The KUP6 subfamily transporters regulated directly via an abscisic acid signaling complex act as key factors in osmotic adjustment by balancing potassium homeostasis in both cell growth and drought stress responses.
Abstract
Osmotic adjustment plays a fundamental role in water stress responses and growth in plants; however, the molecular mechanisms governing this process are not fully understood. Here, we demonstrated that the KUP potassium transporter family plays important roles in this process, under the control of abscisic acid (ABA) and auxin. We generated Arabidopsis thaliana multiple mutants for K+ uptake transporter 6 (KUP6), KUP8, KUP2/SHORT HYPOCOTYL3, and an ABA-responsive potassium efflux channel, guard cell outward rectifying K+ channel (GORK). The triple mutants, kup268 and kup68 gork, exhibited enhanced cell expansion, suggesting that these KUPs negatively regulate turgor-dependent growth. Potassium uptake experiments using 86radioactive rubidium ion (86Rb+) in the mutants indicated that these KUPs might be involved in potassium efflux in Arabidopsis roots. The mutants showed increased auxin responses and decreased sensitivity to an auxin inhibitor (1-N-naphthylphthalamic acid) and ABA in lateral root growth. During water deficit stress, kup68 gork impaired ABA-mediated stomatal closing, and kup268 and kup68 gork decreased survival of drought stress. The protein kinase SNF1-related protein kinases 2E (SRK2E), a key component of ABA signaling, interacted with and phosphorylated KUP6, suggesting that KUP functions are regulated directly via an ABA signaling complex. We propose that the KUP6 subfamily transporters act as key factors in osmotic adjustment by balancing potassium homeostasis in cell growth and drought stress responses.
INTRODUCTION
Plants have evolved systems to control growth and development under various environmental stresses. The control of cell expansion plays an essential role in water stress responses and plant growth (Maggio et al., 2006; Zonia and Munnik, 2007). Cell growth caused by cell expansion is regulated primarily by turgor pressure, which is the physical force against the cell wall, and is maintained by osmotic regulation via osmotically active substances, such as potassium ions (K+), sugars, and amino acids. K+ is an essential element in plant growth, and K+ uptake and efflux affect plant productivity and control cell water potential and turgor in osmotic regulation. K+ affects osmotic pressure in the root xylem (root pressure), which drives long-distance sap flow from roots to shoots (Lebaudy et al., 2007). During water deficit stress, osmotic stress sensing and signaling are pivotal to plant water status and lead to rapid changes in gene expression (Yamaguchi-Shinozaki and Shinozaki, 2006; Osakabe et al., 2011) and turgor-dependent stomatal closing, which responds to hydraulic properties in the xylem (Maggio et al., 2006; Hedrich, 2012; Roelfsema et al., 2012).
Plant hormones coordinate adaptive changes in cellular osmotic regulation. Abscisic acid (ABA) regulates various molecular events in response to water deficit stress and plant growth. Under water deficit stress, ABA induces the activation of anion channels, such as SLAC1, which causes depolarization of the plasma membrane of guard cells (Levchenko et al., 2005; Negi et al., 2008; Vahisalu et al., 2008; Geiger et al., 2009b). There are two types of anion channels, S-type and R-type, both of which are involved in controlling guard cell movements (Hedrich, 2012). The depolarization of the plasma membrane decreases the activity of inward K+ channels, such as KAT1/KAT2, and activates outward K+ channels, such as the guard cell outward rectifying K+ channel, GORK, resulting in K+ efflux from guard cells. Anion and K+ efflux from guard cells leads to loss of guard cell turgor and causes stomatal closing (Schroeder and Hagiwara, 1989; Pei et al., 1997; Ache et al., 2000; Hosy et al., 2003; Kim et al., 2010). SLAC1 is directly activated by Snf1-related protein kinase 2 (SRK2E/OST1/SnRK2.6), which is involved in the ABA signaling complex of the ABA receptor PYR family, and PP2Cs (Geiger et al., 2009b; Lee et al., 2009b) or by the calcium-dependent protein kinases, CPK21 and CPK23 (Geiger et al., 2010), and CPK3 and CPK6 (Brandt et al., 2012; Scherzer et al., 2012). SRK2E also inhibits KAT1 activity by phosphorylation (Sato et al., 2009). These studies have suggested that the mechanism of stomatal movement involves additional transporters, which have functionally redundant roles in this pathway (Hosy et al., 2003).
K+ uptake and efflux are controlled by various types of channels and transporters (Véry and Sentenac, 2003). The Arabidopsis thaliana genome contains multigene families of K+ channels and transporters that have distinct or redundant functions (Mäser et al., 2001), presumably due to high- and low-affinity K+ transport activity, tissue/cellular-specific gene expression, and protein subcellular localization (Véry and Sentenac, 2003; Lebaudy et al., 2007). Classical studies proposed that the two distinct types of K+ transport systems, high- and low-affinity, control K+ uptake in plant roots (Epstein, 1966). The KUP/HAK/KT family transporters were identified as candidate high-affinity K+ transporters (Fu and Luan, 1998; Kim et al., 1998; Gierth and Mäser, 2007; Grabov, 2007). The Shaker family K+ channels include AKT1, which forms a functional K+ channel by heteromerization with a Shaker-type subunit, AKTC1, and mediates K+ uptake in roots (Hirsch et al., 1998; Li et al., 2006; Xu et al., 2006; Geiger et al., 2009a); KAT1, which is involved in K+ uptake during stomatal opening in leaves (Anderson et al., 1992); and GORK, which functions in K+ efflux in stomatal closing (Hosy et al., 2003). Recent studies have suggested that the KUP/HAK/KT family transporters are potentially involved in K+ homeostasis and osmotic regulation in plants. Knocking out KUP4/TINY ROOT HAIR1 (TRH1) in Arabidopsis impaired root hair elongation (Rigas et al., 2001), suggesting that it functions in tissue/cellular-specific cell expansion. KUP4/TRH1 is also involved in root-specific auxin distribution via unknown mechanisms (Vicente-Agullo et al., 2004). The semidominant mutant of KUP2/SHORT HYPOCOTYL3 (SHY3) causes a short hypocotyl phenotype (Reed et al., 1998), which results from a reduction of cell size, suggesting a major function for KUPs in turgor-dependent cell growth (Elumalai et al., 2002). However, osmotic regulation via these factors and the molecular mechanisms that affect plant growth and water use efficiency have not been fully elucidated.
Here, we examined the stress-responsive K+ transport systems and found that KUP6 and its homologs, KUP8 and KUP2/SHY3, together with the K+ efflux channel GORK, have critical roles in osmotic regulation for growth and water stress responses. We generated triple mutants, kup2 kup6 kup8 and kup6 kup8 gork, which showed enhanced cell expansion and auxin responses in lateral root formation. During water deficit stress, the triple mutant plants had impaired ABA-mediated stomatal closing and significantly decreased tolerance to drought stress. Furthermore, KUP6 interacted with the ABA-activated SnRK2-type protein kinase SRK2E, resulting in phosphorylation of the KUP6 C-terminal domain. On the basis of these results, we conclude that the KUP6 subfamily transporters act as key factors in K+ homeostasis in both cell growth and drought stress responses mediated by ABA signaling.
RESULTS
The Stress-Responsive Potassium Transporter KUP6
To explore new candidate transport systems involved in osmotic regulation in plant cells, we focused on stress-responsive membrane factors that were upregulated under water deficit stress and/or ABA treatment in the transcriptome (Maruyama et al., 2009). From these, we selected a KUP/HAK/KT family K+ transporter gene, KUP6, which was responsive to water deficit stress (Maruyama et al., 2009). This gene was recently found to be upregulated in Arabidopsis suspension cells by ABA (Böhmer and Schroeder, 2011). We then performed quantitative RT-PCR (qRT-PCR) to identify the induction profile of KUP6 under various stress and ABA conditions (Figure 1A). KUP6 was highly induced by osmotic stress and ABA treatment. Next, we characterized the induction patterns of KUP6 and its homologs, KUP2/SHY3 and KUP8 (see Supplemental Figure 1 and Supplemental Data Set 1 online), in roots or shoots of Arabidopsis (see Supplemental Figure 2 online). We also characterized the expression patterns of an ABA-responsive K+ channel gene, GORK (Becker et al., 2003). KUP6 and GORK were highly induced by drought stress in shoots (see Supplemental Figure 2 online). The stress/ABA-responsive expression patterns of these two genes were corroborated by the public microarray data in Genevestigator (https://www.genevestigator.ethz.ch/) (see Supplemental Figure 3 online).
Figure 1.
KUP2/SHY3, KUP6, and KUP8 Negatively Control Plant Growth.
(A) Expression levels of KUP6 under abiotic stress and ABA treatments. The value for wild-type Arabidopsis ecotype Columbia (Co) at 0 h was set to 1.0, and all data were normalized accordingly. To normalize the expression levels, 18S rRNA was amplified as an internal control. Values are means and sd (n = 3).
(B) GUS activity in transgenic Arabidopsis plants expressing KUP6pro:GUS. Bars = 50 µm.
(C) and (D) GFP fluorescence in mesophyll protoplasts (C) and root cells in transgenic plants (D) expressing 35S:KUP6-GFP. Bars = 5 µm in (C) and 10 µm in (D).
(E) Ten-day-old kup68, kup268, and kup68g plants grown in soil pots. Bars = 5 mm.
(F) Rosette leaves of 14-d-old mutant plants grown in soil pots. Bars = 5 mm.
(G) Leaf mesophyll cells of 7-d-old mutant plants grown on GM agar plates. Leaves were treated with chloral hydrate and then viewed with a microscope. Bars = 50 µm.
(H) Fresh weight of the shoots of 14-d-old mutant plants grown on GM agar plates. Values are means and sd (n = 25).
(I) Cell areas of 7-d-old kup mutants grown on GM agar plates. Relative cell areas were calculated from the microscope image data using ImageJ (National Institute of Health; http://rsb.info.nih.gov/ij). Values are means and sd (n = 25). Asterisks indicate statistically significant differences between the wild-type and mutant plants, determined by Student’s t tests. *P value < 0.0001.
