Abstract
Th17 CD4+ cells promote inflammation and autoimmunity. Here we report that Th17 cell frequency is reduced in ob/ob mice that are genetically deficient in the adipokine leptin, and that the administration of leptin to ob/ob mice restored Th17 cell numbers to values comparable to those found in wild type animals. Leptin promoted Th17 responses in normal human CD4+ T cells and in mice, both in vitro and in vivo, by inducing RORγt transcription. Leptin also increased Th17 responses in (NZB × NZW)F1 lupus-prone mice, whereas its neutralization in those autoimmune-prone mice inhibited Th17 responses. Since Th17 cells play an important role in the development and maintenance of inflammation and autoimmunity, these findings envision the possibility to modulate abnormal Th17 responses via leptin manipulation, and reiterate the link between metabolism/nutrition and susceptibility to autoimmunity.
Introduction
Leptin is an adipokine that has structural characteristics of the long-chain helical cytokine family (that includes IL-3, IL-6 and IL-12) (1) and binds to a receptor that shares homology with the IL-6 receptor (2). Although the most apparent characteristic of leptin is to control metabolism and energy expenditure, leptin has additional activities that include the ability to modulate immune responses (3). In innate immunity, leptin facilitates the activation of NK cells, chemotaxis of neutrophils, and the secretion of TNF-α, IL-6 and IL-12 from macrophages (4). In adaptive immunity, leptin stimulates the proliferation of naïve T cells and inhibits CD4+CD25+FOXP3+ regulatory T cells (Tregs) (5). Importantly, leptin accelerates the development and progression of autoimmune diseases including experimental autoimmune encephalomyelitis (EAE), antigen-induced arthritis, and experimentally induced colitis (6). Conversely, ob/ob mice - that have a genetic deficiency of leptin due to a mutation in the leptin gene (2) - have a reduced susceptibility to develop autoimmunity and display elevated numbers of peripheral Tregs (7).
In systemic lupus erythematosus (SLE), a chronic autoimmune disease that is characterized by the presence of multiple autoantibodies and elevated numbers of autoreactive CD4+ T cells (8), leptin is abnormally elevated (9). Moreover, an altered regulation of metabolism has been suggested to contribute to the pathogenesis of SLE (10-11). Another pro-inflammatory cytokine, IL-17, is also significantly increased in SLE patients (12-14). IL-17 promotes inflammation and autoimmune responses in several animal models of autoimmune diseases (15-17) and is produced by the Th17 subset of CD4+ T helper (Th) cells (16).
Here we describe a link, both at the molecular and cellular levels, between leptin and IL-17. Leptin deficiency associated with a reduced frequency of Th17 cells that was restored to levels comparable to those found in wild type (WT) animals after administration of leptin. We also found that leptin facilitated Th17 responses by inducing RORγt transcription in CD4+ T cells.
This capacity of leptin to promote Th17 cell differentiation identifies leptin as a new target for the modulation of Th17 immune reactivity in normal and pathologic conditions.
Materials and Methods
Mice
C57BL6/J (B6) wild-type (WT) mice, leptin-deficient B6ob/ob (ob/ob), leptin receptor-deficient B6db/db (db/db) and (NZB × NZW)F1 (NZB/W) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). RORγt-/- (B6-Rorctm3Litt × CD4-Cre) mice were from Taconic (Hudson, NY). The mice were maintained at the University of California Los Angeles (UCLA) with a 12 hr light/dark cycle and were 8-to-12 weeks old and age-matched when used for the experiments, under approved protocols, in accordance with institutional and federal regulations.
Human studies
Blood was drawn from SLE patients that fulfilled the American College of Rheumatology criteria for the classification of SLE, and from healthy matched donors. All SLE patients had stable disease and a therapeutic steroid dose < 10 mg/day. The study was conducted according to the principles of the Declaration of Helsinki and was approved by the Institutional Review Board of UCLA. SLE patients with comorbid conditions were excluded from the investigation.
