Two Ca2+-dependent protein kinases (CPK11 and CPK24) mediate the Ca2+-dependent regulation of inward K+ channels in Arabidopsis pollen tubes and negatively regulate tube growth. CPK11 phosphorylates CPK24 as part of a kinase cascade that transduces the Ca2+ signal and regulates Shaker pollen inward K+ channel activity. This study provides insight into the Ca2+-dependent regulation of K+ channels.
Abstract
Potassium (K+) influx into pollen tubes via K+ transporters is essential for pollen tube growth; however, the mechanism by which K+ transporters are regulated in pollen tubes remains unknown. Here, we report that Arabidopsis thaliana Ca2+-dependent protein kinase11 (CPK11) and CPK24 are involved in Ca2+-dependent regulation of the inward K+ (K+in) channels in pollen tubes. Using patch-clamp analysis, we demonstrated that K+in currents of pollen tube protoplasts were inhibited by elevated [Ca2+]cyt. However, disruption of CPK11 or CPK24 completely impaired the Ca2+-dependent inhibition of K+in currents and enhanced pollen tube growth. Moreover, the cpk11 cpk24 double mutant exhibited similar phenotypes as the corresponding single mutants, suggesting that these two CDPKs function in the same signaling pathway. Bimolecular fluorescence complementation and coimmunoprecipitation experiments showed that CPK11 could interact with CPK24 in vivo. Furthermore, CPK11 phosphorylated the N terminus of CPK24 in vitro, suggesting that these two CDPKs work together as part of a kinase cascade. Electrophysiological assays demonstrated that the Shaker pollen K+in channel is the main contributor to pollen tube K+in currents and acts as the downstream target of the CPK11-CPK24 pathway. We conclude that CPK11 and CPK24 together mediate the Ca2+-dependent inhibition of K+in channels and participate in the regulation of pollen tube growth in Arabidopsis.
INTRODUCTION
Pollen tube growth, which is crucial for sexual reproduction in higher plants, is a rapid and highly polarized process (Wilhelmi and Preuss, 1999; Hepler et al., 2001; Feijó et al., 2004). Inorganic ion dynamics play important roles in regulating the rate and direction of pollen tube growth (Holdaway-Clarke and Hepler, 2003; Michard et al., 2009). Four major ions, Ca2+, H+, K+, and Cl−, are involved in the regulation of pollen tube growth (Feijó et al., 1995; Taylor and Hepler, 1997). Among these ions, Ca2+ plays a pivotal role. An apical [Ca2+]cyt gradient is essential for tube growth and the modulation of cell polarity (Miller et al., 1992; Malhó and Trewavas, 1996). The tip [Ca2+]cyt oscillates at same frequency as pollen tube growth rate (Holdaway-Clarke et al., 1997; Messerli and Robinson, 1997; Messerli et al., 2000). Some Ca2+ binding proteins, such as calmodulins, calcium-dependent protein kinases (CDPKs), and cytoskeletal proteins, sense Ca2+ signals and trigger downstream responses during pollen germination and pollen tube growth (Moutinho et al., 1998; Snowman et al., 2002; Harper et al., 2004; Rato et al., 2004; Myers et al., 2009). In particular, CDPKs are emerging as important regulators of pollen tube growth. In maize (Zea mays), a pollen-specific CDPK involved in the regulation of the actin cytoskeleton is required for both pollen germination and pollen tube growth (Estruch et al., 1994). CDPK can also phosphorylate actin-depolymerizing factor, leading to actin cytoskeleton remodeling (Allwood et al., 2001; Feijó et al., 2004). Two petunia (Petunia hybrida) CDPKs have been reported to regulate pollen tube elongation and growth polarity (Yoon et al., 2006). Myers et al. (2009) presented genetic evidence that CPK17 and CPK34 are essential for pollen fitness and pollen tube growth in Arabidopsis thaliana.
A number of Ca2+-regulated cellular activities, such as cytoskeleton dynamics, exocytosis, and endocytosis, are directly or indirectly involved in the regulation of pollen tube growth (Roy et al., 1999; Hwang et al., 2005; Helling et al., 2006; Konrad et al., 2011). Based on the observation that Ca2+ is known to regulate the activity of ion channels in many other physiological processes, Ca2+ and its downstream components are assumed to play crucial roles in modulating ion channel activity during pollen tube growth. The activity or membrane localization of Shaker K+ channel is modulated by calcineurin B-like proteins (CBLs) and CBL-interacting protein kinases (CIPKs), which plays important roles in K+ uptake and distribution (Xu et al., 2006; Held et al., 2011). In guard cells, Ca2+ signals can be transduced from some CDPKs or other Ca2+ sensor proteins to various types of ion channels, such as K+in channels, nonselective Ca2+-permeable channels, and S-type anion channels, to regulate stomatal movements (Schroeder and Hagiwara, 1989; Li et al., 1998; Berkowitz et al., 2000; Mori et al., 2006; Geiger et al., 2010; Zou et al., 2010). Recently, Cl− channels and outward K+ channels were reported to be regulated by intracellular Ca2+ in pollen tubes (Tavares et al., 2011; Wu et al., 2011).
Potassium is required for both pollen germination and tube growth (Weisenseel and Jaffe, 1976; Feijó et al., 1995; Fan et al., 2001; Mouline et al., 2002). K+ is present in relatively large amounts and regulates several basic cellular parameters, such as turgor and membrane potential during pollen tube growth (Holdaway-Clarke and Hepler, 2003). By means of patch-clamp analysis, the inward and the outward K+ channels were identified in pollen grain protoplasts and pollen tube protoplasts (Obermeyer and Kolb, 1993; Fan et al., 1999, 2001, 2003; Griessner and Obermeyer, 2003; Qu et al., 2007; Wu et al., 2011). Shaker pollen inward K+ channel (SPIK; also named AKT6) is expressed specifically in pollen and pollen tubes. Disruption of SPIK strongly reduces the inward rectifying K+ channel activity and results in impaired pollen tube growth (Mouline et al., 2002). Arabidopsis TPK4, a member of tandem-pore K+ channel (TPK/KCO) family, was reported to be responsible for a marginal proportion of pollen tube K+ conductance and function in the control of membrane potential (Becker et al., 2004). Besides K+ channels, some transporters from the cation/proton exchanger (CHX) or K+ transporter/K+ uptake protein/high affinity K+ transporter (KT/KUP/HAK) family expressed preferentially or specifically in pollen and pollen tubes may also play important roles in H+ fluxes and K+ homeostasis (Sze, et al., 2004; Pina et al., 2005; Bock et al., 2006; Wang et al., 2008; Song et al., 2009). CHX21 and CHX23, which have K+ transporter activity, are essential for pollen tube guidance toward the micropyle (Lu et al., 2011). Recently, the role of K+ in pollen tube bursting, which is crucial for fertilization, has been investigated (Franklin-Tong, 2010; Wu et al., 2011). For example, K+ channel Zea mays 1 (KZM1) is activated by defensin-like protein Embryo Sac 4 secreted from the synergid cells to facilitate K+ influx and sperm release after the burst of pollen tubes (Amien et al., 2010).
Previous studies showed that inward K+ (K+in) channels in the pollen grain are insensitive to [Ca2+]cyt elevation (Fan et al., 1999, 2001). Considering some differences observed in lily (Lilium longiflorum) between the pollen grains and pollen tubes in terms of K+ channel properties (Griessner and Obermeyer, 2003), the Ca2+ sensitivities of K+in channels in pollen tubes cannot be postulated based on measurements in pollen grains.
In this study, we developed a modified method to isolate protoplasts from Arabidopsis pollen tubes for patch clamp recordings and demonstrated that the whole-cell K+in currents of pollen tube protoplasts were significantly inhibited by [Ca2+]cyt elevation. Two Arabidopsis CDPKs, CPK11 and CPK24, are both involved in the Ca2+-dependent regulation of K+in channels. Subsequent phenotype analysis of pollen tube growth indicated that CPK11 and CPK24 function as negative regulators of pollen tube elongation. The interaction between CPK11 and CPK24 is specifically localized to the plasma membrane. CPK11 can phosphorylate CPK24, suggesting that these two CDPKs work together as a kinase cascade similar to a mitogen-activated protein kinase (MAPK) pathway. We showed that SPIK, which plays crucial roles in K+ uptake during pollen tube elongation, is the downstream target of the CDPKs.
RESULTS
The K+in Currents of Pollen Tube Protoplasts Are Sensitive to Cytosolic Free Ca2+ Elevation
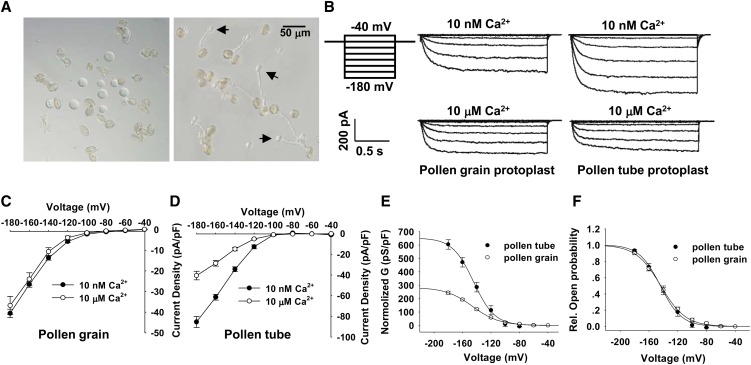
Previous electrophysiology studies showed that [Ca2+]cyt had no affect on K+in currents of pollen gain protoplasts of Brassica and Arabidopsis (Fan et al., 1999, 2001). Similar results were observed in our study with [Ca2+]cyt in the range from 10 nM to 10 μM (Figures 1B and 1C). This study investigated whether the K+in channels at the plasma membrane of pollen tubes are regulated by changes of [Ca2+]cyt. The prior studies successfully established methods for isolating pollen tube protoplasts from lily (Griessner and Obermeyer, 2003; Dutta and Robinson, 2004), pear (Pyrus pyrifolia) (Qu et al., 2007), and tobacco (Nicotiana tabacum) (Michard et al., 2011). In this study, we developed a modified method for isolating pollen tube protoplasts from Arabidopsis (Figure 1A, right panel) and performed patch clamp recordings on the pollen tube protoplasts in the whole-cell configuration. The time- and voltage-dependent inward currents were observed under hyperpolarization conditions (Figure 1B, right panel). The normalized current amplitudes in pollen tube protoplasts were nearly twice those in pollen grain protoplasts when membrane potential was clamped to −180 millivolt (mV) (Figures 1C and 1D). Tail current analysis showed that the measured reversed potential was −37 mV (see Supplemental Figure 1 online), close to the theoretical equilibrium potential for potassium (Ek = −56.1 mV). This result indicates that the currents we recorded were mainly caused by K+ movement across the plasma membrane. The Boltzmann fitting method was applied to analyze the biophysical properties of K+in channels in both pollen grains and pollen tubes. Normalized maximal conductance (Gmax) of K+in channel in pollen tubes was nearly twice that in pollen grains (Figure 1E), while there was no difference in the voltage dependence between the two types of K+ currents (Figure 1F). Importantly, when [Ca2+]cyt was increased from 10 nM to 10 μM, K+in currents recorded from pollen tube protoplasts were significantly inhibited, almost decreasing by 50% when membrane potential was clamped to −180 mV (Figure 1D). The results demonstrated that the K+in channels in pollen tubes were sensitive to elevation of [Ca2+]cyt, which was in contrast with the response of K+in channels in pollen grains (Figure 1C).