To characterize the tissue/cellular-specific expression patterns of KUP6, we generated transgenic Arabidopsis plants expressing a KUP6 promoter:GUS (for β-glucuronidase) construct. GUS activity was detected in the root tips, guard cells, and vascular tissues, especially in the pericycle (Figure 1B). The public microarray data in Genevestigator showed higher expression levels of KUP6, KUP8, and GORK in guard cell protoplasts and the pericycle (see Supplemental Figure 4 online). The transient expression of a KUP6-GFP (for green fluorescent protein) fusion protein in Arabidopsis protoplasts and transgenic plants expressing a 35S cauliflower mosaic virus (CaMV) promoter:KUP6-GFP construct indicated that KUP6-GFP is localized in the plasma membrane (Figures 1C and 1D).
kup2 kup6 kup8 and kup6 kup8 gork Triple Mutants Show Enhanced Cell Expansion
To identify the physiological functions of the K+ transport system in plants mediated by KUPs and GORK, we isolated T-DNA insertion mutants of kup6-1, kup8-1, gork-2, and kup2-8 (see Supplemental Figures 5A and 5B online). The single mutant plants kup2-8, kup6-1, kup8-1, and gork-2 (see Supplemental Figure 5C online) did not show severe phenotypes, as in other reported kup2 null mutants, whereas shy3-1/kup2-1 showed smaller cell and plant size due to a dominant gain-of-function mutation (Elumalai et al., 2002). We first focused on the stress-inducible KUP6 and its homolog KUP8 and generated the kup68 double mutant. Observing that kup68 plants formed larger bodies than wild-type plants (Figure 1E), we then generated triple mutants, kup268 and kup68g (kup68 gork) to identify the redundant functions of these transport systems in plant growth regulation. The kup268 and kup68g plants exhibited more apparent phenotypes; they had large plant bodies (Figures 1E, 1F, and 1H) that resulted from increased cell expansion (Figures 1G and 1I). They did not show any significant changes in developmental processes, morphology, anatomy, or cell numbers (data not shown). The data suggest that KUP2/SHY3, KUP6, KUP8, and GORK appear to be involved in the negative regulation of turgor pressure–dependent growth and that the triple mutations of KUP2, KUP6, and KUP8 had a greater effect on cell expansion than those of KUP6, KUP8, and GORK.
KUP2, KUP6, and KUP8 Negatively Affected High-Affinity K+ Transport
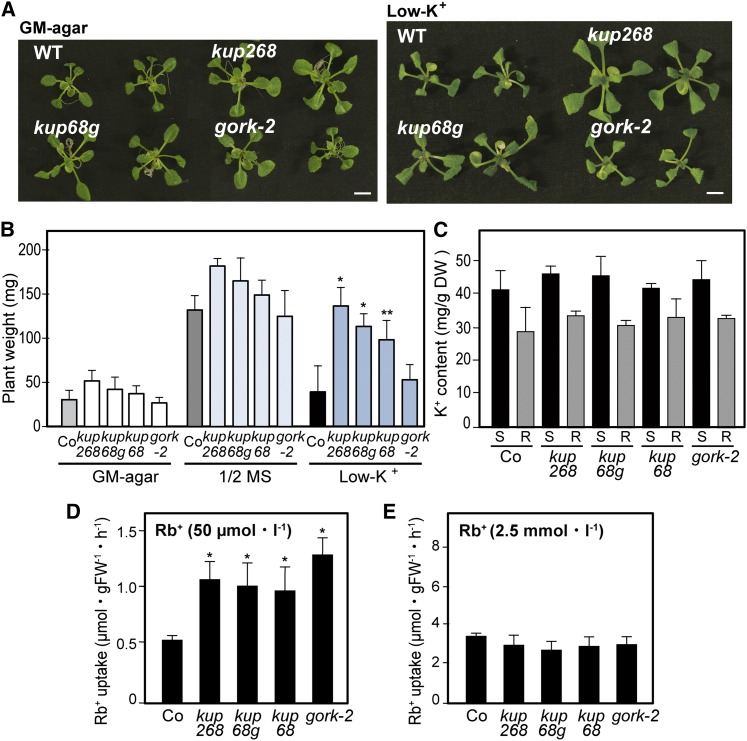
Previous studies indicated that several transport systems, such as the K+ channel AKT1 and the KUP/HAK/KT K+ transporter HAK5, are involved in the major high-affinity K+ uptake into roots (Hirsch et al., 1998; Xu et al., 2006; Pyo et al., 2010). The K+ channels mediate low-affinity transport during membrane depolarization and operate as high-affinity K+ transport systems upon hyperpolarization (Geiger et al., 2009a). The single mutant and double mutant plants of AKT1 and HAK5 failed to grow in low-K+ conditions (Xu et al., 2006; Pyo et al., 2010). To characterize the contributions of the KUP6, KUP8, and KUP2/SHY3 transporters in K+ homeostasis, we examined the K+-dependent growth of the mutants. Young kup68, kup268, kup68g, and gork-2 seedlings grown on germination medium (GM) agar for 10 d were transferred to K+ deficient or half-strength Murashige and Skoog (MS) liquid media and cultured for 5 d. Under the low-K+ conditions, the growth of the wild type was inhibited, whereas compared with the plants cultured on half-strength MS, kup68, kup268, and kup68g plants were not greatly affected (Figures 2A and 2B). The gork-2 plants also showed a similar tendency of low-K+ tolerance (Figures 2A and 2B). We also examined the phenotypes of the mutant plants in high-K+ conditions (100 to 150 mM KCl-containing media), but they did not show any differences compared with the wild type (data not shown). We then measured K+ content in the shoots and roots of the mutants and found no significant differences compared with the wild type (Figure 2C).
Figure 2.
KUP2, KUP6, and KUP8 Negatively Affect High-Affinity K+ Uptake.
(A) Young kup68, kup268, kup68g, and gork-2 seedlings grown on GM plates for 10 d (left) were transferred to K+-deficient liquid media for 5 d (right). Bar = 0.5 cm. WT, the wild type.
(B) Whole-plant weight of mutants grown on GM agar plates for 10 d and then transferred to half-strength MS or K+-deficient liquid media for 5 d. Values are means and sd (n = 9).
(C) K+ contents of Arabidopsis mutants kup68, kup268, kup68g, gork-2, and wild-type (Co). The potassium contents were measured with an inductively coupled plasma optical emission spectrometer (S, shoot; R, root). Values are means and sd (n = 30). DW, dry weight.
(D) and (E) K+ uptake in the roots of the mutants incubated in K+-deficient media using 86Rb+ as a tracer of K+. 86Rb+ uptake at 50 µM external Rb+ (D); 86Rb+ uptake at 2.5 mM external Rb+ (E). Values are means and sd (n = 9). FW, fresh weight.
Asterisks indicate statistically significant differences between the wild-type and mutant plants, determined by Student’s t tests. *P < 0.001 and **P < 0.05.
[See online article for color version of this figure.]
Since the K+ efflux channel GORK is expressed in the root epidermis (Becker et al., 2003; Demidchik et al., 2010), the growth of the mutants in low K+ might result from altered K+ transport. We investigated root K+ uptake in the mutants and wild type incubated in K+-deficient media, using 86Rb+ as a tracer for K+ (Figures 2D and 2E). The high-affinity 86Rb+ uptake in kup68, kup268, kup68g, and gork-2 plants was significantly higher than in the wild type (Figures 2D), whereas the low-affinity uptake was unaffected in these mutants (Figure 2E). These short-term 86Rb+ uptake experiments suggested that the KUP transporters might be involved in the K+ efflux system in roots, similarly to GORK.
kup268 and kup68 gork Enhanced Auxin Responses in Lateral Root Formation
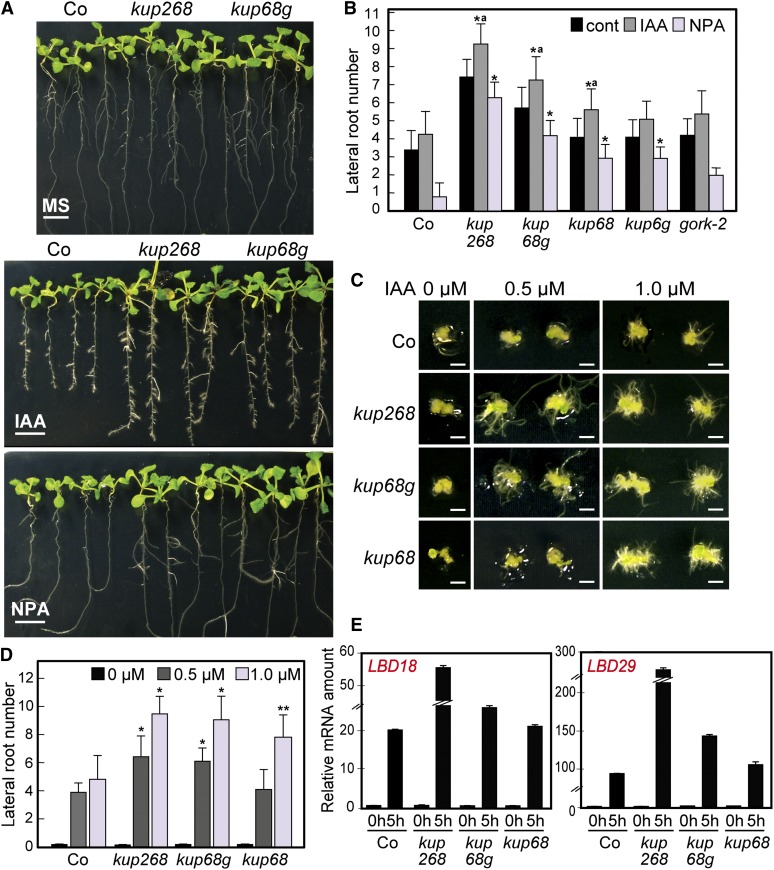
Our results suggest that KUP2/SHY3, KUP6, and KUP8 are functionally involved in the K+ efflux transport system and that disruption of their functions might cause increased cell sizes. Auxin is essential for plant growth and development, affecting cell expansion and division via the activation of ion fluxes, including K+, by factors such as the auxin binding protein ABP1 (Philippar et al., 2004; Braun et al., 2008; Tromas et al., 2010). We characterized the auxin sensitivities of the kup mutants in lateral root (LR) formation. kup268 and kup68g significantly enhanced LR formation in response to exogenous auxin (indole-3-acetic acid [IAA]) and decreased sensitivity to 1-N-naphthylphthalamic acid (NPA), an inhibitor of auxin transport (Figures 3A and 3B). The single mutants kup6-1 and kup8-1 exhibited similar but weaker phenotypes (see Supplemental Figures 5D and 5E online).