Cell preparation
For the human studies, peripheral blood mononuclear cells (PBMC) were prepared by Ficoll gradient. After red blood cell (RBC) lysis, PBMC were washed prior to culture in AIM-V medium (Invitrogen, Carslbad, CA) supplemented with penicillin and streptomycin. For the mouse studies, explanted spleens were teased into single cell suspensions and filtered through a 70 μm cell strainer. After RBC lysis, splenocytes were either cultured in vitro or used for the purification of CD4+ T cells by negative selection using magnetic beads (Miltenyi Biotec, Auburn, CA), according to the manufacturer's instructions. Mouse PBMC were obtained from peripheral blood and cultured in HL-1 medium (Lonza, Walkersville, MD) supplemented with 2% mouse serum (Sigma-Aldrich, St. Louis, MO), penicillin and streptomycin, 2mM glutamine and 5 ×10-5 M 2-β mercaptoethanol.
Flow cytometry
For intracellular staining, cells were incubated for 4–5 hr with 25 ng/ml PMA (Sigma-Aldrich), 2 μg/ml ionomycin (Sigma-Aldrich) and 10 μg/ml brefeldin A (eBioscience, San Diego, CA) at 37° C/5% CO2. After surface staining with fluorescent-labeled anti-CD4, anti-RORγt (eBioscience) and anti-Ob-R Ab (R&D Systems, Minneapolis, MN), cells were resuspended in fixation/permeabilization buffer (eBioscience) for intracellular staining with fluorescent-labeled anti-IL-17 (eBioscience) and anti-Stat3/anti-pStat3 Ab (BD Biosciences, San Jose, CA). Flow cytometry was performed on a FACSCalibur™ instrument (BD Biosciences) and analysis was done using FlowJo software (Tree Star Inc., Ashland, OR).
ELISA
Plasma IL-17 or leptin in human and murine samples were measured using ELISA kits from R&D Systems. Measurements were done according to the manufacturer's instructions.
Leptin reagents
Recombinant mouse leptin and human leptin were purchased from R&D Systems. Anti-leptin Ab was purchased from Cell Sciences (Canton, MA).
Leptin treatment
For in vivo experiments, ob/ob mice or NZB/W mice were injected i.p. twice daily with leptin dissolved in 200 μl saline at a dose of 1 μg/g body weight for 10 days. Age-and sex-matched mice received similar volumes of saline with the same schedule. A group of mice received 150 μg anti-leptin Ab or isotype-matched control Ab i.p. twice, at two days intervals. On day 10, PBMC and splenocytes were collected for flow cytometry analyses. For in vitro experiments with mouse cells, splenocytes were cultured in complete medium or under Th17 polarizing conditions (2 μg/ml anti-CD3 Ab, 2 μg/ml anti-CD28 Ab, 40 U/ml IL-2, 20 ng/ml IL-6, 5 ng/ml TGFβ, 10 μg/ml anti-IL-4 Ab, 10 μg/ml anti-INFγ Ab) for 4 days in a 37°C/5% CO2 incubator. Leptin was added during the last 18 hours before cells underwent intracellular cytokine staining and flow cytometry. For the in vitro experiments with human cells, PBMC were stimulated with Dynabeads CD3/CD28 T Cell Expander (Invitrogen) plus 20 ng/ml IL-6 and 5 ng/ml TGF-β for 4 days in a 37°C/5% CO2 for Th17 polarization. Human leptin was added during the last 18 hours prior to flow cytometry analyses.
Plasmids and retrovirus production
The RORγt (RORγt-IRES-GFP) and control pMIG (IRES-GFP) plasmids (retrovirus-based vectors containing GFP under the regulation of an internal ribosome entry site) were kindly provided by Dr. Littman (18). Phoenix cells were transfected with 4 μg of plasmids using Lipofectamin 2000 (Invitrogen) on day 0. Viral supernatant was collected on day 2-3 and supplemented with 8 μg/ml polybrene (Sigma) before use.
Cell cultures and retroviral transduction
5 × 105/well CD4+ T cells that had been negatively sorted from RORγt-/- mouse splenocytes using magnetic beads were cultured in 96-well-flat bottom plates containing anti-CD3 Ab (2 μg/ml), anti-CD28 Ab (2 μg/ml), and mouse IL-2 (40 U/ml) on day 0. For viral transduction, on days 1 and 2, viral supernatant was added and cells were spun at 2,500 rpm for 1.5 hr at 30° C. After spin infection, the cells were put in culture media in the presence of anti-CD3 Ab/anti-CD28 Ab/IL-2, and harvested on day 5 for staining and flow cytometry. Cultures had scalar concentrations of leptin (or medium only) added during the last 18 hours.
Statistical analyses
The t test was used for two group analyses, and Kruskal-Wallis ANOVA was used for analyses of three or more groups using GraphPad Prizm software (San Diego, CA). Results are expressed as mean ± SD. P<0.05 was considered statistically significant.
Results and Discussion
Mice that are genetically deficient in leptin/leptin receptor have a reduced frequency of Th17 cells
Leptin-deficient ob/ob and leptin receptor (Ob-R)-deficient db/db mice had reduced numbers of peripheral Th17 cells among PBMC and splenocytes in comparison to WT mice (Supplemental Figure 1a-d). In line with this finding, ob/ob and db/db mice had lower plasma IL-17 levels than WT mice (Supplemental Figure 1e), suggesting that an impaired leptin/leptin receptor axis associated with reduced IL-17 responses in vivo. This result is consistent with the observation that in obesity, which is characterized by elevated leptinemia, there is an increase in plasma concentration of IL-17 (19).
Leptin increases Th17 cell frequency in vitro and in vivo
To investigate the above link between leptin and Th17 cells, we cultured ob/ob splenocytes with recombinant leptin. A dose-dependent increase in the number of IL-17+ T cells was observed in the presence of scalar doses of leptin (Fig. 1a-c). A concomitant upregulation of surface expression of Ob-R was also found by flow cytometry (not shown). To extend these findings to an in vivo system, we treated ob/ob mice with recombinant leptin or saline (as control). Leptin-treated mice had the expected weight loss (P<0.01, not shown) associated with the inhibition of appetite by leptin (2). Interestingly, leptin treatment associated with an expansion of Th17 cells among splenocytes (Fig. 1d-e) and PBMC (Fig. 1f), together with an upregulation of the surface expression of Ob-R in Th17 cells and an increase in the phosphorylation of Stat3 (which is activated by leptin) by flow cytometry (not shown).
Figure 1.
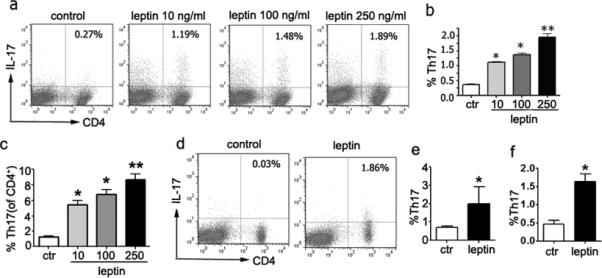
Leptin increases Th17 cell frequency in vitro and in vivo. Splenocytes from untreated ob/ob mice were stimulated under Th17 polarizing conditions and leptin was added at scalar doses during the last 18 hours of culture. The figure shows representative (a) and cumulative data from total splenocytes (b) and gated CD4+ T cells (c) from 8 mice in three independent experiments. (d-e) Representative (d) and cumulative data (e) from two experiments on splenocytes from ob/ob mice (n = 10) that were treated in vivo with leptin or saline (as control), according to the protocol described in the Materials and Methods. (f) IL-17+ T cells in PBMC from ob/ob mice treated with leptin (n = 10 per group, two experiments). *P<0.01, **P<0.007.
