To protect photosynthetic organisms from photodamage, excess energy in photosystem II must be dissipated. In C. reinhardtii, two alternative mechanisms (energy thermal dissipation [qE] and kinase-mediated migration of light harvesting proteins [qT]) synergistically modulate photoprotection via the reversible migration of LHCSR3, a key qE effector in this alga, between photosystems II and I during qT.
Abstract
Absorption of light in excess of the capacity for photosynthetic electron transport is damaging to photosynthetic organisms. Several mechanisms exist to avoid photodamage, which are collectively referred to as nonphotochemical quenching. This term comprises at least two major processes. State transitions (qT) represent changes in the relative antenna sizes of photosystems II and I. High energy quenching (qE) is the increased thermal dissipation of light energy triggered by lumen acidification. To investigate the respective roles of qE and qT in photoprotection, a mutant (npq4 stt7-9) was generated in Chlamydomonas reinhardtii by crossing the state transition–deficient mutant (stt7-9) with a strain having a largely reduced qE capacity (npq4). The comparative phenotypic analysis of the wild type, single mutants, and double mutants reveals that both state transitions and qE are induced by high light. Moreover, the double mutant exhibits an increased photosensitivity with respect to the single mutants and the wild type. Therefore, we suggest that besides qE, state transitions also play a photoprotective role during high light acclimation of the cells, most likely by decreasing hydrogen peroxide production. These results are discussed in terms of the relative photoprotective benefit related to thermal dissipation of excess light and/or to the physical displacement of antennas from photosystem II.
INTRODUCTION
When photosynthetic organisms are exposed to light intensity overwhelming their photosynthetic capacity, they initiate a variety of mechanisms to protect their photosynthetic machinery from photodamage. All these processes are associated with photosystem II (PSII) and are collectively referred to as nonphotochemical quenching (NPQ) (Horton et al., 1996). This term comprises (1) state transitions (i.e., the reversible association of chlorophyll binding complexes with PSII [in state 1] and photosystem I [PSI; in state 2]) (Bonaventura and Myers, 1969; Allen, 1992); (2) high energy–dependent quenching (qE) (i.e., enhanced thermal energy dissipation occurring when the chloroplast is energized by a high rate of photosynthetic electron flow); and (3) photoinhibition, dismantling the photosystems photodamaged by high light.
State transitions are regulated by protein phosphorylation catalyzed by a membrane-bound kinase, STATE TRANSITION7 (STT7 in Chlamydomonas reinhardtii or STN7 in higher plants; Rochaix, 2007). The kinase is activated in response to redox changes in the electron flow chain. Reduction of the plastoquinone (PQ) pool, either by unbalanced PSII/PSI activity (in favor of the former) or by the chlororespiratory chain, activates the kinase through a mechanism that requires plastoquinol2 binding to the cytochrome b6f complex (Wollman, 2001). The phosphorylated light-harvesting complex II (Pi-LHCII) is disconnected from PSII and becomes a PSI antenna. The process is reversible because the kinase is inactivated by oxidation of the plastoquinol (i.e., when the PSII/PSI balance is modified in favor of the latter). This results in Pi-LHCII dephosphorylation by a phosphatase (Pribil et al., 2010; Shapiguzov et al., 2010), which seems to have constitutive but low activity. Dephosphorylated LHCII moves back to PSII, leading to state 1.
The second phenomenon is qE, the pH-dependent component of NPQ, which corresponds to an increased thermal deactivation of the PSII antenna. In higher plants and green algae, qE is related to the accumulation of two carotenoids (antheraxanthin and zeaxanthin), produced by deepoxidation of violaxanthin during the so-called xanthophyll cycle (Yamamoto et al., 1962). Moreover, qE in plants is facilitated by the PSII subunit Photosystem II subunit S (PSBS), which acts as a pH-driven amplifier of carotenoid-mediated energy quenching (Niyogi, 1999). The last component of NPQ, photoinhibition, represents a slowly reversible quenching, generally ascribed to the occurrence of photoinhibition (i.e., the damage to the PSII reaction center [Aro et al., 1993] that occurs when the other photoprotective responses cannot successfully alleviate the light stress). More recently, it has been proposed that this phase also reflects a long-lasting quenching process, possibly related to conformational changes in the PSII antenna (Dall’Osto et al., 2005).
In higher plants, qE is by far the most prominent photoprotective response (Horton et al., 1996). Conversely, quenching due to state transitions (qT) does not provide significant protection from light stress, as indicated by the comparative analysis of the fitness of Arabidopsis thaliana mutants lacking state transitions and/or qE under variable environmental conditions in the field (Frenkel et al., 2007). This is likely because state transitions are of limited amplitude in higher plants. They are thought to adjust the relative absorption capacity of the two photosystems to optimize light use (Allen, 1992). On the other hand, state transitions are a prominent phenomenon in C. reinhardtii, where up to 80% of the light-harvesting complexes can reversibly migrate between PSII and PSI following redox-driven phosphorylation (Delosme et al., 1996). This difference can be explained by the observation that only a few LHCIIs are phosphorylated in plants, while both the minor (CHLOROPHYLL PROTEIN [CP]) and major (LIGHT HARVESTING COMPLEX II [LHCII]) antennas are targeted in C. reinhardtii (Minagawa, 2011). Because of the large change in the PSII and PSI absorption capacity, state transitions in C. reinhardtii do not only serve the purpose of balancing the absorption of the two photosystems. It has been proposed instead that they may play another role in this alga (i.e., enhancing PSI activity in order to favor cyclic electron flow around PSI to increase ATP production) (Vallon et al., 1991; Cardol et al., 2009). Consistent with this hypothesis, a supercomplex containing PSI, the cytochrome b6f complex, FERREDOXIN NADP REDUCTASE, and PROTON GRADIENT REGULATOR5 LIKE1, a protein involved in cyclic flow (Dal Corso et al., 2008; Tolleter et al., 2011), was recently purified from state 2 acclimated cells (Iwai et al., 2010).
An additional role for state transitions can be envisaged in this alga. The psbS gene is present but not expressed under normal growth conditions (Bonente et al., 2008). In C. reinhardtii, the extent of qE is modulated by LHCSR3 (Peers et al., 2009; Bonente et al., 2011; Petroutsos et al., 2011), a member of the light-harvesting complex stress-related (LHCSR) protein family, formerly known as LI818 (Savard et al., 1996). LHCSR genes are only found in algae and mosses (Elrad and Grossman, 2004; Nymark et al., 2009). In C. reinhardtii, LHCSR3 is induced after a prolonged exposure (several hours) to high light stress (Peers et al., 2009). During this period, photodamage can take place because of the lack of photoprotection. State transitions, which develop in a few minutes (Delepelaire and Wollman, 1985), could play an active role in counterbalancing high light stress in this alga by transferring a large part of the PSII antenna to PSI, which is a very efficient quencher of absorbed energy.
To investigate this possibility, and to assess the respective roles of state transitions and qE in high light acclimation in C. reinhardtii, we employed a genetic approach to generate a double mutant lacking both state transitions and qE. By comparing fluorescence quenching, photosynthetic activity, and reactive oxygen species (ROS) production in the wild type, single mutants deficient in state transitions (stt7; Depège et al., 2003) and qE (npq4; Peers et al., 2009), and in the double mutant (npq4 stt7-9), we show that both qE and qT act together during the development of NPQ in this alga. In particular, while qE could be the major photoprotective response in steady state (as in higher plants), state transitions seem to play a prominent role during the early phase of NPQ induction, probably by preventing hydrogen peroxide (H2O2) production, but also provide some protection in steady state conditions. We interpret this result in terms of the possible benefit provided by the reduced size of the PSII antenna in state 2, but also by the diminished reactivity of the PSI-generated electron with molecular oxygen due to the generation of the cyclic PSI supercomplex (Iwai et al., 2010).
RESULTS
Generation of a Double Mutant Impaired in State Transitions and qE in C. reinhardtii
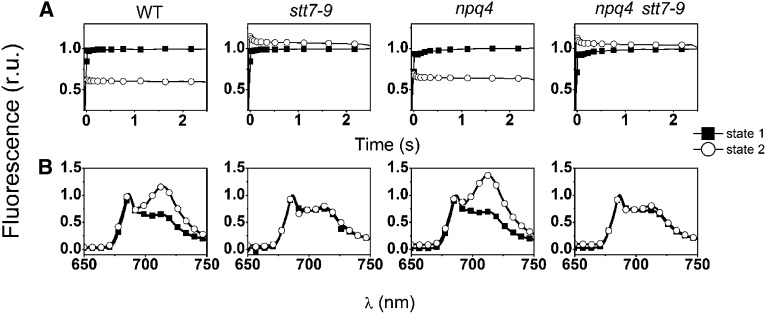
To address the relative role of NPQ-qE and NPQ-qT in light acclimation of C. reinhardtii, we produced a double mutant impaired in both qE and state transitions. This was done by crossing two mutants. One was the state transition–deficient strain stt7-9, a clone that bears the same mutation as the stt7 mutant (Depège et al., 2003) but that has a higher mating capacity (Cardol et al., 2009). The second was npq4, a mutant in which the qE response is largely impaired, due to the disruption of the LHCSR3.1 and LHCSR3.2 genes (Peers et al., 2009). We isolated five clones (npq4 stt7-9, clones #2, #4, #7, #14, and #32) lacking both state transitions and qE, as detected by an in vivo fluorescence screen, and their characteristics are presented in Supplemental Figures 1 and 2 online. Two of them (clones #2 and #7) were further analyzed. To assess state transition capacity, maximum fluorescence yield was recorded at room temperature upon a 2.5-s illumination on cells acclimated in state 1 and in state 2 (Figure 1; see Supplemental Figure 1 online). State 1 was induced by placing the cells in darkness under strong aeration and state 2 by adding the ionophore carbonylcyanide-p-trifluoromethoxyphenyl hydrazine (FCCP; 5 µM) to the incubation medium in the dark. This compound uncouples respiration, thereby lowering the cellular ATP content. This leads in turn to the activation of glycolysis and, therefore, to the accumulation of NADPH, which can reduce the PQ pool via the chlororespiratory chain (reviewed in Wollman, 2001). A reduced PQ pool activates the STT7 kinase, triggering the transfer of a large fraction of Pi-LHCII to PSI, which acts as a strong energy quencher (Delosme et al., 1996). Ultimately, this results in a drop in the maximal fluorescence yield in state 2 conditions (Figure 1A; see Supplemental Figures 1A and 1C online), which was present in the two strains containing an active kinase (the wild type and npq4) but absent in stt7-9 (as expected because of the disruption of STT7) and in the five npq4 stt7-9 clones. In these strains, a small fluorescence increase was observed in state 2 conditions, a phenomenon already observed upon reduction of the PQ pool in strains impaired in state transitions (Hohmann-Marriott et al., 2010).
Figure 1.
State Transition Phenotype of the Different Strains.
Cells were harvested in the exponential phase and resuspended in minimum HS medium at a concentration of 2 × 107 cells mL−1. r.u., relative units.
(A) Fluorescence induction curves. Traces were recorded in the presence of 10 µM DCMU, in the wild type (WT), stt7-9 (lacking the STT7 kinase), npq4 (lacking LHCSR3), and npq4 stt7-9 clones. Strains were placed either in state 1 (solid squares) or in state 2 (open circles) conditions by placing them in darkness under strong aeration (for state 1) or by incubating them with 5 µM FCCP in the dark for 20 min (for state 2). A decrease of the Fm level under state 2 conditions is indicative of a decrease in the size of the PSII antenna due to LHCII migration to PSI. Similar results were obtained when cells were placed in state 1 by illumination in the presence of 20 µM DCMU.
(B) Low-temperature (77 K) fluorescence emission spectra of cells under state 1 (closed squares) and state 2 (open circles) inducing conditions. A high ratio between the fluorescence emission band at 715 nm (PSI) and at 685 nm + 695 nm (PSII) is indicative of a transition to state 2 due to enhanced energy collection by PSI, following LHCII migration from PSII. PSII emission was normalized to 1 in all genotypes.
Measurements of fluorescence emission at cryogenic temperatures confirmed the state transition phenotype of the selected clones (Figure 1B; see Supplemental Figures 1B and 1D online). Unlike in state 1, the fluorescence spectrum was dominated by PSI emission (λ = 715 nm) in the wild-type and npq4 strains under state 2 promoting conditions. This reflects the increased absorption capacity of this complex, induced by LHCII binding (Bonaventura and Myers, 1969). On the other hand, in the same conditions, the PSII emission bands (λ = 685 and 695 nm) were predominant in the stt7-9 strain, where LHCII remained associated with PSII, and in the selected clones, confirming their lack of state transition phenotypes.
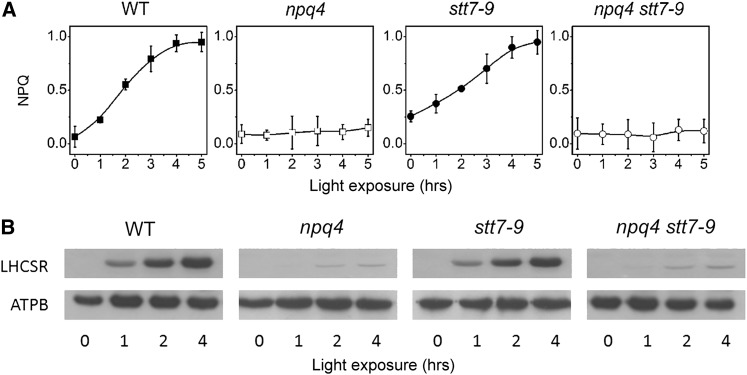
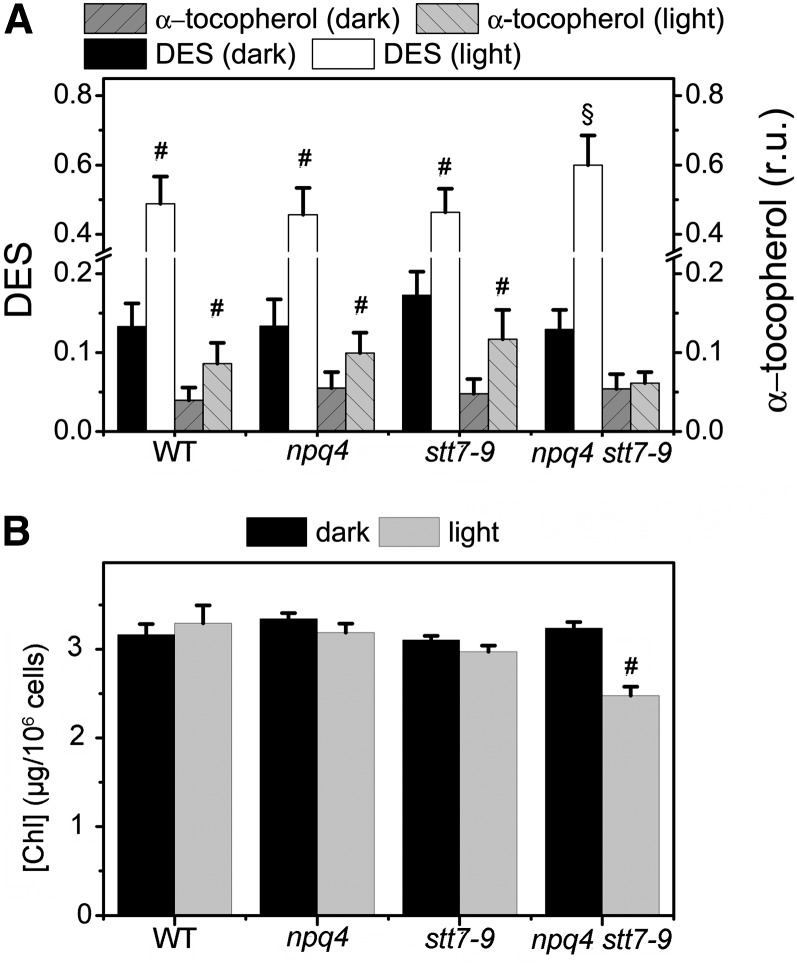
In parallel, progeny from the stt7 × npq4 cross were analyzed for their capacity to develop qE in high light (Figure 2; see Supplemental Figure 2 online). Cells were grown mixotrophically in a Tris-acetate-phosphate (TAP) medium at low light (50 µmol photons m−2 s−1) to prevent the accumulation of LHCSR3 (Peers et al., 2009). They were harvested in the exponential phase, resuspended in a minimum high-salt (HS) medium (without CO2 supply), and then transferred to high light (500 µmol photons m−2 s−1) to induce LHCSR3 accumulation (Peers et al., 2009). Fluorescence quenching was measured at different times during high-light exposure, along with the accumulation of LHCSR3, which was evaluated by immunoblotting with a specific antibody. Figure 2 shows that quenching was induced after a few hours of high-light exposure in the wild type and the stt7-9 strains, in parallel with the accumulation of LHCSR3. A small NPQ was seen in the npq4 as well as in the putative npq4 stt7-9 clones (Figure 2A; see Supplemental Figure 2A online), in which LHCSR3 did not accumulate in high light (Figure 2B; see Supplemental Figure 2B online). In these clones, the absence of qE was not due to impaired carotenoid deepoxidation (Figure 3; see also Peers et al., 2009 for npq4). In fact, a slightly higher deepoxidation was observed in the double mutant at the end of illumination (Figure 3A). In both the npq4 and npq4 stt7-9 mutants, a lower intensity band cross-reacting with the anti-LHCSR antibody was observed at the end of the illumination period (Figure 2B). Because no quenching increase was associated with the induction of this protein, and because of its apparent lower molecular weight (Peers et al., 2009; Bonente et al., 2011), we tentatively ascribed this band to LHCSR1, an LHCSR isoform that seems to play a minor role in qE development in cells fully acclimated to high light (Peers et al., 2009).
Figure 2.
NPQ Induction in Wild-Type, stt7-9, npq4, and npq4 stt7-9 Strains.
Cells were harvested in the exponential phase and resuspended in minimum HS medium at a concentration of 2 × 107 cells mL−1. WT, the wild type.
(A) NPQ efficiency. Cells were exposed to high light (500 µmol photons m−2 s−1, white light) without external carbon dioxide addition for the indicated times and then briefly (10 min) dark adapted. NPQ capacity was evaluated from the (Fm − Fm′)/Fm′ parameter (Bilger and Björkman, 1990) using a fast imaging setup. Values represent mean ± se (n = 4 biological replicates).
(B) LHCSR accumulation. Cells were harvested at the indicated times, and samples were analyzed by immunoblotting with an anti-LHCSR antibody. ATPB (β-subunit of the CF0Fi ATPase) is shown as loading control. One microgram of chlorophyll was loaded in each lane.
Figure 3.
Pigment Composition of the Different Strains in the Dark and after High-Light Exposure.
(A) Carotenoids and α-tocopherol content. C. reinhardtii cells were grown as described in Methods and harvested either in the dark or after high-light (500 µmol photos m−2 s−1, white light) exposure for 4 h. The cells were centrifuged and the pellet was resuspended in methanol. After three consecutive extractions, the supernatant was used to estimate pigment content by HPLC analysis. DES indicates the deepoxidation ratio ([zeaxanthin] + 1/2 [antheraxanthin])/([zeaxanthin] + [antheraxanthin] + [violaxanthin]). α-Tocopherol content is expressed as relative units (r.u.; after normalization to the lutein content). Values represent mean ± se (n = 3 biological replicates). WT, the wild type.
(B) Cellular chlorophyll (Chl) a+b content in dark and high light–treated cells. Values represent mean ± se (n = 4 to 6 biological replicates).
Statistical comparison was performed using one-way analysis of variance (ANOVA) followed by the Tukey multiple comparison test (P < 0.05). Symbols in the graph denote significant differences: #, between light and dark; §, from the wild type, npq4, and stt7-9.