Figure 3.
kup268 and kup68 gork Plants Showed Enhanced Auxin Responses and Decreased Sensitivity to an Auxin Transport Inhibitor in LR Formation.
(A) LR formation of kup68, kup268, kup68g, and gork-2 plants in the presence of 1.0 µM IAA and 10 µM NPA. The plants were sown on GM plates for 5 d and after germination were transferred to IAA- or NPA-containing media and cultured for 4 d (Co, wild-type Arabidopsis ecotype Co). Bars = 0.5 cm.
(B) The mutant plants grown in the presence of 1.0 µM IAA and 10 µM NPA. Values are means and sd (n = 25). Asterisks indicate statistically significant differences between the wild-type and mutant plants, determined by Student’s t tests. *P < 0.0001. “a” indicates a statistically significant difference between the control and auxin treatment in each mutant, determined by Student’s t tests (P < 0.05). There are no significant differences (P > 0.05) between the control and NPA treatment in each kup mutant.
(C) Root regeneration assay to estimate the auxin-mediated root regeneration in callus of the mutant plants in the presence of IAA. Bars = 0.2 cm.
(D) Root numbers in the root regeneration assay of kup68, kup268, and kup68g callus in IAA-containing media. Values are means and sd (n = 25). Asterisks indicate statistically significant differences between the wild-type and mutant plants, determined by Student’s t tests. *P < 0.001 and **P < 0.01.
(E) The expression levels of auxin-responsive transcription factors LBD18 and LBD29 in mutant plants determined by qRT-PCR. The 10-d-old plants were treated with 1.0 µM IAA for 5 h before total RNA was isolated. The value for wild-type (Co) at 0 h was set to 1.0, and all data were normalized accordingly. No significant differences were detected between genotypes at 0 h. To normalize the expression levels, 18S rRNA was amplified as an internal control. Values are means and sd (n = 3).
Next, we questioned whether the auxin hypersensitive phenotypes of the mutants are affected in earlier processes in cell division and differentiation. Sugimoto et al. (2010) recently reported that ectopic activation of LR development is a common callus formation mechanism in tissue culture systems. We performed callus formation and subsequent root regeneration assays to estimate the auxin-mediated root regeneration in the mutants. In the presence of IAA, the root regeneration rates of kup268 and kup68g greatly increased, and kup68 also showed similar auxin hypersensitivity (Figures 3C and 3D).
To understand the molecular mechanisms of auxin hypersensitivity in the mutants, we then characterized the expression levels of auxin-responsive transcription factors LBD18 and LBD29, which regulate LR formation (Okushima et al., 2007). The expression levels of these two genes were enhanced in the triple mutants in the presence of IAA (Figure 3E).
kup268 and kup68 gork Decreased ABA-Mediated Inhibition of LR Formation
ABA and osmotic stresses cause reduction of LR formation (De Smet et al., 2006; Shkolnik-Inbar and Bar-Zvi, 2010; Galvan-Ampudia and Testerink, 2011). The upregulation of KUP6 and GORK genes by stress and ABA allowed us to predict their main roles under stress conditions. Thus, we characterized the inhibition of LR formation by ABA in the mutants. The kup multiple mutants exhibited higher numbers of LRs in the ABA and osmotic stress treatments (Figures 4A to 4C). kup6-1 and kup8-1 exhibited similar trends (see Supplemental Figure 5E online). Together with auxin hypersensitivity, the ABA/osmotic stress insensitivity of the mutants in LR formation suggested that KUP6, KUP8, KUP2/SHY3, and GORK have negative roles in LR formation under the control of ABA and auxin.
Figure 4.
KUP2, KUP6, and KUP8 Positively Affect ABA and Osmotic Stress Responses in LR Formation.
LR formation of kup68, kup268, kup68g, and gork-2 plants in the presence of ABA ([A] and [B]), 100 mM NaCl (C), and 200 mM mannitol (C). The plants were sown on GM plates and then transferred to ABA-, NaCl-, or mannitol-containing media and cultured for 4 d. Values are means and sd (n = 25). Asterisks indicate statistically significant differences between wild-type and mutant plants, determined by Student’s t tests. *P < 0.0001 and **P < 0.005. There are no significant differences (P > 0.05) between the control and ABA, NaCl, or mannitol treatment in each mutant. Bar = 0.5 cm.
[See online article for color version of this figure.]
kup268 and kup68 gork Decreased ABA-Mediated Stomatal Closing
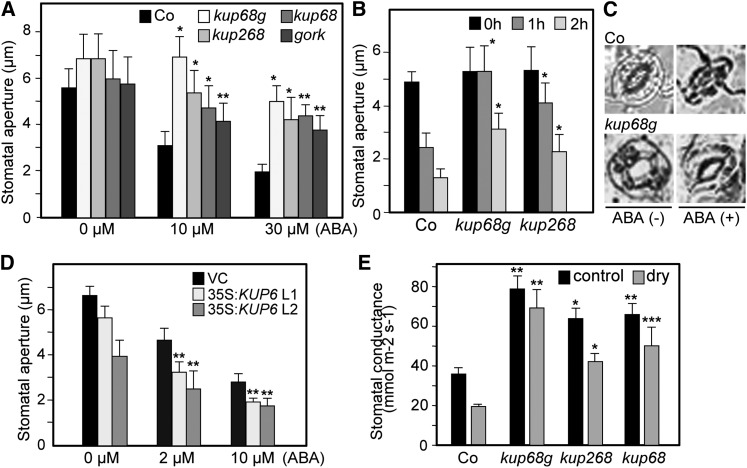
In water stress conditions, ABA controls turgor pressure via K+ transport in guard cells to enhance stomatal closure. ABA induces the activation of outward K+ channels (GORK), resulting in K+ efflux from guard cells, which leads to loss of guard cell turgor and stomatal closing (Hosy et al., 2003; Kim et al., 2010). However, the involvement of additional transporters has been presumed in this process (Hosy et al., 2003). We examined the roles of the KUP6 subfamily in ABA-mediated stomatal closing and found that kup68g strongly impaired ABA sensitivity in stomatal closing (Figures 5A to 5C). To investigate KUP6 gain of function in stress responses, we then generated transgenic plants overexpressing KUP6 (35S:KUP6) (see Supplemental Figure 6 online). The 35S:KUP6 plants weighed slightly less than the controls, but this was not statistically significant (see Supplemental Figure 6 online). Their stomatal cells had a tendency to close more rapidly than those of the controls (Figure 5D). We also measured the stomatal conductance of the mutants during dehydration stress. kup68g showed higher stomatal conductance in both normal and stress conditions (Figure 5E). These data suggested that the KUPs function in ABA-induced stomatal closing.
Figure 5.
Responses of kup268, kup68 gork, and KUP6-Overexpressing Plants to ABA-Mediated Stomatal Closure.
(A) Effects of various concentrations of ABA on stomatal closure in the kup68, kup268, kup68g, and gork-2 mutants. Epidermal peels were treated with or without ABA for 1 h after stomatal preopening under light for 2 h, and the stomatal aperture was measured by microscope. Values are means and sd (n = 50).
(B) Effects of ABA on stomatal closure in the kup mutants at various treatment times. Values are means and sd (n = 50).
(C) Guard cells of kup68g and control wild-type plants treated with or without 10 µM ABA for 1 h. Bars = 30 µm.
(D) Effects of various concentrations of ABA on stomatal closure in KUP6-OXs.
(E) The stomatal conductance of kup mutants in both normal and water-deficit stress conditions (SWC = 20 to 30%). Values are means and sd (n = 20).
Asterisks indicate statistically significant differences between the wild-type and mutant plants, determined by Student’s t tests. *P < 0.0001, **P < 0.001, and ***P < 0.01.
kup268 and kup68 gork Decreased Tolerance to Water Deficit Stress
ABA-mediated stomatal closing reduces leaf water loss during drought stress. To investigate the role of KUPs in this process, we measured the fresh weight loss in detached leaves from kup268, kup68g, and 35S:KUP6 plants (Figures 6A and 6B). The kup68g plants exhibited significantly increased water loss rates compared with the wild type and other mutant plants, whereas KUP6 overexpression resulted in decreased water loss in the transgenic plants (Figures 6A and 6B; see Supplemental Figure 6 online). Figures 6C to 6E show the survival rates of the kup268 and kup68g mutant plants grown in soil pots that were exposed to water deficit stress by not watering them for 2 weeks (30 to 40% RH, soil water content [SWC] = 10 to 15%), after which they were watered and cultured for a week. Both kup68g and kup268 plants had significantly lower survival rates under water-deficit conditions compared with the control plants, the single mutant gork-2, and a K+ uptake channel mutant akt1, the three of which exhibited similar responses (Figure 6E). In these tests, we sowed the seeds at different times to optimize the plant sizes (Figure 6F). Similar plant weights were observed among the kup268, kup68g, and wild-type plants, but their growth stages were different (e.g., the flowering of wild-type plants was earlier than kup268 and kup68g) (Figures 6C and 6F) as a result of sowing the wild-type seeds earlier than the mutants. The kup68g and kup268 plants also showed significantly lower survival rates than the wild type when they were sowed on the same day and differed in size (see Supplemental Figure 7C online). The decreased survival rates of kup68g and kup268 plants during water stress were related to the lower relative water content (RWC) of the mutant leaves compared with the wild type (SWC = 20 to 30%) (Figure 6G). The water use efficiencies (WUEs), the ratio between biomass produced and total water used, of kup68g and kup268 during water deficit stress did not change under the stress conditions, whereas those of the wild-type control increased during stress (Figure 6H). By contrast, the 35S:KUP6 transgenic plants showed improved tolerance to drought (Figures 6I and 6J).
Figure 6.
KUP2/SHY3, KUP6, and KUP8 Coordinately Maintain the Water Status during Water Deficit Stress.
(A) and (B) Transpirational water loss in kup and gork mutants (A) and 35S:KUP6 (B) at the indicated time points after leaf detachment. Water loss is expressed as a percentage of the initial fresh weight. Values are means and sd of five samples of three leaves each. VC (vector control plants), the control transgenic plants carrying the 35S vector.