Figure 1.
Ca2+ Sensitivity of the K+in Currents Recorded from Arabidopsis Pollen Grain Protoplasts and Pollen Tube Protoplasts.
(A) Photographs of pollen grain protoplast (left) and pollen tube protoplast (right) isolation. Pollen tube protoplasts emerging from pollen tubes during the enzymatic digestion are indicated with arrows.
(B) Typical K+in currents recorded from pollen grain protoplasts (middle column) and pollen tube protoplasts (right column) with the addition of 10 nM or 10 μM free Ca2+ in the pipette solution. Voltage protocols, current, and time scale bars are shown in the left column.
(C) and (D) Current-voltage relationship of the K+in currents recorded from pollen grain protoplasts (C) and pollen tube protoplasts (D) with the addition of 10 nM or 10 μM free Ca2+ in the pipette solution. The data are derived from the recordings as shown in (B) and are presented as means ± se (n ≥ 8). The replicates for each treatment shown in (C) are 14 (10 nM Ca2+) and 8 (10 μM Ca2+), and the replicates for each treatment shown in (D) are 16 (10 nM Ca2+) and 13 (10 μM Ca2+).
(E) The G-V relationship of the steady state currents recorded from pollen grain protoplasts and pollen tube protoplasts. The G was normalized and calculated as normalized G = G/Cm, where Cm is the membrane capacitance of the protoplast. The data are presented as means ± se (n ≥ 6). G-V curves were best fitted with the Boltzmann function.
(F) The relative open probability (G/Gmax) of K+in channels in pollen grains and pollen tubes plotted against the test voltage. The solid lines represent the best fits according to the Boltzmann function: G/Gmax = 1/(1+exp((Vm − V1/2)/S)). G is the cord conductance and calculated as G = I/(Vm − EK). S is a slope factor equivalent to RT/zF, where z represents the apparent gating charge. The data are presented as means ± se (n ≥ 6).
CPK11 and CPK24 Are Involved in Ca2+-Dependent Regulation of the K+in Currents of Pollen Tube Protoplasts
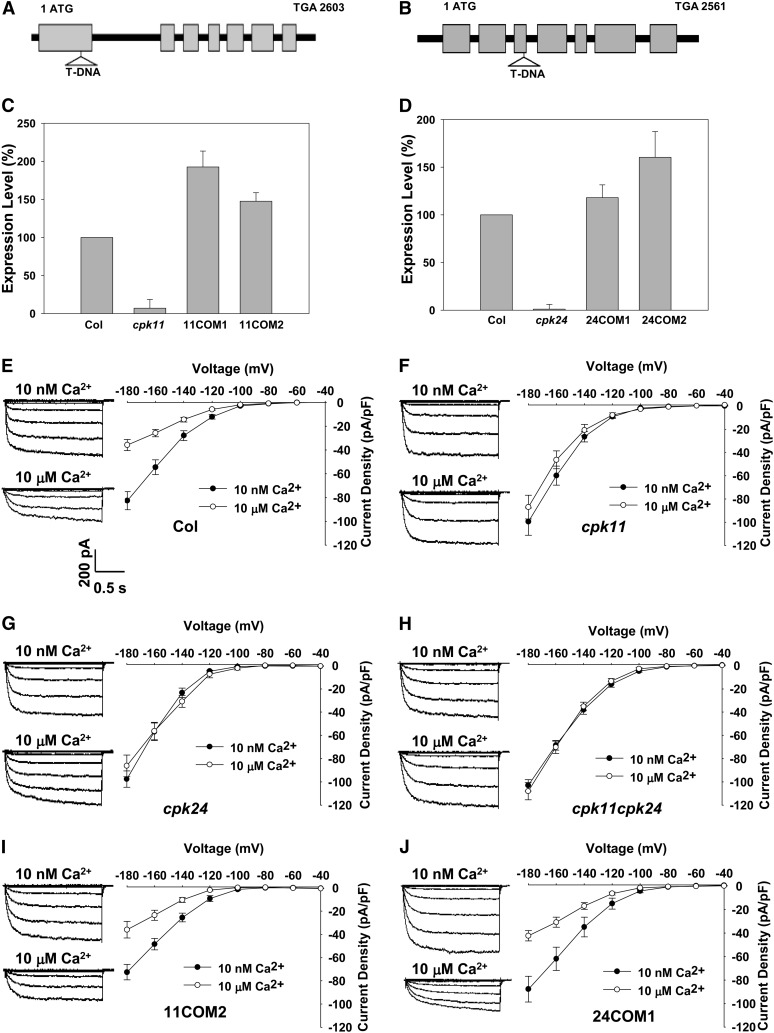
CDPKs have been demonstrated to participate in the Ca2+ regulation of K+in channels in guard cells (Li et al., 1998; Berkowitz et al., 2000; Zou et al., 2010). It was hypothesized that some CDPKs may also be involved in the Ca2+ inhibition of K+in currents in pollen tube protoplasts. Based on expression profiling data, 16 CDPK isoforms are expressed in pollen grains or pollen tubes (Becker et al., 2003; Pina et al., 2005; Wang et al., 2008). The homozygous T-DNA insertion mutants for 13 CDPKs out of these 16 pollen-expressed CDPKs were obtained. In vitro pollen germination assays revealed that the mutants for six CDPKs displayed differences in pollen germination or pollen tube growth compared with wild-type plants. Using patch-clamp techniques, we tested the Ca2+ sensitivity of pollen tube K+in channel in these six CDPK mutants. The hypothesis was confirmed in two T-DNA insertion lines, cpk11 (Salk_054495) and cpk24 (Salk_015986) (Figure 2). The T-DNA insertions (Figures 2A and 2B) caused the disruption in their transcription for both genes (Figures 2C and 2D). In contrast with the Ca2+-dependent inhibition of K+in currents in the pollen tube protoplasts of wild-type plants (Figure 2E), the K+in currents recorded from cpk11 (Figure 2F) and cpk24 (Figure 2G) were not inhibited when the [Ca2+]cyt was increased from 10 nM to 10 μM. The complementation lines that showed similar transcriptional expression of CPK11 and CPK24 compared with wild-type plants (Figures 2C and 2D) were analyzed with the patch clamp technique. Similar to wild-type plants, K+in currents in the pollen tube protoplasts of all of these complementation lines were inhibited by the increased [Ca2+]cyt (Figures 2I and 2J; see Supplemental Figure 2A online). All these results demonstrate that the Ca2+-dependent inhibition of K+in channels requires the presence of both CPK11 and CPK24.
Figure 2.
Effect of Cytosolic Free Ca2+ on the K+in Currents of Pollen Tube Protoplasts Isolated from Various Plant Materials.
(A) and (B) Gene structures and T-DNA insertion sites in cpk11 and cpk24 mutants. The T-DNA fragment was inserted in the first exon of the CPK11 genomic DNA and in the third exon of the CPK24 genomic DNA. Black boxes denote exons, and solid lines represent introns and untranslated regions.
(C) Real-time PCR analysis of CPK11 expression in the wild type (Columbia [Col]), cpk11 mutant, and two complementation lines (11COM1 and 11COM2). Each data bar represents the means ± se (n = 3).
(D) Real-time PCR analysis of CPK24 expression in the wild type (Col), cpk24 mutant, and two complementation lines (24COM1 and 24COM2). Each data bar represents the means ± se (n = 3).
(E) to (J) Current-voltage relationship of the K+in currents in pollen tube protoplasts isolated from various plant materials with the addition of 10 nM or 10 μM Ca2+ in the pipette solution, including wild-type Col (E), cpk11 mutant (F), cpk24 mutant (G), double mutant cpk11 cpk24 (H), complementation line 11COM2 for cpk11 (I), and complementation line 24COM1 for cpk24 mutant (J). The data points represent the means ± se (n ≥ 8). Typical whole-cell recordings are shown on the left of I-V curves. Current and time scale bars are shown in (E), and voltage protocols are the same as those described in Figure 1B.
Considering that disruption of either CPK11 or CPK24 could completely impair the Ca2+-dependent inhibition of K+in currents, CPK11 and CPK24 might be involved in the same signaling pathway. To test this notion, K+in currents in the pollen tube protoplasts of the cpk11 cpk24 double mutants were further analyzed. The results showed that the double mutants exhibited a similar K+in current phenotype as single mutants in the presence of 10 nM or 10 μM free Ca2+ in the pipette solution (Figure 2H), suggesting that there was no function redundancy between CPK11 and CPK24 and that both CDPKs were involved in the same pathway directing the Ca2+-dependent regulation of pollen tube K+in channels.