Fluorescence Quenching Dynamics in C. reinhardtii Wild Type and Mutants Impaired in State Transitions and qE
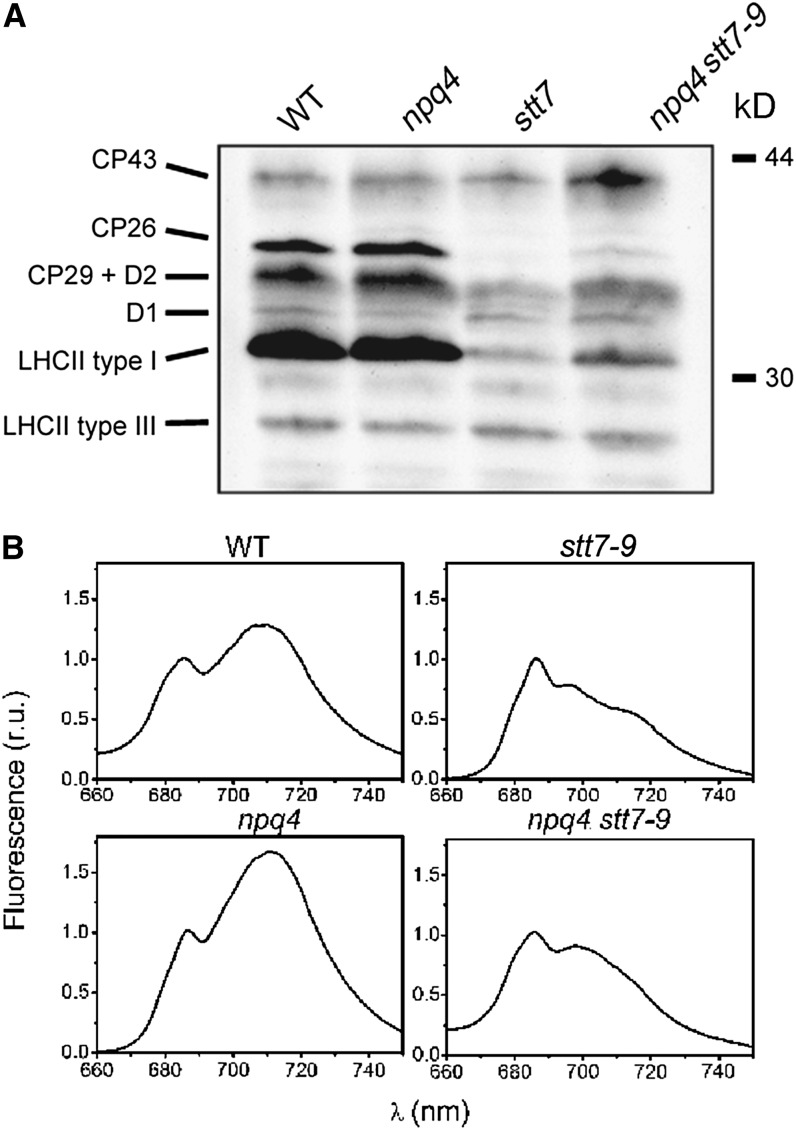
To further evaluate the effect of this treatment on NPQ, fluorescence quenching dynamics were assayed in cells that were exposed to high light for 4 h and then vigorously aerated in the dark for 15 min. Normally, this treatment is sufficient to allow for a complete relaxation of NPQ and reversion of cells to state 1 (Figure 1). However, this was not the case for wild-type cells that had been subjected to a shift from mixotrophic conditions in low light to photoautotrophy in high light. We found that CP26, CP29, and LHCII type I (LhcbM3/-4/-6/-8/-9) were heavily phosphorylated in wild-type cells (as shown by immunoblotting using an antiphosphothreonine antibody; Figure 4A). In parallel, we observed changes in the 77K fluorescence emission spectra, which confirmed the occurrence of state 2 in the wild-type and npq4 strains (Figure 4B).
Figure 4.
Protein Phosphorylation and State Transition Phenotype in Wild-Type, stt7, npq4, and npq4 stt7-9 Mutant Cells upon High-Light Treatments.
Cells were harvested in the exponential phase and resuspended in minimum HS medium. They were exposed to high light (500 µmol of photons m−2 s−1, white light) for 4 h and then briefly (15 min) dark adapted. WT, the wild type.
(A) Phosphorylation status of the PSII antennae, including CP26, CP29, and LHCII type I (LhcbM3/-4/-6/-8/-9) in high light–treated cells. Protein phosphorylation was measured by immunoblotting with antiphosphothreonine antibody. Cells were shock frozen at the time of sampling and pelleted by centrifugation before protein extraction. Total proteins from the 2 × 106 cells were loaded in each lane.
(B) Low-temperature (77 K) fluorescence emission spectra in high light–treated cells. A high ratio between the fluorescence emission band at 715 nm (PSI) and at 685 nm + 695 nm (PSII) is indicative of a transition to state 2 due to enhanced energy collection by PSI, following LHCII migration from PSII. PSII emission was normalized to 1 in all genotypes. r.u., relative units.
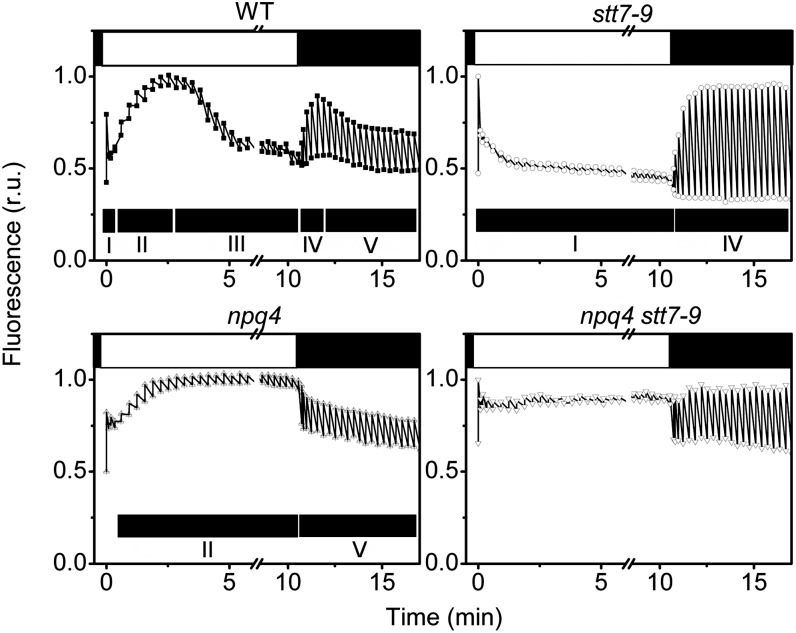
When the light was switched on, we observed NPQ (Figure 5) in agreement with earlier results (Peers et al., 2009; see also Figure 2). However, the fluorescence quenching pattern observed after high-light treatment turned out to be extremely complex (Figure 5) and different from the one normally seen in plants. In high light–treated wild-type cells, light exposure first leads to the development of a fast fluorescence quenching (phase I, Figure 5), which is completed in ∼30 s. This phase is followed by a fluorescence recovery (phase II, Figure 5) that continues for around 3 min, and then by a second slower fluorescence quenching (phase III, Figure 5), until a steady state level is reached. When the light is switched off, quenching relaxation is observed (phase IV, Figure 5), and this is replaced by a slower decline (phase V, Figure 5), down to the initial dark level. In cells constitutively locked in state 1 (stt7-9), the fluorescence quenching profile is different: both the fluorescence rise (phase II) in high light and its decline in the dark (phase V) are abolished (Figure 5). Therefore, illumination led to the onset of a fast quenching (phase I), which rapidly reached a steady state level. The cells almost completely recover in the dark (phase IV). In the qE-deficient npq4 strain, phases II and V are maintained, while phases I, III, and IV are largely depressed (Figure 5). In the double mutant, all the phases observed in the wild type and in the single mutants are largely abolished. This indicates that the complex kinetics measured in wild-type cells is due to the superimposition of qE and state transitions (Figure 5). The comparison between the different genotypes suggests that phases I and IV, which are observed only in the strains capable of accumulating LHCSR3 (the wild type and stt7-9), should correspond to the development of qE in high light and to its relaxation in the dark, respectively. Phases II and V are inhibited by the stt7 mutation, suggesting a role for state transitions. Fluorescence changes are associated with the phosphorylation state of LHCII proteins, including the minor CPs (Allen, 1992; Takahashi et al., 2006; Rochaix, 2007). Thus, we tested the occurrence of phosphorylation-associated qT during the dark–high light–dark incubation by immunoblotting wild-type cells with an antiphosphothreonine antibody. We found that the phosphorylation level of LHCII slowly decreased upon illumination, confirming the occurrence of a state 2-to-1 transition during phase II (see Supplemental Figure 3A online). However, LHCII dephosphorylation also continued during phase III. When the light was switched off, LHCII phosphorylation recovered with a time course largely correlating with phase V.
Figure 5.
Fluorescence Dynamics in High Light–Treated C. reinhardtii Cells.
Cells were harvested in the exponential phase and resuspended in minimum HS medium at a cell concentration of 2 × 107 mL−1. They were exposed to high light (500 µmol of photons m−2 s−1, white light) for 4 h and then briefly (15 min) dark adapted before measuring their fluorescence dynamics upon exposure to actinic light (700 µmol photons m−2 s−1, λ = 520 nm). Closed box, dark; open box, actinic light on. Spikes represent maximum fluorescence emission achieved upon illumination with saturating pulses. Black bars indicate phases I through V. See text for more details. r.u., relative units; WT, the wild type.
We also employed chemical inhibitors to investigate the nature of NPQ in C. reinhardtii. qE is selectively suppressed by the dissipation of the ΔpH using the K+/H+ exchanger nigericin (Horton et al., 1996). Therefore, it is possible to exploit the different ΔpH sensitivity of these two processes to deconvolute the qE (i.e., of the nigericin sensitive) and qT (i.e., of the nigericin insensitive) components of NPQ starting from fluorescence traces measured in wild-type cells under control or nigericin poisoned conditions (see Supplemental Figure 3B online). We found a strong correlation between the kinetics of qT estimated by this approach (see Supplemental Figure 3C online) and the changes in the phosphorylation profile measured by immunoblotting. Therefore, we conclude that phases II and V correspond to a state 2-to-1 transition in the light and to the recovery of state 2 in the dark, respectively. In agreement with previous assignments based on the comparative analysis of fluorescence kinetics in the four genotypes (Figure 5), we also confirm that phase I represents the onset of qE-type quenching, while phase IV corresponds to its relaxation in the dark. To further investigate if phase II and IV could be due to state transitions, we employed the respiratory-deficient mutant dum22, which has been previously shown to be in state 2 in the dark (Cardol et al., 2003; see Supplemental Figure 4 online). Phase II is clearly observed in low light TAP-grown dum22 (see Supplemental Figure 4A online) and is absent in the wild-type cells grown in a similar fashion. This provides another piece of evidence that the phase II pattern observed in high light–grown cells is due to the cells being in state 2 in the dark.
Altogether, the data of Figures 4 and 5 and Supplemental Figures 3 and 4 online show that exposure of cells for several hours to high light, as required to induce LHCSR3, not only provides cells with the capacity to develop qE but also induces a transition to state 2 in the dark as previously observed in the green alga Dunaliella tertiolecta (Casper-Lindley and Björkman, 1998). Both phenomena (qE and transition to state 2 in the dark) were largely absent in cells that were exposed to low light (see Supplemental Figure 5 online).
