Abstract
Objective
The objective of this article is to broadly review the scientific literature and summarize the most up-to-date findings on ovarian cancer health disparities worldwide and in the United States (U.S.).
Methods
The present literature on disparities in ovarian cancer was reviewed. Original research and relevant review articles were included.
Results
Ovarian cancer health disparities exist worldwide and in the U.S. Ovarian cancer disproportionately affect African American women at all stages of the disease, from presentation through treatment, and ultimately increased mortality and decreased survival, compared to non-Hispanic White women. Increased mortality is likely to be explained by unequal access to care and non-standard treatment regimens frequently administered to African American women, but may also be attributed to genetic susceptibility, acquired co-morbid conditions and increased frequency of modifiable risk factors, albeit to substantially lesser extent. Unequal access to care is, in turn, largely a consequence of lower socioeconomic status and lack of private health insurance coverage among the African American population.
Conclusions
Our findings suggest the need for policy changes aimed at facilitating equal access to quality medical care. At the same time, further research is necessary to fully resolve racial disparities in ovarian cancer.
Keywords: Ovarian cancer, Race, Health disparities
Introduction
Ovarian cancer is the sixth most common cancer and the seventh cause of death from cancer in women worldwide [1]. However, this malignancy takes even greater toll on females in the United States (U.S.), where it is the fifth leading cause of cancer-related deaths in women (after lung, breast, colorectal, and pancreatic cancers) and is the most common type of gynecological malignancy [1]. Although ovarian cancer accounts for only 3% of all cancers in U.S. women, this malignancy is disproportionally deadly due to the absence of either specific symptoms or effective screening and early detection strategies, leading to over 70% of patients being diagnosed with stage III and IV tumors, which generally have a poor prognosis even with aggressive and immediate treatment. Indeed, the average relative 5-year survival rates for stage III and IV tumors are 35% and 20%, respectively [2].
In addition to being disproportionally deadly, ovarian cancer is a striking example of racial-related health disparity. Worldwide, the highest incidences are observed in non-Hispanic White, followed by Hispanic, Asian and African women; however, mortalities are higher in Africa where access to accurate diagnostics and sophisticated treatments is limited. In the U.S., ovarian cancer incidences follow the worldwide trend; however, mortality is increased in American women of African descent despite presumable equal access to medical care. In addition, African American women present with more advanced tumors [3–10], tend to have a higher prevalence of unstaged or not classified tumors [4,11,12], are reportedly being undertreated or untreated [13–16], and have shorter disease-free survival [2]. These factors may predispose women of African descent that are diagnosed with ovarian cancer to higher death rates (71%) compared to women of European (66%), Hispanic (59%) or Asian (50%) descent [1]. Though the exact causes of racial disparities in ovarian cancer remain unclear, they are likely to be multifaceted [9,17]. Numerous reports elucidate racial disparities across the entire continuum of the disease; however, attempts to gather the separate, sporadic information into a comprehensive, “big picture” perspective have been rare. The goal of this manuscript is to review the scientific literature and summarize the most up-to-date findings on ovarian cancer racial health disparities. This manuscript will thus facilitate an understanding of the various ovarian cancer health disparities in the U.S. and worldwide, supporting an effort to eradicate them.
Ovarian cancer incidence
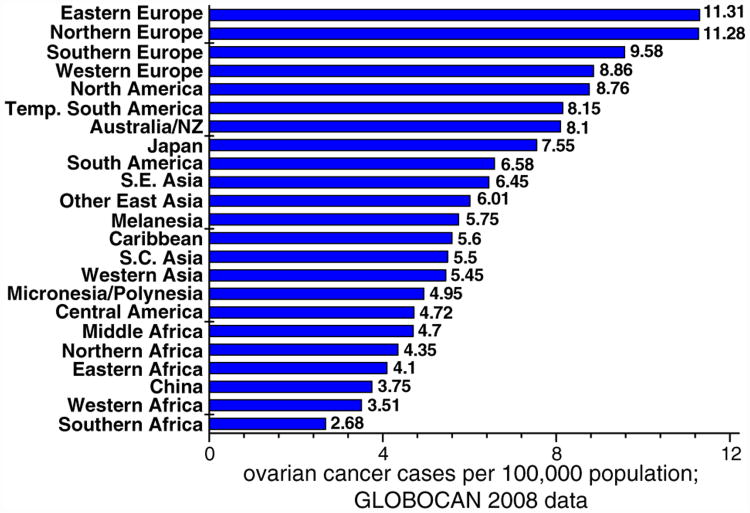
A striking disparity in ovarian cancer incidence is observable worldwide. The highest incidences are reported in Europe and North America (Canada and the United States). The lowest incidences are noted in China and Africa. According to the GLOBOCAN 2008 database [18], the incidence rates range from over 11 cases per 100,000 in Europe to less than 3 cases per 100,000 in Africa. The majority of South American, Asian, and Caribbean countries are at the intermediate range of 4–8 cases per 100,000 (Fig. 1). Because ovarian cancer is generally a disease of older women, reduced life expectancy observed in the developing countries, may potentially lead to decreased ovarian cancer rates. However, confounding factors such as access and quality of care and early detection significantly complicate teasing out the direct effect of life expectancy on ovarian cancer. It appears, with the information that is available, that other factors (unrelated to life expectancy) influence worldwide disparities observed in ovarian cancer incidence and mortality.
Fig. 1.
Incidence of ovarian cancer by world region. GLOBOCAN 2008 data. Complete list of countries included in the world regions, is shown in Appendix A. NZ: New Zealand; S.E. Asia: South-Eastern Asia; S.C. Asia: South-Central Asia.
Parkin et al. [19] presented a comprehensive review of ovarian cancer incidence in 23 world regions during the period of 1983-1993. By comparing the data by Parkin et al. [19] with the most recent Globocan data (Fig. 1), it could be concluded that, although individual world regions might have slightly changed positions in relation to each other, global ovarian cancer incidences and disparities around the world have not changed significantly in the last 30 years.