CPK11 and CPK24 Regulate Pollen Tube Elongation
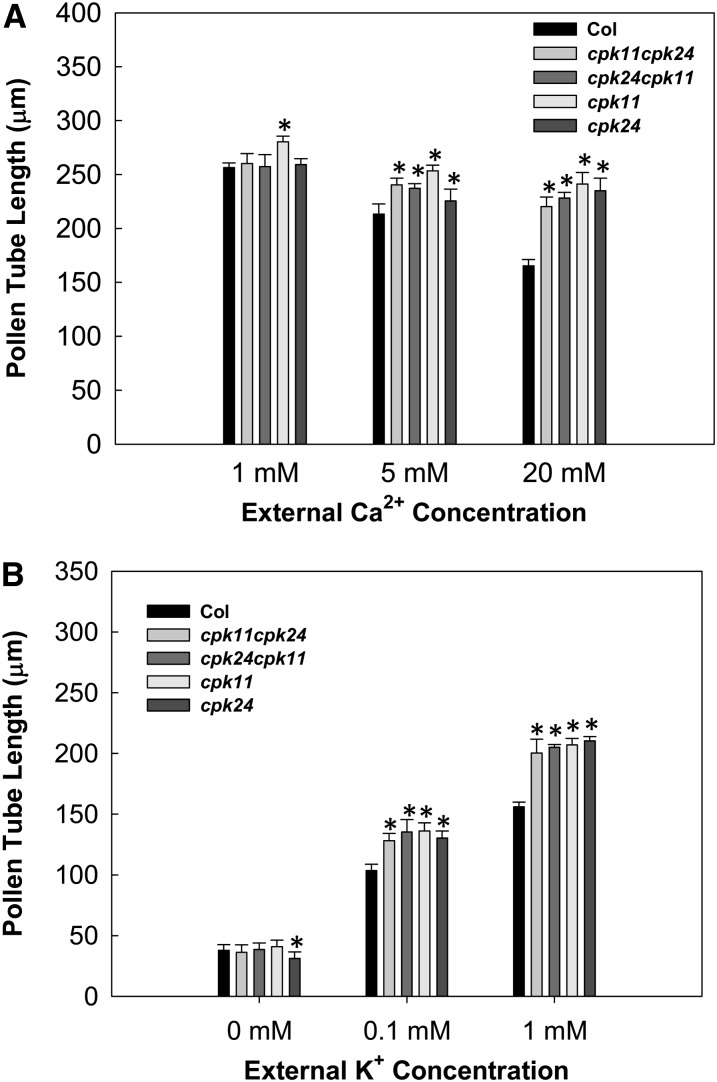
It is well known that Ca2+ is an important factor in the regulation of pollen tube growth (Feijó et al., 1995; Taylor and Hepler, 1997; Franklin-Tong, 1999). To test whether CPK11 and CPK24 are involved in the Ca2+ signaling response during pollen tube growth, in vitro pollen tube growth assays were performed with different external Ca2+ concentrations. As shown in Figure 3A, an increase of the external Ca2+ concentration from 1 to 5 mM or 20 mM had significant inhibitory effects on the growth of wild-type pollen tubes. By contrast, the increase of external Ca2+ concentration did not significantly affect the pollen tube growth of cpk11, cpk24, or the double mutants (Figure 3A), suggesting that the Ca2+-dependent inhibition on pollen tube growth depends on the presence of both CPK11 and CPK24. Representative images of pollen tube growth under a high concentration (20 mM) of Ca2+ are shown in Supplemental Figure 3.
Figure 3.
Effect of External Ca2+ and K+ on Pollen Tube Growth.
(A) Effect of external Ca2+ on pollen tube length. Pollen grains were germinated on solidified medium containing 1 mM K+ and the indicated concentrations of Ca2+. Each data bar represents the means ± se of three replicates. More than 80 pollen tubes were measured for the average pollen tube length in each replicate. Asterisks indicate significant difference from Columbia (Col) at P < 0.05 by Student's t test.
(B) Effect of external K+ on pollen tube length. Pollen grains were germinated on solidified medium containing 20 mM Ca2+ and the indicated concentrations of K+. Each data bar represents the means ± se of three replicates. The average length of more than 80 pollen tubes was in each replicate. Asterisks indicate significant difference from Columbia (Col) at P < 0.05 by Student’s t test.
Considering the finding that CPK11 and CPK24 are involved in the Ca2+-dependent regulation of K+in channels in pollen tube protoplasts, we further tested if Ca2+-regulated pollen tube growth was correlated with K+ uptake during pollen tube growth. By culturing pollen in medium containing different concentrations of K+, we compared pollen tube lengths between wild-type plants and various mutants (Figure 3B). The pollen tubes of all plant materials displayed similar lengths in the absence of external K+ in the medium (Figure 3B). When the external [K+] was increased to 0.1 or 1 mM K+, the pollen tube lengths of cpk11 and cpk24 mutants as well as of the double mutants were much greater than those of wild-type plants (Figure 3B). In addition, no significant difference in pollen tube length was observed between complementation lines and wild-type plants in all of these in vitro pollen germination assays (see Supplemental Figures 2B and 2C online). These results together with our electrophysiological experiments (Figure 2) suggest that both CPK11 and CPK24, as negative regulators of K+ influx, are involved in the Ca2+-dependent regulation of pollen tube growth.
CPK11 Can Phosphorylate CPK24
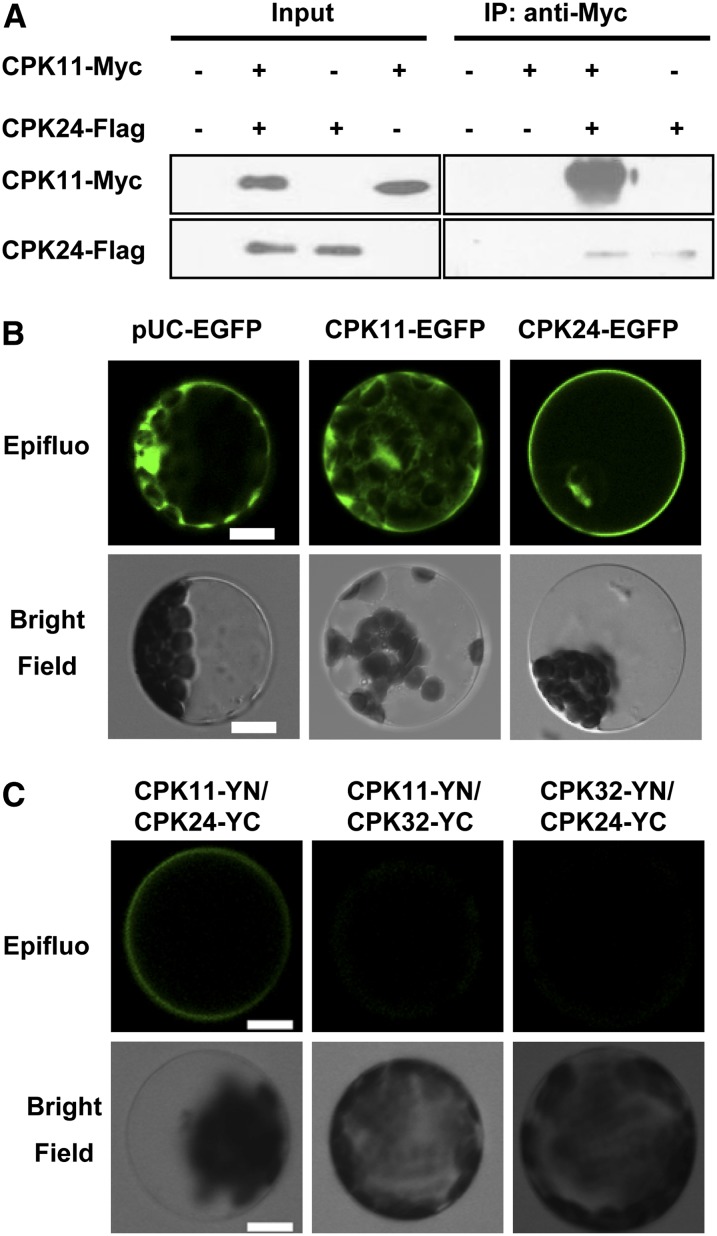
According to the results of patch clamping experiments and pollen tube growth measurements, we hypothesized that CPK11 and CPK24 are involved in the same regulatory pathway. Thus, we further tested if these two kinases interact with each other. In the presented coimmunoprecipitation (Co-IP) assays, combinations of Flag-CPK24 and Myc-CPK11 were cotransformed into the tobacco leaves. As shown in Figure 4A, immunoprecipitation of CPK24 with Flag-coupled Sepharose yielded a coimmunoprecipitating band of 58 kD corresponding to the tagged CPK11 that was labeled with the anti-myc antibody. This Co-IP assay result demonstrated the interaction between CPK11 and CPK24. To further confirm this result in vivo, bimolecular fluorescence complementation (BiFC) assays were performed. CPK11 was localized to the cytoplasm and nucleus as described previously (Dammann et al., 2003; Rodriguez Milla et al., 2006; Zhu et al., 2007), and CPK24 was localized to the plasma membrane and nucleus (Figure 4B). Our BiFC assays (Figure 4C) showed not only that CPK11 and CPK24 interacted each other, but also that the CPK11/CPK24 complex was specifically localized to the plasma membrane of the cotransformed Arabidopsis mesophyll protoplasts. To test the interaction specificity between CPK11 and CPK24, another pollen-expressed CDPK, CPK32, was selected as a negative control. As shown in Figure 4C, when either CPK11 or CPK24 was substituted with CPK32, the interaction between the two CPKs disappeared or was significantly weakened.
Figure 4.
Assays to Detect an Interaction between CPK11 and CPK24.
(A) Co-IP assays showing an interaction between CPK11 and CPK24 expressed in tobacco leaves. Extracted protein samples were incubated with anti-Flag–coupled agarose. The immunoprecipitates were analyzed with anti-Myc antibodies against Myc-CPK11.
(B) Subcellular localization of CPK11 and CPK24 in Arabidopsis mesophyll protoplasts. Protoplasts expressing pUC-EGFP were used as the control. Bars = 10 μm.
(C) BiFC assays of the in vivo interaction between CPK11 and CPK24. CPK11-YN/CPK32-YC and CPK32-YN/CPK24-YC expressions were used as the controls. Bars = 10 μm.