Phase III of fluorescence quenching is particularly interesting. This phase is seen in high light–treated cells (Figure 5) but not in low light–acclimated ones (see Supplemental Figure 5 online). It is also evident in low light–grown dum22 cells (see Supplemental Figure 4A online), where its appearance correlates with increased LHCSR3 accumulation (see Supplemental Figure 4C online) and xanthophyll deepoxidation (see Supplemental Figure 4D online), when compared with the wild-type cells in the same conditions. Phase III is abolished by the npq4 mutation (Figure 5) as well as by nigericin incubation to dissipate the ΔpH in both high light–treated cells (see Supplemental Figures 3B and 6 online) and low light–treated dum22 cells (see Supplemental Figure 4B online). Thus, this phase should correspond to a qE type of NPQ. However, according to our deconvolution of the kinetics of qT, and to measurements of the dynamics of LHCII phosphorylation, this phase also corresponds to the end of the state 2-to-1 transition, which had already started during phase II. Phase III therefore comprises both a qT and a qE type of quenching. The observation of different qE phases (phases I and III) in relation to the occurrence of state transitions suggests that the quenching capacity of C. reinhardtii could be different in states 1 and 2. To understand the relationship between the two phenomena, we analyzed possible links between qT and LHCSR3. Since this protein is presumably a part of the PSII antenna, we reasoned that it could reversibly migrate between the two photosystems during state transitions. This hypothesis was tested by measuring changes in the association of this protein with the photosystems in state 1 and state 2 conditions. We first induced LHCSR3 synthesis in cells containing His-tagged versions of PSII or PSI by exposing them to high light for 4 h as in Figure 2. Then, we acclimated these cells to either state 1 or state 2 (state 1: light + DCMU; state 2: FCCP). PSI and PSII supercomplexes were then isolated and analyzed for the presence of LHCSR3 by immunoblot analysis. Figure 6 shows that a significant amount of LHCSR3 was associated with PSI in state 2 as well as the PSII antenna, including the major trimeric LHCII (LhcbM6), the minor monomeric LHCII proteins (CP26 and CP29), and the cytochrome b6f complex, as previously shown in the case of the cyclic PSI supercomplex (Iwai et al., 2010). Conversely, LHCSR3 was almost exclusively bound to PSII in state 1 as well as the PSII antenna, including major and minor LHCII proteins. This indicates that LHCSR3 is also a part of the mobile fraction of the PSII antenna during state transitions. Its migration between the two photosystems could therefore modulate energy quenching in PSII, by changing the number of quenching effectors bound to this complex.
Figure 6.
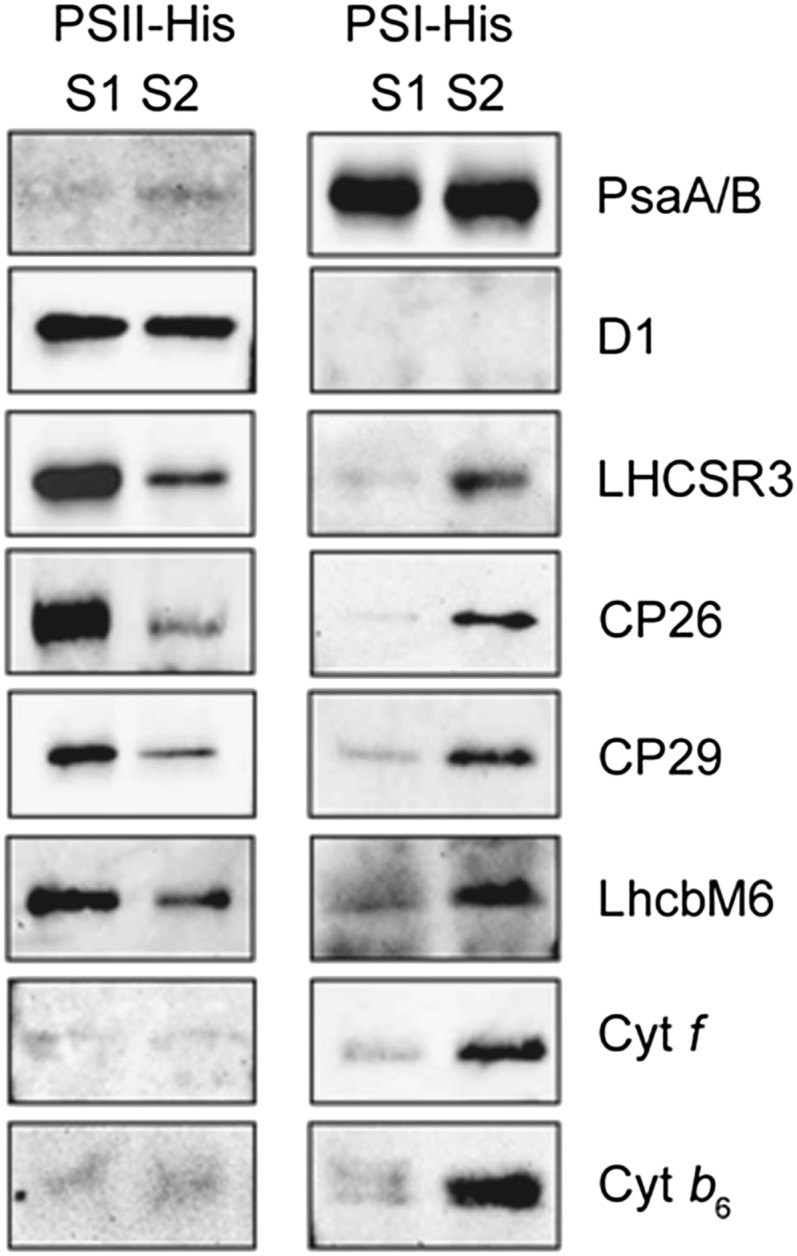
LHCSR3 Migrates between PSII and PSI during State Transitions.
Cells were placed either in state 1 (S1; 20 µM DCMU) or in state 2 (S2; 5 µM FCCP in the dark) conditions immediately after high-light incubation (500 µmol photons m−2 s−1, white light) for 4 h. PSI and PSII supercomplexes were isolated as described in Methods, and their protein composition was tested by immunoblotting with the specific antibodies. PSII-His and PSI-His supercomplex proteins (0.5 µg) were loaded in the respective lanes. Cyt, cytochrome.
Role of State Transitions and qE in Photoprotection of C. reinhardtii
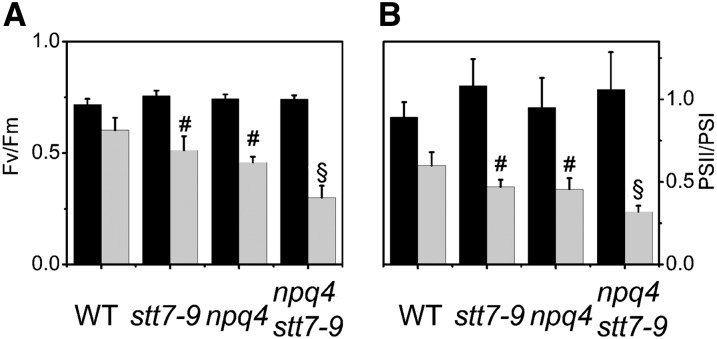
A major role of NPQ in photosynthetic organisms is to protect the components of the electron flow chain, and PSII in particular, from light damage. By showing that both LHCSR3-dependent quenching and state transitions are induced in response to high light, our data suggest that both processes could be involved in photoprotection in this alga. This possibility was investigated by measuring changes in PSII activity before and after the high-light treatment required to induce LHCSR3 accumulation. We observed that the variable fluorescence/maximum fluorescence parameter, which reflects the maximum quantum efficiency of PSII, was decreased to a greater extent in the single mutants than in the wild type after exposure to 500 µmol photons m−2 s−1 for 4 h (Figure 7A). This suggests that some photoinhibition was occurring in stt7-9 and npq4 during this treatment. The decline of the Fv/Fm was even more pronounced in the double mutant, indicating an increased light sensitivity in this strain. Assessment of PSII and PSI activities by measuring the electrochromic shift (ECS; Bailleul et al., 2010a) confirmed this observation. In the dark, a PSII:PSI ratio of ∼1 was found in all strains, in agreement with previous estimates (Cardol et al., 2009). Conversely, a stronger decrease of the PSII photochemical activity was seen in npq4 stt7-9 when compared with the other genotypes after incubation in high light (Figure 7B). This indicates that PSII was more susceptible to high-light stress in the double than in the single mutants and the wild type. Consistent with this, besides changes due to state transitions, a lower PSII:PSI ratio was observed in npq4 compared with the wild type and npq4 stt7-9 compared with stt7-9 at cryogenic temperatures after high-light exposure (Figure 4B). The deleterious effects of the double mutation were confirmed when the analysis of the effects of high light on the photosynthetic properties of four strains was extended to 12 h (see Supplemental Figure 7 online). In this case, we observed some additional accumulation of LHCSR3, confirming the role of this protein in the long-term photoprotection of C. reinhardtii, as well as some increased photoinhibition, which was enhanced in the double mutant up to 8 h of high-light exposure.
Figure 7.
PSI and PSII Activities in Wild-Type, npq4, stt7-9, and npq4 stt7-9 Cells.
Cells were harvested in the exponential phase and resuspended in minimum HS medium at a cell concentration of 2 × 107 mL−1. WT, the wild type. Black columns, dark-adapted cells; gray columns, cells exposed to high light (500 µmol photons m−2 s−1, white light) for 4 h and then shortly (10 min) dark adapted. Values represent means ± se (n = 3 to 6 biological replicates). Statistical comparison was performed using one-way ANOVA followed by the Tukey multiple comparison test (P < 0.05). Symbols in the graph denote significant differences: #, from the wild type; §, from the wild type, npq4, and stt7-9.
(A) PSII efficiency. PSII quantum yield was monitored as Fv/Fm using an imaging fluorescence setup.
(B) Functional PSII:PSI ratio from the ECS signal. PSII and PSI activities were measured as described in Methods.
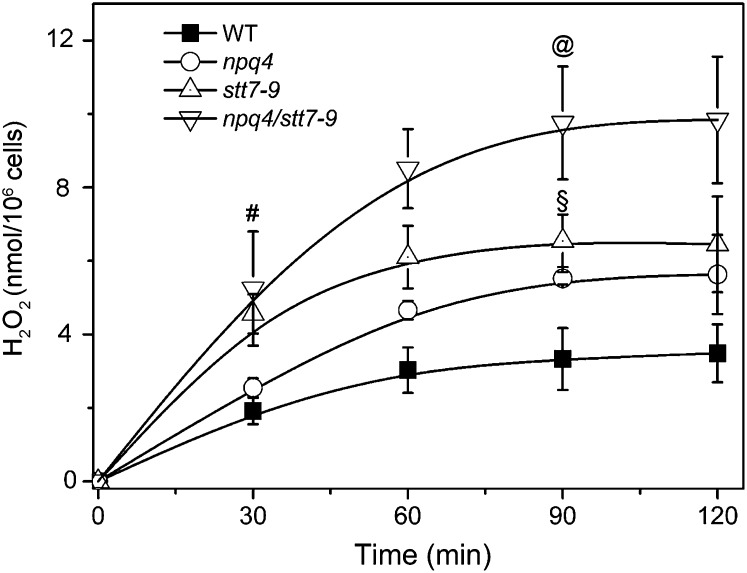
Under conditions where the electron flow capacity is saturated, photoinhibition is triggered by the accumulation of ROS, which are produced at different levels in the photosynthetic electron flow chain (Krieger-Liszkay and Trebst, 2006). To understand the reasons for the increased photosensitivity of the npq4 stt7-9 mutant, we measured ROS levels in the wild type, the single mutants, and in the double mutant during the first hours of high-light exposure. We first evaluated the accumulation of H2O2 in the four genotypes and found that this species was produced in all strains during exposure to high light (Figure 8). However, differences were seen between the different strains in both the rate and the extent of H2O2 accumulation. The amount of H2O2 was lower in wild-type than in npq4 and stt7-9 cells. Conversely, H2O2 accumulation was increased in the double mutant, possibly explaining its higher photosensitivity. Moreover, we observed that stt7-9 and the double mutant accumulated more H2O2 than the npq4 and wild-type strains at the beginning of illumination. This suggests that state transitions may be of particular importance in preventing H2O2 generation during the first phase of high-light exposure (i.e., before the induction of qE). To provide a more complete scenario of ROS production in C. reinhardtii, we also tried to measure 1O2 generation in the different genotypes analyzed in this work using the specific dye DanePy oxalate (Fischer et al., 2007). Unfortunately, the sensitivity of this dye was insufficient to detect 1O2 in vivo unless enhancers (bromoxynil or methylene blue) were added to the cells (see Supplemental Figure 8 online). Nonetheless, we detected lower amounts of α-tocopherol in high light–treated npq4 stt7-9 cells when compared with the other genotypes (Figure 3A). α-Tocopherol acts as an efficient scavenger of intracellular 1O2 (Krieger-Liszkay and Trebst, 2006; Li et al., 2012). It may be that its lower concentration in this strain is due to its rapid consumption to counteract the increased generation of this ROS. The observation of some chlorophyll bleaching after high-light exposure (Figure 3B) is also consistent with the idea that ROS production was increased in the double mutant.