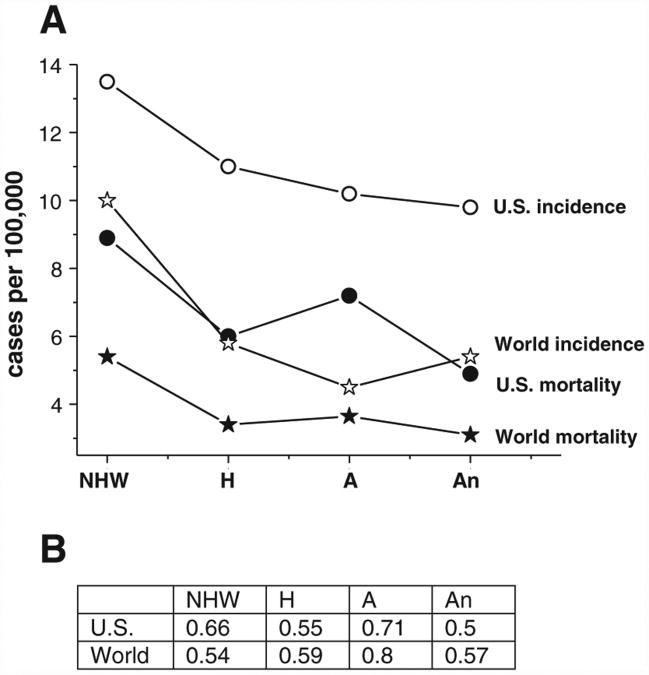
Both the worldwide and U.S. trends reveal the highest incidence in the non-Hispanic White women, followed by Hispanic, African and Asian women (Fig. 2A, open symbols). However, absolute incidence rates are proportionally higher in the U.S. for every racial group (Fig. 2A, open symbols), compared with the worldwide rates. Although no comprehensive explanation to this phenomenon exists at this time, it is plausible to suggest that environmental factors that influence an average U.S. female (lower childbearing, diets higher in saturated fats and caloric load and decreased physical activity), as well as increased life expectancy combined with improved detection strategies, might be at least partially responsible for the observed increase in ovarian cancer incidence. Additional research is required to fully elucidate the grounds for increased susceptibility of U.S. females to ovarian cancer.
Fig. 2.
A. Ovarian cancer incidence (open symbols) and mortality (closed symbols) rates worldwide (stars) and in the U.S. (circles). B. Mortality/incidence (M/I) ratios. Average worldwide rates were calculated using the GLOBOCAN 2008 database [18]: NHW (Non-Hispanic White) women — Northern, Central, Eastern and Western Europe;H (Hispanic) women — Central and South Americas and Mexico; A (African) women — North and Middle Africa; An (Asian) women — South-East, South-Central and East Asia.
Ovarian cancer mortality
Worldwide ovarian cancer absolute mortality is by large proportional to its incidence; however, developed countries tend to demonstrate improved mortality/incidence (M/I) ratios due to advancements in detection and treatment (Fig. 2B). In general, African countries have intermediate to low absolute mortality rates, but high M/I ratios; this might be explained by late diagnosis and an unavailability of adequate treatment that includes sophisticated surgeries and chemotherapy regimens. The M/I ratios in the majority of Asian and South American countries are comparable to those observed in Europe and the U.S. (0.5–0.6).
In the U.S., African American women have the highest M/I ratio (0.71), followed by non-Hispanic White (0.66), Hispanic (0.55), and Asian (0.5) women (Fig. 2A, closed symbols). Notably, African American women tend to demonstrate higher stage-by-stage and age-normalized mortality rates, compared to women of any other racial and ethnic groups [2,4,5]. This disparity may be attributed to certain factors that disproportionally affect American women of African descent, including inherited (genetic) factors, as well as socio-cultural determinants, lifestyle, and other modifiable risk factors. Alternatively, the risk factors for ovarian cancer may be the same across all racial and ethnic groups, and the differences in the attributable risk create the disparities. These issues will be discussed in the further sections of this review.
Screening, risk factors and early detection
Ovarian cancer is highly treatable when detected early (stage I) while the tumor is confined to the ovary (ovaries), with a relative 5-year survival rate exceeding 90% [20]. However, early diagnosis and screening are difficult due to lack of disease-specific symptoms (hence, ovarian cancer is sometimes referred as a “silent killer”), and because no screening method has been proven effective in improving survival [21,22]. Despite the universal lack of reliable screening methods, developed countries tend to be at an advantage due to better overall medical care and wide access to modern imaging techniques. However, it is not uncommon for the developing countries to fail to diagnose ovarian cancer until it has metastased, or misdiagnose it for a different type of cancer or even a different disease [23]. Of note, delayed diagnosis as well as misdiagnosis of ovarian cancer are also happening in the U.S. [24], albeit with lesser frequency.
A recent study suggests that in the U.S. lower rates of private health insurance coverage adversely affect a woman's chances of being diagnosed with early stage ovarian cancer [8]. In this study, African American women were less likely to be diagnosed with an early stage disease com pared to White women (OR=0.78) due to decreased rates of private health insurance coverage. In addition, privately insured women were more likely (OR=1.6) to present with a stage I disease, compared to uninsured or Medicaid insured women. Moorman et al. [9] reported that a history of endometriosis, later age at menarche and a history of breastfeeding did not seem to be risk-informative (regarding ovarian cancer) in African Americans. The authors especially reinforced the existence of marked differences in the prevalence of certain risk factors in women of European and African descent. For instance, African Americans were significantly more likely to have had tubal ligation, be obese, and less likely have used hormonal contraceptives. In addition, the protective effect of later age at menarche and history of breastfeeding was not evident in African Americans. Concurrently, the lower incidence of ovarian cancer in African American women may be partially explained by the higher rates of hysterectomy in that particular racial group compared to Caucasian women (OR=3.52) [25]. Evidence is suggestive that hysterectomy may be protective of ovarian cancer [26,27].