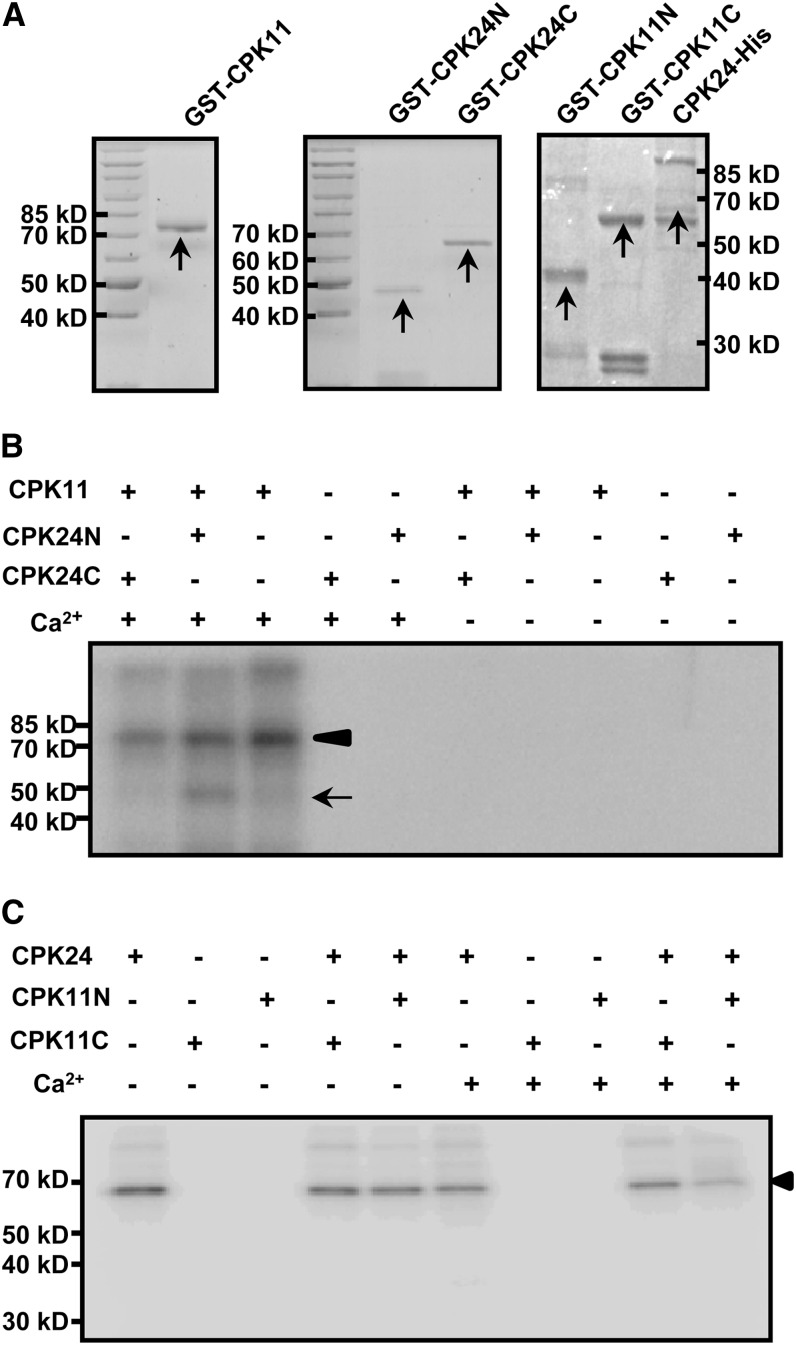
An in vitro protein phosphorylation assay further demonstrated the interaction between these two proteins. Full-length, recombinant CPK11 was produced as an 81-kD glutathione S-transferase (GST) fusion protein (Figure 5A, left panel), and Ca2+ was found to be required for the autophosphorylation activity of CPK11 (Figure 5B). CPK24 was divided into two parts, the N-terminal region (CPK24N, 48 kD) and the C-terminal region (CPK24C, 66 kD) (Figure 5A, middle panel), with the cleavage at the Asn-195 residue, which is located in the middle of the kinase domain. CPK24N contained an N-terminal variable domain and half of the kinase domain, and CPK24C contained the remaining parts. Neither of the GST fusions (CPK24N and CPK24C) displayed kinase activity (Figure 5B). The results showed that CPK11 can phosphorylate the CPK24N (48 kD) but not the CPK24C (66 kD), and the phosphorylation was Ca2+ dependent (Figure 5B). According to the truncated CPK24 variants used in the phosphorylation assays, we generated two truncated CPK11 variants, CPK11N (N-terminal, from Met-1 to Pro-153 residue; Figure 5A, right panel) and CPK11C (C-terminal, from Glu-154 to the end; Figure 5A, right panel). Neither of the truncated CPK11 variants displayed kinase activity, and both of them could not be phosphorylated by CPK24 (Figure 5C). Furthermore, according to the truncated CPK24 variant phosphorylated by CPK11, we also generated the homologous truncated variant of CPK32 as a control in the phosphorylation assays. The results showed that CPK11 was not able to phosphorylate CPK32, indicating that the phosphorylation of CPK24 by CPK11 is specific (see Supplemental Figure 4 online).
Figure 5.
Phosphorylation of CPK24 by CPK11 in Vitro.
(A) Coomassie blue–stained recombinant proteins of CPK11 and CPK24. Purified GST-CPK11 (left panel), GST-CPK24N (N-terminal, from Met-1 to Asn-195 residue; middle panel), GST-CPK24C (C-terminal, from Phe-196 to the end; middle panel), CPK24-His (right panel), GST-CPK11N (N-terminal, from Met-1 to Pro-153 residue; right panel), and GST-CPK11C (C-terminal, from Glu-154 to the end, right panel) are indicated with arrows in Coomassie blue–stained gels.
(B) Phosphorylation of the N terminus of CPK24 by CPK11. Each lane represents an independent reaction and contains 2 μg purified protein (kinase:substrate = 1:1). The arrowhead shows the autophosphorylation of CPK11, and the arrow shows the phosphorylation of CPK24 in the N terminus.
(C) CPK24 cannot phosphorylate CPK11 in vitro. The autoradiogram shows that neither of the truncated CPK11 variants (GST-CPK11N and GST-CPK11C) could be phosphorylated by CPK24 in vitro. The arrowhead shows the autophosphorylation of CPK24.
The Shaker K+ Channel SPIK Is the Downstream Target of CPK11 and CPK24
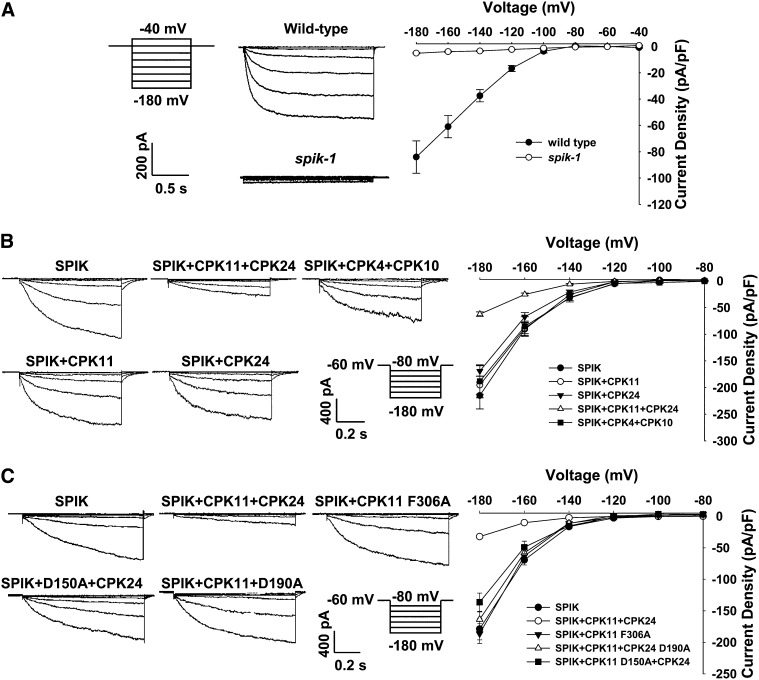
It has been reported that disruption of the Shaker potassium channel SPIK (AKT6) impaired K+ channel activity in pollen (Mouline et al., 2002). In this study, pollen tube protoplasts were isolated from the spik-1 mutant pollen for patch clamp recording. As shown in Figure 6A, the time- and voltage-dependent K+in currents recorded in pollen tube protoplasts of wild-type plants were completely absent in spik-1 pollen tube protoplasts, indicating that SPIK is the main contributor to K+in currents in Arabidopsis pollen tubes. Considering that CPK11 and CPK24 are involved in the Ca2+-dependent regulation of K+in channels in pollen tubes, we further hypothesized that SPIK may be the downstream target of CPK11 and CPK24. Since the K+ channel activity of SPIK had been identified in COS cells (Mouline et al., 2002), we also used COS cells for patch clamping to test if CPK11 and CPK24 could regulate SPIK in vitro. As shown in Figure 6B, the currents recorded from COS cells expressing SPIK alone were approximately 3 times larger than those in cells coexpressing SPIK, CPK11, and CPK24 when the membrane potential was clamped to −180 mV. Coexpression of either CPK11 or CPK24 with SPIK did not result in a significant change in SPIK-mediated K+ currents. Furthermore, as a control experiment, substitution of both CPK11 and CPK24 with two other CDPKs, CPK4 and CPK10, had no effect on the SPIK currents. In addition, we constructed inactive forms of both kinases and the constitutively active version of CPK11 and analyzed the effect of these CDPK variants on K+ currents in COS cells. As shown in Figure 6C, coexpression of CPK11 and the inactive form of CPK24 (CPK24 D190A) with SPIK had no effect on the SPIK currents, and similar results were obtained for the coexpression of CPK24 and the inactive version of CPK11 (CPK11 D150A) with SPIK. In vitro kinase assays showed that the D150A and D190A substitutions led to the inactivation of CPK11 and CPK24, respectively (see Supplemental Figures 4 and 5A online). In the absence of CPK24, constitutively active CPK11 (CPK11 F306A; Rodriguez Milla et al., 2006) also had no effect on SPIK currents in COS cells.
Figure 6.
Regulation of the SPIK-Mediated K+in Currents by CPK11 and CPK24.
(A) Representative K+in currents recorded in pollen tube protoplasts of wild-type plants and the spik-1 mutant. The data points for the current-voltage curves are presented as means ± se (the wild type, n = 7; spik-1, n = 14).
(B) Whole-cell recordings of K+in currents in COS cells expressing different combinations of SPIK and CDPKs. The data points for the current-voltage curves are presented as means ± se (SPIK, n = 12; SPIK+CPK11+CPK24, n = 16; SPIK+CPK11, n = 7; SPIK+CPK24, n = 8; SPIK+CPK4+CPK10, n = 8).
(C) Whole-cell recordings of K+in currents in COS cells expressing different combinations of SPIK and the variants of CDPK. The data points for the current-voltage curves are presented as means ± se (SPIK, n = 15; SPIK+CPK11+CPK24, n = 15; SPIK+CPK11 F306A, n = 19; SPIK+CPK11+CPK24 D190A, n = 22; SPIK+CPK11 D150A+CPK24, n = 12).
To test the specificity of SPIK inhibition by CPK11/CPK24, another K+ channel was used as a control. AKT1 is a close homolog of SPIK in the Shaker channel family. However, when expressed alone in COS cells, AKT1 did not show any K+in currents. Therefore, we used another K+ channel Os-AKT1 as control. Os-AKT1 is an AKT1 homolog from rice (Oryza sativa) and can mediate K+in currents in COS cells. The control experiment was performed by coexpressing CPK11 and CPK24 with Os-AKT1. The results showed that Os-AKT1–mediated K+ currents were not affected by the presence of the two CDPKs in COS cells (see Supplemental Figure 6 online). All these results support the notion that that SPIK may acts as the downstream target of the CPK11/CPK24-related pathway.