(C) to (F) The survival rates of kup268 and kup68g mutant plants grown in soil pots and exposed to water deficit stress by not watering them for 2 weeks. Survival rates were determined as the number of visible green plants after rehydration ([D] and [E]). The mutant plants exposed to water deficit stress were grown in soil pots, in which the seeds were sowed at different times to optimize plant weight (F). Arrows indicate developing flower buds in the wild-type plants (C). Values are means and sd (n = 25) from four independent experiments.
(G) and (H) The leaf RWC (G) and the WUE (H) were determined in plants exposed to water deficit stress by withholding water (SWC = 20 to 30%). Values are means and sd (n = 25).
(I) and (J) Drought tolerance of the 35S:KUP6 plants. Survival rates were determined described as in (D), but after 3 weeks of water stress. Values are means and sd (n = 25). VC, vector control plants. Asterisks indicate statistically significant differences between the wild-type and mutant plants, determined by Student’s t tests. *P < 0.0005 and **P < 0.05.
SRK2E/OST1/SnRK2.6 Physically Interacts with and Phosphorylates KUP6
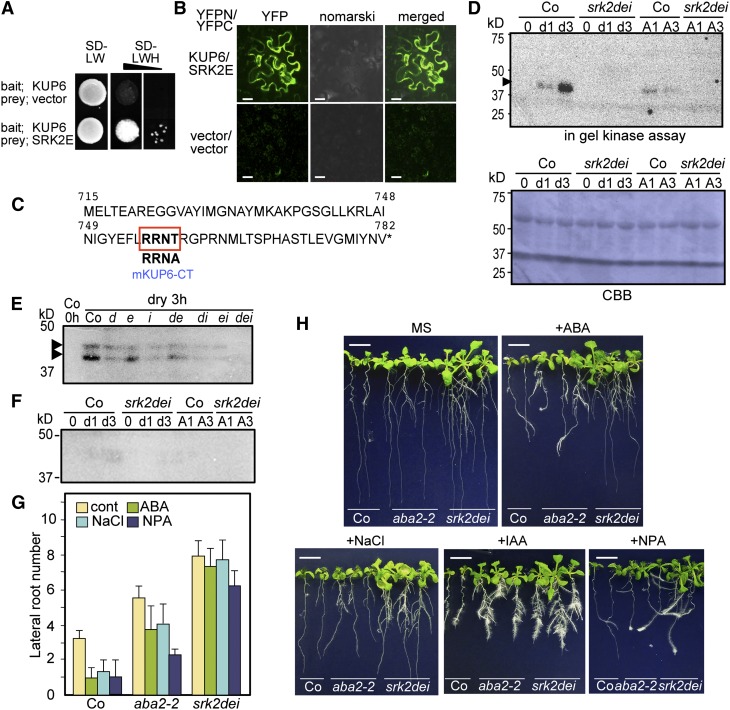
ABA activates downstream signaling by PYR/RCAR-mediated inactivation of PP2Cs and the activation of SnRK2s. SRK2E/OST1/SnRK2.6 modifies membrane transport systems in guard cells by phosphorylating SLAC1 and NADPH oxidase and inactivating a K+ inward channel, KAT1 (Sirichandra et al., 2009; Hubbard et al., 2012). We investigated whether SRK2E targets KUP6 to control K+ transport during drought stress. We tested for protein interaction between SRK2E and full-length KUP6 using a split-ubiquitin membrane yeast two-hybrid system and bimolecular fluorescence complementation (BiFC) in Nicotiana benthamiana (Figures 7A and 7B) and found that SRK2E interacted with the full-length KUP6 in both yeast and plant cells.
Figure 7.
KUP6 Is Phosphorylated by SnRK2 and Regulated by ABA Signaling.
(A) and (B) Interactions between KUP6 and the ABA-activated SNF1-related protein kinase 2E (SRK2E/OST1/SnRK2.6) in a split-ubiquitin yeast two-hybrid system (A) and BIFC in N. benthamiana (B). SD, synthetic defined medium; L, leucine; W, tryptophan; H, histidine.
(C) Amino acid sequences of the KUP6 C-terminal region (KUP6-CT) and the amino acid–substituted mKUP6-CT used for the in-gel kinase assay. The putative target motif for SRK2E, R-X-X-S/T, is indicated.
(D) In-gel kinase assay of recombinant GST-tagged KUP6-CT with proteins extracted from the wild type and srk2dei triple mutant at 0, 1, and 3 h after treatment with drought stress and 50 µM ABA. CBB, Coomassie blue.
(E) In-gel kinase assay of KUP6-CT with proteins extracted from the wild type and srk2 single or multiple mutants after treatment with drought stress. d, srk2d; e, srk2e; i, srk2i; de, srk2de; di, srk2di; ei, srk2ei; dei, srk2dei.
(F) In-gel kinase assay of recombinant GST-tagged mKUP6-CT with the same protein extracts as in (D).
(G) and (H) LR formation of aba2-2 and srk2dei plants was tested in the presence of 30 µM ABA, 120 mM NaCl, 10 µM NPA, and 1.0 µM IAA. Values are means and sd (n = 12). Bars = 0.5 cm.
When the C-terminal regions of KUP6 and KUP8 were used as bait in the yeast two-hybrid system, KUP proteins interacted strongly with SRK2E and weakly with SRK2D (see Supplemental Figure 8 online). We then focused on the extended C-terminal cytosolic region of KUP6, in which one putative SnRK2 target phosphorylation motif, R-X-X-S/T, was found (Figure 7C). To determine whether the KUP6 C-terminal region (KUP6-CT) is phosphorylated by SRK2E, we performed an in-gel kinase assay with recombinant KUP6-CT or a mutated KUP6-CT fragment fused with glutathione S-transferase (GST) as substrates (Figure 7C) using protein extracted from the wild type and the srk2 dei triple mutant, which disrupts three SnRK2s and strongly impairs ABA responses (Nakashima et al., 2009; Umezawa et al., 2009). One or two bands around 40 to 42 kD were detected in the drought- or ABA-treated wild-type extracts as phosphorylated products, and these bands were from SnRK2 activity because they were not present in the srk2dei sample (Figures 7D and 7E). Furthermore, the phosphorylation of the target motif was decreased by the amino acid substitution (T759A) in mKUP6-CT (Figure 7F). These results indicated that KUP6-CT was phosphorylated by the SnRK2 kinase in ABA-mediated signaling during water deficit stress.
To examine whether the activity of KUPs in vivo is functionally correlated with ABA signaling, we tested the LR formation of srk2 mutants in the presence of ABA, NaCl, and NPA. The srk2 dei plants showed similar sensitivities to ABA and auxin as the kup mutants (Figures 7G and 7H; see Supplemental Figure 9 online). aba2-2 plants tended to exhibit decreased ABA, NPA, and NaCl sensitivity in LR formation, suggesting that endogenous ABA levels are required for these responses (Figure 7G). To test whether auxin directly affected the phosphorylation of KUP6 by SRK2s, we performed an in-gel kinase assay with histone or KUP6-CT as substrates using protein extracted from plants treated with ABA, drought stress, and IAA. The pretreatment or coexistence with IAA did not affect the phosphorylation strength (see Supplemental Figure 9 online), suggesting that auxin does not directly affect the ABA- and drought stress–induced phosphorylation.
DISCUSSION
Plants need to reallocate energy to increase stress tolerance and adjust growth and development during stress-inducing conditions (Ahuja et al., 2010; Skirycz and Inzé 2010). Stomatal responses and root system architecture and growth are critical to improve WUE during stress. The direct activation of ion transport systems by ABA signaling through PYR/RCAR-mediated phosphorylation is the key step in stomatal closing. The phosphorylation activity of the ABA-activated protein kinase SnRK2 is linked to the activation of S-type anion channels and the inhibition of K+ influx channels (Hubbard et al., 2012). In this study, we determined that KUP6 interacted with SRK2E in yeast and plant cells and the conserved sequence motif in the KUP6 C terminus was phosphorylated by SnRK2 in the in-gel kinase assay under water stress conditions (Figure 7). These data suggest the possibility that KUP6 is a novel SRK2E target in the control of water stress responses and that its K+ efflux activity is also directly mediated by ABA signaling (see Supplemental Figure 10 online).
It has been shown that AtKUP1 and KUP2 can complement the potassium transport deficiency of an Escherichia coli mutant and that the transgenic Arabidopsis suspension cells overexpressing AtKUP1 showed increased Rb+ uptake (Kim et al., 1998). The study indicated that AtKUP1 encodes high-affinity potassium uptake activity in vivo (Kim et al., 1998). Contrary to this, the semidominant mutant of KUP2/SHY3 causes a reduction in cell size, suggesting the function of KUP2 is turgor reduction in plant cells (Elumalai et al., 2002). K+ homeostasis in plant cells is controlled by the balance of influx and efflux that responds to variations in external supplies of K+ (Szczerba et al., 2009). For instance, with low K+ concentrations in media, the steady state K+ level detected in root hairs was relatively higher, suggesting K+ uptake and release is balanced in root cells (Ivashikina et al., 2001). Transient K+ efflux is induced by the application of elicitors and K+ reuptake is then induced to regain apoplastic K+ concentrations (Ivashikina et al., 2001). The inactivation of the root hair K+ channel is voltage dependent and modulated by changes in external K+ concentration (Ivashikina et al., 2001). Thus, the K+ uptake and efflux system in the root are dependent on the biochemical equilibrium, and similar mechanisms have also been observed in other nutrient transport systems (Crawford and Glass, 1998; Narang et al., 2000; Segonzac et al., 2007). In our study, when KUP2, KUP6, KUP8, and GORK were disrupted in mutant plants, K+ homeostasis in the mutant roots was affected and the relative transient K+ uptake tended to rise compared with the wild type (Figure 2). Further investigation to directly determination their activity is needed to clarify the changes in K+ homeostasis in the mutants.
LRs in Arabidopsis are initiated from cell division in pericycle cells, which are adjacent to the xylem (Benková and Bielach, 2010; Yadav et al., 2010). Auxin stimulates the asymmetric cell division of the pericycle and enhances LR primordium development and emergence, whereas ABA and osmotic stress repress LR emergence (Fukaki and Tasaka, 2009). ABA-deficient and ABA signaling mutants show increased LR formation under stress conditions (De Smet et al., 2006; Xiong et al., 2006; Galvan-Ampudia and Testerink, 2011). Our data showed that SRK2D/E/I and KUP2/6/8 mutants exhibited significant ABA/osmotic stress insensitivity in LR formation (Figures 4 and 7). We also showed that the triple mutants enhanced IAA responses and reduced responses to NPA (Figure 3). Furthermore, KUP mutations enhanced the expression of LBD genes, which control LR formation (Figure 3) (Okushima et al., 2007; Lee et al., 2009a). Together, KUP2/SHY3, KUP6, and KUP8 act in LR initiation and development in the auxin and ABA antagonistic crosstalk signaling pathways. The similar insensitivity to osmotic stress in LR formation of srk2 mutants suggests that the KUPs negatively affect LR formation under the direct control of ABA signaling.
Recent studies have suggested that ABA plays important roles in LR formation in crosstalk with auxin. Mutation in ABA INSENSITIVE3, a B3-type transcription factor, reduced responses to the auxin inhibitor NPA (Brady et al., 2003) and auxin-induced LR formation was inhibited in overexpressors of ABI4 (an AP2 transcription factor) as a result of reduced expression of the auxin efflux carrier protein PIN1 (Shkolnik-Inbar and Bar-Zvi, 2010). The ABA-deficient mutants aba2-1 and aba3-1 showed impaired responses to the auxin transport inhibitors NPA and 2,3,5-triiodobenzoic acid (Deak and Malamy, 2005). Here, insensitivity to ABA/osmotic stress and increased sensitivity to auxin were also observed in kup68g. In roots, the inward Shaker K+ channel AKT1 is activated by K+ deficiency and controls K+ uptake (Hirsch et al., 1998). The outward shaker K+ channel, GORK, is expressed in the guard cells and root epidermis (Becker et al., 2003) and controls K+ efflux from root cells during stress, accompanied by reactive oxygen species generation (Demidchik et al., 2010). Expression of KUP6 and KUP8 was detected mainly in the pericycle (Figure 1; see Supplemental Figure 4 online), whereas GORK was expressed mainly in the epidermis in roots (Becker et al., 2003) and KUP2/SHY3 expression has been reported in various tissues (Elumalai et al., 2002). These transporters and channels might coordinately control K+ homeostasis across continuous root tissues from the epidermis to the stele. The disruption of KUPs and/or GORK might positively affect K+ uptake in the pericycle and vasculature, and the enhanced LR formation in the triple mutants might be caused by a local excess of K+ in the pericycle cells, resulting in LR formation. Our results also show that the KUP mutations promote cell expansion, which is driven by increased turgor. However, LR initiation is based on cell division associated with differential K+ channel expression, which in turn leads to a reduction in turgor (Sano et al., 2009). Electrophysiological studies have revealed a cell cycle dependency of K+ channel activity and a reduced K+ uptake in dividing tobacco (Nicotiana tabacum) Bright Yellow-2 cells (Sano et al., 2009). Among four Shaker-like K+ channel genes expressed, NKT1, an inwardly rectifying K+ channel that mediates K+ uptake, is transcriptionally induced during the G1 phase, while the transcripts of an outward K+ channel, NTORK1, dominate the S phase (Sano et al., 2009). These studies suggest that K+ uptake is required for proper cell cycle progression. In our results, the KUP mutations might enhance K+ uptake during the early phases of cell cycle in the pericycle resulting in cell division for LR formation. The SRK2E/OST1/SnRK2.6 gene is expressed in both the guard cells and vascular tissues of leaves and roots (Mustilli et al., 2002). KUP6 phosphorylation by SRK2E and other SRK2s (e.g., SRK2D; see Supplemental Figure 8 online), in roots is likely the main control system of KUP6 activity in LR formation.
Water deficit stress affects water status in cells and stomatal responses, which is pivotal in leaf turgor control (Hsiao, 1973). ABA induces stomatal closure under water deficit stress. Anion efflux causes membrane depolarization, and this activates K+ efflux by GORK in the guard cells. However, studies of the gork-1 mutant have shown the possibility of other K+ efflux systems being involved in stomatal closing (Hosy et al., 2003). Here, kup68g plants exhibited decreased ABA sensitivity in stomatal closing and tolerance to drought stress (Figures 5 and 6). These results suggest that KUP6 and KUP8 are novel factors that function in ABA-mediated stomatal closure.
The triple mutant kup268 also showed decreased survival after water deficit stress, although the stomatal responses to ABA of kup268 were weaker than those of kup68g (Figures 5 and 6). The WUE of both triple mutants was not affected by water deficit stress (Figure 6), suggesting that the disruption of K+ transport impaired their adaptive responses to the stress. These results also suggest that the K+ efflux in root systems is modulated by KUP2, KUP6, and KUP8 and also affects water status in the whole plant during water deficit stress. Stomatal aperture is controlled by the water potential in the water-conducting system, and stomatal closing has been suggested to directly respond to the hydraulic properties within the xylem during stress (Kramer and Boyer, 1995; Ache et al., 2010). Under drought conditions, water deficit stress causes a xylem sap pH increase in several plant species (Schachtman and Goodger, 2008), and stomatal closure is induced by cytosolic alkalization of guard cells in response to ABA (Gonugunta et al., 2008; Islam et al., 2010; Wang et al., 2012). The KUP family members are thought to function as K+/H+ symporters (Grabov, 2007). Recently, the tonoplast-localized Na+, K+/H+ antiporters, NHX1 and NHX2, have been shown to function in K+ uptake at the tonoplast and regulate stomatal function (Barragán et al., 2012). NHX1 and NHX2 regulate the intravacuolar K+ concentration and vacuolar pH and facilitate cell expansion (Bassil et al., 2011). The KUP6 subfamily might also have roles in the maintenance of pH and water status during stress in both roots and guard cells. In conclusion, our results suggest that the K+ transport system via the KUP6 subfamily is regulated by SnRKs in the maintenance of plant water status and has a potential role in tolerance to water stress and plant growth during such stress.
METHODS
Plant Materials and Growth Conditions
Arabidopsis thaliana (Columbia-0 and Wassilewskija-2) T-DNA insertion mutants kup2-8 (SAIL_504_A07), kup6-1 (SALK_086950), kup8-1 (SALK_001070), gork-2 (SALK_082258) (Columbia-0 background), and akt1-1 (Wassilewskija-2 background) were obtained from the ABRC. The srk2 single or multiple mutants were described previously (Nakashima et al., 2009; Umezawa et al., 2009). The multiple mutants were generated by crossing kup6-1, kup8-1, gork-2, and kup2-8. Homozygous individuals were isolated in the F2 progeny by PCR genotyping. The expression levels of the mutant genes were confirmed by RT-PCR. The mutants and the transgenic plants expressing 35S:KUP6, 35S:KUP6-GFP, and KUP6pro:GUS were sown on GM composed of 1× MS salts, 1× Gamborg’s B-5 vitamin solution (Sigma-Aldrich), and 0.5 g/L of MES with 1% Suc and grown at 2500 lux at 22°C under a 16-h-light/8-h-dark photoperiod (Osakabe et al., 2010). Transgenic and control plants were grown on GM agar plates containing kanamycin (20 mg/L).
Construction of Transgenic Plants
The KUP6 cDNA was cloned into a pGreenII containing a modified 35S CaMV promoter with the omega sequence of the Tobacco mosaic virus included and a double HA-His tag in the C terminus to produce a HA/His fusion (Osakabe et al., 2010) (35S:KUP6). The KUP6 cDNA was also cloned into a pGreenII that contained the 35S CaMV promoter and a GFP fusion in the C terminus (Yamada et al., 2010) (35S:KUP6-GFP). The KUP6 4 kb promoter region and a partial region of exon IV were amplified by PCR (primers are listed in Supplemental Table 1 online) and cloned into pGreenII that contained a GUS fusion in the C terminus (Yamada et al., 2010) (KUP6pro:GUS). The transgenic plants expressing 35S:KUP6, 35S:KUP6-GFP, and KUP6pro:GUS were generated using Agrobacterium tumefaciens as described previously (Osakabe et al., 2005). We obtained 30 transgenic T2 lines, from which homozygous lines were identified in the T3 and used for further experiments.
Root Growth Assay
Sterilized seeds were germinated on GM medium. Five-day-old seedlings were then transferred to fresh MS medium (1% Suc) with various concentrations of IAA, NPA, ABA, NaCl, and mannitol. LR growth was measured after 3 to 5 d. Root regeneration assays were performed as described previously (Sugimoto et al., 2010). Cotyledons were excised from seedlings at 10 d after germination, cut into two pieces, and cultured on callus induction media composed of Gamborg’s B-5 salt mixture (Sigma-Aldrich), 1× Gamborg’s B-5 vitamin solution, 0.5 g/L of MES, 2% Glc, 0.25 mg/L of kinetin, and 0.5 mg/L of 2,4-D for 5 d. The segments were then transferred to root induction media composed of the same salts as callus induction media but with the plant hormones changed to various concentrations of IAA. All experiments were repeated five times.
86Rb+ Uptake and Potassium Contents
86Rb+ was used as a tracer for physiological analysis of K+ uptake in roots and Rb+ uptake activity of K+ transporters and channels. Plants germinated in GM media were transferred to uptake solution consisting of K+-free solution supplemented with nonradiolabeled RbCl (0.05 mM) and cultured for 5 d, as described previously with slight modifications (Gierth et al., 2005; Pyo et al., 2010). Plants were transferred to K+-free solution containing 2.5 mM (low-affinity uptake) or 50 µM (high-affinity) nonradiolabeled RbCl, and then 86Rb+ (Perkin-Elmer) was added at a concentration of 0.005 μCi/nmol Rb+ and plants were incubated for 30 min. The plants were then blotted with paper and immediately washed three times for 2 min in 4°C washing solution (K+-free nutrient solution supplemented with 1.75 mM nonradiolabeled RbCl). Plants were separated into roots and shoots and surface dried, and the fresh weights of the roots and shoots were determined. The radioactivity was measured with a scintillation counter (LS 6500; Beckman Coulter). All experiments were repeated three times.
K+ contents in plants were measured with an atomic absorption spectrometer (AA-6200; Shimadzu) in emission mode. Plants were dissected into shoots and roots for drying. Samples (5 to 10 mg) were carefully weighed into Teflon cups and decomposed by nitric acid in Teflon bombs heated at 150°C for 10 h. The sample solutions were made up to 100 mL with 0.1 M nitric acid before analysis. The mean values of the K+ concentrations (mg/g) were calculated.