Leptin induces RORγt transcription in CD4+ T cells
Since leptin increased Th17 cell frequency in vitro and in vivo (Fig. 1), we tested whether leptin could promote Th17 differentiation. RORγt (retinoic acid receptor related orphan nuclear hormone receptor family) is a transcription factor that acts as a master regulator in the differentiation of Th17 cells (19-20). To investigate the effects of leptin on the expression of RORγt, we evaluated RORγt expression in negatively sorted RORγt-/- CD4+ T cells infected with RORγt-expressing vector or control after culture in the presence of scalar doses of leptin. As shown in Fig. 2a-b, leptin increased RORγt expression and IL-17 production in a dose-dependent fashion in CD4+ T cells. The surface expression of Ob-R was concomitantly upregulated on CD4+ T cells that differentiated into Th17 cells, together with an increased Stat3 phosphorylation by flow cytometry (not shown). Leptin also increased RORγt expression and IL-17 production in CD4+ T cells from ob/ob mice (Fig. 2c).
Figure 2.
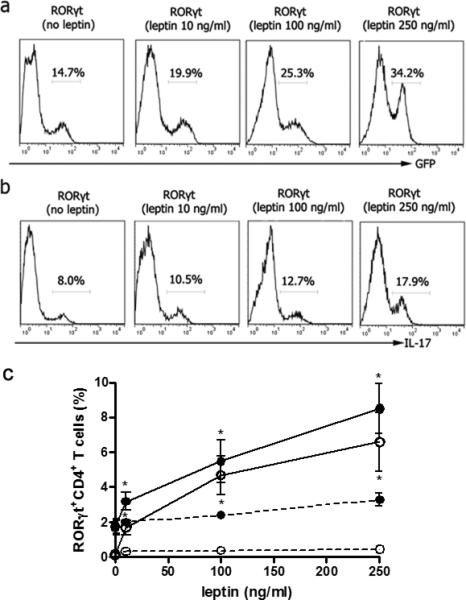
Leptin increases RORγt transcription and IL-17 production in CD4+ T cells. Sorted RORγt-/- CD4+ T cells were stimulated with anti-CD3/CD28 Ab for 24 hours, transduced with retrovirus encoding RORγt, and restimulated with anti-CD3/CD28 Ab and scalar doses of leptin for 18 hours. The figure shows the flow cytometry results of RORγt (GFP) (a) and IL-17 expression (b) in the presence of scalar doses of leptin. c. Flow cytometry for RORγt expression in ob/ob CD4+ T cells cultured under polarizing (closed circles) or non-polarizing (open circles) conditions in the presence of scalar doses of leptin during the last 18 hours, stimulated (straight lines) or not (dashed lines) with PMA/ionomycin *P<0.05 in the comparison between polarizing and non-polarizing conditions.
Leptin promotes Th17 responses in human CD4+ T cells
In the presence of increasing concentrations of leptin, Th17 cell numbers increased in a dose-dependent manner in cultures of healthy human PBMC (Supplemental Figure 2a-b). IL-17 was concomitantly higher in culture supernatants (Supplemental Figure 2c). Moreover, a positive correlation between plasma leptin and IL-17 was found in SLE patients but not in healthy matched controls (Supplemental Figure 2d).
Leptin promotes Th17 responses in NZB/W lupus-prone mice
Recent work has suggested that Th17 cells can contribute to the pathogenesis of SLE in humans (12-13) and in lupus animal models (21-22) - where IL-17 blockade has been proven beneficial in reducing disease manifestations (23).
When NZB/W splenocytes were cultured in the presence of scalar doses of leptin, a dose-dependent increase in Th17 cell frequency was observed (Fig. 3a-b). In vivo, the administration of leptin to NZB/W mice associated with an increased expression of Ob-R on Th17 cells by flow cytometry (not shown) and an increased number of peripheral Th17 cells, whereas treatment with anti-leptin Ab associated with a reduction in the number of Th17 cells (Fig. 3c-d). Thus, leptin promoted - and leptin blockade inhibited – Th17 responses in NZB/W lupus mice.
Figure 3.