DISCUSSION
The Downstream Target of the Two CDPKs
K+in currents across the plasma membrane of pollen tube consist of two components (Obermeyer and Blatt, 1995; Griessner and Obermeyer, 2003; Becker et al., 2004). One component was defined as the hyperpolarization-activated, time-, and voltage-dependent inward rectifier. This component is contributed by SPIK in Arabidopsis (Mouline et al., 2002) and is responsible for 90% of pollen tube K+in currents (Figure 6A). The other was defined as instantaneously activated, voltage-independent conductance that is mediated by TPK4 in Arabidopsis (Becker et al., 2004). K+in currents mediated by TPK4-like channels contributed to 20 to 30% of the total K+in currents across the pollen grain and pollen tube plasma membrane in previous reports (Mouline et al., 2002; Becker et al., 2004), but they were not more than 10% of the whole K+in currents in our experiments (Figure 6A). The difference could result from differences in the experimental methods, such as those used for protoplast isolation or the electrophysiological recording mode. According to our patch clamping results, the decreased currents caused by an elevation of [Ca2+]cyt were ∼50% of the whole K+in currents recorded under the control condition, being several times greater than the total TPK4-mediated K+ currents. Thus, the Ca2+-inhibited K+in currents in pollen tube protoplasts were mainly mediated by SPIK, but not by TPK4-like channels, and SPIK must be the downstream target of the CPK11/CPK24-mediated pathway. This conclusion was further demonstrated by our patch clamping results obtained from COS cells (Figure 6B). Considering that the direct interaction between CPK24 (CPK11) and SPIK was not detected in our experiments, other unknown intermediate components could exist in this regulatory pathway. K+ influx has been reported to occur in the apical domain of pollen tube (Messerli et al., 1999); however, Michard et al. (2009) show that the K+ influx can be detected in the tube shank region, but not in the tip. If one also considers the tip-focused gradient of free Ca2+, our experimental evidence may support Michard’s notion on the spatial patterning of K+ influx and explain why the elevated apical [Ca2+]cyt activates the CDPKs and subsequently inhibits the K+in channel activity.
CDPKs are known to be involved in the modulation of many types of ion channels. A tonoplast-located Cl− channel is activated by a purified CDPK in broad bean (Vicia faba) guard cells (Pei et al., 1996). KAT1 can be phosphorylated by CDPK from broad bean guard cells (Li et al., 1998) and inhibited by a soybean (Glycine max) CDPK in Xenopus laevis oocytes (Berkowitz et al., 2000). CPK3 and CPK6 are essential factors for the abscisic acid– and Ca2+-dependent activation of S-type anion channels in guard cells (Mori et al., 2006). The guard cell anion channel SLAC1 is activated by CPK23 with distinct Ca2+ affinities (Geiger et al., 2010). CPK10 was shown to be involved in abscisic acid– and Ca2+-dependent inhibition of the K+in channels in guard cells (Zou et al., 2010). This study demonstrated that CPK11 and CPK24 work together to mediate Ca2+-dependent inhibition of SPIK activity in the pollen tubes, which further increases our understanding of the CDPK-mediated regulatory mechanisms of ion channels.
CPK11 and CPK24 Regulate Pollen Tube Elongation by Modulating K+ Influx
Pollen tubes use K+ to maintain the osmotic concentration and membrane potential and to regulate the turgor pressure that induces pollen tubes to burst during fertilization (Holdaway-Clarke and Hepler, 2003; Franklin-Tong, 2010). It is known that K+ influx promotes pollen tube growth (Fan et al., 2001; Mouline et al., 2002; Boavida and McCormick, 2007). In this study, in vitro pollen tube growth assays showed that pollen tubes of cpk11 and cpk24 mutants grew faster than those of the wild type at a relatively high external Ca2+ concentration (Figure 3A). Moreover, the enhanced pollen tube growth of the mutants was correlated with the external K+ concentration (Figure 3B). These results suggest that the Ca2+-dependent inhibition of pollen tube growth may rely on the presence of both CPK11 and CPK24, which function as negative regulators of K+-related pollen tube growth (Figure 3). This notion was further supported by our electrophysiological results showing that CPK11 and CPK24 coordinately regulate the K+in channel activity in pollen tubes. The combination of the tube growth phenotype and electrophysiological results leads us to conclude that CPK11 and CPK24 may regulate pollen tube elongation by modulating the K+ influx.
Previous studies identified some CDPKs involved in the regulation of pollen tube growth. In petunia, Pi-CDPK1 regulates the polarity of pollen tubes and Ca2+ homeostasis in the pollen tube tip, whereas Pi-CDPK2 inhibits tube growth with no influence on polarity (Yoon et al., 2006). Both CPK17 and CPK34 maintain the tube growth rate and facilitate the response to tropism cues in pollen tube growth (Myers et al., 2009). Together, these findings suggest that different pollen-expressed CDPK isoforms regulate different aspects of pollen tube growth.
CPK11 Phosphorylates CPK24
It is well known that many signaling networks in living plant cells are regulated by protein phosphorylations that are performed by protein kinases (Hrabak et al., 2003). Protein kinases translate the signal by phosphorylating various downstream substrates, including transcription factors, ion channels, and cytoskeleton proteins (Nakagami et al., 2005; Kudla et al., 2010). The function or activity of a protein kinase is also frequently regulated by phosphorylation that is performed by other protein kinases, resulting in amplification of signals or crosstalk between different pathways (Harper et al., 2004; Ludwig et al., 2005). For example, a MAPK cascade is minimally composed of three kinase modules, mitogen-activated protein kinase kinase kinase–mitogen-activated protein kinase kinase–MAPK, that are linked in various ways to upstream receptors and downstream targets (Ichimura et al., 2002; Jonak et al., 2002; Rodriguez et al., 2010). Besides MAPK cascades, the regulation of some other protein kinases has been reported to be achieved by transphosphorylation. Some residues in the activation loop of SOS2 (CIPK24) can be phosphorylated by unknown protein kinases (Guo et al., 2001; Gong et al., 2002). At least one CDPK from tobacco (CDPK2) requires transphosphorylation by unknown kinases to become fully activated (Romeis et al., 2000, 2001). In this study, the interaction between CPK11 and CPK24 was identified, and CPK11 could phosphorylate the N terminus of CPK24 in vitro, suggesting that these two CDPKs work together as a novel kinase cascade similar to the MAPK pathway. This finding improves our understanding of the mechanism by which CDPKs act in plant signaling.
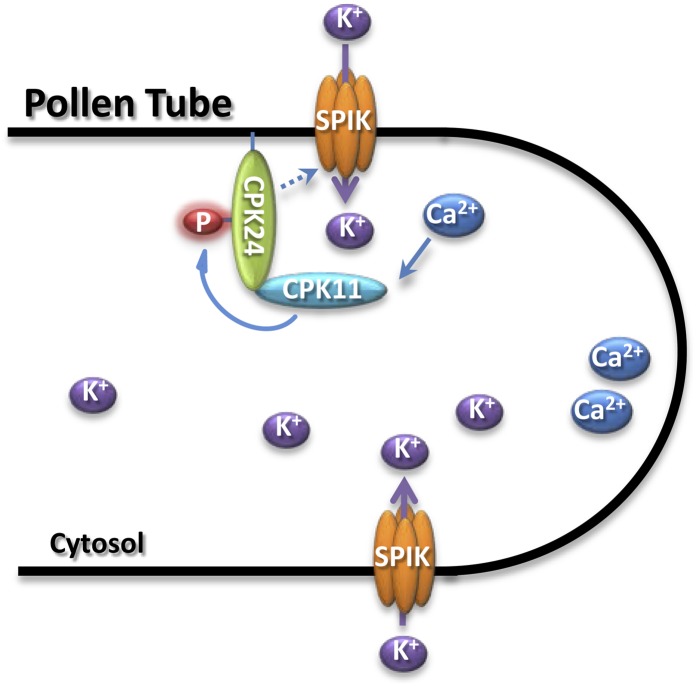
Although it is generally believed that CDPK activity is Ca2+ dependent, it has been reported that different CDPKs showed different calcium dependencies for their kinase activity. For example, the isoforms from subgroup 3 (including CPK24) of the CDPK family exhibit low or no calcium dependence on substrate phosphorylation, although they can still bind calcium (Boudsocq et al., 2012). In our experiments, we also found that CPK24 activity is independent of calcium (see Supplemental Figure 5A online). The calcium independence of some CDPKs raises the question of the role of calcium in CDPK regulation, suggesting possible alternative CDPK activation mechanisms besides calcium binding. CPK11 is almost inactive in the absence of calcium, and its kinase activity is shown to be highly Ca2+ dependent (Figure 5B). The calcium dependence of a CDPK may be correlated to its function in sensing the calcium signature. The difference in calcium dependence between CPK11 and CPK24 led us to propose a hypothetical working model as shown in Figure 7. During pollen tube growth, although CPK24 fails to effectively sense the calcium signature, the fine tuning of calcium signature may be sensed by CPK11. Ca2+-activated CPK11 subsequently phosphorylates CPK24, transduces the calcium signals, and regulates SPIK activity.
Figure 7.
Working Model for the Regulation of the SPIK Channel by the CPK11 and CPK24 Signaling Cascade in Arabidopsis Pollen Tubes.
CPK11 and CPK24 are involved in the Ca2+-dependent regulation of the K+in channels in Arabidopsis pollen tubes. During pollen tube growth, cytoplasmic Ca2+ elevation activates CPK11, and the activated CPK11 subsequently phosphorylates CPK24. This kinase cascade transduces the calcium signals and regulates SPIK activity and pollen tube growth.
Differences in Ca2+ Sensitivity of the K+in Channels between Pollen Grains and Pollen Tubes
Comparing the K+in currents recorded from pollen grain protoplasts and pollen tube protoplasts of Arabidopsis, we observed significant differences in the properties of K+in channels, such as current density, normalized Gmax, and Ca2+ sensitivity (Figure 1). The current density and normalized Gmax of K+in channels in pollen tubes are significantly larger than those in pollen grains, suggesting that the amount or activity of K+in channels may be different between these two types of protoplasts. Previous studies revealed that K+in currents recorded from pollen grain protoplasts were not sensitive to internal Ca2+ (Fan et al., 1999, 2001), and similar results were observed in this study (Figure 1C). However, elevation of [Ca2+]cyt results in the significant inhibition of K+in currents in pollen tube protoplasts (Figure 1D). This difference in Ca2+ sensitivity of K+in channels between pollen grains and pollen tubes might be ascribed to the differences of functions and/or expression patterns of some components in the Ca2+ signaling pathway. Although quantitative real-time PCR results showed that there was no significant change in CPK11 transcription between pollen grains and pollen tubes, the transcripts of CPK24 in pollen tubes were ∼20 times as abundant as those in pollen grains (see Supplemental Figure 7 online). The transcriptional change of CPK24 may be associated with the difference in Ca2+ sensitivity of the K+in channels between pollen grains and pollen tubes.