Physiological Analyses in Stress Responses
Determination of survival rates, SWC, leaf RWC, and WUE under water deficit stress conditions was performed as described previously (Osakabe et al., 2010; Yoo et al., 2010) with slight modifications. Four-week-old plants grown in soil pots at 22°C under a 16-h-light/8-h-dark photoperiod in 30 to 40% RH were subjected to dehydration stress by lack of watering. Pots were irrigated with water to saturation and weighed at the start of water deficit stress treatment (initial weight) and then periodically throughout the stress treatment period. Relative SWC was calculated as (final fresh weight – dry weight)/(initial weight – dry weight) × 100. After 14 to 21 d of withholding water, the plants were watered for another week and then photographed. The surviving green plants were counted. Twenty-five plants were used in each experiment, and all experiments were repeated five times.
Leaf RWC of fully expanded leaves from 3-week-old plants grown in pots was determined under control (in which pots were watered to saturation) and water deficit stress conditions. Leaf fresh weight was measured immediately after detachment. Leaves were then placed onto dishes filled with deionized water for 24 h, blotted with paper, and then weighed (leaf turgid weight). Leaves were then dried at 70°C for 2 d and reweighed (leaf dry weight). Leaf RWC was calculated as (fresh weight − dry weight)/(turgid weight − dry weight) × 100. Twenty-five plants were used in each experiment, and all experiments were repeated three times.
WUE was calculated as final shoot dry weight divided by total water loss over the culture period. Culture pots were placed in containers and covered with plastic wrap as described previously (Yoo et al., 2010). Individual pots were weighed before and after irrigation with water every 3 to 4 d. Water loss from control pots (without plants) was subtracted from treatment pots. Shoot dry weight was determined at the end of the experiment. Twenty-five plants were used in each experiment and all experiments were repeated three times.
Analysis of fresh weight loss was performed using detached rosette leaves of plants at the same developmental stage and size as 3-week-old controls. Three leaves per plant were excised and maintained in a growth chamber in 30% RH. Fresh weights were measured at the indicated time periods, and 10 plants of each genotype and transgenic line were analyzed in independent experiments repeated five times.
Stomatal Movement Assay
ABA-induced stomatal closing assays were performed as described previously with slight modifications (Osakabe et al., 2005, 2010). Epidermal peels from leaves of 4-week-old plants grown at 22°C under 16 h light/8 h dark in 50% RH were incubated for 2 h in a solution containing 10 mM KCl, 0.2 mM CaCl2, and 10 mM MES-KOH, pH 6.15, under white light (300 μmol m−2 s−1). The peeled strips were subsequently incubated in a solution containing the same buffer plus ABA. Guard cells were photographed under a light microscope. Fifty stomatal apertures were measured for each experiment. All experiments were repeated three times.
Histochemical Analyses for GUS Activity and GFP Imaging
Histochemical analysis of GUS activity in the KUP6pro:GUS transgenic plants was performed as described previously (Osakabe et al., 2005). After GUS staining, root tissues were dehydrated, embedded in Technovit 7100 resin (Kulzer), and cut with a sliding microtome. Root cross sections and other plant tissues were then viewed with a Leica M205C-SP microscope. GFP fluorescence (excitation filter, 514 nm; emission filter band-pass, 530/30 nm) of the 35S:KUP6-GFP plants was observed with a LSM5 PASCAL confocal laser scanning microscope (Zeiss).
RNA Extraction and qRT-PCR
Total RNA was extracted with Trizol reagent (Invitrogen) from 3-week-old Arabidopsis grown on GM agar plates treated with or without ABA or IAA. Real-time quantitative PCR was performed as described previously (Osakabe et al., 2010). The primers for qRT-PCR are listed in Supplemental Table 1 online.
In-Gel Kinase Assay
A 204-bp DNA fragment located in the KUP6 C terminus (KUP6-CT) and the mutated KUP6-CT (mKUP6) were amplified by PCR (using primers listed in Supplemental Table 1 online) to generate GST fusions. mKUP6 was generated by introducing a single mutation into KUP6-CT by substituting Thr-759 with Ala. KUP6-CT or mKUP6CT was cloned in frame into the pGEX4T-1 plasmid, and the GST fusion from Escherichia coli Rosetta (DE3) was purified. The extraction of proteins from srk2 mutants and wild-type plants treated with ABA, IAA, and drought stress was performed as previously described (Nakashima et al., 2009; Yoshida et al., 2002). Phosphorylation of the purified polypeptides was analyzed by in-gel kinase activity assays (Nakashima et al., 2009: Umezawa et al., 2009).
Split-Ubiquitin Membrane Yeast Two-Hybrid Assay and BiFC
The full-length cDNAs of KUP6 and SRK2E/OST1 were cloned in frame with either the C-terminal (Cub) or N-terminal (NubG) subdomain of ubiquitin and then introduced into yeast strain NMY32, as described previously (Iyer et al., 2005). C-terminal regions of KUP6 (1812 to 2348 nucleotides) and KUP8 (1826 to 2346 nucleotides) cloned into pGADT7 were also used to detect interactions with SnRK2s cloned into pGBKT7 (Umezawa et al., 2009) in the yeast strain AH109 by the general yeast two-hybrid system (Clontech).
The full-length cDNAs of KUP6 and SRK2E/OST1 were cloned into 35S-SPYNE and 35S-SPYCE vectors to generate BiFC constructs (Walter et al., 2004). Agrobacterium was used for infiltration of Nicotiana benthamiana leaves as described previously (Walter et al., 2004). The yellow fluorescent protein fluorescence in the lower epidermis cells 4 d after infiltration was analyzed by confocal microscopy (Zeiss Pascal).
Accession Numbers
Arabidopsis Genome Initiative locus identifiers for the genes described in this article are as follows: KUP2/SHY3 (At2g40540), KUP6 (At1g70300), KUP8 (At5g14880), GORK (At5g37500), AKT1 (At2g26650), SRK2E (At4g33950), SRK2D (At3g50500), SRK2I (At5g66880), LBD18 (At2g45420), and LBD29 (At3g58190).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Phylogenetic Analysis of KUP in Various Species.
Supplemental Figure 2. The Expression Levels of the KUP/HAK/KT Family Genes, KUP2/SHY3, KUP6, KUP8, and an ABA-Responsive Potassium Channel, GORK, in Arabidopsis Shoots and Roots during Abiotic Stress and ABA Treatments.
Supplemental Figure 3. The Expression Patterns of KUP/HAK/KT and Shaker Family Members under Various Stimuli in the Public Microarray Data Provided by Genevestigator (https://www.genevestigator.ethz.ch/).
Supplemental Figure 4. The Tissue-Specific Expression Patterns of KUP2/SHY3, KUP6, KUP8, and GORK in the Public Microarray Data Provided by Genevestigator (https://www.genevestigator.ethz.ch/).
Supplemental Figure 5. T-DNA Insertion Mutant Plants of KUP and GORK Genes.
Supplemental Figure 6. The KUP6-Overexpressing Transgenic Arabidopsis Plants.
Supplemental Figure 7. The Measurements of Water Loss and Drought Tolerance of the KUP Mutants.
Supplemental Figure 8. Yeast Two-Hybrid Analysis of SnRK2s (pGBKT7) and C-Terminal Regions of KUP6 and KUP8 (pGADT7).
Supplemental Figure 9. ABA and NPA Sensitivity in the LR Formation of srk2 Mutants and the in-Gel Kinase Assay Using Wild-Type Plants with Drought Stress, ABA, and Auxin Treatments.
Supplemental Figure 10. Model of Osmotic Regulation via KUPs and GORK under Normal Growth and Water Deficit Stress Conditions and Stomatal Responses via KUP6 and GORK.
Supplemental Table 1. Primer Pairs Used in RT-PCR, Quantitative RT-PCR, and KUP6SCT and KUP6pro:GUS Construction.
Supplemental Data Set 1. Text File of the Alignment Used to Generate the Phylogenetic Tree in Supplemental Figure 1.
Acknowledgments
We thank Kaori Amano, Kyouko Yoshiwara, Masami Toyoshima (Japan International Research Center for Agricultural Sciences), and Hiroko Kobayashi (RIKEN) for their technical assistance. This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas (22119004) and a Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (No. 17078003 to K.Y.-S.), by a Grant-in-Aid for Scientific Research C from the Japan Society for the Promotion of Science (21580125 to Y.O.), and by the Program for Promotion of Basic and Applied Researches for Innovations in Bio-oriented Industry of Japan.
AUTHOR CONTRIBUTIONS
Y.O. designed and performed research, analyzed the data, and wrote the article. N.A., T.U., S.K., K.N., H.T., H.O., K.Y., S.-U.S., and M.A. performed research. E.Y. and K.S. analyzed the data. K.Y.-S. designed the research and wrote the article.
Glossary
- ABA
abscisic acid
- GUS
β-glucuronidase
- GFP
green fluorescent protein
- CaMV
cauliflower mosaic virus
- GM
germination medium
- MS
Murashige and Skoog
- LR
lateral root
- IAA
indole-3-acetic acid
- NPA
1-N-naphthylphthalamic acid
- SWC
soil water content
- RWC
relative water content
- WUE
water use efficiency
- BiFC
bimolecular fluorescence complementation
- GST
glutathione S-transferase
- qRT-PCR
quantitative RT-PCR
References
- Ache P., Bauer H., Kollist H., Al-Rasheid K.A., Lautner S., Hartung W., Hedrich R. (2010). Stomatal action directly feeds back on leaf turgor: New insights into the regulation of the plant water status from non-invasive pressure probe measurements. Plant J. 62: 1072–1082 [DOI] [PubMed] [Google Scholar]
- Ache P., Becker D., Ivashikina N., Dietrich P., Roelfsema M.R., Hedrich R. (2000). GORK, a delayed outward rectifier expressed in guard cells of Arabidopsis thaliana, is a K+-selective, K+-sensing ion channel. FEBS Lett. 486: 93–98 [DOI] [PubMed] [Google Scholar]
- Ahuja I., de Vos R.C., Bones A.M., Hall R.D. (2010). Plant molecular stress responses face climate change. Trends Plant Sci. 15: 664–674 [DOI] [PubMed] [Google Scholar]
- Anderson J.A., Huprikar S.S., Kochian L.V., Lucas W.J., Gaber R.F. (1992). Functional expression of a probable Arabidopsis thaliana potassium channel in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 89: 3736–3740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barragán V., Leidi E.O., Andrés Z., Rubio L., De Luca A., Fernández J.A., Cubero B., Pardo J.M. (2012). Ion exchangers NHX1 and NHX2 mediate active potassium uptake into vacuoles to regulate cell turgor and stomatal function in Arabidopsis. Plant Cell 24: 1127–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassil E., Tajima H., Liang Y.C., Ohto M.A., Ushijima K., Nakano R., Esumi T., Coku A., Belmonte M., Blumwald E. (2011). The Arabidopsis Na+/H+ antiporters NHX1 and NHX2 control vacuolar pH and K+ homeostasis to regulate growth, flower development, and reproduction. Plant Cell 23: 3482–3497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker D., Hoth S., Ache P., Wenkel S., Roelfsema M.R., Meyerhoff O., Hartung W., Hedrich R. (2003). Regulation of the ABA-sensitive Arabidopsis potassium channel gene GORK in response to water stress. FEBS Lett. 554: 119–126 [DOI] [PubMed] [Google Scholar]
- Benková E., Bielach A. (2010). Lateral root organogenesis - From cell to organ. Curr. Opin. Plant Biol. 13: 677–683 [DOI] [PubMed] [Google Scholar]
- Böhmer M., Schroeder J.I. (2011). Quantitative transcriptomic analysis of abscisic acid-induced and reactive oxygen species-dependent expression changes and proteomic profiling in Arabidopsis suspension cells. Plant J. 67: 105–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady S.M., Sarkar S.F., Bonetta D., McCourt P. (2003). The ABSCISIC ACID INSENSITIVE 3 (ABI3) gene is modulated by farnesylation and is involved in auxin signaling and lateral root development in Arabidopsis. Plant J. 34: 67–75 [DOI] [PubMed] [Google Scholar]
- Brandt B., Brodsky D.E., Xue S., Negi J., Iba K., Kangasjärvi J., Ghassemian M., Stephan A.B., Hu H., Schroeder J.I. (2012). Reconstitution of abscisic acid activation of SLAC1 anion channel by CPK6 and OST1 kinases and branched ABI1 PP2C phosphatase action. Proc. Natl. Acad. Sci. USA 109: 10593–10598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun N., Wyrzykowska J., Muller P., David K., Couch D., Perrot-Rechenmann C., Fleming A.J. (2008). Conditional repression of AUXIN BINDING PROTEIN1 reveals that it coordinates cell division and cell expansion during postembryonic shoot development in Arabidopsis and tobacco. Plant Cell 20: 2746–2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford N.M., Glass A.D.M. (1998). Molecular and physiological aspects of nitrate uptake in plants. Trends Plant Sci. 3: 389–395 [Google Scholar]
- Deak K.I., Malamy J. (2005). Osmotic regulation of root system architecture. Plant J. 43: 17–28 [DOI] [PubMed] [Google Scholar]
- Demidchik V., Cuin T.A., Svistunenko D., Smith S.J., Miller A.J., Shabala S., Sokolik A., Yurin V. (2010). Arabidopsis root K+-efflux conductance activated by hydroxyl radicals: Single-channel properties, genetic basis and involvement in stress-induced cell death. J. Cell Sci. 123: 1468–1479 [DOI] [PubMed] [Google Scholar]
- De Smet I., Zhang H., Inzé D., Beeckman T. (2006). A novel role for abscisic acid emerges from underground. Trends Plant Sci. 11: 434–439 [DOI] [PubMed] [Google Scholar]
- Elumalai R.P., Nagpal P., Reed J.W. (2002). A mutation in the Arabidopsis KT2/KUP2 potassium transporter gene affects shoot cell expansion. Plant Cell 14: 119–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E. (1966). Dual pattern of ion absorption by plant cells and by plants. Nature 212: 1324–1327 [Google Scholar]
- Fu H.H., Luan S. (1998). AtKuP1: A dual-affinity K+ transporter from Arabidopsis. Plant Cell 10: 63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukaki H., Tasaka M. (2009). Hormone interactions during lateral root formation. Plant Mol. Biol. 69: 437–449 [DOI] [PubMed] [Google Scholar]
- Galvan-Ampudia C.S., Testerink C. (2011). Salt stress signals shape the plant root. Curr. Opin. Plant Biol. 14: 296–302 [DOI] [PubMed] [Google Scholar]
- Geiger D., Becker D., Vosloh D., Gambale F., Palme K., Rehers M., Anschuetz U., Dreyer I., Kudla J., Hedrich R. (2009a). Heteromeric AtKC1-AKT1 channels in Arabidopsis roots facilitate growth under K+-limiting conditions. J. Biol. Chem. 284: 21288–21295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D., Scherzer S., Mumm P., Marten I., Ache P., Matschi S., Liese A., Wellmann C., Al-Rasheid K.A., Grill E., Romeis T., Hedrich R. (2010). Guard cell anion channel SLAC1 is regulated by CDPK protein kinases with distinct Ca2+ affinities. Proc. Natl. Acad. Sci. USA 107: 8023–8028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D., Scherzer S., Mumm P., Stange A., Marten I., Bauer H., Ache P., Matschi S., Liese A., Al-Rasheid K.A., Romeis T., Hedrich R. (2009b). Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphatase pair. Proc. Natl. Acad. Sci. USA 106: 21425–21430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierth M., Mäser P. (2007). Potassium transporters in plants—Involvement in K+ acquisition, redistribution and homeostasis. FEBS Lett. 581: 2348–2356 [DOI] [PubMed] [Google Scholar]
- Gierth M., Mäser P., Schroeder J.I. (2005). The potassium transporter AtHAK5 functions in K+ deprivation-induced high-affinity K+ uptake and AKT1 K+ channel contribution to K+ uptake kinetics in Arabidopsis roots. Plant Physiol. 137: 1105–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonugunta V.K., Srivastava N., Puli M.R., Raghavendra A.S. (2008). Nitric oxide production occurs after cytosolic alkalinization during stomatal closure induced by abscisic acid. Plant Cell Environ. 31: 1717–1724 [DOI] [PubMed] [Google Scholar]
- Grabov A. (2007). Plant KT/KUP/HAK potassium transporters: Single family - multiple functions. Ann. Bot. (Lond.) 99: 1035–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich R. (2012). Ion channels in plants. Physiol. Rev. 92: 1777–1811 [DOI] [PubMed] [Google Scholar]
- Hirsch R.E., Lewis B.D., Spalding E.P., Sussman M.R. (1998). A role for the AKT1 potassium channel in plant nutrition. Science 280: 918–921 [DOI] [PubMed] [Google Scholar]
- Hosy E., et al. (2003). The Arabidopsis outward K+ channel GORK is involved in regulation of stomatal movements and plant transpiration. Proc. Natl. Acad. Sci. USA 100: 5549–5554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao T.C. (1973). Plant responses to water stress. Annu. Rev. Plant Physiol. 24: 519–570 [Google Scholar]
- Hubbard K.E., Siegel R.S., Valerio G., Brandt B., Schroeder J.I. (2012). Abscisic acid and CO2 signalling via calcium sensitivity priming in guard cells, new CDPK mutant phenotypes and a method for improved resolution of stomatal stimulus-response analyses. Ann. Bot. (Lond.) 109: 5–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M.M., Hossain M.A., Jannat R., Munemasa S., Nakamura Y., Mori I.C., Murata Y. (2010). Cytosolic alkalization and cytosolic calcium oscillation in Arabidopsis guard cells response to ABA and MeJA. Plant Cell Physiol. 51: 1721–1730 [DOI] [PubMed] [Google Scholar]
- Ivashikina N., Becker D., Ache P., Meyerhoff O., Felle H.H., Hedrich R. (2001). K+ channel profile and electrical properties of Arabidopsis root hairs. FEBS Lett. 508: 463–469 [DOI] [PubMed] [Google Scholar]
- Iyer K., Bürkle L., Auerbach D., Thaminy S., Dinkel M., Engels K., Stagljar I. (2005). Utilizing the split-ubiquitin membrane yeast two-hybrid system to identify protein-protein interactions of integral membrane proteins. Sci. STKE 2005: pl3. [DOI] [PubMed] [Google Scholar]
- Kim E.J., Kwak J.M., Uozumi N., Schroeder J.I. (1998). AtKUP1: An Arabidopsis gene encoding high-affinity potassium transport activity. Plant Cell 10: 51–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.H., Böhmer M., Hu H., Nishimura N., Schroeder J.I. (2010). Guard cell signal transduction network: Advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu. Rev. Plant Biol. 61: 561–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer, P.J., and Boyer, J.S. (1995). Water Relations of Plants and Soils. (San Diego, CA: Academic Press). [Google Scholar]
- Lebaudy A., Véry A.A., Sentenac H. (2007). K+ channel activity in plants: Genes, regulations and functions. FEBS Lett. 581: 2357–2366 [DOI] [PubMed] [Google Scholar]