Figure 8.
H2O2 Production during High-Light Exposure of C. reinhardtii Cells.
Cells were harvested in the exponential phase and resuspended in minimum HS medium at a concentration of 5 × 106 cells mL−1. They were exposed to high light (500 µmol of photons m−2 s−1, white light), and samples were collected at different time points. H2O2 production was assessed as described in Methods. Values represent means ± se (n = 4 to 6 biological replicates). Statistical comparison was performed using one-way ANOVA followed by the Tukey multiple comparison test (P < 0.05). Symbols in the graph denote significant differences: #, from stt7-9 and npq4-stt7-9; §, from the wild type and npq4 stt7-9; @, from the wild type, npq4, and npq4 stt7-9. WT, the wild type.
DISCUSSION
In this work, we studied the development of state transitions and of qE during high-light acclimation of the green alga C. reinhardtii and their respective roles in protecting the photosynthetic machinery from photodamage. Using a combined biochemical, biophysical, and genetic approach, we first demonstrated that high-light treatment for several hours (without any additional carbon dioxide source) not only induces qE (Figure 2), in agreement with previous data (Peers et al., 2009; Bonente et al., 2011; Petroutsos et al., 2011), but also results in transition to state 2 that is not reversed by strong agitation (oxygenation) in the dark for 15 min after the treatment (Figures 4 and 5; see Supplemental Figure 3 online). This suggests that the two processes are linked in C. reinhardtii. Consistent with this possibility, cells that are maintained in low light cannot develop qE and state 2 (see Supplemental Figure 5 online). Next, we revealed that both state transitions and qE are involved in protecting C. reinhardtii cells from high-light stress. This conclusion is supported by the increased light sensitivity of the npq4 stt7-9 double mutant, which displays a higher PSII photosensitivity (Figure 7; see Supplemental Figure 7 online), possibly because of enhanced ROS production during the high-light exposure (Figure 8). The occurrence of significant light stress in this strain is also confirmed by the slight increase in its deepoxidation state (Figure 3A), the reduced α-tocopherol content in high light (Figure 3A), possibly to counteract 1O2 accumulation, and the bleaching of chlorophyll (Figure 3B). The observation of a tight link between qE and state transitions seems different from previous data reported in C. reinhardtii (Peers et al., 2009). However, this apparent contradiction can be explained by the fact that the cells were systematically exposed to 5 min of far-red light to ensure transitions into state 1 before the NPQ measurements (Peers et al., 2009). This may have prevented the observation of fluorescence changes related to state transitions during illumination, in contrast with the data shown in this work (Figure 5).
Metabolic Links between qE and qT in C. reinhardtii
The concomitant occurrence of qE and qT in high light suggests that both responses could be induced by the same metabolic signal(s) in the cell. Information concerning the nature of the photosynthetic step responsible for the induction of qE and qT in high light can be obtained from the kinetic analysis of the NPQ response presented in Figure 5. There, we show that high light–treated cells are in state 2 in the dark (Figure 5; see also Figure 4 and Supplemental Figure 3 online) and revert to state 1 upon illumination (phase II). This behavior can be rationalized based on previous knowledge of the mechanisms of state transition. The first step in the activation of state 2 transition is the reduction of the PQ pool (Wollman, 2001), either via PSII photochemistry or via a nonphotochemical (chlororespiratory) process. A net reduction of the PQ pool is expected in high light because the rate of PQH2 generation by PSII becomes faster than the rate of PQH2 oxidation by the cytochrome b6f complex at this intensity. Thus, high-light exposure should promote the transition to state 2 instead of the transition to state 1 as observed in Figure 5. On the other hand, previous work has shown that the state 2 transition is inhibited by high light (Rintamäki et al., 2000; Vink et al., 2004), either due to the negative redox control exerted by the ferredoxin/thioredoxin system on STT7/STN7 kinase (Rintamäki et al., 2000) or to a conformational change within the PSII antenna that prevents its phosphorylation by the active kinase (Vink et al., 2004). Both mechanisms can equally account for the rapid recovery of state 1 observed upon illumination of the cells (phase II). No such inhibitory effect is expected upon dark adaptation, since both mechanisms are light dependent (Aro and Ohad, 2003). Therefore, both hypotheses are compatible with the high phosphorylation levels seen in wild-type and npq4 cells after high-light exposure (Figure 4; see Supplemental Figure 3 online). High-light treatments in the absence of any exogenous carbon source, as required to induce LHCSR3 (Peers et al., 2009), should lead to a limitation of photosynthetic activity by carbon assimilation at the level of the Calvin cycle, thereby promoting NADPH accumulation. After illumination, this NADPH can be consumed by the chlororespiratory pathway in reducing the PQ pool and thereby promoting a rapid state 2 transition. This could explain the origin of phase V. Consistent with this, exposure of C. reinhardtii cells to high light enhances the capacity for nonphotochemical reduction of the PQ pool at the expense of stromal reducing equivalents (Houyoux et al., 2011).
qE and qT Dynamics during Induction of NPQ in C. reinhardtii
The tight relationship between state transitions and qE in high light–treated cells is not only evident in the dark but also during illumination. This is shown by the existence of two fluorescence quenching phases in high light–treated wild-type cells: The first one (phase I) likely corresponds to the development of qE in state 2 cells, while the second one (phase III) most likely represents an additional quenching phase linked to the attainment of state 1. Indeed, this phase can be ascribed to a qE type of quenching, based on its absence in the qE-lacking strain npq4 (Figure 5) and on its sensitivity to the dissipation of the ΔpH by the addition of the ionophore nigericin (see Supplemental Figure 6 online). However, phase III also comprises a state 2-to-1 transition, as indicated by measurements of protein phosphorylation kinetics in the light (see Supplemental Figure 3 online). The observation that this phase is slower than phase I (i.e., qE onset in state 2) may suggest that qE development is slower in state 1 than in state 2. However, this possibility is not consistent with the observation that the rate of fluorescence quenching at the onset of illumination is similar in wild-type and stt7 cells, which are in states 2 and 1, respectively. Moreover, the different rates of phases I and III cannot be explained by a different accumulation of LHCSR3 and zeaxanthin, which accumulated to a maximum extent during the high-light treatment that precedes the measurement of NPQ (Figures 2 and 3, respectively). Under these conditions, lumen acidification becomes the only time-limiting process for qE onset (Joliot and Finazzi, 2010), leading in principle to a very fast quenching response (Johnson et al., 2009). To explain the different qE kinetics measured in phases I and III, we propose instead that phase III represents the signature of the dynamics of LHCSR3 binding to PSII during the state 2-to-1 transition. LHCSR3 reversibly binds PSI in state 2, possibly because of its phosphorylation by STT7 (Bonente et al., 2011) (i.e., the same kinase involved in PSII antenna phosphorylation) (Depège et al., 2003). Therefore, the observation that qE quenching increases (phase III) at the end of the antenna movement to PSII (state 1 transition, phase II + phase III) suggests that the reassociation of LHCSR3 to PSII during a state 2-to-1 transition could be slower than the binding of the antenna complexes to the reaction center. This would explain why the fluorescence emission is first increased by the state 1 transition (phase II) due to the increased PSII absorption capacity, and then decreased (phase III) due to the reassociation of LHCSR3 with PSII. This latter process should amplify fluorescence quenching in PSII by increasing the number of quenching effectors bound to the PSII supercomplex, as already shown in the case of the plant qE effector PSBS (Li et al., 2002).
Photoprotective Role of State Transitions in C. reinhardtii
As discussed above, the phenotypic analysis of the npq4 stt7-9 double mutant suggests that both qE and qT counteract photodamage in high light. In particular, measurements of ROS production suggest that state transitions play a photoprotective role during the early phase of light stress (i.e., when qE has not yet developed). This role is illustrated by the enhanced H2O2 production (Figure 8) observed in the two strains lacking STT7 (stt7-9 and npq4 stt7-9) during the first hours of illumination. This observation is in contrast with the kinetic analysis of NPQ (Figure 5), which shows that both wild-type and npq4 cells rapidly reach state 1 upon high-light exposure (phase II). Accordingly, no differences are expected between the four genotypes, which should all be in state 1 after a few minutes of illumination (either because of the inactivation of STT7 or because of the inhibition of LHCII phosphorylation by the mechanisms discussed above). However, this incoherence can be tackled by considering the fact that recovery of state 1 is not complete in high light. In steady state photosynthesis, a fraction of the photosynthetic chains is always in state 2 (Forti et al., 2003), possibly to ensure the proper balance between NADPH and ATP production. The existence of this fraction, which is only expected in the STT7-containing strains (the wild type and npq4), could explain why only a lower amount of H2O2 is produced by these strains during the early phase of high-light exposure (i.e., before the induction of qE).