The age at presentation/diagnosis for African American compared to Caucasian women have been reported inconsistently, with some studies reporting African American women to be slightly younger [2,5,6,10,28,29], and others — slightly older [4,11]. The basis for an in consistency in age is unclear. However, younger age may indicate biologically aggressive and/or genetic (as opposed to sporadic) disease, while older age is associated with poorer survival due to age-related co-morbid conditions. The latter is especially important since African American women, compared to their Caucasian counterparts, tend to report higher co-morbidity indices [12,14], and because co-morbid conditions were shown to adversely affect survival of cancer patients [30,31].
Other frequently mentioned disparities between Caucasian and African American women include a higher fraction of later stage or distant disease at presentation [3–10], unstaged or not classified tumors [4,6,11,12], as well as the lower frequency of well-differentiated disease [9] and a higher degree and frequency of lymph node involvement [10,14]. This evidence suggests that African American women have an increased likelihood to present with a later stage or aggressive cancer, for which the current treatment merely aims to palliate the symptoms.
Although no significant differences in ovarian cancer histology or tumor type were found between African American and Caucasian patients [5], a small study by Boyce et al. [32] found that non-Caucasian ethnicity (in this study, African and Mexican American) was associated with increased incidence of granulosa cell tumors (GCT) (OR=8.49). Due to a small sample size in the current study, additional research is needed to further confirm this finding and evaluate its relevance to ovarian cancer racial disparity.
Apart from biological differences, numerous socio-economic disparities were noted between Caucasian and African American ovarian cancer patients. African American women tend to belong to lower socio economic status (SES) groups [3,12,14], carry lower levels of educational attainment [14,16], have an increased risk of being uninsured or reliant on public health insurance [34], reside in an economically disadvantaged area [14,16], and be unmarried [6,10,12,14,16]. All aforementioned factors are associated with poorer survival mainly due to restricted, limited or delayed access to quality medical care [34], and thus inevitably influence racial disparity.
Taken together, these data suggest that African American women affected by ovarian cancer, tend to already be at a disadvantage at disease diagnosis. However, non-standard treatment regimens, frequently ad ministered to African American women, may further influence survival in that particular racial group. These treatment disparities are reviewed in the next section.
Genetics
It has been established that approximately 10% of all ovarian cancers are directly attributed to specific genetic alterations [35]. However, it was suggested that the genetic component contributing to the development of ovarian cancer is even more prominent, and extensive studies aimed to map ovarian cancer genome are ongoing [36–43]. It is known that specific deleterious mutations in the BRCA1 and BRCA2 genes, which account for about 5–13% of all ovarian cancers, impose the lifetime risk as high as 20–65% on their carriers (compared to 1.4–2.5% risk for a woman from general population with no affected relatives) [44]. This mutation follows autosomal dominant pattern of inheritance and is called hereditary breast and ovarian cancer syndrome (HBOC) [28]. In addition, women with hereditary nonpolyposis colorectal cancer (Lynch syndrome, mutations in the mismatch-repair (MMR) genes) have about 10–12% lifetime risk of developing ovarian cancer [45]. MSH2, MLH1 and MSH6 are the most studied MMR genes and account for approximately 1–2% of all epithelial ovarian cancer cases [46]. Of note, the genetic variance in MMR genes and the risk of ovarian cancer have only been studied in predominantly Caucasian (NHW) populations. Whether the genetic variance in MMR genes contributes to the racial disparity in ovarian cancer, remains to be elucidated.
Women that are deemed to be “high-risk” (that is, having a family history of ovarian cancer, or being from a family affected with one of the mentioned above syndromes, or diagnosed with an early onset colorectal, breast, uterine or endometrial cancer) are advised to undergo genetic testing to rule out their personal carrier status. If necessary, regular screenings and risk-reductive measures are administered [47].
In the US, high-risk African American women are generally less likely to undergo genetic counseling and testing (OR=0.28; 95% CI: 0.09–0.89) compared to White women [48]. In addition, a substantially greater fraction of Caucasian women report having heard about genetic testing, compared to African American women (48% versus 31%, respectively) [49]. Olaya et al. [50] determined that only a personal history of breast cancer and higher level of education were statistically significant predictors of BRCA test use; however, African Americans tend to have lower levels of educational attainment which may undermine their likelihood of being tested. All authors came to a uniform conclusion that awareness of genetic testing for cancer susceptibility is considerably lower among minority U.S. populations, and the benefit of predictive genetic testing will not be fully realized until every racial and ethnic group takes equal and full advantage of it.
Schildkraut et al. [51] have reported that short CAG repeat length in the androgen receptor (AR) gene increases ovarian cancer risk 2-fold in African American, but not Caucasian, women. The authors have concluded that observed difference may be due to the rarity of short CAG alleles in Caucasian population or could reflect racial differences in disease etiology. In their other study, Schildkraut et al. [38] have observed a modest increase in ovarian cancer risk in Caucasian, but not African American, women that carried a single nucleotide polymorphism (SNP) rs2287498, that is located in exon 2 of the neighboring TP53 gene WDR79. Although the African American sample size was small, these findings suggest racial differences in ovarian cancer etiology and reinforce the need for involvement of women representing diverse racial and ethnic backgrounds into ovarian cancer genetic research.
It is also possible that African American and Caucasian women harbor different profiles of deleterious genetic mutations. For in stance, African Americans present with predominantly BRCA2 mutations (80%) as compared to Caucasians (BRCA1 mutations=69%) [52]. In addition, more African American (46%) than Caucasian (12%) women had variants of uncertain significance. It was reported [28] that women of African ancestry had a significantly higher prevalence of deleterious BRCA1/2 mutations compared to women of Western European ancestry (15.6% versus 12.1%, respectively). In addition, African American women were younger than Caucasian women (45.9 versus 50 years). Whether these genetic variations affect racial disparity in ovarian cancer remains to be elucidated.
Treatment
In the developed countries (within the scope of this review includes U.S. and Europe, i.e. mostly Caucasian women), surgery is the gold standard of any stage ovarian cancer treatment. In later stages when the tumor has spread outside of the ovary (ovaries), chemotherapy has shown to improve survival [53]. Lymphadenectomy and lymph node sampling improve survival in patients at any stage [54,55]. Despite the continuous improvements in the sophisticated treatment modalities, survival is poor in developed countries. The situation is even worse in developing countries, where all ovarian cancer patients may get the same type of standard treatment, or no specific treatment at all [56].
At present, the U.S. seems to be the only country that reports ovarian cancer treatment disparities. Among these, the risk of receiving delayed treatment [13,14], non-standard treatment regimens [14,57] or no treatment at all [6,15] was greatest in African American patients. Administration of non-standard treatment regimens includes treatment of early stage African American patients with surgery alone, while Caucasian patients were treated with a combination of surgery plus chemotherapy [14]. Among the later stage ovarian cancer patients, African American women were more often administered chemotherapy without surgery, while Caucasian patients received both treatments. The authors also noted that while Caucasian women were equally likely to receive guide line therapy regardless of insurance status, the absence of private insurance was a strong predictor of a non-standard treatment in African American women.
Similarly, African American women were less likely to be treated with a combination of surgery and chemotherapy for any stage of disease, compared to Caucasian women (33% vs 44% for early; and 61% vs 70% for late stage cancer) [3]. In addition, Caucasian women were more likely to be treated with adjuvant chemotherapy (OR=1.33) compared to African American women. Merrill et al. [11] noted that African American women were less likely to receive any surgery at all (OR=0.42 compared to the Caucasian group), even after adjusting for age, marital status, and tumor stage and tumor grade at diagnosis. Williams et al. [6] reached a similar conclusion after adjusting for all possible confounding factors, finding that African Americans diagnosed with regional or distant-stage disease were more likely to receive no treatment (OR=1.22) and less likely to receive surgery and/or a combination of surgery and chemotherapy (OR=0.79), compared to Caucasian women. Barnholtz-Sloan et al. [58] noted that African American women are less likely than Caucasian women to receive surgery (76% versus 83% in 1997, respectively). In a separate study, Barnholtz-Sloan et al. [10] also observed African American women were approximately 40% less likely to receive surgery as part of their treatment compared to Caucasian women, despite overall more aggressive disease at presentation. Goff et al. [59] reported in their comprehensive study of surgical treatment predictors that African American race was significantly associated with decreased likelihood of surgical treatment, compared to Caucasian race (OR=0.66). Wright et al. [13] emphasized the finding that African American ethnicity alone was associated with a more than double likelihood of delayed chemotherapy after surgery, compared to Caucasian ethnicity.
It was also reported that African American, compared to Caucasian, women with ovarian cancer were less likely to undergo lymphadenectomy (23% vs 27%, respectively). In addition, when lymphadenectomy was performed, less positive lymph nodes were identified in African American, compared to Caucasian, women (5 versus 7, respectively). In a separate publication, Chan et al. [60] reported a similar finding when only 32.7% of African American patients had a lymphadenectomy, compared to 42.7% of Caucasian patients. Lymphadenectomy is shown to correlate with better survival, especially for late stage patients. In addition to treatment disparity per se, Aranda et al. [61] reported that African American women with ovarian cancer have substantially lower odds of being treated by a high-volume provider, compared to Caucasian women (OR=0.7). The authors attributed this effect to decreased prevalence of private insurance coverage among African American women, and suggested that selective referral to high-volume providers should be considered to improve treatment outcomes and reduce the disparity. Bristow et al. [62] reported that compared to Caucasian women, African Americans had a lower likelihood of hysterectomy (OR=0.53, 95%CI=0.42–0.66), colon resection (OR=0.65, 95%CI=0.48–0.87), lymphadenectomy (OR=0.67, 95%CI=0.50–0.91), and surgery by a high-volume surgeon (OR=0.55, 95%CI=0.44–0.69).
This section can conclude that significant racial disparities exist in the administration of ovarian cancer treatment in the US. Unfortunately, there is no data available on this topic from the rest of the world. It is noteworthy that an increasing diversity of the US population provides both the need and the means to collect and analyze such data. However, other countries that experience growing population diversity (notably, Europe) may also be able to contribute. It is the authors' strong belief that in-depth global research will help identify the causes and thus alleviate the treatment disparities in ovarian cancer — racial or otherwise.
Survival
It was reported [2,63] that survival of African American women was lower in all age groups, especially ages 50–69 (38.6% versus 50.6% 5-year relative survival, respectively), compared to Caucasian women. McGuire et al. [4] reported that death rates were significantly elevated among African Americans compared to Caucasians (OR=1.14), especially in the 50–69 age group. Chan et al. [5] reported that African American race (OR=1.095–1.27) was an independent prognostic factor for worse disease-specific survival, even after adjusting for age, stage, grade, and histology. Albain et al. [64] reported that African American patients with ovarian cancer had significantly worse overall survival and persistently demonstrated increased mortality rates (OR=1.21– 2.24) after adjusting for all possible covariates, despite the uniform and standardized treatment assignment. Morgan et al. [33] concluded that overall survival was worse for African American patients; however, normalizing for stage and insurance status eliminated the disparity in survival. Kim et al. [16] concluded that even after adjusting for all possible covariates, African American women had a two-fold increased risk of dying from ovarian cancer (OR=2.2). Howe et al. [65] concluded that survival among Caucasian women with ovarian cancer in the U.S. is better than survival among African American women (50.1% vs. 47.5). Barnholtz-Sloan et al. [58] concluded that African American women continued to have the worst prognosis with a 5-year relative survival at all times, as did older and unmarried women of any race. Interestingly, of the women who did have surgery, African Americans still had a slightly decreased survival compared with Caucasian women and women of any other racial and ethnic group. Barnholtz-Sloan et al. [10] also noted that the crude median survival for African American women was nearly 1 year less than for Caucasian women (22 months versus 32 months, respectively), but also that African American women were at a 30% increased risk of death from any cause when adjusting for all other prognostic variables. Terplan et al. [66] have reported that African American women had increased overall mortality after adjustment for major confounders (OR 1.31; CI: 1.26–1.37), compared to their White counterparts.
However, there are also encouraging reports of equivalent survival between African American and Caucasian patients when both groups undergo uniform treatment regimens. Winter III et al. [67] noted the median survival for African Americans with advanced ovarian cancer was poorer than that for Caucasian women (22 versus 32 months, respectively), yet the difference disappeared after adjustment for disparities in treatment. A similar conclusion was drawn by Temkin et al. [68] and Terplan et al. [69] who noted no differences in median overall survival between African American and Caucasian women (37.2 versus 34.1 months, respectively) in a single, large, equal-access institution. Farley et al. [70,71] reported similar results with late stage epithelial ovarian cancer patients that were assigned to receive a standard treatment by paclitaxel and cisplatin chemotherapy. The median disease-free survival was 16.2 and 16.1 months for African American and Caucasian women, respectively. O'Malley et al. [72] found no association of race with survival or receipt of different treatment regimens. In the cited study, only age and co-morbid conditions were significant predictors of survival. Bristow et al. [73] concluded that upon administration of the uniform treatment regimens (including surgery and chemotherapy) there was no difference in survival between women of African and European descent (47 compared to 50 months, respectively; p=0.56). Finally, Du et al. [12] reported neither all-cause, nor cancer-specific mortality differed between races after controlling for patient characteristics and ensuring equal treatment.
Conclusions and future directions
Ovarian cancer health disparities exist worldwide and in the U.S. Ovarian cancer worldwide mortalities are generally proportional to incidences observed in a given population; however, in the U.S., women of African descent experience the highest mortality of all racial and ethnic groups, despite one of the lowest ovarian cancer incidences. Ovarian cancer disparities disproportionately affect African American women at all stages of the disease, from presentation through treatment, and ultimately increased mortality and decreased survival, compared to non-Hispanic White women (Table 1). Increased mortality is likely to be explained by unequal access to care and non-standard treatment regimens frequently administered to African American women, but may also be attributed to genetic susceptibility, acquired co-morbid conditions and increased frequency of modifiable risk factors, albeit to substantially lesser extent. Unequal access to care is, in turn, largely a consequence of lower SES and lack of private health insurance coverage among the African American population. This conclusion suggests the need for policy changes aimed at facilitating equal access to quality medical care. At this same time, further research is necessary to resolve racial disparities in ovarian cancer.
Table 1.
Disparities affecting African American women, diagnosed with ovarian cancer (compared to Caucasian women).
| Stage of the disease | Disparities | References |
|---|---|---|
| Risk factors and early detection | 1. Lower SES, rates of private health insurance and educational levels; higher likelihood of being unmarried. | [8] |
| 2. More likely to have had tuballigation and hysterectomy, be obese and less likely to ever have used oral contraceptives. | [9,25] | |
| 3. Protective effect of later age at menarche and history of ever breastfeeding is not evident. | [9] | |
| 4. Different profiles and/or frequencies of deleterious BRCA1/2 mutations. | [28,52] | |
| 5. Decreased use of genetic counseling by high-risk women. | [48–50] | |
| Presentation | 1. Age disparity (either slightly younger or slightly older). | [2,5,10,28,29] or [4,11] |
| 2. Higher comorbidity indices. | [12,14] | |
| 3. Increased proportions of later staged, unstaged or not classified tumors, lower frequency of well-differentiated disease and higher degree and frequency of lymph node involvement. | [3–10,4,11,12,9,10,14,32] | |
| 4. Increased incidence of granulosa cell tumors. | [3,12,14,14,16,33,14,16,10,12,14,16] | |
| 5. Socio-cultural: lower SES and levels of educational attainment, increased risk of being uninsured or having only public insurance, reside in an economically disadvantaged area, and be unmarried. | ||
| Treatment | 1. Delayed treatment. | [13,14] |
| 2. Increased risk of receiving non-standard treatment: reduced odds of receiving surgery in general and/or surgery+chemotherapy. | [3,6,10,11,13,14,57–59,62] | |
| 3. Less likely to undergo lymphadenectomy; if they do, usually less positive lymph nodes identified. | [5,60,62] | |
| 4. Increased risk of receiving no treatment (OR=1.17 or 1.22) | [6,15] | |
| 5. Less likely to receive care by the high-volume provider | [61,62] | |
| Survival | Elevated mortality and worse survival | [2,4,5,10,16,33,68,64–66] |
| No disparity with the receipt of equal guideline therapy | [12,67–73] |
Supplementary Material
Highlights.
Ovarian cancer incidences are highest in Europe and North America, and lowest in Africa and Asia.
Mortality to incidence ratios are highest in Africa and in the U.S. women of African descent.
Further research is necessary to fully elucidate and resolve racial disparities in ovarian cancer.
Acknowledgments
The authors gratefully acknowledge Dr. Nagi B. Kumar, Ms. Kyle Dalton and Mr. Richard Tanner for their insights on the contents of this manuscript. GC is supported by the Department of Defense W81XWH-11-1-0376. EKA is supported by National Institute of Health (NIH) R25T CA147832. Additional sources of funding include NIH R01-CA142081 and R01-CA76016 (JMS); R01 CA149429-01 and P20 MD003375-01 (CMP).
Footnotes
Conflict of interest: All authors declare that they have no conflict of interest.
Appendix A. Supplementary data: Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ygyno.2012.12.016.
References
- 1.Ahmedin J, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Kosary C. Cancer of the ovary. In: Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, et al., editors. SEER survival monograph: cancer survival among adults: US SEER program, 1988–2001, patient and tumor characteristics. Bethesda (Maryland): National Cancer Institute, SEER Program; 2007. [Google Scholar]
- 3.Parham G, Phillips JL, Hicks ML, Andrews N, Jones WB, Shingleton HM, et al. The National Cancer Data Base report on malignant epithelial ovarian carcinoma in African-American women. Cancer. 1997;80(4):816–26. [PubMed] [Google Scholar]
- 4.McGuire V, Jesser CA, Whittemore AS. Survival among U.S. women with invasive epithelial ovarian cancer. Gynecol Oncol. 2002;84:399–403. doi: 10.1006/gyno.2001.6536. [DOI] [PubMed] [Google Scholar]
- 5.Chan JK, Zhang M, Hu JM, Shin JY, Osann K, Kapp DS. Racial disparities in surgical treatment and survival of epithelial ovarian cancer in United States. J Surg Oncol. 2008;97:103–7. doi: 10.1002/jso.20932. [DOI] [PubMed] [Google Scholar]
- 6.Williams VL, Stockwell HG, Hoffman MS, Barnholtz-Sloan JS. Racial differences in treatment modalities among female residents of Florida diagnosed with epithelial ovarian cancer. J Gynecol Surg. 2010;26(1):15–22. [Google Scholar]
- 7.Cress RD, O'Malley CD, Leiserowitz GS, Campleman SL. Patterns of chemotherapy use for women with ovarian cancer: a population-based study. J Clin Oncol. 2003;21(8):1530–5. doi: 10.1200/JCO.2003.08.065. [DOI] [PubMed] [Google Scholar]
- 8.Morris CR, Sands MT, Smith LH. Ovarian cancer: predictors of early-stage diagnosis. Cancer Causes Control. 2010;21:1203–11. doi: 10.1007/s10552-010-9547-0. [DOI] [PubMed] [Google Scholar]
- 9.Moorman PG, Palmieri RT, Akushevich L, Berchuck A, Schildkraut JM. Ovarian cancer risk factors in African-American and white women. Am J Epidemiol. 2009;170(5):598–606. doi: 10.1093/aje/kwp176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnholtz-Sloan JS, Tainsky MA, Abrams J, Severson RK, Qureshi F, Jacques SM, et al. Ethnic differences in survival among women with ovarian carcinoma. Cancer. 2002;94(6):1886–93. doi: 10.1002/cncr.10415. [DOI] [PubMed] [Google Scholar]
- 11.Merrill RM, Anderson AE, Merrill JG. Racial/ethnic differences in the use of surgery for ovarian cancer in the United States. Adv Med Sci. 2010;55(1):93–8. doi: 10.2478/v10039-010-0021-8. [DOI] [PubMed] [Google Scholar]
- 12.Du XL, Sun CC, Milam MR, Bodurka DC, Fang S. Ethnic differences in socioeconomic status, diagnosis, treatment, and survival among older women with epithelial ovarian cancer. Int J Gynecol Cancer. 2008;18:660–9. doi: 10.1111/j.1525-1438.2007.01081.x. [DOI] [PubMed] [Google Scholar]
- 13.Wright JD, Doan T, McBride R, Jacobson JS, Hershman DL. Variability in chemo therapy delivery for elderly women with advanced stage ovarian cancer and its impact on survival. Br J Cancer. 2008;98:1197–203. doi: 10.1038/sj.bjc.6604298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harlan LC, Clegg LX, Trimble EL. Trends in surgery and chemotherapy for women diagnosed with ovarian cancer in the United States. J Clin Oncol. 2003;21(18):3488–94. doi: 10.1200/JCO.2003.01.061. [DOI] [PubMed] [Google Scholar]
- 15.Terplan M, Smith EJ, Temkin SM. Race in ovarian cancer treatment and survival: a systematic review with meta-analysis. Cancer Causes Control. 2009;20:1139–50. doi: 10.1007/s10552-009-9322-2. [DOI] [PubMed] [Google Scholar]
- 16.Kim S, Dolecek TA, Davis FG. Racial differences in stage at diagnosis and survival from epithelial ovarian cancer: a fundamental cause of disease approach. Soc Sci Med. 2010;71:274–81. doi: 10.1016/j.socscimed.2010.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brawley OW. Is race really a negative prognostic factor for cancer? JNCI. 2009;101(14):970–1. doi: 10.1093/jnci/djp185. [DOI] [PubMed] [Google Scholar]
- 18.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM, GLOBOCAN . Cancer incidence and mortality worldwide: IARC CancerBase No 10 [Internet] Lyon, France: International Agency for Research on Cancer; 2010. Available from: http://globocan.iarc.fr. [Google Scholar]
- 19.Parkin M, Bray F, Ferlay J, Pisani P. Global cancer statistics. CA Cancer J Clin. 2002;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 20.Kurman RJ, Visvanathan K, Roden R, Shih IM. Early detection and treatment of ovarian cancer: shifting from early stage to minimal volume of disease based on a new model of carcinogenesis. Am J Obstet Gynecol. 2008;198(4):351–6. doi: 10.1016/j.ajog.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clarke-Pearson DL. Screening for ovarian cancer. N Engl J Med. 2009;361:170–7. doi: 10.1056/NEJMcp0901926. [DOI] [PubMed] [Google Scholar]
- 22.Brown PO, Palmer C. The preclinical natural history of serous ovarian cancer: defining the target for early detection. PLoS Med. 2009;6(7):e1000114. doi: 10.1371/journal.pmed.1000114. http://dx.doi.org/10.1371/journal.pmed.1000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sankaranarayanan R. Worldwide burden of gynaecological cancer: the size of the problem. Best Pract Res Clin Obstet Gynaecol. 2006;20(2):207–25. doi: 10.1016/j.bpobgyn.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Vine MF, Calingaert B, Berchuck A, Schildkraut JM. Characterization of prediagnostic symptoms among primary epithelial ovarian cancer cases and controls. Gynecol Oncol. 2003;90(1):75–82. doi: 10.1016/s0090-8258(03)00175-6. [DOI] [PubMed] [Google Scholar]
- 25.Bower JK, Schreiner PJ, Sternfeld B, Lewis CE. Black–White differences in hysterectomy prevalence: the CARDIA study. Am J Public Health. 2009;99(2):300–7. doi: 10.2105/AJPH.2008.133702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hankinson SE, Hunter DJ, Colditz GA, Willett WC, Stampfer MJ, Rosner B, et al. Tubal ligation, hysterectomy, and risk of ovarian cancer. JAMA. 1993;270(23):2813–8. [PubMed] [Google Scholar]
- 27.Permuth-Wey J, Sellers TA. Epidemiology of ovarian cancer. Verma M, editor. Methods in molecular biology, 472 Cancer epidemiology. 2009:413–38. doi: 10.1007/978-1-60327-492-0_20. http://dx.doi.org/10.1007/978-1-60327-492-0. [DOI] [PubMed]
- 28.Hall MJ, Reid JE, Burbidge LA, Pruss D, Deffenbaugh AM, Frye C, et al. BRCA1 and BRCA2 mutations in women of different ethnicities undergoing testing for hereditary breast–ovarian cancer. Cancer. 2009;115(10):2222–33. doi: 10.1002/cncr.24200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karami S, Young HA, Henson DE. Earlier age at diagnosis: another dimension in cancer disparity? Cancer Detect Prev. 2007;31:29–34. doi: 10.1016/j.cdp.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Tetsche MS, Norgaard M, Jacobsen J, Wogelius P, Sorensen HT. Comorbidity and ovarian cancer survival in Denmark, 1995–2005: a population-based cohort study. Int J Gynecol Cancer. 2008;18(3):421–7. doi: 10.1111/j.1525-1438.2007.01036.x. [DOI] [PubMed] [Google Scholar]
- 31.Extermann M. Interaction between comorbidity and cancer. Cancer Control. 2007;14(1):13–22. doi: 10.1177/107327480701400103. [DOI] [PubMed] [Google Scholar]
- 32.Boyce EA, Costaggini I, Vitonis A, Feltmate C, Muto M, Berkowitz R, et al. The epidemiology of ovarian granulosa cell tumors: a case–control study. Gynecol Oncol. 2009;115:221–5. doi: 10.1016/j.ygyno.2009.06.040. [DOI] [PubMed] [Google Scholar]
- 33.Morgan MA, Behbakht K, Benjamin I, Berlin M, King SA, Rubin SC. Racial differences in survival from gynecologic cancer. Obstet Gynecol. 1996;88(6):914–8. doi: 10.1016/s0029-7844(96)00342-0. [DOI] [PubMed] [Google Scholar]
- 34.Goss E, Lopez AM, Brown CL, Wollins DS, Brawley OW, Raghavan D. American Society of Clinical Oncology policy statement: disparities in cancer care. J Clin Oncol. 2009;27:2881–5. doi: 10.1200/JCO.2008.21.1680. [DOI] [PubMed] [Google Scholar]
- 35.Berek JS, Bast RC. Ovarian cancer. In: Kufe DW, Pollock RE, Weichselbaum RR, Bast RCJ, Gansler TS, Holland JS, Frei E, editors. Cancer medicine 6. London: BC Decker Inc; 2003. [Google Scholar]
- 36.Goode EL, Chenevix-Trench G, Song H, Ramus SJ, Notaridou M, Lawrenson K, et al. A genome-wide association study identifies susceptibility loci for ovarian cancer at 2q31 and 8q24. Nat Genet. 2010;42:874–9. doi: 10.1038/ng.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Landen CN, Birrer MJ, Sood AK. Early events in the pathogenesis of epithelial ovarian cancer. J Clin Oncol. 2008;26(6):995–1005. doi: 10.1200/JCO.2006.07.9970. [DOI] [PubMed] [Google Scholar]
- 38.Schildkraut JM, Goode EL, Clyde MA, Iversen ES, Moorman PG, Berchuk A, et al. Single nucleotide polymorphisms in the TP53 region and susceptibility to invasive epithelial ovarian cancer. Cancer Res. 2009;69:2349. doi: 10.1158/0008-5472.CAN-08-2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peedicayil A, Vierkant RA, Shridhar V, Schildkraut JM, Armasu S, Hartmann LC, et al. Polymorphisms in TCEAL7 and risk of epithelial ovarian cancer. Gynecol Oncol. 2009;114(2):260–4. doi: 10.1016/j.ygyno.2009.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.White KL, Vierkant RA, Phelan CM, Fridley B, Anderson S, Knutson K, et al. Polymorphisms in NF-κB inhibitors and risk of epithelial ovarian cancer. BMC Cancer. 2009;9:170. doi: 10.1186/1471-2407-9-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goode EL, Fridley BL, Vierkant RA, Cunningham JM, Phelan CM, Anderson S, et al. Candidate gene analysis using imputed genotypes: cell cycle single-nucleotide polymorphisms and ovarian cancer risk. Cancer Epidemiol Biomarkers Prev. 2009;18(3):935–44. doi: 10.1158/1055-9965.EPI-08-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goode EL, Maurer MJ, Sellers TA, Phelan CM, Kalli KR, Fridley BL, et al. Inherited determinants of ovarian cancer survival. Clin Cancer Res. 2010;16(3):995–1007. doi: 10.1158/1078-0432.CCR-09-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peedicayil A, Vierkant RA, Hartmann LC, Fridley BL, Fredericksen ZS, White KL, et al. Risk of ovarian cancer and inherited variants in relapse-associated genes. PLoS One. 2010;5(1):e8884. doi: 10.1371/journal.pone.0008884. http://dx.doi.org/10.1371/journal.pone.0008884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.King MC, Marks JH, Mandell JB. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302(5645):643–6. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- 45.Schmeler KM, Lynch HT, Chen LM, Munsell MF, Soliman PT, Clark MB, et al. Prophylactic surgery to reduce the risk of gynecologic cancers in the Lynch syndrome. N Engl J Med. 2006;354:261–9. doi: 10.1056/NEJMoa052627. [DOI] [PubMed] [Google Scholar]
- 46.Harley I, Rosen B, Risch HA, Siminovitch K, Beiner ME, McLaughlin J, et al. Ovarian cancer risk is associated with a common variant in the promoter sequence of the mismatch repair gene MLH1. Gynecol Oncol. 2008;109(3):384–7. doi: 10.1016/j.ygyno.2007.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nelson HD, Huffman LH, Fu R, Harris EL. Genetic risk assessment and BRCA mutation testing for breast and ovarian cancer susceptibility: systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2005;143(5):362–79. doi: 10.7326/0003-4819-143-5-200509060-00012. [DOI] [PubMed] [Google Scholar]
- 48.Armstrong K, Micco E, Carney A, Stopfer J, Putt M. Racial differences in the use of BRCA1/2 testing among women with a family history of breast or ovarian cancer. JAMA. 2005;293:1729–36. doi: 10.1001/jama.293.14.1729. [DOI] [PubMed] [Google Scholar]
- 49.Pagán JA, Su D, Li L, Armstrong K, Asch DA. Racial and ethnic disparities in awareness of genetic testing for cancer risk. Am J Prev Med. 2009;37(6):524–30. doi: 10.1016/j.amepre.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 50.Olaya W, Esquivel P, Wong JH, Morgan JW, Freeberg A, Roy-Chowdhury S, et al. Disparities in BRCA testing: when insurance coverage is not a barrier. Am J Surg. 2009;198:562–5. doi: 10.1016/j.amjsurg.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 51.Schildkraut JM, Murphy SK, Palmieri RT, Iversen ES, Moorman PG, Huang Z, et al. Trinucleotide repeat polymorphisms in the androgen receptor gene and risk of ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:473. doi: 10.1158/1055-9965.EPI-06-0868. [DOI] [PubMed] [Google Scholar]
- 52.Haffty BG, Silber A, Matloff E, Chung J, Lannin D. Racial differences in the incidence of BRCA1 and BRCA2 mutations in a cohort of early onset breast cancer patients: African American compared to white women. J Med Genet. 2006;43:133–7. doi: 10.1136/jmg.2005.034744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zeimet AG, Reimer D, Concin N, Braun S, Marth C. Primary chemotherapy and maintenance therapy in epithelial ovarian cancer. MEMO. 2008;1:99–102. [Google Scholar]
- 54.Angioli R, Plotti F, Palaia I, Calcagno M, Montera R, Cafà EV, et al. Update on lymphadenectomy in early and advanced ovarian cancer. Curr Opin Obstet Gynecol. 2008;20(1):34–9. doi: 10.1097/GCO.0b013e3282f2fd68. [DOI] [PubMed] [Google Scholar]
- 55.Kim HS, Ju W, Jee BC, Kim YB, Park NH, Song YS, et al. Systematic lymphadenectomy for survival in epithelial ovarian cancer: a meta-analysis. Int J Gynecol Cancer. 2010;20(4):520–8. doi: 10.1111/IGC.0b013e3181d6de1d. [DOI] [PubMed] [Google Scholar]
- 56.Sterling L, van Lonkhuijzen L, Nyangena J, Orango E, Strother M, Busakhala N, et al. Protocol development for ovarian cancer treatment in Kenya: a brief report. Int J Gynecol Cancer. 2011;2(2):424–7. [PubMed] [Google Scholar]
- 57.Chase D, Fedewa S, Chou TS, Chen A, Ward E, Brewster WR. Disparities in the allocation of treatment in advanced ovarian cancer: are there certain patient characteristics associated with nonstandard therapy? Obstet Gynecol. 2012;119(1):68–77. doi: 10.1097/AOG.0b013e31823d4006. [DOI] [PubMed] [Google Scholar]
- 58.Barnholtz-Sloan JS, Schwartz AG, Qureshi F, Jacques S, Malone J, Munkarah AR. Ovarian cancer: changes in patterns at diagnosis and relative survival over the last three decades. Am J Obstet Gynecol. 2003;189:1120–7. doi: 10.1067/s0002-9378(03)00579-9. [DOI] [PubMed] [Google Scholar]
- 59.Goff BA, Matthews BJ, Larson EH, Andrilla HA, Wynn M, Lishner DM, et al. Predictors of comprehensive surgical treatment in patients with ovarian cancer. Cancer. 2007;109:2031–42. doi: 10.1002/cncr.22604. [DOI] [PubMed] [Google Scholar]
- 60.Chan JK, Munro EG, Cheung MK, Husain A, Teng NN, Berek JS, et al. Association of lymphadenectomy and survival in stage I ovarian cancer patients. Obstet Gynecol. 2007;109:12–9. doi: 10.1097/01.AOG.0000249610.95885.ef. [DOI] [PubMed] [Google Scholar]
- 61.Aranda MA, McGory M, Sekeris E, Maggard M, Ko K, Zingmond DS. Do racial/ethnic disparities exist in the utilization of high-volume surgeons for women with ovarian cancer? Gynecol Oncol. 2008;111:166–72. doi: 10.1016/j.ygyno.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bristow RE, Zahurak ML, Ibeanu OA. Racial disparities in ovarian cancer surgical care: a population-based analysis. Gynecol Oncol. 2011;121(2):364–8. doi: 10.1016/j.ygyno.2010.12.347. [DOI] [PubMed] [Google Scholar]
- 63.Bristow RE, Palis BE, Chi DS, Cliby WA. The National Cancer Database report on advanced-stage epithelial ovarian cancer: Impact of hospital surgical case volume on overall survival and surgical treatment paradigm. Gynecol Oncol. 2010;118(3):262–7. doi: 10.1016/j.ygyno.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 64.Albain KS, Unger JM, Crowley JJ, Coltman CA, Jr, Hershman DL. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst. 2009;101:984–92. doi: 10.1093/jnci/djp175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Howe HL, Tung KH, Coughlin S, Jean-Baptiste R, Hotes J. Race/ethnic variations in ovarian cancer mortality in the United States, 1992–1997. Cancer. 2003;97(10):2686–93. doi: 10.1002/cncr.11350. [DOI] [PubMed] [Google Scholar]
- 66.Terplan M, Schluterman N, McNamara EJ, Tracy K, Temkin SM. Have racial disparities in ovarian cancer increased over time? An analysis of SEER data. Gynecol Oncol. 2010;125(1):19–24. doi: 10.1016/j.ygyno.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 67.Winter WE, III, