (A) using the COI1 antibody (α-COI1).
(C) Phenotype of 10-d-old seedlings grown on Murashige and Skoog (MS) medium without (top panel) or with (middle panel) MeJA (25 μM). The bottom panel shows average root lengths of seedlings pictured in the middle panel. Error bars represent sd (n > 100). JA, jasmonate.
(D) Analysis of stamen length (top panel), anther dehiscence (second panel), and the pollen viability in the wild type, coi1-1, and coi1:HiCOI1. Fluorescein diacetate and propidium iodide were used to stain pollen grains with viable pollen staining green, while inviable pollen stains red (third panel). Pollen grains of the wild type and coi1:HiCOI1 germinate normally (bottom panel).
(E) The coi1:HiCOI1 and wild-type plants similarly exhibited normal seed production. The top panel shows representative primary inflorescences. The middle panel shows representative siliques. Seed numbers counted from mature siliques are shown in the bottom panel. Error bars represent sd (n = 10).
(F) Anthocyanin accumulation in Col-0, coi1-1, and coi1:HiCOI1 treated with MeJA (25 μM). Error bars represent sd (n = 6).
We further conducted genetic crosses of coi1:HiCOI1 with ask1-1 and axr6-1/+ mutants to generate plants that constantly overexpress the COI1 gene in a background with compromised ASK1 or CUL1 function (ask1:HiCOI1 or axr6:HiCOI1). Indeed, the COI1 transcripts in ask1:HiCOI1 and axr6:HiCOI1 were maintained at a high level similar to coi1:HiCOI1 (Figures 4A and 4B). However, immunoblot analysis showed that the levels of COI1 protein were greatly reduced in the ask1:HiCOI1 and axr6:HiCOI1 lines (Figures 4A and 4B). These data further suggested that COI1 was regulated at a posttranslational level and that COI1 is stabilized by the SCFCOI1 core subunits ASK1 and CUL1 in Arabidopsis.
Figure 4.
Stable and High COI1 Transcription Failed to Increase COI1 abundance in ask1-1 and axr6-1/+.
The protein level of HA-tagged COI1 was determined for the leaves of 4-week-old ask1:HiCOI1 (A) and axr6:HiCOI1 (B) by immunoblot analysis using anti-HA antibody (top panel). The COI1 transcript level was determined by RT-PCR (middle panel) and real-time quantitative PCR analysis (bottom panel) using the leaves of 4-week-old plants. Error bars represent sd (n = 3). coi1:HiCOI1, as described in Figure 3, served as reference sample.
COI1 Stability Is Regulated by SCFCOI1 in Vitro
Having shown that SCFCOI1 is essential for COI1 stability in Arabidopsis (Figures 1 to 4), we used in vitro assays to further verify that COI1 stability is dependent on the SCFCOI1 complex. Myc-tagged COI1 (Myc-COI1) was expressed in a Nicotiana benthamiana transient expression system, purified with anti-Myc affinity beads and evaluated for its bioactivity as assayed for its interaction with JAZ in a coronatine-dependent manner (Figure 5A). Myc-COI1 protein was then mixed with total protein extracts prepared from ask1-1, axr6-1/+, or wild-type plants, and Myc-COI1 stability was assessed by immunoblot analysis after the treatment at 22°C for various periods of time up to 8 h.
Figure 5.
In Vitro Analysis of the Regulatory Role for SCFCOI1 in the COI1 Stability.
(A) Myc-tagged COI1 transiently expressed in N. benthamiana was extracted from tobacco leaves (Myc-COI1 ext), purified with Anti-c-Myc Agarose Affinity Gel (Purified Myc-COI1), and subsequently assessed for its ability to interact with recombinant JAZ1-His protein in presence of coronatine at various concentrations (0, 0.01, 0.1, 1, and 10 μM) in a pull-down assay. Total protein extracts from tobacco leaves infiltrated with empty expression vector is shown as the control. The samples were purified by SDS-PAGE and immunoblotted with the anti-Myc antibody (top panel). The PVDF membrane was stained with Memstain to visualize the recombinant JAZ1-His protein in the nickel-nitrilotriacetic acid (Ni-NTA) affinity resin (bottom panel).
(B) The purified Myc-COI1 protein was added to total crude protein extracts from ask1-1 (ask1 Crude) and Arabidopsis Landsberg erecta plants (wild type [WT] Crude), incubated at 22°C for the indicated time periods, and subjected to immunoblot analysis with anti-Myc antibody (α-Myc). The PVDF membrane was stained with Memstain to visualize the large subunit of ribulose-1,5-bisphosphate as a control for equal loading (Loading Control).
(C) The purified Myc-COI1 protein was added to total crude protein extracts from axr6-1/+ plants (axr6 Crude) or Columbia wild-type control plants (WT Crude), incubated at 22°C for the indicated time periods, and subjected to immunoblot analysis with anti-Myc antibody (α-Myc).
As shown in Figures 5B and 5C, the Myc-COI1 protein degraded more rapidly in either ask1-1 and axr6-1/+ extracts compared with the wild type. These data demonstrated that both SCF core components ASK1 and CUL1 are necessary for Myc-COI1 stability, implying that an intact SCF complex is required for maintaining COI1 levels. Furthermore, as the Myc-COI1 protein was used for the in vitro assays, these data confirmed that Myc-COI1 stability is regulated at the posttranslational level and not at the level of transcription or translation.
All data, obtained from our in vivo and in vitro assays (Figures 1 to 5), collectively point to the COI1 protein being stabilized by the SCFCOI1 complex.
COI1 Is Degraded via the 26S Proteasome Pathway in an SCFCOl1-Independent Manner
Recent studies reported that degradation of the Arabidopsis F-box proteins TIR1, EBF1/2, and ZTL are mediated by proteasome-dependent proteolysis (Stuttmann et al., 2009; An et al., 2010; Ito et al., 2012); we further investigated the mechanism involved in COI1 protein degradation. Total protein extracts from 4-week-old coi1:HiCOI1 plants were used to assess COI1 stability in presence of the specific proteasome inhibitors MG132 and PS341 and a mixture of inhibitors against a broad range of proteases. COI1 degradation was attenuated by MG132 and PS341 (Figures 6A and 6B) but not by the nonspecific protease inhibitors (Figure 6A), suggesting that COI1 may be degraded via the 26S proteasome pathway. As ubiquitin/proteasome degradation is an ATP-dependent process (Etlinger and Goldberg, 1977), we were not surprised to find that ATP treatment significantly enhanced the COI1 degradation rate (Figure 6C).
Figure 6.
COI1 Is Degraded via the 26S Proteasome Pathway.
(A) Total protein extracts from the wild-type Arabidopsis Columbia were incubated at 22°C with DMSO (Mock), MG132 (50 μM), PS341 (50 μM), or protease inhibitor mixture (1× protease inhibitor cocktail from Roche) for 0 (start), 4, and 8 h, separated by SDS-PAGE, and immunoblotted with COI1 antibody (top panel). The PVDF membrane was stained with Memstain to visualize the large subunit of ribulose-1,5-bisphosphate as a loading control (bottom panel).
(B) Quantitative analysis of the relative abundance of COI1 in the presence of proteasome inhibitors. Total protein extracts from wild-type plants were incubated at 22°C with DMSO (Mock), MG132 (50 μM), or PS341 (50 μM) for the time periods indicated. The abundance of COI1 at the start point (0-h incubation) was set to 100 as a reference for calculating its relative abundance after various incubation periods. Error bars represent sd. The experiment was repeated three times.
(C) The total protein extracts were incubated at 22°C with 5 mM ATP or solvent (Mock) for the indicated time periods and immunoblotted with COI1 antiserum (α-COI1).
[See online article for color version of this figure.]
Mutations in RPT5a, an important component of the 26S proteasome (Rubin et al., 1998), attenuated the degradation of many proteins (Sakamoto et al., 2011). Here, we found that the level of COI1 protein was moderately increased in the rpt5a-4 mutant compared with wild-type plants (Figure 7A). The rpt5a-4 mutant was moderately but clearly more sensitive to jasmonate as indicated by enhanced expression of the typical jasmonate-responsive gene VSP1 (Figure 7B) and accumulation of anthocyanin (Figure 7C). Taken together, these results further suggest that the COI1 degradation was mediated by the 26S proteasome pathway.
Figure 7.
The COI1 Protein Level Is Elevated in the 26S Proteasome Mutant rpt5a-4.
(A) Immunoblot analysis of COI1 with COI1 antiserum (α-COI1) in the rpt5a-4 (rpt5a) mutant and wild-type Arabidopsis Columbia plants (the wild type [WT]).
(B) Real-time quantitative PCR analysis of VSP1 (VSP) transcripts in 8-d-old seedlings grown on Murashige and Skoog medium (0) or Murashige and Skoog medium supplemented with 25 μM MeJA (25). Error bars represent sd (n = 6).
(C) Anthocyanin accumulation in 8-d-old seedlings grown on Murashige and Skoog medium (0) or Murashige and Skoog medium supplemented with MeJA (5 and 25 μM). The o.d. of (535 to 650 nm)/g fresh weight was regarded as the Y value (error bars indicate se). Error bars represent sd (n = 6).
Many F-box proteins are recruited by their respective SCF complexes for ubiquitination and degradation via the 26S proteasome pathway (Galan and Peter, 1999; He et al., 2005). To examine whether the COl1 degradation is dependent on SCFCOI1, we used the in vitro assay to evaluate stability of the truncated Myc-tagged COI1 lacking the F-box motif (Myc-COI1ΔF). We found that Myc-COI1ΔF was degraded very rapidly and abolished completely after 4-h incubation with the total protein extracts from the wild type (Figures 8A and 8B) and coi1-1 where the SCFCOl1 complexes are absent (Figure 8B), suggesting that COI1 was not recruited by SCFCOI1 for degradation. Together with the observation that the presence of the proteasome inhibitor MG132 significantly attenuated degradation of Myc-COI1ΔF (Figure 8C), our data collectively demonstrated that degradation of COI1 is mediated by the 26S proteasome pathway in an SCFCOl1-independent manner.
Figure 8.
COI1 Is Degraded via the 26S Proteasome Pathway in an SCFCOl1-Independent Manner.
(A) Purified Myc-COI1 and Myc-COI1 without the F-box motif (Myc-COI1ΔF) were incubated with total crude protein extracts prepared from the wild-type Arabidopsis Columbia, incubated at 22°C for the indicated time periods, and subjected to immunoblot analysis with anti-Myc antibody (α-Myc). The PVDF membrane was stained with Memstain to visualize the large subunit of ribulose-1,5-bisphosphate as a control for equal loading (Loading Control).
(B) The purified Myc-COI1ΔF protein were incubated with total crude protein extracts prepared from the wild type (wild type [WT] Crude) or the coi1-1 plants (coi1 Crude) at 22°C and subjected to immunoblot analysis with anti-Myc antibody (α-Myc).
(C) Purified Myc-COI1 lacking the F-box motif (Myc-COI1ΔF) was added to total protein extracts from wild-type plants, incubated at 22°C with 50 μM MG132 (MG132) or solvent (Mock) for the indicated time periods, and immunoblotted with anti-Myc antibody.
The 297th Lys Residue of COI1 Is a Ubiquitination Site
Having shown that COI1 is degraded through the ubiquitin/26S proteasome pathway, we sought to identify possible ubiquitination sites in COI1. Isolated Flag-tagged COI1 was enriched and purified using anti-Flag agarose affinity gel from the total protein extracts of the MG132-treated coi1:HiCOI1 seedlings. The purified COI1 protein was separated on SDS-PAGE; the gel band of interest was excised, alkylated, in-gel digested with trypsin, and subjected to liquid chromatography–tandem mass spectrometry (LC-MS/MS) analysis.