The observation that the impairment of state transitions enhances H2O2 production is intriguing. The opposite would be expected because PSI turnover is increased in state 2, owing to the largely enhanced absorption capacity. This higher turnover capacity should translate into an increased H2O2 production under the conditions employed here, where the availability of CO2 should be limiting to photosynthesis. This apparent contradiction can be explained considering (1) that a part of the H2O2 generated in the chloroplast is produced by PSII. This is consistent with recent data showing that this ROS can be produced directly by PSII, although to a very small extent (Pospíšil, 2012). Therefore, transition to state 2 may reduce the generation of H2O2 by reducing the excitation pressure on this complex. On the other hand, (2) recent data have shown that the state 2 transition in C. reinhardtii leads to the formation of supercomplexes containing PSI, FERREDOXIN NADP REDUCTASE, PROTON GRADIENT REGULATOR5 LIKE1, and the cytochrome b6f (Iwai et al., 2010). These supercomplexes should be able to sequester the soluble electron carriers (plastocyanin and ferredoxin) within a thermodynamically confined space, to enhance the efficiency of cyclic electron flow around PSI (Iwai et al., 2010). By doing so, it could also lower the probability of side reactions between PSI acceptors and molecular oxygen, thereby directly reducing the yield of H2O2 generation. Removal of both mechanisms in strains locked in state 1 could enhance the production of H2O2, thereby increasing the probability of damaging the photosynthetic apparatus. This negative effect could be amplified in a genetic background where production of other ROS species is also enhanced, such as npq4. Although the detection limits of DanePy in the absence of bromoxynil are too low to reliably measure 1O2, other cellular responses (pigment bleaching, α-tocopherol consumption, and photoinhibition) suggest that this is the case at least in the npq4 stt7-9 strain. This is in agreement with the notion that by accumulating a large amount of excited state in the antenna, decreased qE could increase the concentration of the dangerous triplet state of chlorophylls, which is the main source of 1O2 (Krieger-Liszkay and Trebst, 2006). Consistent with this hypothesis, data obtained in intact chloroplasts from Arabidopsis indicate that 1O2 production is higher in a mutant lacking PSBS (Roach and Krieger-Liszkay, 2012).
Physiology of State Transitions in C. reinhardtii Revisited
Previous data in higher plants have documented that state transitions do not play any relevant role in the high-light stress response (Frenkel et al., 2007) but are only involved in the short-term acclimation to a changing low light environment (Bellafiore et al., 2005). The different results observed in higher plants and in C. reinhardtii likely reflect the observation that state transitions are by far larger in this alga than in plants. The reasons for the different extent of qT between the two types of organism have been largely debated (reviewed in Wollman, 2001; Finazzi, 2005; Rochaix, 2007; Minagawa, 2011). In the frame of the observations presented here, it is tempting to speculate that one possible reason for the larger capacity of state transitions in C. reinhardtii is its involvement in photoprotection. As discussed above, induction of the qE response during a low-light to high-light transition is a much slower process in this alga than in plants, owing to the longer time required to accumulate LHCSR3 during high-light exposure (Peers et al., 2009, Figure 2; this work). The occurrence of a state 2 transition during this period may alleviate high-light pressure on PSII by reducing H2O2 production (likely via the establishment of the PSI-cytochrome b6f supercomplex) but also by reducing its light-harvesting capacity (via the transfer of a large fraction of the PSII antennas to the rather efficient energy quencher reaction center of PSI). Consistent with this possibility, the LHCSR proteins that modulate the qE response are constitutively expressed in other microalgae, such as the diatom Phaeodactylum tricornutum (Nymark et al., 2009; Bailleul et al., 2010b; Zhu and Green, 2010), while state transitions are absent (Ting and Owens, 1993). In the moss Physcomitrella patens, both PSBS and LHCSR3 contribute to qE (Alboresi et al., 2010), but the LHCSR isoform that modulates quenching is also constitutively expressed.
Altogether, it appears that a large state transition capacity may have evolved to provide photosynthetic organisms with a high degree of flexibility in coping with abiotic stresses. Indeed, the possibility of modulating the PSI and PSII absorption to a large extent (by displacing most of the LHCII) and to regulate the ATP/NADPH synthesis capacity via changes in the linear versus cyclic electron flow ratio (Cardol et al., 2009; Iwai et al., 2010) can provide a clear benefit in high-light conditions when the electron flow chain is overreduced. The same system could also be useful under nutrient deprivation. Indeed, a systematic transition to state 2 is observed upon nutrient starvation in C. reinhardtii (i.e., a condition where the electron flow capacity is restricted and light is absorbed in excess), paving the way to photoinhibition (reviewed in Finazzi, 2005). By providing benefits in terms of ROS production (Figure 8), the large displacement of LHCII between PSII and PSI could mediate the first algal responses to stress and contributes to some protection also in steady state (see Supplemental Figure 7 online). On the other hand, qE would maintain an essential role in protecting cells from high light, in agreement with earlier results (Peers et al., 2009). Interestingly, LHCSR3 transcripts also accumulate under nutrient stress conditions in C. reinhardtii (Zhang et al., 2004; Naumann et al., 2007), suggesting that both layers of photoprotection are required to boost the fitness of the cells under nonpermissive conditions.
METHODS
Cells and Growth Conditions
Several Chlamydomonas reinhardtii wild-type strains were studied in this work. The 137c and 222+ strains are derived from strain 137C. 4A+ is also derived from 137C (gift from J.-D. Rochaix, University of Geneva). The npq4 strain is a mutant lacking a functional copy of LHCSR3.1 and LHCSR3.2 (Peers et al., 2009). stt7-9 is a clone allelic to stt7 (Depège et al., 2003), which can be easily crossed, unlike the original strain (gift from J.-D. Rochaix; Cardol et al., 2009). The double mutant npq4 stt7-9 clones were obtained by crossing the npq4 mt− mutant with a stt7-9 mt+ mutant using standard procedures. Five npq4 stt7-9 clones were isolated (see Supplemental Figures 1 and 2 online). The dum22 strain is a mitochondrial DNA deletion mutant lacking the left telomere, cob, and part of nd4 (Cardol and Remacle, 2008). Genetically modified C. reinhardtii strains PSII-His (Cullen et al., 2007) and PSI-His (Gulis et al., 2008), carrying a 6x His-tag at the C terminus of psbH and the N terminus of psaA, respectively, were used to isolate thylakoid membranes. C. reinhardtii cells were routinely cultivated at 50 µmol photons m−2 s−1 in mixotrophic (in a TAP medium) conditions.
Fluorescence Measurements
To evaluate state transitions (Figure 1; see Supplemental Figure 1 online), algae were grown mixotrophically, harvested in the exponential phase, and resuspended to a cell density of 2 × 107 cells mL−1 in HS medium (Sueoka, 1960). Two milliliters was loaded onto a multiwell plate (24 wells) and subjected to continuous shaking (by putting a glass bead into each well and depositing the plate on a shaker) in the dark or illuminated in the presence of the PSII inhibitor DCMU (20 µM) to obtain state 1. State 2 was achieved by incubating the cells with FCCP (5 µM) for 20 min (Bulte et al., 1990). Fluorescence emissions were measured at 77K using a Speedzen MX fluorescence imaging setup (JBeamBio) or a charge-coupled device spectrophotometer (JBeamBio).
Fluorescence Quenching Measurements
Algae in minimum HS medium were exposed to high-light intensity (500 µmol of photons m−2 s−1) white light provided by a WH 100 light-emitting diode panel (JBeamBio) to induce the accumulation of LHCSR3. The NPQ response was measured using a Speedzen MX fluorescence imaging setup (JBeamBio). Excitation was done in the blue range (λ = 450 nm) using short pulses (10 µs). Emission was measured in the near far red. Saturating pulses (duration 250 ms) were provided by a green (λ = 520 nm) light-emitting diode array. Measurements were done 15 min after high-light exposure to ensure relaxation of the NPQ induced by the preillumination. The actinic light (λ = 520 nm) intensity was 700 µmol of photons m−2 s−1. NPQ was calculated as (Fm − Fm′)/Fm′ (Bilger and Björkman, 1990), where Fm and Fm′ are the maximum fluorescence emission level in the dark and light, respectively, measured after exposure to a saturating pulse of light, the intensity of which was 2700 µmol of photons m−2 s−1.
ROS Production
For H2O2 measurements, cells were exposed to 500 µmol photons m−2 s−1 provided by a 125-W cool fluorescent lamp. One milliliter of culture was diluted onefold and combined with 10 units of horseradish peroxidase and 0.5 µM Amplex Red (Invitrogen) forming the fluorescent resorufin product (excitation, 518 nm; emission, 583 nm). C. reinhardtii cells were removed by brief centrifugation before measurement. H2O2 was quantified against a standard curve. Measurements at time 0 were subtracted from all other time points. DanePy fluorescence was measured in the same conditions as those of Fischer et al. (2007).
Spectroscopy Analysis
Spectroscopy analysis was performed in vivo using a JTS-10 spectrophotometer (BioLogic). Changes in the amount of functional photosynthetic complexes were evaluated measuring the ECS spectral change, a shift in the pigment absorption bands that is linearly correlated to the number of light-induced charge separations within the reaction centers (Witt, 1979). Functional PSI and PSII content was estimated from changes in the amplitude of the fast phase of the ECS signal (at 520 to 546 nm) upon excitation with a saturating laser flash (520 nm, 5-ns duration). PSII contribution was calculated from the decrease in the signal amplitude upon the addition of DCMU (20 µM) and hydroxylamine (2 mM) to irreversibly block PSII charge separation. Conversely, PSI was estimated as the fraction of the signal that was insensitive to these inhibitors (Bailleul et al., 2010a).
Biochemical Analysis
Whole-Cell Analysis
C. reinhardtii cells were shock frozen at the time of sampling and pelleted later by centrifugation at 16,000g for 2 min. Proteins were extracted by resuspending the pellet in 100 μL of extraction buffer (60 mM DTT, 60 mM Na2CO3, 2% [v/v] SDS, and 12% [w/v] Suc) and collected in the supernatant after centrifugation at 16,000g for 10 min. Proteins were separated on 12.5% denaturing polyacrylamide gels (Laemmli, 1970), transferred onto nitrocellulose membrane (0.45 μm; Whatman), and revealed by immunodetection with the ECL+ detection kit (GE Healthcare).
Isolation of Thylakoid Membranes and Purification of PSII and PSI Supercomplexes
C. reinhardtii cells were harvested after an illumination at 500 µmol photons m−2 s−1 in HS medium for 4 h and disrupted twice by BioNeb (Glas-col) at 7.5 kilogram force cm−2. Thylakoid membranes were isolated as described previously (Tokutsu et al., 2009) with a slight modification: The buffer used for preparing thylakoid membranes contained 25 mM MES, 0.33 M Suc, 5 mM EDTA, and 1.5 mM NaCl, pH 6.5. Purification of PSII supercomplex and PSI supercomplex was done using the respective His-tagged strains as described previously by Iwai et al. (2008, 2010), except that n-dodecyl-α-d-maltoside (α-DM; Anatrace) was used to solubilize the thylakoid membranes. Thylakoid membranes were adjusted to 0.4 mg chlorophyll mL−1 in thylakoid preparation buffer and solubilized with 1.0% (w/v) α-DM on ice for 5 min in the dark (Tokutsu et al., 2012). The solubilized membranes were applied to a column with ProBond resin (Invitrogen), which was preequilibrated with the same buffer supplemented with 0.02% (w/v) α-DM. The column was washed with the same buffer supplemented with 15 mM imidazole and 0.02% (w/v) α-DM until the flow-through solution became clear. The photosystem supercomplexes were eluted with the same buffer supplemented with 250 mM imidazole and 0.02% α-DM. SDS-PAGE and immunoblotting were conducted as described previously (Tokutsu et al., 2009).
Antibodies
Antibodies used to detect the polypeptides in the supercomplexes were as described previously (Iwai et al., 2010). Anti-LHCSR antibody was a kind gift of M. Guertin (Université Laval, Canada) to K.K.N., antiphosphothreonine antibodies were from Zymed Lab, and anti-ATPB was obtained from Agrisera.
Pigment Analysis
The method used to analyze and quantify carotenoids is detailed by Fraser et al. (2000). C. reinhardtii cells were frozen and resuspended in methanol. After three consecutive extractions, the supernatant was used to estimate pigment content by HPLC analysis using a C30 reverse-phase column (250 × 4.6 mm) manufactured by YMC Co. and purchased from Interchim. The following mobile phases used were methanol (A), water/methanol (20/80 by volume) containing 0.2% (w/v) ammonium acetate (B), and tert-methyl butyl ether (C). The gradient used was 95% A/5% B isocratically for 12 min, a step to 80% A/5% B/15% C at 12 min, followed by a linear gradient to 30% A/5% B/65% C by 30 min (Fraser et al., 2000). Identification of pigments was achieved by comparing retention times and spectral properties of authentic standards. Quantification was achieved by evaluating the area below each peak using software provided by the manufacturer. Tocopherols were measured using the same method as for pigments, using a fluorescence detector instead of a diode array detector.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: STN7, AAO63768.1; STT7, AAO63768.1; and LCCSR3.2, EDP01087.1.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. State Transition Phenotype of the Different Strains.
Supplemental Figure 2. High-Energy Quenching Phenotype of the Different Strains.
Supplemental Figure 3. Dynamics of Protein Phosphorylation and Fluorescence Quenching in High Light–Treated C. reinhardtii Wild-Type Cells.
Supplemental Figure 4. Fluorescence Quenching Dynamics in dum22 Cells.
Supplemental Figure 5. Fluorescence Dynamics in C. reinhardtii Cells in Low Light.
Supplemental Figure 6. Sensitivity of Fluorescence Quenching to the Presence of a Proton Gradient in C. reinhardtii.
Supplemental Figure 7. Long-Term High-Light Acclimation in C. reinhardtii.
Supplemental Figure 8. In Vivo Detection of 1O2 Production by DanePy in C. reinhardtii.
Acknowledgments
G.A., G.F., M.K., C.B., D.P., and D.S.-B. thank financial support from Agence Nationale de la Recherche Grant “phytadapt” NT09_567009. G.F. and J.M. thank the Japanese Society of Technology–Centre National de la Recherche Scientifique cooperative program on Marine Genomics and Marine Biology for support. R.T. and J.M. acknowledge financial support from the Research Fellowship for Young Scientists (21001384) and the NEXT Program initiated by the Council for Science and Technology Policy Grant (GS026), respectively. K.K.N. and G.P. were supported by a grant from the Chemical Sciences, Geosciences, and Biosciences Division, Office of Basic Energy Sciences, Office of Science, U.S. Department of Energy (Field Work Proposal number 449B). K.K.N. is an investigator of the Howard Hughes Medical Institute and the Gordon and Betty Moore Foundation. P.C. and F.F. are junior and senior research associates of the Fonds de la Recherche Scientifique, respectively. This work was also supported by Fonds de la Recherche Scientifique grants (MIS F.4520 and FRFC 2.4597) and by ANR-09-BLAN-0055-01 to A.K.-L. T.R. was supported by the European Union FP7 Marie Curie Initial Training Network HARVEST (FP7 Project 238017). This study was carried out under the National Institute for Basic Biology Cooperative Research Program for the Okazaki Large Spectrograph number 12-513. We thank James Connorton (Commissariat à l’Energie Atomique) for critical reading of the article and Marion Fargier for help during the first phase of this study.
AUTHOR CONTRIBUTIONS
G.A., R.T., T.R., G.P., P.C., J.G.-B., D.S.-B., M.K., C.B., F.F., F.-A.W., K.K.N., A.K.-L., J.M., and G.F. designed the research. G.A., R.T., T.R., G.P., P.C., J.G.-B., D.S.-B., M.K., C.B., A.K.-L., and G.F. performed the research. G.A., R.T., T.R., P.C., M.K., D.P., C.B., A.K.-L., J.M., and G.F. analyzed data. A.K.-L., J.M., and G.F. wrote the article.
Glossary
- PSII
photosystem II
- NPQ
nonphotochemical quenching
- PSI
photosystem I
- qE
high energy–dependent quenching
- PQ
plastoquinone
- PQH2
plastoquinol
- Pi-LHCII
phosphorylated light-harvesting complex II
- PSBS
photosystem II subunit S
- qT
quenching due to state transitions
- ROS
reactive oxygen species
- H2O2
hydrogen peroxide
- FCCP
carbonylcyanide-p-trifluoromethoxyphenyl hydrazine
- TAP
Tris-acetate-phosphate
- HS
high salt
- Fv/Fm
variable fluorescence / maximum fluorescence
- ECS
electrochromic shift
- α-DM
n-dodecyl-α-d-maltoside
- ANOVA
analysis of variance
References
- Alboresi A., Gerotto C., Giacometti G.M., Bassi R., Morosinotto T. (2010). Physcomitrella patens mutants affected on heat dissipation clarify the evolution of photoprotection mechanisms upon land colonization. Proc. Natl. Acad. Sci. USA 107: 11128–11133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J.F. (1992). Protein phosphorylation in regulation of photosynthesis. Biochim. Biophys. Acta 1098: 275–335 [DOI] [PubMed] [Google Scholar]
- Aro E.M., Ohad I. (2003). Redox regulation of thylakoid protein phosphorylation. Antioxid. Redox Signal. 5: 55–67 [DOI] [PubMed] [Google Scholar]
- Aro E.M., Virgin I., Andersson B. (1993). Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochim. Biophys. Acta 1143: 113–134 [DOI] [PubMed] [Google Scholar]
- Bailleul B., Cardol P., Breyton C., Finazzi G. (2010a). Electrochromism: A useful probe to study algal photosynthesis. Photosynth. Res. 106: 179–189 [DOI] [PubMed] [Google Scholar]
- Bailleul B., Rogato A., de Martino A., Coesel S., Cardol P., Bowler C., Falciatore A., Finazzi G. (2010b). An atypical member of the light-harvesting complex stress-related protein family modulates diatom responses to light. Proc. Natl. Acad. Sci. USA 107: 18214–18219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellafiore S., Barneche F., Peltier G., Rochaix J.D. (2005). State transitions and light adaptation require chloroplast thylakoid protein kinase STN7. Nature 433: 892–895 [DOI] [PubMed] [Google Scholar]
- Bilger W., Björkman O. (1990). Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth. Res. 25: 173–186 [DOI] [PubMed] [Google Scholar]
- Bonente G., Ballottari M., Truong T.B., Morosinotto T., Ahn T.K., Fleming G.R., Niyogi K.K., Bassi R. (2011). Analysis of LhcSR3, a protein essential for feedback de-excitation in the green alga Chlamydomonas reinhardtii. PLoS Biol. 9: e1000577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonente G., Passarini F., Cazzaniga S., Mancone C., Buia M.C., Tripodi M., Bassi R., Caffarri S. (2008). The occurrence of the psbS gene product in Chlamydomonas reinhardtii and in other photosynthetic organisms and its correlation with energy quenching. Photochem. Photobiol. 84: 1359–1370 [DOI] [PubMed] [Google Scholar]
- Bonaventura C., Myers J. (1969). Fluorescence and oxygen evolution from Chlorella pyrenoidosa. Biochim. Biophys. Acta 189: 366–383 [DOI] [PubMed] [Google Scholar]
- Bulte L., Gans P., Rebeille F., Wollman F.A. (1990). ATP control on state transitions in vivo in Chlamydomonas reinhardtii. Biochim. Biophys. Acta 1020: 72–80 [Google Scholar]
- Cardol P., Alric J., Girard-Bascou J., Franck F., Wollman F.A., Finazzi G. (2009). Impaired respiration discloses the physiological significance of state transitions in Chlamydomonas. Proc. Natl. Acad. Sci. USA 106: 15979–15984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardol P., Gloire G., Havaux M., Remacle C., Matagne R., Franck F. (2003). Photosynthesis and state transitions in mitochondrial mutants of Chlamydomonas reinhardtii affected in respiration. Plant Physiol. 133: 2010–2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardol, P., and Remacle, C. (2008). The mitochondrial genome. In The Chlamydomonas Source Book 3, Vol. 2, Organellar and Metabolic Processes, D. Stern and E.E. Harris eds (Kidlington, UK; Burlington, MA; San Diego, CA: Academic Press, Elsevier), pp. 445–468. [Google Scholar]
- Casper-Lindley C., Björkman O. (1998). Fluorescence quenching in four unicellular algae with different light-harvesting and xanthophyll-cycle pigments. Photosynth. Res. 56: 277–289 [Google Scholar]
- Cullen M., Ray N., Husain S., Nugent J., Nield J., Purton S. (2007). A highly active histidine-tagged Chlamydomonas reinhardtii photosystem II preparation for structural and biophysical analysis. Photochem. Photobiol. Sci. 6: 1177–1183 [DOI] [PubMed] [Google Scholar]
- DalCorso G., Pesaresi P., Masiero S., Aseeva E., Schünemann D., Finazzi G., Joliot P., Barbato R., Leister D. (2008). A complex containing PGRL1 and PGR5 is involved in the switch between linear and cyclic electron flow in Arabidopsis. Cell 132: 273–285 [DOI] [PubMed] [Google Scholar]
- Dall’Osto L., Caffarri S., Bassi R. (2005). A mechanism of nonphotochemical energy dissipation, independent from PsbS, revealed by a conformational change in the antenna protein CP26. Plant Cell 17: 1217–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delepelaire P., Wollman F.A. (1985). Correlations between fluorescence and phosphorylation changes in thylakoid membranes of Chlamydomonas reinhardtii in vivo: A kinetic analysis. Biochim. Biophys. Acta 809: 277–283 [Google Scholar]
- Delosme R., Olive J., Wollman F.A. (1996). Changes in light energy distribution upon state transitions: An in vivo photoacoustic study of the wild type and photosynthesis mutants from Chlamydomonas reinhardtii. Biochim. Biophys. Acta 1273: 150–158 [Google Scholar]
- Depège N., Bellafiore S., Rochaix J.D. (2003). Role of chloroplast protein kinase Stt7 in LHCII phosphorylation and state transition in Chlamydomonas. Science 299: 1572–1575 [DOI] [PubMed] [Google Scholar]
- Elrad D., Grossman A.R. (2004). A genome’s-eye view of the light-harvesting polypeptides of Chlamydomonas reinhardtii. Curr. Genet. 45: 61–75 [DOI] [PubMed] [Google Scholar]
- Finazzi G. (2005). The central role of the green alga Chlamydomonas reinhardtii in revealing the mechanism of state transitions. J. Exp. Bot. 56: 383–388 [DOI] [PubMed] [Google Scholar]
- Fischer B.B., Krieger-Liszkay A., Hideg E., Snyrychová I., Wiesendanger M., Eggen R.I.L. (2007). Role of singlet oxygen in chloroplast to nucleus retrograde signaling in Chlamydomonas reinhardtii. FEBS Lett. 581: 5555–5560 [DOI] [PubMed] [Google Scholar]
- Forti G., Furia A., Bombelli P., Finazzi G. (2003). In vivo changes of the oxidation-reduction state of NADP and of the ATP/ADP cellular ratio linked to the photosynthetic activity in Chlamydomonas reinhardtii. Plant Physiol. 132: 1464–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser P.D., Pinto M.E., Holloway D.E., Bramley P.M. (2000). Technical advance: Application of high-performance liquid chromatography with photodiode array detection to the metabolic profiling of plant isoprenoids. Plant J. 24: 551–558 [DOI] [PubMed] [Google Scholar]
- Frenkel M., Bellafiore S., Rochaix J.D., Jansson S. (2007). Hierarchy amongst photosynthetic acclimation responses for plant fitness. Physiol. Plant. 129: 455–459 [Google Scholar]
- Gulis G., Narasimhulu K.V., Fox L.N., Redding K.E. (2008). Purification of His(6)-tagged photosystem I from Chlamydomonas reinhardtii. Photosynth. Res. 96: 51–60 [DOI] [PubMed] [Google Scholar]
- Hohmann-Marriott M.F., Takizawa K., Eaton-Rye J.J., Mets L., Minagawa J. (2010). The redox state of the plastoquinone pool directly modulates minimum chlorophyll fluorescence yield in Chlamydomonas reinhardtii. FEBS Lett. 584: 1021–1026 [DOI] [PubMed] [Google Scholar]
- Horton P., Ruban A.V., Walters R.G. (1996). Regulation of light harvesting in green plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47: 655–684 [DOI] [PubMed] [Google Scholar]
- Houyoux P.A., Ghysels B., Lecler R., Franck F. (2011). Interplay between non-photochemical plastoquinone reduction and re-oxidation in pre-illuminated Chlamydomonas reinhardtii: A chlorophyll fluorescence study. Photosynth. Res. 110: 13–24 [DOI] [PubMed] [Google Scholar]
- Iwai M., Takahashi Y., Minagawa J. (2008). Molecular remodeling of photosystem II during state transitions in Chlamydomonas reinhardtii. Plant Cell 20: 2177–2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai M., Takizawa K., Tokutsu R., Okamuro A., Takahashi Y., Minagawa J. (2010). Isolation of the elusive supercomplex that drives cyclic electron flow in photosynthesis. Nature 464: 1210–1213 [DOI] [PubMed] [Google Scholar]
- Johnson M.P., Pérez-Bueno M.L., Zia A., Horton P., Ruban A.V. (2009). The zeaxanthin-independent and zeaxanthin-dependent qE components of nonphotochemical quenching involve common conformational changes within the photosystem II antenna in Arabidopsis. Plant Physiol. 149: 1061–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joliot P.A., Finazzi G. (2010). Proton equilibration in the chloroplast modulates multiphasic kinetics of nonphotochemical quenching of fluorescence in plants. Proc. Natl. Acad. Sci. USA 107: 12728–12733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger-Liszkay A., Trebst A. (2006). Tocopherol is the scavenger of singlet oxygen produced by the triplet states of chlorophyll in the PSII reaction centre. J. Exp. Bot. 57: 1677–1684 [DOI] [PubMed] [Google Scholar]
- Laemmli U.K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- Li X.P., Muller-Moule P., Gilmore A.M., Niyogi K.K. (2002). PsbS-dependent enhancement of feedback de-excitation protects photosystem II from photoinhibition. Proc. Natl. Acad. Sci. USA 99: 15222–15227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z.R., Keasling J.D., Niyogi K.K. (2012). Overlapping photoprotective function of vitamin E and carotenoids in Chlamydomonas. Plant Physiol. 158: 313–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa J. (2011). State transitions—The molecular remodeling of photosynthetic supercomplexes that controls energy flow in the chloroplast. Biochim. Biophys. Acta 1807: 897–905 [DOI] [PubMed] [Google Scholar]
- Naumann B., Busch A., Allmer J., Ostendorf E., Zeller M., Kirchhoff H., Hippler M. (2007). Comparative quantitative proteomics to investigate the remodeling of bioenergetics pathways under iron deficiency in Chlamydomonas reinhardtii. Proteomics 21: 3964–3979 [DOI] [PubMed] [Google Scholar]
- Niyogi K.K. (1999). Photoprotection revisited: Genetic and molecular approaches. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50: 333–359 [DOI] [PubMed] [Google Scholar]
- Nymark M., Valle K.C., Brembu T., Hancke K., Winge P., Andresen K., Johnsen G., Bones A.M. (2009). An integrated analysis of molecular acclimation to high light in the marine diatom Phaeodactylum tricornutum. PLoS ONE 4: e7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peers G., Truong T.B., Ostendorf E., Busch A., Elrad D., Grossman A.R., Hippler M., Niyogi K.K. (2009). An ancient light-harvesting protein is critical for the regulation of algal photosynthesis. Nature 462: 518–521 [DOI] [PubMed] [Google Scholar]
- Petroutsos D., Busch A., Janssen I., Trompelt K., Bergner S.V., Weinl S., Holtkamp M., Karst U., Kudla J., Hippler M. (2011). The chloroplast calcium sensor CAS is required for photoacclimation in Chlamydomonas reinhardtii. Plant Cell 23: 2950–2963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribil M., Pesaresi P., Hertle A., Barbato R., Leister D. (2010). Role of plastid protein phosphatase TAP38 in LHCII dephosphorylation and thylakoid electron flow. PLoS Biol. 8: e1000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pospíšil P. (2012). Molecular mechanisms of production and scavenging of reactive oxygen species by photosystem II. Biochim. Biophys. Acta 1817: 218–231 [DOI] [PubMed] [Google Scholar]
- Rintamäki E., Martinsuo P., Pursiheimo S., Aro E.M. (2000). Cooperative regulation of light-harvesting complex II phosphorylation via the plastoquinol and ferredoxin-thioredoxin system in chloroplasts. Proc. Natl. Acad. Sci. USA 97: 11644–11649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach T., Krieger-Liszkay A. (2012). The role of the PsbS protein in the protection of photosystems I and II against high light in Arabidopsis thaliana. Biochim. Biophys. Acta 1817: 2158–2165 [DOI] [PubMed] [Google Scholar]
- Rochaix J.D. (2007). Role of thylakoid protein kinases in photosynthetic acclimation. FEBS Lett. 581: 2768–2775 [DOI] [PubMed] [Google Scholar]
- Savard F., Richard C., Guertin M. (1996). The Chlamydomonas reinhardtii LI818 gene represents a distant relative of the cabI/II genes that is regulated during the cell cycle and in response to illumination. Plant Mol. Biol. 32: 461–473 [DOI] [PubMed] [Google Scholar]
- Shapiguzov A., Ingelsson B., Samol I., Andres C., Kessler F., Rochaix J.D., Vener A.V., Goldschmidt-Clermont M. (2010). The PPH1 phosphatase is specifically involved in LHCII dephosphorylation and state transitions in Arabidopsis. Proc. Natl. Acad. Sci. USA 107: 4782–4787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueoka N. (1960). Mitotic replication of deoxyribunucleic acid in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 46: 83–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Iwai M., Takahashi Y., Minagawa J. (2006). Identification of the mobile light-harvesting complex II polypeptides for state transitions in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 103: 477–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting C.S., Owens T.G. (1993). Photochemical and nonphotochemical fluorescence quenching processes in the diatom Phaeodactylum tricornutum. Plant Physiol. 101: 1323–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokutsu R., Iwai M., Minagawa J. (2009). CP29, a monomeric light-harvesting complex II protein, is essential for state transitions in Chlamydomonas reinhardtii. J. Biol. Chem. 284: 7777–7782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokutsu R., Kato N., Bui K.-H., Ishikawa T., Minagawa J. (2012). Revisiting the supramolecular organization of photosystem II in Chlamydomonas reinhardtii. J. Biol. Chem. 287: 31574–31581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolleter D., et al. (2011). Control of hydrogen photoproduction by the proton gradient generated by cyclic electron flow in Chlamydomonas reinhardtii. Plant Cell 23: 2619–2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallon O., Bulte L., Dainese P., Olive J., Bassi R., Wollman F.A. (1991). Lateral redistribution of cytochrome b6/f complexes along thylakoid membranes upon state transitions. Proc. Natl. Acad. Sci. USA 88: 8262–8266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink M., Zer H., Alumot N., Gaathon A., Niyogi K.K., Herrmann R.G., Andersson B., Ohad I. (2004). Light-modulated exposure of the light-harvesting complex II (LHCII) to protein kinase(s) and state transition in Chlamydomonas reinhardtii xanthophyll mutants. Biochemistry 43: 7824–7833 [DOI] [PubMed] [Google Scholar]
- Witt H.T. (1979). Energy conversion in the functional membrane of photosynthesis. Analysis by light pulse and electric pulse methods. The central role of the electric field. Biochim. Biophys. Acta 505: 355–427 [DOI] [PubMed] [Google Scholar]
- Wollman F.A. (2001). State transitions reveal the dynamics and flexibility of the photosynthetic apparatus. EMBO J. 20: 3623–3630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H.Y., Nakayama T.O., Chichester C.O. (1962). Studies on the light and dark interconversions of leaf xanthophylls. Arch. Biochem. Biophys. 97: 168–173 [DOI] [PubMed] [Google Scholar]
- Zhang Z.D., Shrager J., Jain M., Chang C.W., Vallon O., Grossman A.R. (2004). Insights into the survival of Chlamydomonas reinhardtii during sulfur starvation based on microarray analysis of gene expression. Eukaryot. Cell 3: 1331–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S.H., Green B.R. (2010). Photoprotection in the diatom Thalassiosira pseudonana: Role of LI818-like proteins in response to high light stress. Biochim. Biophys. Acta 1797: 1449–1457 [DOI] [PubMed] [Google Scholar]