- Lee H.W., Kim N.Y., Lee D.J., Kim J. (2009a). LBD18/ASL20 regulates lateral root formation in combination with LBD16/ASL18 downstream of ARF7 and ARF19 in Arabidopsis. Plant Physiol. 151: 1377–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.C., Lan W., Buchanan B.B., Luan S. (2009b). A protein kinase-phosphatase pair interacts with an ion channel to regulate ABA signaling in plant guard cells. Proc. Natl. Acad. Sci. USA 106: 21419–21424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levchenko V., Konrad K.R., Dietrich P., Roelfsema M.R., Hedrich R. (2005). Cytosolic abscisic acid activates guard cell anion channels without preceding Ca2+ signals. Proc. Natl. Acad. Sci. USA 102: 4203–4208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Kim B.G., Cheong Y.H., Pandey G.K., Luan S. (2006). A Ca2+ signaling pathway regulates a K+ channel for low-K response in Arabidopsis. Proc. Natl. Acad. Sci. USA 103: 12625–12630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio A., Zhu J.K., Hasegawa P.M., Bressan R.A. (2006). Osmogenetics: Aristotle to Arabidopsis. Plant Cell 18: 1542–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K., et al. (2009). Metabolic pathways involved in cold acclimation identified by integrated analysis of metabolites and transcripts regulated by DREB1A and DREB2A. Plant Physiol. 150: 1972–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäser P., et al. (2001). Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol. 126: 1646–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustilli A.C., Merlot S., Vavasseur A., Fenzi F., Giraudat J. (2002). Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 14: 3089–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K., Fujita Y., Kanamori N., Katagiri T., Umezawa T., Kidokoro S., Maruyama K., Yoshida T., Ishiyama K., Kobayashi M., Shinozaki K., Yamaguchi-Shinozaki K. (2009). Three Arabidopsis SnRK2 protein kinases, SRK2D/SnRK2.2, SRK2E/SnRK2.6/OST1 and SRK2I/SnRK2.3, involved in ABA signaling are essential for the control of seed development and dormancy. Plant Cell Physiol. 50: 1345–1363 [DOI] [PubMed] [Google Scholar]
- Narang R.A., Bruene A., Altmann T. (2000). Analysis of phosphate acquisition efficiency in different Arabidopsis accessions. Plant Physiol. 124: 1786–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negi J., Matsuda O., Nagasawa T., Oba Y., Takahashi H., Kawai-Yamada M., Uchimiya H., Hashimoto M., Iba K. (2008). CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature 452: 483–486 [DOI] [PubMed] [Google Scholar]
- Okushima Y., Fukaki H., Onoda M., Theologis A., Tasaka M. (2007). ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. Plant Cell 19: 118–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osakabe Y., Kajita S., Osakabe K. (2011). Genetic engineering of woody plants: Current and future targets in a stressful environment. Physiol. Plant. 142: 105–117 [DOI] [PubMed] [Google Scholar]
- Osakabe Y., Maruyama K., Seki M., Satou M., Shinozaki K., Yamaguchi-Shinozaki K. (2005). Leucine-rich repeat receptor-like kinase1 is a key membrane-bound regulator of abscisic acid early signaling in Arabidopsis. Plant Cell 17: 1105–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osakabe Y., Mizuno S., Tanaka H., Maruyama K., Osakabe K., Todaka D., Fujita Y., Kobayashi M., Shinozaki K., Yamaguchi-Shinozaki K. (2010). Overproduction of the membrane-bound receptor-like protein kinase 1, RPK1, enhances abiotic stress tolerance in Arabidopsis. J. Biol. Chem. 285: 9190–9201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Z.M., Kuchitsu K., Ward J.M., Schwarz M., Schroeder J.I. (1997). Differential abscisic acid regulation of guard cell slow anion channels in Arabidopsis wild-type and abi1 and abi2 mutants. Plant Cell 9: 409–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippar K., Ivashikina N., Ache P., Christian M., Lüthen H., Palme K., Hedrich R. (2004). Auxin activates KAT1 and KAT2, two K+-channel genes expressed in seedlings of Arabidopsis thaliana. Plant J. 37: 815–827 [DOI] [PubMed] [Google Scholar]
- Pyo Y.J., Gierth M., Schroeder J.I., Cho M.H. (2010). High-affinity K+ transport in Arabidopsis: AtHAK5 and AKT1 are vital for seedling establishment and postgermination growth under low-potassium conditions. Plant Physiol. 153: 863–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J.W., Elumalai R.P., Chory J. (1998). Suppressors of an Arabidopsis thaliana phyB mutation identify genes that control light signaling and hypocotyl elongation. Genetics 148: 1295–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigas S., Debrosses G., Haralampidis K., Vicente-Agullo F., Feldmann K.A., Grabov A., Dolan L., Hatzopoulos P. (2001). TRH1 encodes a potassium transporter required for tip growth in Arabidopsis root hairs. Plant Cell 13: 139–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelfsema M.R., Hedrich R., Geiger D. (2012). Anion channels: Master switches of stress responses. Trends Plant Sci. 17: 221–229 [DOI] [PubMed] [Google Scholar]
- Sano T., Kutsuna N., Becker D., Hedrich R., Hasezawa S. (2009). Outward-rectifying K+ channel activities regulate cell elongation and cell division of tobacco BY-2 cells. Plant J. 57: 55–64 [DOI] [PubMed] [Google Scholar]
- Sato A., Sato Y., Fukao Y., Fujiwara M., Umezawa T., Shinozaki K., Hibi T., Taniguchi M., Miyake H., Goto D.B., Uozumi N. (2009). Threonine at position 306 of the KAT1 potassium channel is essential for channel activity and is a target site for ABA-activated SnRK2/OST1/SnRK2.6 protein kinase. Biochem. J. 424: 439–448 [DOI] [PubMed] [Google Scholar]
- Schachtman D.P., Goodger J.Q. (2008). Chemical root to shoot signaling under drought. Trends Plant Sci. 13: 281–287 [DOI] [PubMed] [Google Scholar]
- Scherzer S., Maierhofer T., Al-Rasheid K.A., Geiger D., Hedrich R. (2012). Multiple calcium-dependent kinases modulate ABA-activated guard cell anion channels. Mol. Plant 5: 1409–1412 [DOI] [PubMed] [Google Scholar]
- Schroeder J.I., Hagiwara S. (1989). Cytosolic calcium regulates ion channels in the plasma membrane of Vicia faba guard cells. Nature 338: 427–430 [Google Scholar]
- Segonzac C., Boyer J.C., Ipotesi E., Szponarski W., Tillard P., Touraine B., Sommerer N., Rossignol M., Gibrat R. (2007). Nitrate efflux at the root plasma membrane: Identification of an Arabidopsis excretion transporter. Plant Cell 19: 3760–3777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shkolnik-Inbar D., Bar-Zvi D. (2010). ABI4 mediates abscisic acid and cytokinin inhibition of lateral root formation by reducing polar auxin transport in Arabidopsis. Plant Cell 22: 3560–3573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirichandra C., Gu D., Hu H.C., Davanture M., Lee S., Djaoui M., Valot B., Zivy M., Leung J., Merlot S., Kwak J.M. (2009). Phosphorylation of the Arabidopsis AtrbohF NADPH oxidase by OST1 protein kinase. FEBS Lett. 583: 2982–2986 [DOI] [PubMed] [Google Scholar]
- Skirycz A., Inzé D. (2010). More from less: Plant growth under limited water. Curr. Opin. Biotechnol. 21: 197–203 [DOI] [PubMed] [Google Scholar]
- Sugimoto K., Jiao Y., Meyerowitz E.M. (2010). Arabidopsis regeneration from multiple tissues occurs via a root development pathway. Dev. Cell 18: 463–471 [DOI] [PubMed] [Google Scholar]
- Szczerba M.W., Britto D.T., Kronzucker H.J. (2009). K+ transport in plants: Physiology and molecular biology. J. Plant Physiol. 166: 447–466 [DOI] [PubMed] [Google Scholar]
- Tromas A., Paponov I., Perrot-Rechenmann C. (2010). AUXIN BINDING PROTEIN 1: Functional and evolutionary aspects. Trends Plant Sci. 15: 436–446 [DOI] [PubMed] [Google Scholar]
- Umezawa T., Sugiyama N., Mizoguchi M., Hayashi S., Myouga F., Yamaguchi-Shinozaki K., Ishihama Y., Hirayama T., Shinozaki K. (2009). Type 2C protein phosphatases directly regulate abscisic acid-activated protein kinases in Arabidopsis. Proc. Natl. Acad. Sci. USA 106: 17588–17593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahisalu T., Kollist H., Wang Y.F., Nishimura N., Chan W.Y., Valerio G., Lamminmäki A., Brosché M., Moldau H., Desikan R., Schroeder J.I., Kangasjärvi J. (2008). SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature 452: 487–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Véry A.A., Sentenac H. (2003). Molecular mechanisms and regulation of K+ transport in higher plants. Annu. Rev. Plant Biol. 54: 575–603 [DOI] [PubMed] [Google Scholar]
- Vicente-Agullo F., Rigas S., Desbrosses G., Dolan L., Hatzopoulos P., Grabov A. (2004). Potassium carrier TRH1 is required for auxin transport in Arabidopsis roots. Plant J. 40: 523–535 [DOI] [PubMed] [Google Scholar]
- Walter M., Chaban C., Schütze K., Batistic O., Weckermann K., Näke C., Blazevic D., Grefen C., Schumacher K., Oecking C., Harter K., Kudla J. (2004). Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J. 40: 428–438 [DOI] [PubMed] [Google Scholar]
- Wang Y., Papanatsiou M., Eisenach C., Karnik R., Williams M., Hills A., Lew V.L., Blatt M.R. (2012). Systems dynamic modeling of a guard cell CI- channel mutant uncovers an emergent homeostatic network regulating stomatal transpiration. Plant Physiol. 160: 1956–1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L., Wang R.G., Mao G., Koczan J.M. (2006). Identification of drought tolerance determinants by genetic analysis of root response to drought stress and abscisic acid. Plant Physiol. 142: 1065–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Li H.D., Chen L.Q., Wang Y., Liu L.L., He L., Wu W.H. (2006). A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell 125: 1347–1360 [DOI] [PubMed] [Google Scholar]
- Yadav S.R., Bishopp A., Helariutta Y. (2010). Plant development: Early events in lateral root initiation. Curr. Biol. 20: R843–R845 [DOI] [PubMed] [Google Scholar]
- Yamada K., Osakabe Y., Mizoi J., Nakashima K., Fujita Y., Shinozaki K., Yamaguchi-Shinozaki K. (2010). Functional analysis of an Arabidopsis thaliana abiotic stress-inducible facilitated diffusion transporter for monosaccharides. J. Biol. Chem. 285: 1138–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K., Shinozaki K. (2006). Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu. Rev. Plant Biol. 57: 781–803 [DOI] [PubMed] [Google Scholar]
- Yoo C.Y., Pence H.E., Jin J.B., Miura K., Gosney M.J., Hasegawa P.M., Mickelbart M.V. (2010). The Arabidopsis GTL1 transcription factor regulates water use efficiency and drought tolerance by modulating stomatal density via transrepression of SDD1. Plant Cell 22: 4128–4141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R., Hobo T., Ichimura K., Mizoguchi T., Takahashi F., Aronso J., Ecker J.R., Shinozaki K. (2002). ABA-activated SnRK2 protein kinase is required for dehydration stress signaling in Arabidopsis. Plant Cell Physiol. 43: 1473–1483 [DOI] [PubMed] [Google Scholar]
- Zonia L., Munnik T. (2007). Life under pressure: Hydrostatic pressure in cell growth and function. Trends Plant Sci. 12: 90–97 [DOI] [PubMed] [Google Scholar]