By database searching, we identified a peptide ion with the mass corresponding to the modified peptide (297-KLDLLYALLETEDHCTLIQK-316) with an addition of 114 D (mass of Gly-Gly), suggesting that the 297th Lys residue of COI1 is the ubiquitination site. This has been confirmed by the MS/MS spectrum (Figure 9A), in which masses of fragment ions matched to predicted b and y ions of the modified peptides with ubiquitination at the 297th Lys residue. Based on the COI1 crystal structure, the 297th Lys residue is localized within the 9th tandem Leucine-rich repeat in the COI1 C-terminal horseshoe-shaped solenoid domain (Figure 9B) (Sheard et al., 2010).
Figure 9.
The 297th Lys Residue of COI1 Is a Ubiquitination Site.
(A) The MS/MS spectrum of a doubly charged ion at mass-to-charge ratio (m/z) 844.1127 for MH22+ corresponding to the mass of the ubiquitination peptide (297-KLDLLYALLETEDHCTLIQK-316) with the first Lys residue was modified. The labeled peaks correspond to masses of b and y ions of the modified peptides. Observation of the addition of 114 D (mass of Gly-Gly) for all b ions confirms that 297th Lys residue of COI1 is the ubiquitination site.
(B) A diagrammatic representation of the mutant site of K297A in the COI1 protein. The structure of the COI1 protein is shown as a ribbon and the 297th Lys residue of COI1 is labeled.
(C) and (D) The effect of the K297A mutation on COI1 stability by in vitro assays. Myc-COI1 or Myc-COI1K297A was transiently expressed in N. benthamiana leaves, purified with anti-c-Myc agarose affinity gel, added to total protein extracts from the wild-type Arabidopsis Columbia plants, incubated at 22°C (C) or 27°C (D) for the indicated time periods, and subjected to immunoblot analysis with anti-Myc antibody. The PVDF membrane was stained with Memstain to visualize the large subunit of ribulose-1,5-bisphosphate as a loading control.
[See online article for color version of this figure.]
To experimentally verify that the 297th Lys residue of COI1 is a ubiquitination site, we investigated whether changing the 297th COI1 residue from Lys to Ala, by generating Myc-COI1 (Myc-COI1K297A), could attenuate COI1 degradation. The Myc-COI1K297A protein was transiently expressed in tobacco plants, purified with anti-Myc affinity beads, and added to the total wild-type protein extracts. COI1 degradation was subsequently assessed by immunoblot analysis with anti-Myc antibody. Myc-COI1K297A was marginally more stable compared with the Myc-COI1 protein when incubated at 22°C (Figure 9). When the incubation temperature was increased to 27°C, the degradation of Myc-COI1 protein accelerated and was not detectable by 8 h of incubation, whereas Myc-COI1K297A was still observed. A higher incubation temperature more clearly exposed the differences in protein stability (Figure 9D), which further demonstrated that the K297A mutation improved the stability of Myc-COI1, suggesting that the 297th Lys residue is an active ubiquitination site.
DISCUSSION
SCF complexes, an important class of ubiquitin E3 ligases, have been extensively studied with respect to their roles in substrate recognition for ubiquitin-mediated proteolysis (Petroski and Deshaies, 2005; Hua and Vierstra, 2011). In plants, the SCF complexes SCFCOI1, SCFTIR1, SCFEBF1/2, SCFSLEEPY (and SCFGID2), SCFFKF1, SCFSKP2A, and SCFZTL are responsible for ubiquitination and subsequent degradation of their respective substrate JAZ proteins: auxin/indole-3-acetic acids, EIN3/EIL1, DELLAs, CDF1, E2FC/DPB, and TOC1 (Lechner et al., 2006; Santner and Estelle, 2010; Shan et al., 2012). In this study, we revealed a function of SCFCOI1 by showing that it is responsible for stabilization of the F-box protein COI1 in Arabidopsis.
We found that COI1 abundance is strictly maintained at a proper and stable level in Arabidopsis (Figure 1A). Mutations in either ASK1 or CUL1 clearly reduced COI1 protein stability in Arabidopsis (Figures 1C, 2A, and 2B), and when COI1 transcripts were increased 10-fold in transgenic Arabidopsis lines, they were unable to significantly elevate the COI1 protein level (Figures 3 and 4). The in vitro assays showed that purified COI1 protein was unstable and quickly degraded in total protein extracts from the mutant plants where either of the core SCFCOI1 subunits ASK1 or CUL1 was compromised (Figures 5B and 5C). These data collectively suggest that integrity of SCFCOI1 plays an essential role in maintaining COI1 stability in Arabidopsis. Consistent with this, a gel filtration assay showed that that the majority of COI1 is assembled into the complex in Arabidopsis (Feng et al., 2003; data not shown). It would be interesting to determine whether other SCF complexes, such as SCFTIR1 involved in auxin response, have a similar novel function to stabilize their F-box proteins in Arabidopsis.
We observed that the ASK1 mutation severely decreased, but did not entirely abolish, the COI1 protein in the ask1-1 mutant plants. The small amount of COI1 protein that remained in the ask1-1 mutant plants was possibly stabilized by other ASKs-based SCFCOI1 complexes, as other ASK proteins were also reported to interact with COI1 (Xu et al., 2002; Takahashi et al., 2004). In our yeast assays when ASK1 was coexpressed with COI1 (Figure 1B), ASK1 preferentially interacted with COI1, leading to an increased COI1 protein level. However, in Arabidopsis, it may not necessarily follow that overexpression of ASK1 would obviously increase the COI1 protein abundance, as is known to interact with many other Arabidopsis F-box proteins (Risseeuw et al., 2003;Lechner et al., 2006), thereby limiting its availability for interaction with COI1.
Degradation of many F-box proteins is dynamically regulated by an autocatalytic mechanism. Such F-box proteins, including Grr1, Cdc4, Met30, and FWD1, are recruited by their respective SCF complexes, including SCFGrr1, SCFCdc4, SCFMet30, and SCFFWD1, for ubiquitination and subsequent degradation in a proteasome-dependent manner (Galan and Peter, 1999; He et al., 2005). Mutations occurring in their F-box motifs have been shown to attenuate ubiquitination and degradation of these F-box proteins (Galan and Peter, 1999; He et al., 2005). In this study, we demonstrated that deleting the F-box motif did not attenuate, but on the contrary accelerated the degradation of COI1 (Figure 8A). This implies that COI1 degradation is not subjected to an autocatalytic mechanism. Furthermore, we found that the 26S proteasome inhibitors MG132 and PS341 effectively stabilized COI1 protein (Figure 6) and that COI1 accumulated in the 26S proteasome mutant rpt5a-4 (Figure 7). In addition, we showed that the 297th Lys residue of COI1 is one of the active ubiquitination sites (Figure 9). These results suggest that COI1 is recruited by another, unidentified E3 ligase, but not by SCFCOI1 itself, for ubiquitination and subsequent degradation via 26S proteasome pathway. Previous studies showed that the F-box proteins TIR1, EBF1/2, and ZTL were degraded via the ubiquitin-proteasome pathway in Arabidopsis (Kim et al., 2007; Stuttmann et al., 2009; An et al., 2010). It remains to be elucidated whether the degradation of these Arabidopsis F-box proteins shares this mechanism with COI1 or employs the more common autocatalytic mechanism.
Our results provide new mechanistic insights into how plant cells regulate COI1 abundance. We propose that COI1 protein is strictly regulated by a dynamic balance between SCFCOI1-mediated stabilization and 26S proteasome–mediated degradation and maintained at a protein level essential to exert its proper functions in Arabidopsis (Figure 10). In the wild type, the COI1 protein is stabilized by interacting with ASK1 and further stabilized by assembly into the SCFCOI1 complex. COI1 levels were lower in the cul1 mutants axr6-1 and axr6-2, where reduced CUL1 activity potentially limited the assembly of SCFCOI1. The most rapid degradation of COI1 occurred in ask1-1 mutants where the COI1-ASK interaction was completely abolished. Free COI1 proteins without interaction with ASKs, including the mutated COI1 proteins that are unable to associate with ASKs and the overexpressed COI1 proteins in the transgenic lines, are predicted to be recruited by a yet unidentified E3 ligase, but not by SCFCOI1, for targeted ubiquitination and subsequent degradation via the 26S proteasome. Characterization of this unidentified E3 ligase would provide a new insight into molecular mechanism regulating the COI1 protein to satisfy the cellular demands for the SCFCOI1 activity essential for jasmonate-mediated plant defense and development.
Figure 10.
A Simplified Model for Regulation of the Dynamic Balance between COI1 Stabilization and Destabilization in Arabidopsis.
The COI1 protein is stabilized by interaction with ASK to form the COI1-ASK heterodimers (such as COI1-ASK1), which are further stabilized by the assembly of SCFCOI1 complexes (such as ASK1-based SCFCOI1) via interaction with CUL1. The mutation in CUL1 in axr6-1/+, or dissociation of CUL1 from the SCF complex (Hua and Vierstra, 2011), releases the COI1-ASK heterodimer and might also affect the interaction between COI1 and ASK proteins. The dissociated COI1 protein in the absence of interaction with ASK proteins, including the transgenically overexpressed COI1 proteins and the mutated COI1 proteins that are unable to interact with ASKs, is recruited by an unidentified E3 ligase for ubiquitination and subsequent degradation via the 26S proteasome pathway. COI1 protein is maintained at a proper and stable level in Arabidopsis through a combination of SCFCOI1-mediated stabilization and the 26S proteasome–mediated degradation.
METHODS
Plant Materials and Growth Conditions
The Arabidopsis thaliana mutants coi1-1, axr6-1 and axr6-2, rpt5a-4, and ask1-1 were gifts from J. Turner, M. Estelle, H. Huang, and H. Ma, respectively. Arabidopsis seeds were surface sterilized and plated on Murashige and Skoog medium with 2% Suc and 7 g/L agar, chilled at 4°C in the dark for 3 d, and then placed in a growth room under a 16-h-light (21 to 23°C)/8-h-dark (16 to 19°C) photoperiod for 10 d. For adult plants, seedlings from plates were transferred to soil and grown to maturity at 22°C under a 16-h-light/8-h-dark cycle.
Nicotiana benthamiana seeds were directly sown into soil and grown under similar growth conditions.
Plasmid Constructs
The yeast expression construct pBridge-COI1 was generated by cloning the COI1 cDNA into multiple cloning site 2 of the pBridge vector (Clontech). The yeast coexpression constructs pBridge-ASK1-COI1 and pBridge-JAZ1-COI1 were generated by cloning the ASK1 or JAZ1 cDNA, respectively, into the multiple cloning site 1 of the construct pBridge-COI1.
For the constructs used in the N. benthamiana transient expression system (Liu et al., 2010), the COI1 cDNA fragment was in-frame tagged with the Myc epitope at the 5′ terminus and driven by the CaMV 35S promoter in the plant binary vector (Xu et al., 2002) to generate the Myc-COI1 construct. The Myc-COI1ΔF and Myc-COI1K297A constructs were similarly generated. The point mutation of COI1K297A was introduced by site-directed mutagenesis using a fast mutagenesis kit (Transgen).
In Figures 3 and 4, the COI1 cDNA fragment was in-frame tagged with the Flag epitope at the 5′ terminus and the HA epitope at the 3′ terminus and driven by the CaMV 35S promoter in the plant binary vector (Xu et al., 2002) to generate the Flag-COI1-HA construct. The Arabidopsis-expressed Flag-COI1-HA fusion proteins were detected with anti-HA antibody (Sigma-Aldrich; H6908; Figures 3 and 4).
All the primers using in generation of the plasmid constructs are shown in Supplemental Table 1 online.
Expression of COI1 Protein in Yeast
The constructs pBridge-ASK1-COI1 and pBridge-JAZ1-COI1 were transformed into the yeast strain AH109. The yeast cultures were grown on SD synthetic medium lacking Trp and Met at 30°C for 16 h. Whole-cell lysates were prepared using Yeast Protein Extraction Reagent (Y-PER; Thermo), followed by centrifugation at 14,000g for 15 min. The supernatants of whole-cell lysates were separated by SDS-PAGE, transferred to polyvinylidene fluoride (PVDF) membrane, and detected with anti-COI1 antiserum (Xu et al., 2002).
Transient Expression of COI1 in N. benthamiana
Leaves of 4-week-old N. benthamiana were inoculated with Agrobacterium tumefaciens GV3101 strain carrying the Myc-tagged COI1, Myc-COI1K297A, or Myc-tagged COI1ΔF constructs following a previously described method (Liu et al., 2010). Two days after infiltration, total protein was extracted from infiltrated leaves with extraction buffer (50 mM Tris-Cl, pH 7.8, 100 mM NaCl, 10% [v/v] glycerol, and 20 mM β-mercaptoethanol) and incubated with Anti-c-Myc Agarose Affinity Gel (Sigma-Aldrich) at 4°C for 3 to 4 h. After washing, the Myc-tagged proteins were eluted with 50 μg/mL Myc peptide (Sigma-Aldrich).
A pull-down assay was used to determine the activity of purified Myc-COI1 as described previously (Yan et al., 2009).
In Vitro Protein Degradation Assay
For the in vitro degradation assay of Myc-COI1, Myc-COI1ΔF, and Myc-COI1K297A described in Figures 5B, 5C, 8, 9C, and 9D, 5 μg of purified protein was added to 900 μL of total crude protein extracts (1 mg/mL) prepared from young rosette leaves of 4-week-old plants, as indicated. The reaction mixture was incubated at 22°C for the time periods indicated in the figures, separated by SDS-PAGE, transferred to PVDF membrane, and detected with anti-Myc antibody (Roche).
In Figures 6A to 6C, the total protein extracts (1 mg/mL) from 4-week-old wild-type young rosette leaves were incubated at 22°C for indicated time periods with or without MG132 (50 μM), PS341 (50 μM), 1× protease inhibitor cocktail (Roche), or ATP (5 mM), separated by SDS-PAGE, transferred to PVDF membrane, and detected with the anti-COI1 antiserum (Xu et al., 2002).
Anthocyanin Measurement, Pollen Viability, and Pollen Germination
Eight-day-old Arabidopsis seedlings grown on Murashige and Skoog medium supplemented with MeJA at the indicated concentrations were used for anthocyanin measurements following the method described previously (Shan et al., 2009). The anthocyanin content is presented as (A535–A650)/ g fresh weight. The experiment was repeated three times.
Pollen grains were resuspended in 6 μg/mL fluorescein diacetate and 0.5 μg/mL propidium iodide stain for microscopy to assess pollen viability (Regan and Moffatt, 1990).
The germination medium (10% [w/v] Suc, 0.01% boric acid, 1 mM MgSO4, 5 mM CaCl2, and 5 mM KCl, pH 7.5, with 1.5% agar) was used for the pollen germination assay (Song et al., 2011).
RT-PCR and Real-Time Quantitative PCR
The total RNA extracts were treated with DNase I (NEB) according to the manufacturer’s instructions of the Reverse Transcriptase M-MLV (RNase H; TaKaRa). RT-PCR amplifications were performed with a limited number of cycles, ensuring the amplifications were within the linear range, and amplified transcripts were detected by ethidium bromide staining on 2% (w/v) agarose gels. ACTIN1 was used as an internal control.
Real-time quantitative PCR analysis was performed using the ABI 7500 real-time PCR system and the RealMasterMix (SYBR Green I; TaKaRa). ACTIN8 was used as the internal control.
Primer pairs used for RT-PCR and real-time quantitative PCR analysis are listed in Supplemental Table 1 online.
MS Analysis
Total protein was extracted from 30 g of young rosette leaves of 4-week-old plants transgenic for the Flag-COI1-HA construct (coi1:Hi-COI1) with extraction buffer (50 mM Tris-Cl, pH 7.8, 100 mM NaCl, 25 mM imidazole, 10% [v/v] glycerol, 0.1% [v/v] Tween 20, and 20 mM β-mercaptoethanol) in the presence of MG132. The total protein extracts were concentrated and incubated with anti-Flag agarose affinity gel (Sigma-Aldrich) for 8 h at 4°C with gentle rocking. After washing, the purified Flag-tagged COI1 proteins were denatured at 98°C and separated by SDS-PAGE. The gel bands at the approximate size of Flag-tagged COI1 were excised, reduced with 10 mM DTT, and alkylated with 55 mM iodoacetamide (Calbiochem). Then the in-gel digestion was performed with trypsin (Promega) in 50 mM ammonium bicarbonate at 37°C overnight. The peptides were extracted twice with 1% (v/v) trifluoroacetic acid in 50% (v/v) acetonitrile aqueous solution for 30 min.
For LC-MS/MS analysis, the trypsin-digested sample was separated by a 65-min gradient elution at a flow rate of 0.25 µL/min with the EASY-nLCII integrated nano-HPLC system (Proxeon), which directly interfaced with the Thermo LTQ-Orbitrap Velos mass spectrometer. The analytical column was a homemade fused silica capillary column (75 µm i.d., 150-mm length; Upchurch) packed with C-18 resin (300 Å, 5 µm; Varian). Mobile phase A consisted of 0.1% (v/v) formic acid, and mobile phase B consisted of 100% acetonitrile and 0.1% formic acid. The LTQ-Orbitrap mass spectrometer was operated in the data-dependent acquisition mode using Xcalibur 2.0.7 software with a single full-scan mass spectrum in the Orbitrap (400 to 1800 mass-to-charge ratio; 30,000 resolution) followed by six data-dependent MS/MS scans in the ion trap at 35% normalized collision energy. The MS/MS spectra from each LC-MS/MS run were searched against the selected databases using an in-house Mascot or Proteome Discovery searching algorithm.
Accession Numbers
The Arabidopsis Genome Initiative accession numbers for the genes and gene products mentioned in this article are as follows: COI1 (At2g39940), ASK1 (At1g75950), AtVSP1 (AT5G24780), AtCUL1 (At4g02570), RPT5a (At3g05530), ACTIN1 (AT2G37620), and ACTIN8 (AT1G49240).
Supplemental Data
The following material is available in the online version of this article.
Supplemental Table 1. List of Constructs and Primers Used in This Study.
Acknowledgments
This work was financially supported by the grants from the National Science Foundation of China (91017012, 31230008 and 31200214) and the Ministry of Agriculture (2011ZX08011-006).
AUTHOR CONTRIBUTIONS
J.Y. and H.L. performed all the experiments. S.L. and R.Y. performed degradation in vitro and LC-MS/MS assay, respectively. H.D. and Q.X. provided useful advice in mass spectrometry analysis. J.Y. and D.X. designed the experiments and wrote the article.
Glossary
- CHX
cycloheximide
- MeJA
jasmonic acid methyl ester
- HA
hemagglutinin
- CaMV
cauliflower mosaic virus
- LC-MS/MS
liquid chromatography–tandem mass spectrometry
- PVDF
polyvinylidene fluoride
References
- An F.Y., et al. (2010). Ethylene-induced stabilization of ETHYLENE INSENSITIVE3 and EIN3-LIKE1 is mediated by proteasomal degradation of EIN3 binding F-box 1 and 2 that requires EIN2 in Arabidopsis. Plant Cell 22: 2384–2401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J.M. (1991). Jasmonic acid-dependent increase in storage protein in vegetative storage protein in soybean tissue-culture. J. Plant Growth Regul. 10: 5–10 [Google Scholar]
- Cheng Z.W., Sun L., Qi T.C., Zhang B.S., Peng W., Liu Y.L., Xie D.X. (2011). The bHLH transcription factor MYC3 interacts with the Jasmonate ZIM-domain proteins to mediate jasmonate response in Arabidopsis. Mol. Plant 4: 279–288 [DOI] [PubMed] [Google Scholar]
- Cheong J.J., Choi Y.D. (2003). Methyl jasmonate as a vital substance in plants. Trends Genet. 19: 409–413 [DOI] [PubMed] [Google Scholar]
- Chini A., Fonseca S., Fernández G., Adie B., Chico J.M., Lorenzo O., García-Casado G., López-Vidriero I., Lozano F.M., Ponce M.R., Micol J.L., Solano R. (2007). The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448: 666–671 [DOI] [PubMed] [Google Scholar]
- del Pozo J.C., Boniotti M.B., Gutierrez C. (2002). Arabidopsis E2Fc functions in cell division and is degraded by the ubiquitin-SCF(AtSKP2) pathway in response to light. Plant Cell 14: 3057–3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etlinger J.D., Goldberg A.L. (1977). A soluble ATP-dependent proteolytic system responsible for the degradation of abnormal proteins in reticulocytes. Proc. Natl. Acad. Sci. USA 74: 54–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer E.E., Alméras E., Krishnamurthy V. (2003). Jasmonates and related oxylipins in plant responses to pathogenesis and herbivory. Curr. Opin. Plant Biol. 6: 372–378 [DOI] [PubMed] [Google Scholar]
- Farmer E.E., Ryan C.A. (1992). Octadecanoid precursors of jasmonic acid activate the synthesis of wound-inducible proteinase inhibitors. Plant Cell 4: 129–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S., Ma L., Wang X., Xie D., Dinesh-Kumar S.P., Wei N., Deng X.W. (2003). The COP9 signalosome interacts physically with SCF COI1 and modulates jasmonate responses. Plant Cell 15: 1083–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Calvo P., et al. (2011). The Arabidopsis bHLH transcription factors MYC3 and MYC4 are targets of JAZ repressors and act additively with MYC2 in the activation of jasmonate responses. Plant Cell 23: 701–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan J.M., Peter M. (1999). Ubiquitin-dependent degradation of multiple F-box proteins by an autocatalytic mechanism. Proc. Natl. Acad. Sci. USA 96: 9124–9129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray W.M., Kepinski S., Rouse D., Leyser O., Estelle M. (2001). Auxin regulates SCF(TIR1)-dependent degradation of AUX/IAA proteins. Nature 414: 271–276 [DOI] [PubMed] [Google Scholar]
- Guo H.W., Ecker J.R. (2003). Plant responses to ethylene gas are mediated by SCF(EBF1/EBF2)-dependent proteolysis of EIN3 transcription factor. Cell 115: 667–677 [DOI] [PubMed] [Google Scholar]
- He Q., Cheng P., He Q.Y., Liu Y. (2005). The COP9 signalosome regulates the Neurospora circadian clock by controlling the stability of the SCFFWD-1 complex. Genes Dev. 19: 1518–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmann H., Estelle M. (2002). Plant development: Regulation by protein degradation. Science 297: 793–797 [DOI] [PubMed] [Google Scholar]
- Hellmann H., Hobbie L., Chapman A., Dharmasiri S., Dharmasiri N., del Pozo C., Reinhardt D., Estelle M. (2003). Arabidopsis AXR6 encodes CUL1 implicating SCF E3 ligases in auxin regulation of embryogenesis. EMBO J. 22: 3314–3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko A., Ciechanover A. (1998). The ubiquitin system. Annu. Rev. Biochem. 67: 425–479 [DOI] [PubMed] [Google Scholar]
- Ho M.S., Ou C., Chan Y.R., Chien C.T., Pi H. (2008). The utility F-box for protein destruction. Cell. Mol. Life Sci. 65: 1977–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie L., McGovern M., Hurwitz L.R., Pierro A., Liu N.Y., Bandyopadhyay A., Estelle M. (2000). The axr6 mutants of Arabidopsis thaliana define a gene involved in auxin response and early development. Development 127: 23–32 [DOI] [PubMed] [Google Scholar]
- Howe G.A., Lightner J., Browse J., Ryan C.A. (1996). An octadecanoid pathway mutant (JL5) of tomato is compromised in signaling for defense against insect attack. Plant Cell 8: 2067–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua Z., Vierstra R.D. (2011). The cullin-RING ubiquitin-protein ligases. Annu. Rev. Plant Biol. 62: 299–334 [DOI] [PubMed] [Google Scholar]
- Imaizumi T., Schultz T.F., Harmon F.G., Ho L.A., Kay S.A. (2005). FKF1 F-box protein mediates cyclic degradation of a repressor of CONSTANS in Arabidopsis. Science 309: 293–297 [DOI] [PubMed] [Google Scholar]
- Ito S., Song Y.H., Imaizumi T. (2012). LOV domain-containing F-box proteins: Light-dependent protein degradation modules in Arabidopsis. Mol. Plant 5: 573–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado S., Abraham Z., Manzano C., López-Torrejón G., Pacios L.F., Del Pozo J.C. (2010). The Arabidopsis cell cycle F-box protein SKP2A binds to auxin. Plant Cell 22: 3891–3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado S., Díaz-Triviño S., Abraham Z., Manzano C., Gutierrez C., del Pozo C. (2008). SKP2A, an F-box protein that regulates cell division, is degraded via the ubiquitin pathway. Plant J. 53: 828–841 [DOI] [PubMed] [Google Scholar]
- Katsir L., Schilmiller A.L., Staswick P.E., He S.Y., Howe G.A. (2008). COI1 is a critical component of a receptor for jasmonate and the bacterial virulence factor coronatine. Proc. Natl. Acad. Sci. USA 105: 7100–7105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W.Y., Fujiwara S., Suh S.S., Kim J., Kim Y., Han L.Q., David K., Putterill J., Nam H.G., Somers D.E. (2007). ZEITLUPE is a circadian photoreceptor stabilized by GIGANTEA in blue light. Nature 449: 356–360 [DOI] [PubMed] [Google Scholar]
- Kim W.Y., Geng R.S., Somers D.E. (2003). Circadian phase-specific degradation of the F-box protein ZTL is mediated by the proteasome. Proc. Natl. Acad. Sci. USA 100: 4933–4938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner E., Achard P., Vansiri A., Potuschak T., Genschik P. (2006). F-box proteins everywhere. Curr. Opin. Plant Biol. 9: 631–638 [DOI] [PubMed] [Google Scholar]
- Li Y., Gazdoiu S., Pan Z.Q., Fuchs S.Y. (2004). Stability of homologue of Slimb F-box protein is regulated by availability of its substrate. J. Biol. Chem. 279: 11074–11080 [DOI] [PubMed] [Google Scholar]
- Liu H.X., Stone S.L. (2010). Abscisic acid increases Arabidopsis ABI5 transcription factor levels by promoting KEG E3 ligase self-ubiquitination and proteasomal degradation. Plant Cell 22: 2630–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Zhang Y., Tang S., Zhao Q., Zhang Z., Zhang H., Dong L., Guo H., Xie Q. (2010). An efficient system to detect protein ubiquitination by agroinfiltration in Nicotiana benthamiana. Plant J. 61: 893–903 [DOI] [PubMed] [Google Scholar]
- Más P., Kim W.Y., Somers D.E., Kay S.A. (2003). Targeted degradation of TOC1 by ZTL modulates circadian function in Arabidopsis thaliana. Nature 426: 567–570 [DOI] [PubMed] [Google Scholar]
- McConn M., Creelman R.A., Bell E., Mullet J.E., Browse J. (1997). Jasmonate is essential for insect defense in Arabidopsis. Proc. Natl. Acad. Sci. USA 94: 5473–5477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon J., Parry G., Estelle M. (2004). The ubiquitin-proteasome pathway and plant development. Plant Cell 16: 3181–3195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Y.J., Figueroa P., Browse J. (2011). Characterization of JAZ-interacting bHLH transcription factors that regulate jasmonate responses in Arabidopsis. J. Exp. Bot. 62: 2143–2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroski M.D., Deshaies R.J. (2005). Function and regulation of cullin-RING ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 6: 9–20 [DOI] [PubMed] [Google Scholar]
- Potuschak T., Lechner E., Parmentier Y., Yanagisawa S., Grava S., Koncz C., Genschik P. (2003). EIN3-dependent regulation of plant ethylene hormone signaling by two Arabidopsis F box proteins: EBF1 and EBF2. Cell 115: 679–689 [DOI] [PubMed] [Google Scholar]
- Qi T.C., Song S.S., Ren Q.C., Wu D.W., Huang H., Chen Y., Fan M., Peng W., Ren C.M., Xie D.X. (2011). The jasmonate-ZIM-domain proteins interact with the WD-Repeat/bHLH/MYB complexes to regulate Jasmonate-mediated anthocyanin accumulation and trichome initiation in Arabidopsis thaliana. Plant Cell 23: 1795–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao H., Wang F., Zhao L., Zhou J., Lai Z., Zhang Y., Robbins T.P., Xue Y. (2004). The F-box protein AhSLF-S2 controls the pollen function of S-RNase-based self-incompatibility. Plant Cell 16: 2307–2322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan S.M., Moffatt B.A. (1990). Cytochemical analysis of pollen development in wild-type Arabidopsis and a male-sterile mutant. Plant Cell 2: 877–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren C.M., Pan J.W., Peng W., Genschik P., Hobbie L., Hellmann H., Estelle M., Gao B., Peng J.R., Sun C.Q., Xie D.X. (2005). Point mutations in Arabidopsis Cullin1 reveal its essential role in jasmonate response. Plant J. 42: 514–524 [DOI] [PubMed] [Google Scholar]
- Risseeuw E.P., Daskalchuk T.E., Banks T.W., Liu E., Cotelesage J., Hellmann H., Estelle M., Somers D.E., Crosby W.L. (2003). Protein interaction analysis of SCF ubiquitin E3 ligase subunits from Arabidopsis. Plant J. 34: 753–767 [DOI] [PubMed] [Google Scholar]
- Rubin D.M., Glickman M.H., Larsen C.N., Dhruvakumar S., Finley D. (1998). Active site mutants in the six regulatory particle ATPases reveal multiple roles for ATP in the proteasome. EMBO J. 17: 4909–4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto T., Kamiya T., Sako K., Yamaguchi J., Yamagami M., Fujiwara T. (2011). Arabidopsis thaliana 26S proteasome subunits RPT2a and RPT5a are crucial for zinc deficiency-tolerance. Biosci. Biotechnol. Biochem. 75: 561–567 [DOI] [PubMed] [Google Scholar]
- Santner A., Estelle M. (2010). The ubiquitin-proteasome system regulates plant hormone signaling. Plant J. 61: 1029–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A., Itoh H., Gomi K., Ueguchi-Tanaka M., Ishiyama K., Kobayashi M., Jeong D.H., An G., Kitano H., Ashikari M., Matsuoka M. (2003). Accumulation of phosphorylated repressor for gibberellin signaling in an F-box mutant. Science 299: 1896–1898 [DOI] [PubMed] [Google Scholar]
- Shan X., Zhang Y., Peng W., Wang Z., Xie D. (2009). Molecular mechanism for jasmonate-induction of anthocyanin accumulation in Arabidopsis. J. Exp. Bot. 60: 3849–3860 [DOI] [PubMed] [Google Scholar]
- Shan X.Y., Yan J.B., Xie D.X. (2012). Comparison of phytohormone signaling mechanisms. Curr. Opin. Plant Biol. 15: 84–91 [DOI] [PubMed] [Google Scholar]
- Sheard L.B., et al. (2010). Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature 468: 400–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalle J., Vierstra R.D. (2004). The ubiquitin 26S proteasome proteolytic pathway. Annu. Rev. Plant Biol. 55: 555–590 [DOI] [PubMed] [Google Scholar]
- Somers D.E., Kim W.Y., Geng R.S. (2004). The F-box protein ZEITLUPE confers dosage-dependent control on the circadian clock, photomorphogenesis, and flowering time. Plant Cell 16: 769–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S.S., Qi T.C., Huang H., Ren Q.C., Wu D.W., Chang C.Q., Peng W., Liu Y.L., Peng J.R., Xie D.X. (2011). The Jasmonate-ZIM domain proteins interact with the R2R3-MYB transcription factors MYB21 and MYB24 to affect Jasmonate-regulated stamen development in Arabidopsis. Plant Cell 23: 1000–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick P.E. (1994). Storage proteins of vegetative plant tissues. Annu. Rev. Plant Biol. 45: 303–322 [Google Scholar]
- Staswick P.E., Su W., Howell S.H. (1992). Methyl jasmonate inhibition of root growth and induction of a leaf protein are decreased in an Arabidopsis thaliana mutant. Proc. Natl. Acad. Sci. USA 89: 6837–6840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirnberg P., van De Sande K., Leyser H.M. (2002). MAX1 and MAX2 control shoot lateral branching in Arabidopsis. Development 129: 1131–1141 [DOI] [PubMed] [Google Scholar]
- Stone S.L., Callis J. (2007). Ubiquitin ligases mediate growth and development by promoting protein death. Curr. Opin. Plant Biol. 10: 624–632 [DOI] [PubMed] [Google Scholar]
- Stuttmann J., Lechner E., Guérois R., Parker J.E., Nussaume L., Genschik P., Noël L.D. (2009). COP9 signalosome- and 26S proteasome-dependent regulation of SCFTIR1 accumulation in Arabidopsis. J. Biol. Chem. 284: 7920–7930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N., Kuroda H., Kuromori T., Hirayama T., Seki M., Shinozaki K., Shimada H., Matsui M. (2004). Expression and interaction analysis of Arabidopsis Skp1-related genes. Plant Cell Physiol. 45: 83–91 [DOI] [PubMed] [Google Scholar]
- Thines B., Katsir L., Melotto M., Niu Y., Mandaokar A., Liu G.H., Nomura K., He S.Y., Howe G.A., Browse J. (2007). JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 448: 661–665 [DOI] [PubMed] [Google Scholar]
- Wasternack C. (2007). Jasmonates: An update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann. Bot. (Lond.) 100: 681–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie D.X., Feys B.F., James S., Nieto-Rostro M., Turner J.G. (1998). COI1: An Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 280: 1091–1094 [DOI] [PubMed] [Google Scholar]
- Xu G.X., Ma H., Nei M., Kong H.Z. (2009). Evolution of F-box genes in plants: Different modes of sequence divergence and their relationships with functional diversification. Proc. Natl. Acad. Sci. USA 106: 835–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L.H., Liu F.Q., Lechner E., Genschik P., Crosby W.L., Ma H., Peng W., Huang D.F., Xie D.X. (2002). The SCF(COI1) ubiquitin-ligase complexes are required for jasmonate response in Arabidopsis. Plant Cell 14: 1919–1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J.B., Zhang C., Gu M., Bai Z.Y., Zhang W.G., Qi T.C., Cheng Z.W., Peng W., Luo H.B., Nan F.J., Wang Z., Xie D.X. (2009). The Arabidopsis CORONATINE INSENSITIVE1 protein is a jasmonate receptor. Plant Cell 21: 2220–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y.X., Stolz S., Chételat A., Reymond P., Pagni M., Dubugnon L., Farmer E.E. (2007). A downstream mediator in the growth repression limb of the jasmonate pathway. Plant Cell 19: 2470–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Hu Y., Lodhi M., McCombie W.R., Ma H. (1999). The Arabidopsis SKP1-LIKE1 gene is essential for male meiosis and may control homologue separation. Proc. Natl. Acad. Sci. USA 96: 11416–11421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng N., et al. (2002). Structure of the Cul1-Rbx1-Skp1-F boxSkp2 SCF ubiquitin ligase complex. Nature 416: 703–709 [DOI] [PubMed] [Google Scholar]