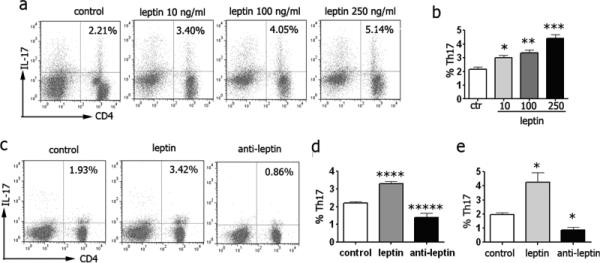
Leptin promotes Th17 responses in NZB/W lupus mice. Representative (a) and cumulative data (b) (n = 8) from NZB/W mouse splenocytes cultured with scalar doses of leptin for 18 hours and stimulated with PMA/iomycin during the last 5 hr. Representative (c) and cumulative data (d) in PBMC (n = 8 per group) or splenocytes (e) (n = 8 per group) from NZB/W mice treated with saline, leptin or anti-leptin Ab (see Materials and Methods for details). *P<0.04, **P<0.006, ***P<0.002, ****P<0.004, *****P<0.02.
In summary, we report a positive association between leptin and Th17 responses that associates with leptin-induced effects on the transcription of the Th17 master regulator RORγt. Together with the findings of a Th17-promoting effect of leptin on collagen-induced arthritis (24) and an inhibitory effect of anti-leptin Ab on Th17 responses in Hashimoto's thyroidits (25), our results suggest a potential for leptin-based manipulation of Th17 responsiveness in those conditions where Th17 cells have detrimental effects on the disease course.
Supplementary Material
Acknowledgements
We are grateful to Dr. Dan Littman (New York University) for providing the RORγt and control pMIG constructs.
Footnotes
This work was supported by the National Institutes of Health grants AR53239 and AI095921 to A.L.C. G.M. is supported by grants from the E.U. Ideas Programme, ERC-Starting Grant 310496, Telethon-JDRF grant GJT08004 and FIRB MERIT Grant RBNE08HWLZ .
Disclosure
The authors declare no conflict of interest.
References
- 1.Zhang F, Basinski MB, Beals JM, Briggs SL, Churgay LM, Clawson DK, DiMarchi RD, Furman TC, Hale JE, Hsiung HM, Schoner BE, Smith DP, Zhang XY, Wery JP, Schevitz RW. Crystal structure of the obese protein leptin-E100. Nature. 1997;387:206–209. doi: 10.1038/387206a0. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 3.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- 4.La Cava A, Matarese G. The weight of leptin in immunity. Nat. Rev. Immunol. 2004;4:371–379. doi: 10.1038/nri1350. [DOI] [PubMed] [Google Scholar]
- 5.De Rosa V, Procaccini C, Calì G, Pirozzi G, Fontana S, Zappacosta S, La Cava A, Matarese G. A key role of leptin in the control of regulatory T cell proliferation. Immunity. 2007;26:241–255. doi: 10.1016/j.immuni.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Matarese G, Leiter EH, La Cava A. Leptin in autoimmunity: many questions, some answers. Tissue Antigens. 2007;70:87–95. doi: 10.1111/j.1399-0039.2007.00886.x. [DOI] [PubMed] [Google Scholar]
- 7.Matarese G, Carrieri PB, La Cava A, Perna F, Sanna V, De Rosa V, Aufiero D, Fontana S, Zappacosta S. Leptin increase in multiple sclerosis associates with reduced number of CD4+CD25+ regulatory T cells. Proc. Natl. Acad. Sci. USA. 2005;102:5150–5155. doi: 10.1073/pnas.0408995102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hahn BH. Antibodies to DNA. N. Engl. J. Med. 1998;338:1359–1368. doi: 10.1056/NEJM199805073381906. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Gonzalez A, Gonzalez-Lopez L, Valera-Gonzalez IC, Cardona-Muñoz EG, Salazar-Paramo M, González-Ortiz M, Martínez-Abundis E, Gamez-Nava JI. Serum leptin levels in women with systemic lupus erythematosus. Rheumatol. Int. 2002;22:138–141. doi: 10.1007/s00296-002-0216-9. [DOI] [PubMed] [Google Scholar]
- 10.Caza TN, Talaber G, Perl A. Metabolic regulation of organelle homeostasis in lupus T cells. Clin. Immunol. 2012;144:200–213. doi: 10.1016/j.clim.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsokos GC. Systemic lupus erythematosus. N. Engl. J. Med. 2011;365:2110–2121. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 12.Crispín JC, Oukka M, Bayliss G, Cohen RA, Van Beek CA, Stillman IE, Kyttaris VC, Juang YT, Tsokos GC. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J. Immunol. 2008;181:8761–8766. doi: 10.4049/jimmunol.181.12.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah K, Lee WW, Lee SH, Kim SH, Kang SW, Craft J, Kang I. Dysregulated balance of Th17 and Th1 cells in systemic lupus erythematosus. Arthritis Res. Ther. 2010;12:R53. doi: 10.1186/ar2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nalbandian A, Crispín JC, Tsokos GC. Interleukin-17 and systemic lupus erythematosus: current concepts. Clin. Exp. Immunol. 2009;157:209–215. doi: 10.1111/j.1365-2249.2009.03944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bettelli E, Carrier Y, Cao W, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector Th17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 16.Bettelli E, Korn T, Kuchroo VK. Th17: the third member of the effector T cell trilogy. Curr. Opin. Immunol. 2007;19:652–657. doi: 10.1016/j.coi.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 18.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 19.Sumarac-Dumanovic M, Stevanovic D, Ljubic A, Jorga J, Simic M, Stamenkovic-Pejkovic D, Starcevic V, Trajkovic V, Micic D. Increased activity of interleukin-23/interleukin-17 proinflammatory axis in obese women. Int. J. Obes. (Lond) 2009;33:151–156. doi: 10.1038/ijo.2008.216. [DOI] [PubMed] [Google Scholar]
- 20.Manel N, Unutmaz D, Littman DR. The differentiation of human TH-17 cells requires transforming growth factor-β and induction of the nuclear receptor RORγt. Nat. Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacob N, Yang H, Pricop L, Liu Y, Gao X, Zheng SG, Wang J, Gao HX, Putterman C, Koss MN, Stohl W, Jacob CO. Accelerated pathological and clinical nephritis in systemic lupus erythematosus-prone New Zealand Mixed 2328 mice doubly deficient in TNF receptor 1 and TNF receptor 2 via a Th17-associated pathway. J. Immunol. 2009;182:2532–2541. doi: 10.4049/jimmunol.0802948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu HC, Yang P, Wang J, Wu Q, Myers R, Chen J, Yi J, Guentert T, Tousson A, Stanus AL. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat. Immunol. 2008;9:166–175. doi: 10.1038/ni1552. [DOI] [PubMed] [Google Scholar]
- 23.Hou LF, He SJ, Li X, Yang Y, He PL, Zhou Y, Zhu FH, Yang YF, Li Y, Tang W, Zuo JP. Oral administration of artemisinin analog SM934 ameliorates lupus syndromes in MRL/lpr mice by inhibiting Th1 and Th17 cell responses. Arthritis Rheum. 2011;63:2445–2455. doi: 10.1002/art.30392. [DOI] [PubMed] [Google Scholar]
- 24.Deng J, Liu Y, Yang M, Wang S, Zhang M, Wang X, Ko KH, Hua Z, Sun L, Cao X, Lu L. Leptin exacerbates collagen-induced arthritis via enhancement of Th17 cell response. Arthritis Rheum. 2012;64:3564–3573. doi: 10.1002/art.34637. [DOI] [PubMed] [Google Scholar]
- 25.Wang S, Baidoo SE, Liu Y, Zhu C, Tian J, Ma J, Tong J, Chen J, Tang X, Xu H, Lu L. T cell-derived leptin contributes to increased frequency of T helper type 17 cells in female patients with Hashimoto's thyroiditis. Clin. Exp. Immunol. 2013;171:63–68. doi: 10.1111/j.1365-2249.2012.04670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