In summary, this study demonstrates that both CPK11 and CPK24 function as negative regulators of K+in channel activity and pollen tube elongation. These two CDPKs work together as part of a kinase cascade to transduce the signal and eventually regulate SPIK activity and pollen tube growth.
METHODS
Plant Growth Conditions and Mutant Identification
Arabidopsis thaliana plants were grown in potting soil mixture (rich soil:vermiculite = 2:1, v/v) and kept in growth chambers at 22°C under an illumination of 120 μmol m−2 s−1 and a 16/8-h daily light/dark cycle. The relative humidity was ∼70% (±5%).
T-DNA insertion lines, cpk11 (Salk_054495) and cpk24 (Salk_015986), were obtained from the ABRC (http://www.Arabidopsis.org/abrc/). The homozygous cpk11 and cpk24 mutants were identified using gene-specific primers (for cpk11, forward primer 5′-CACAGAGAAATCAACCTCCG-3′ and reverse primer 5′-GCAAGCTCCCTAACTACACCT-3′; for cpk24, forward primer 5′-ATTTGGTGCTCTCTCTGC-3′ and reverse primer 5′-AAGGACGGAGATAGATGTGG-3′) and one T-DNA left border primer (Lba1, 5′-GCGTGGACCGCTTGCTGCAACT-3′).
Vector Construction and Arabidopsis Transformation
To generate the constructs for complementation of the mutants, genomic DNA fragments of CPK11 or CPK24 were excised from BAC clones obtained from the ABRC (F15O4 for CPK11 and T9H9 for CPK24) and cloned into the pCAMBIA 1300 vector. Arabidopsis transformation with Agrobacterium tumefaciens (strain GV3101) was performed by the floral dip method (Clough and Bent, 1998).
Real-Time PCR
Total RNA was extracted from pollen grains or pollen tubes with Trizol reagent (Invitrogen). The cDNA was synthesized using Superscript II reverse transcriptase according to the manufacturer’s instructions (Invitrogen). Real-time PCR analysis was performed using SYBR Green PCR Master Mix (Applied Biosystems) in an Applied Biosystems 7500 real-time PCR system. Relative expression levels of CDPKs were determined by normalization to 18S rRNA and presented as mean normalized transcript levels using the comparative cycle threshold method (2−ΔΔCt). Real-time PCR was conducted with CPK11-specific primers (forward primer 5′-TGTCGATGAACAAGCAGCACCA-3′ and reverse primer 5′-ATCGTTCCGCTGTTGTCTGTGT-3′) and CPK24-specific primers (forward primer 5′-AACGGTAACGACCAGTGGATCA-3′ and reverse primer 5′-AAGCAGCTTAGCCCTCTTCCTT-3′).
In Vitro Pollen Germination Assays
In vitro Arabidopsis pollen germination experiments were conducted as described previously (Fan et al., 2001) with minor modifications. The basic medium was composed of 0.8 mM MgSO4, 1.5 mM boric acid, 1% (w/v) agarose, 19.8% (w/v) Suc, 0.05% (w/v) lactalbumin hydrolysate, 10 μM myo-inositol, and 5 mM MES, and the pH was adjusted to 5.8 with Tris. For the treatments with different external Ca2+ or K+ concentrations, different amounts of CaCl2 (1, 5, and 20 mM) or KCl (0, 0.1, and 1 mM) were added to the basic medium. Pollen tube length was measured under a microscope (Olympus IX-71) after incubation for 4.5 h at 23°C. All the experiments were repeated three times and each treatment in one experiment included three independent replicates. For each replicate, more than 80 pollen tubes were measured.
Isolation of Pollen Grain Protoplasts and Pollen Tube Protoplasts
Arabidopsis pollen grain protoplasts were isolated as described previously (Fan et al., 2001). Enzyme solution was prepared by dissolving 1% (w/v) Cellulysin (Calbiochem), 1% (w/v) Cellulase RS (Yakult), 0.2% (w/v) Pectolyase Y-23 (Yakult), 0.2% (w/v) Macerozyme R-10 (Yakult), 0.2% (w/v) PDS, and 0.2% (w/v) BSA in standard solution containing (in mM) 5 CaCl2, 1 KNO3, 0.2 KH2PO4, 1 MgSO4, 0.001 KI, 0.0001 CuSO4, 600 sorbitol, 600 Glc, and 5 MES, and pH was adjusted to 5.8 with Tris.
For isolation of pollen tube protoplasts, 40 to 60 fresh flowers were collected and the pollen was spread uniformly on the surface of solid germination medium (5 mM Ca2+ and 1 mM K+ were added to the basic medium, which was described in the in vitro pollen germination assays) in a 35-mm-diameter Petri dish, followed by the addition of 80 to 120 μL of liquid germination medium (solid germination medium without 1% agarose), which formed a thin liquid layer on the medium surface. It should be noted that the addition of a liquid layer on the medium surface is crucial for pollen tube protoplast isolation, as it prevents pollen tube bursting during the subsequent enzyme digestion. After the pollen was incubated for 2.5 to 3 h at 25°C, the average pollen tube length was between 100 and 150 μm, and 1 mL of the half-strength enzyme solution (as used in the isolation of pollen grain protoplasts) was slowly added to the Petri dish without disturbing the germinated pollen. After 2.5 to 3.5 min, protoplasts emerged from the tip region of the pollen tubes (Figure 1A, right panel). Two milliliters of standard solution (as used to isolate pollen grain protoplasts) was added to the dish to terminate enzyme digestion. The subsequent procedures were the same as those used for pollen grain protoplast isolation described previously (Fan et al., 2001). Because pollen grain protoplasts do not emerge within 15 min, the pollen tube protoplasts would not be contaminated with pollen grain protoplasts. The prepared protoplasts were kept on ice in dark for at least 1.5 h before patch clamp recordings.
Electrophysiological Experiments
Patch clamp whole-cell recordings from pollen grain protoplasts and pollen tube protoplasts were conducted using an Axopatch-200B amplifier (Axon Instruments) at 22°C in dim light. The contents of the bath and pipette solution were the same as described previously (Fan et al., 2001). The normalized whole-cell currents were presented as current densities, the ratio of steady state currents to cell membrane capacitances.
For the patch clamping recordings from COS cells, COS-7 cells (from American Type Culture Collection) were cultured in Dulbecco's modified eagle medium supplemented with 10% fetal calf serum (Gibco) at 37°C in 5% CO2. The cultured COS-7 cells were transfected with different combinations of recombinant plasmids using Lipofectamine LTX transfection reagent (Invitrogen). SPIK was cloned into pIRES2-EGFP (Clontech), and green fluorescent protein fluorescence indicated successful transfection. The vector pBudCE4.1 (Invitrogen) was used for the simultaneous expression of both CPK11 and CPK24 (or both CPK4 and CPK10). DsRed2 sequences were inserted into the pBudCE4.1 vectors, following the sequence of CPK11 or CPK4. A successful transfection results in red fluorescent protein fluorescence due to the expression of DsRed2. To confirm that the cells were successfully transfected, green fluorescent protein and red fluorescent protein were examined using an inverted fluorescence microscope (Olympus IX-71) before patch clamping experiments. Patch-clamp recordings were conducted 36 h after transfection. The contents of the bath solution are the same as described previously (Mouline et al., 2002). The CPK11 and CPK24 variants (CPK11 D150A, F306A, and CPK24 D190A) used in electrophysiological assays were generated using the QuikChange Lightning site-directed mutagenesis kit (Stratagene). The pipette solutions contained (in mM) 150 KCl, 4.83 CaCl2, 3 MgCl2, 5 EGTA, 10 HEPES, and 2.5 ATP, and pH was adjusted to 7.2 with NaOH.
Co-IP Assays
The coding sequences of CPK11 and CPK24 fused with the myc or flag tag (Myc-CPK11 and Flag-CPK24) were cloned into the pCAMBIA1300 vector under control of the SUPER promoter. The combinations of Myc-CPK11 and Flag-CPK24 were cotransformed into tobacco (Nicotiana benthamiana) leaves using the Agrobacterium-mediated infiltration method. Proteins were extracted 4 d after transformation. The extraction buffer contained (in mM) 50 Tris-HCl, pH 7.5, 100 NaCl, 2 EDTA, 1 NaF, 1 NaVO3, 1 PMSF, 10 β-glycerolphosphate, 0.1% (v/v) Triton X-100, and 0.5% (v/v) Nonidet P-40. The supernatant was incubated with 30 μL anti-Flag agarose conjugate (Sigma-Aldrich) for 6 h at 4°C. The coimmunoprecipitation products were washed with the extraction buffer five times and then detected via immunoblot analysis. Both anti-Myc (Sigma-Aldrich) and anti-Flag (Sigma-Aldrich) antibodies were used at 1:8000 dilutions, and chemiluminescence signals were detected by Kodak film.
BiFC Assays
BiFC assays were performed as described previously (Walter et al., 2004). To generate the BiFC vectors, the coding region of CPK11 was cloned via XbaΙ-XhoΙ into pUC-SPYNE, resulting in CPK11-YN, and the coding region of CPK24 was cloned via SpeΙ-SmaΙ into pUC-SPYCE, resulting in CPK24-YC. After incubation for 12 to 18 h at 23°C, the fluorescence of yellow fluorescent protein in the transformed protoplasts was imaged using a confocal laser scanning microscope (Nikon C1).
Phosphorylation Assays
To construct GST fusion proteins, full-length CPK11 was cloned into the pGEX-4T vector via BamHI-XhoI. To create the truncated variants of CDPKs, CPK24, CPK11, and CPK32 were individually divided into two parts with the cleavage at CPK24 N195, CPK11 P153, and CPK32 N192 residues located in the middle of the kinase domain. The DNA fragments of these truncated CDPK variants were cloned into pGEX-4T vectors via SmaΙ-NotΙ for CPK24, BamHI-XhoI for CPK11, and BamHI-SalI for CPK32. Recombinant plasmids were transformed into Escherichia coli BL21 (DE3), and protein expression was induced by the addition of 0.5 mM Isopropyl β-D-1-Thiogalactopyranoside (IPTG). The expressed proteins were extracted after incubation at 22°C for 8 h. Full-length CPK24 was cloned into pcDNA3.1-FLAG via KpnI-NotI. The purified plasmids were transfected into HEK293T cells. CPK24 proteins were purified with Ni2+-nitrilotriacetic acid-agarose from HEK293T cells 36 to 48 h after transfection and used in an in vitro kinase assay.
In vitro phosphorylation assays were performed in 50 μL reaction buffer containing (in mM) 50 Tris-HCl, pH 7.2, 10 MgCl2, 0.45 EGTA, 1 DTT, and 0.55 CaCl2, and CaCl2 was omitted for the control. Phosphorylation was initiated by adding 5 μCi of [32P]ATP, and the reaction was incubated for 20 min at 30°C. Then, the proteins were separated by 10% SDS-PAGE, and the phosphorylated proteins were visualized by autoradiography.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: At1g35670 for CPK11, At2g31500 for CPK24, and At2g25600 for SPIK.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Analysis of Tail Currents Recorded from an Arabidopsis Pollen Tube Protoplast.
Supplemental Figure 2. Pollen Tube Inward K+ Currents Recordings and Pollen Tube Growth Phenotypes of Complementation Lines under Different Ca2+ and K+ Concentrations.
Supplemental Figure 3. Representative Images of Pollen Tube Growth under High Concentration of Ca2+.
Supplemental Figure 4. A Control Experiment for Substrate Specificity of CPK11.
Supplemental Figure 5. In Vitro Kinase Activity Assay for the Variants of CPK24.
Supplemental Figure 6. Current-Voltage Relationship for Os-AKT1 Expressed Alone or Together with CPK11 and CPK24.
Supplemental Figure 7. Transcription Profiles of CPK11 and CPK24 in Arabidopsis Pollen Grains and Pollen Tubes.
Acknowledgments
We thank Hervé Sentenac (French National Institute of Agricultural Research, France) for providing the spik-1 mutant line that was used in our patch clamping experiments. This work was supported by grants from the “973” Project (2011CB915401), the National Natural Science Foundation of China (Nos. 30830013 and 31121002), and the “111” Project (No. B06003).
AUTHOR CONTRIBUTIONS
W.-Z.Z., L.-K.S., L.-N.Z., and W.-H.W. designed the research. L.-N.Z., L.-K.S., W.-Z.Z., and W.Z. performed research and analyzed data. L.-K.S. and W.-H.W. wrote the article. W.-H.W. and Y.W. revised the article.
Glossary
- CDPK
calcium-dependent protein kinase
- SPIK
Shaker pollen inward K+ channel
- K+in
inward K+
- MAPK
mitogen-activated protein kinase
- Co-IP
coimmunoprecipitation
- BiFC
bimolecular fluorescence complementation
- GST
glutathione S-transferase
- Gmax
normalized maximal conductance
References
- Allwood E.G., Smertenko A.P., Hussey P.J. (2001). Phosphorylation of plant actin-depolymerising factor by calmodulin-like domain protein kinase. FEBS Lett. 499: 97–100 [DOI] [PubMed] [Google Scholar]
- Amien S., Kliwer I., Márton M.L., Debener T., Geiger D., Becker D., Dresselhaus T. (2010). Defensin-like ZmES4 mediates pollen tube burst in maize via opening of the potassium channel KZM1. PLoS Biol. 8: e1000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker D., et al. (2004). AtTPK4, an Arabidopsis tandem-pore K+ channel, poised to control the pollen membrane voltage in a pH- and Ca2+-dependent manner. Proc. Natl. Acad. Sci. USA 101: 15621–15626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J.D., Boavida L.C., Carneiro J., Haury M., Feijó J.A. (2003). Transcriptional profiling of Arabidopsis tissues reveals the unique characteristics of the pollen transcriptome. Plant Physiol. 133: 713–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz G., Zhang X., Mercie R., Leng Q., Lawton M. (2000). Co-expression of calcium-dependent protein kinase with the inward rectified guard cell K+ channel KAT1 alters current parameters in Xenopus laevis oocytes. Plant Cell Physiol. 41: 785–790 [DOI] [PubMed] [Google Scholar]
- Boavida L.C., McCormick S. (2007). Temperature as a determinant factor for increased and reproducible in vitro pollen germination in Arabidopsis thaliana. Plant J. 52: 570–582 [DOI] [PubMed] [Google Scholar]
- Bock K.W., Honys D., Ward J.M., Padmanaban S., Nawrocki E.P., Hirschi K.D., Twell D., Sze H. (2006). Integrating membrane transport with male gametophyte development and function through transcriptomics. Plant Physiol. 140: 1151–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudsocq M., Droillard M.J., Regad L., Laurière C. (2012). Characterization of Arabidopsis calcium-dependent protein kinases: Activated or not by calcium? Biochem. J. 447: 291–299 [DOI] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Dammann C., Ichida A., Hong B., Romanowsky S.M., Hrabak E.M., Harmon A.C., Pickard B.G., Harper J.F. (2003). Subcellular targeting of nine calcium-dependent protein kinase isoforms from Arabidopsis. Plant Physiol. 132: 1840–1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta R., Robinson K.R. (2004). Identification and characterization of stretch-activated ion channels in pollen protoplasts. Plant Physiol. 135: 1398–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estruch J.J., Kadwell S., Merlin E., Crossland L. (1994). Cloning and characterization of a maize pollen-specific calcium-dependent calmodulin-independent protein kinase. Proc. Natl. Acad. Sci. USA 91: 8837–8841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L.M., Wang Y.F., Wang H., Wu W.H. (2001). In vitro Arabidopsis pollen germination and characterization of the inward potassium currents in Arabidopsis pollen grain protoplasts. J. Exp. Bot. 52: 1603–1614 [PubMed] [Google Scholar]
- Fan L.M., Wang Y.F., Wu W.H. (2003). Outward K+ channels in Brassica chinensis pollen protoplasts are regulated by external and internal pH. Protoplasma 220: 143–152 [DOI] [PubMed] [Google Scholar]
- Fan L.M., Wu W.H., Yang H.Y. (1999). Identification and characterization of the inward K+ channel in the plasma membrane of Brassica pollen protoplasts. Plant Cell Physiol. 40: 859–865 [DOI] [PubMed] [Google Scholar]
- Feijó J.A., Costa S.S., Prado A.M., Becker J.D., Certal A.C. (2004). Signalling by tips. Curr. Opin. Plant Biol. 7: 589–598 [DOI] [PubMed] [Google Scholar]
- Feijó J.A., Malhó R., Obermeyer G. (1995). Ion dynamics and its possible role during in vitro pollen germination and tube growth. Protoplasma 187: 155–167 [Google Scholar]
- Franklin-Tong N. (2010). Plant fertilization: Bursting pollen tubes! Curr. Biol. 20: R681–R683 [DOI] [PubMed] [Google Scholar]
- Franklin-Tong V.E. (1999). Signaling and the modulation of pollen tube growth. Plant Cell 11: 727–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D., Scherzer S., Mumm P., Marten I., Ache P., Matschi S., Liese A., Wellmann C., Al-Rasheid K.A., Grill E., Romeis T., Hedrich R. (2010). Guard cell anion channel SLAC1 is regulated by CDPK protein kinases with distinct Ca2+ affinities. Proc. Natl. Acad. Sci. USA 107: 8023–8028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong D., Guo Y., Jagendorf A.T., Zhu J.K. (2002). Biochemical characterization of the Arabidopsis protein kinase SOS2 that functions in salt tolerance. Plant Physiol. 130: 256–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griessner M., Obermeyer G. (2003). Characterization of whole-cell K+ currents across the plasma membrane of pollen grain and tube protoplasts of Lilium longiflorum. J. Membr. Biol. 193: 99–108 [DOI] [PubMed] [Google Scholar]
- Guo Y., Halfter U., Ishitani M., Zhu J.K. (2001). Molecular characterization of functional domains in the protein kinase SOS2 that is required for plant salt tolerance. Plant Cell 13: 1383–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J.F., Breton G., Harmon A. (2004). Decoding Ca(2+) signals through plant protein kinases. Annu. Rev. Plant Biol. 55: 263–288 [DOI] [PubMed] [Google Scholar]
- Held K., Pascaud F., Eckert C., Gajdanowicz P., Hashimoto K., Corratgé-Faillie C., Offenborn J.N., Lacombe B., Dreyer I., Thibaud J.B., Kudla J. (2011). Calcium-dependent modulation and plasma membrane targeting of the AKT2 potassium channel by the CBL4/CIPK6 calcium sensor/protein kinase complex. Cell Res. 21: 1116–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helling D., Possart A., Cottier S., Klahre U., Kost B. (2006). Pollen tube tip growth depends on plasma membrane polarization mediated by tobacco PLC3 activity and endocytic membrane recycling. Plant Cell 18: 3519–3534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler P.K., Vidali L., Cheung A.Y. (2001). Polarized cell growth in higher plants. Annu. Rev. Cell Dev. Biol. 17: 159–187 [DOI] [PubMed] [Google Scholar]
- Holdaway-Clarke T.L., Feijó J.A., Hackett G.R., Kunkel J.G., Hepler P.K. (1997). Pollen tube growth and the intracellular cytosolic calcium gradient oscillate in phase while extracellular calcium influx is delayed. Plant Cell 9: 1999–2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdaway-Clarke T.L., Hepler P.K. (2003). Control of pollen tube growth: Role of ion gradients and fluxes. New Phytol. 159: 539–563 [DOI] [PubMed] [Google Scholar]
- Hrabak E.M., et al. (2003). The Arabidopsis CDPK-SnRK superfamily of protein kinases. Plant Physiol. 132: 666–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J.U., Gu Y., Lee Y.J., Yang Z. (2005). Oscillatory ROP GTPase activation leads the oscillatory polarized growth of pollen tubes. Mol. Biol. Cell 16: 5385–5399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura K., Shinozaki K., Tena G., Sheen J., Henry Y., Champion A., Kreis M., Zhang S., Hirt H., Wilson C. MAPK Group (2002). Mitogen-activated protein kinase cascades in plants: A new nomenclature. Trends Plant Sci. 7: 301–308 [DOI] [PubMed] [Google Scholar]
- Jonak C., Okrész L., Bögre L., Hirt H. (2002). Complexity, cross talk and integration of plant MAP kinase signalling. Curr. Opin. Plant Biol. 5: 415–424 [DOI] [PubMed] [Google Scholar]
- Konrad K.R., Wudick M.M., Feijó J.A. (2011). Calcium regulation of tip growth: New genes for old mechanisms. Curr. Opin. Plant Biol. 14: 721–730 [DOI] [PubMed] [Google Scholar]
- Kudla J., Batistic O., Hashimoto K. (2010). Calcium signals: The lead currency of plant information processing. Plant Cell 22: 541–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Lee Y.R.J., Assmann S.M. (1998). Guard cells possess a calcium-dependent protein kinase that phosphorylates the KAT1 potassium channel. Plant Physiol. 116: 785–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Chanroj S., Zulkifli L., Johnson M.A., Uozumi N., Cheung A., Sze H. (2011). Pollen tubes lacking a pair of K+ transporters fail to target ovules in Arabidopsis. Plant Cell 23: 81–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig A.A., Saitoh H., Felix G., Freymark G., Miersch O., Wasternack C., Boller T., Jones J.D.G., Romeis T. (2005). Ethylene-mediated cross-talk between calcium-dependent protein kinase and MAPK signaling controls stress responses in plants. Proc. Natl. Acad. Sci. USA 102: 10736–10741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhó R., Trewavas A.J. (1996). Localized apical increases of cytosolic free calcium control pollen tube orientation. Plant Cell 8: 1935–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerli M.A., Créton R., Jaffe L.F., Robinson K.R. (2000). Periodic increases in elongation rate precede increases in cytosolic Ca2+ during pollen tube growth. Dev. Biol. 222: 84–98 [DOI] [PubMed] [Google Scholar]
- Messerli M.A., Danuser G., Robinson K.R. (1999). Pulsatile influxes of H+, K+ and Ca2+ lag growth pulses of Lilium longiflorum pollen tubes. J. Cell Sci. 112: 1497–1509 [DOI] [PubMed] [Google Scholar]
- Messerli M.A., Robinson K.R. (1997). Tip localized Ca2+ pulses are coincident with peak pulsatile growth rates in pollen tubes of Lilium longiflorum. J. Cell Sci. 110: 1269–1278 [DOI] [PubMed] [Google Scholar]
- Michard E., Alves F., Feijó J.A. (2009). The role of ion fluxes in polarized cell growth and morphogenesis: The pollen tube as an experimental paradigm. Int. J. Dev. Biol. 53: 1609–1622 [DOI] [PubMed] [Google Scholar]
- Michard E., Lima P.T., Borges F., Silva A.C., Portes M.T., Carvalho J.E., Gilliham M., Liu L.H., Obermeyer G., Feijó J.A. (2011). Glutamate receptor-like genes form Ca2+ channels in pollen tubes and are regulated by pistil D-serine. Science 332: 434–437 [DOI] [PubMed] [Google Scholar]
- Miller D.D., Callaham D.A., Gross D.J., Hepler P.K. (1992). Free Ca2+ gradient in growing pollen tubes of Lillium. J. Cell Sci. 101: 7–12 [Google Scholar]
- Mori I.C., Murata Y., Yang Y., Munemasa S., Wang Y.F., Andreoli S., Tiriac H., Alonso J.M., Harper J.F., Ecker J.R. (2006). CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion- and Ca2+-permeable channels and stomatal closure. PLoS Biol. 4: 1749–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouline K., Véry A.A., Gaymard F., Boucherez J., Pilot G., Devic M., Bouchez D., Thibaud J.B., Sentenac H. (2002). Pollen tube development and competitive ability are impaired by disruption of a Shaker K(+) channel in Arabidopsis. Genes Dev. 16: 339–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutinho A., Trewavas A.J., Malhó R. (1998). Relocation of a Ca2+-dependent protein kinase activity during pollen tube reorientation. Plant Cell 10: 1499–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers C., Romanowsky S.M., Barron Y.D., Garg S., Azuse C.L., Curran A., Davis R.M., Hatton J., Harmon A.C., Harper J.F. (2009). Calcium-dependent protein kinases regulate polarized tip growth in pollen tubes. Plant J. 59: 528–539 [DOI] [PubMed] [Google Scholar]
- Nakagami H., Pitzschke A., Hirt H. (2005). Emerging MAP kinase pathways in plant stress signalling. Trends Plant Sci. 10: 339–346 [DOI] [PubMed] [Google Scholar]
- Obermeyer G., Blatt M.R. (1995). Electrical properties of intact pollen grains of Lilium longiflorum: Characteristics of the non-germinating pollen grain. J. Exp. Bot. 46: 803–813 [Google Scholar]
- Obermeyer G., Kolb H.A. (1993). K+ channels in the plasma membrane of lily pollen protoplasts. Bot. Acta 106: 26–31 [Google Scholar]
- Pei Z.M., Ward J.M., Harper J.F., Schroeder J.I. (1996). A novel chloride channel in Vicia faba guard cell vacuoles activated by the serine/threonine kinase, CDPK. EMBO J. 15: 6564–6574 [PMC free article] [PubMed] [Google Scholar]
- Pina C., Pinto F., Feijó J.A., Becker J.D. (2005). Gene family analysis of the Arabidopsis pollen transcriptome reveals biological implications for cell growth, division control, and gene expression regulation. Plant Physiol. 138: 744–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu H.Y., Shang Z.L., Zhang S.L., Liu L.M., Wu J.Y. (2007). Identification of hyperpolarization-activated calcium channels in apical pollen tubes of Pyrus pyrifolia. New Phytol. 174: 524–536 [DOI] [PubMed] [Google Scholar]
- Rato C., Monteiro D., Hepler P.K., Malhó R. (2004). Calmodulin activity and cAMP signalling modulate growth and apical secretion in pollen tubes. Plant J. 38: 887–897 [DOI] [PubMed] [Google Scholar]
- Rodriguez M.C., Petersen M., Mundy J. (2010). Mitogen-activated protein kinase signaling in plants. Annu. Rev. Plant Biol. 61: 621–649 [DOI] [PubMed] [Google Scholar]
- Rodriguez Milla M.A., Uno Y., Chang I.F., Townsend J., Maher E.A., Quilici D., Cushman J.C. (2006). A novel yeast two-hybrid approach to identify CDPK substrates: Characterization of the interaction between AtCPK11 and AtDi19, a nuclear zinc finger protein. FEBS Lett. 580: 904–911 [DOI] [PubMed] [Google Scholar]
- Romeis T., Ludwig A.A., Martin R., Jones J.D.G. (2001). Calcium-dependent protein kinases play an essential role in a plant defence response. EMBO J. 20: 5556–5567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeis T., Piedras P., Jones J.D.G. (2000). Resistance gene-dependent activation of a calcium-dependent protein kinase in the plant defense response. Plant Cell 12: 803–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S.J., Holdaway-Clarke T.L., Hackett G.R., Kunkel J.G., Lord E.M., Hepler P.K. (1999). Uncoupling secretion and tip growth in lily pollen tubes: Evidence for the role of calcium in exocytosis. Plant J. 19: 379–386 [DOI] [PubMed] [Google Scholar]
- Schroeder J.I., Hagiwara S. (1989). Cytosolic calcium regulates ion channels in the plasma membrane of Vicia faba guard cells. Nature 338: 427–430 [Google Scholar]
- Snowman B.N., Kovar D.R., Shevchenko G., Franklin-Tong V.E., Staiger C.J. (2002). Signal-mediated depolymerization of actin in pollen during the self-incompatibility response. Plant Cell 14: 2613–2626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L.F., Zou J.J., Zhang W.Z., Wu W.H., Wang Y. (2009). Ion transporters involved in pollen germination and pollen tube tip-growth. Plant Signal. Behav. 4: 1193–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze H., Padmanaban S., Cellier F., Honys D., Cheng N.H., Bock K.W., Conéjéro G., Li X., Twell D., Ward J.M., Hirschi K.D. (2004). Expression patterns of a novel AtCHX gene family highlight potential roles in osmotic adjustment and K+ homeostasis in pollen development. Plant Physiol. 136: 2532–2547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares B., Dias P.N., Domingos P., Moura T.F., Feijó J.A., Bicho A. (2011). Calcium-regulated anion channels in the plasma membrane of Lilium longiflorum pollen protoplasts. New Phytol. 192: 45–60 [DOI] [PubMed] [Google Scholar]
- Taylor L.P., Hepler P.K. (1997). Pollen germination and tube growth. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48: 461–491 [DOI] [PubMed] [Google Scholar]
- Walter M., Chaban C., Schütze K., Batistic O., Weckermann K., Näke C., Blazevic D., Grefen C., Schumacher K., Oecking C., Harter K., Kudla J. (2004). Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J. 40: 428–438 [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhang W.Z., Song L.F., Zou J.J., Su Z., Wu W.H. (2008). Transcriptome analyses show changes in gene expression to accompany pollen germination and tube growth in Arabidopsis. Plant Physiol. 148: 1201–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenseel M.H., Jaffe L.F. (1976). The major growth current through lily pollen tubes enters as K+ and leaves as H+. Planta 133: 1–7 [DOI] [PubMed] [Google Scholar]
- Wilhelmi L.K., Preuss D. (1999). The mating game: Pollination and fertilization in flowering plants. Curr. Opin. Plant Biol. 2: 18–22 [DOI] [PubMed] [Google Scholar]
- Wu J.Y., Qu H.Y., Shang Z.L., Tao S.T., Xu G.H., Wu J., Wu H.Q., Zhang S.L. (2011). Reciprocal regulation of Ca²+-activated outward K+ channels of Pyrus pyrifolia pollen by heme and carbon monoxide. New Phytol. 189: 1060–1068 [DOI] [PubMed] [Google Scholar]
- Xu J., Li H.D., Chen L.Q., Wang Y., Liu L.L., He L., Wu W.H. (2006). A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell 125: 1347–1360 [DOI] [PubMed] [Google Scholar]
- Yoon G.M., Dowd P.E., Gilroy S., McCubbin A.G. (2006). Calcium-dependent protein kinase isoforms in Petunia have distinct functions in pollen tube growth, including regulating polarity. Plant Cell 18: 867–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S.Y., et al. (2007). Two calcium-dependent protein kinases, CPK4 and CPK11, regulate abscisic acid signal transduction in Arabidopsis. Plant Cell 19: 3019–3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J.J., Wei F.J., Wang C., Wu J.J., Ratnasekera D., Liu W.X., Wu W.H. (2010). Arabidopsis calcium-dependent protein kinase CPK10 functions in abscisic acid- and Ca2+-mediated stomatal regulation in response to drought stress. Plant Physiol. 154: 1232–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]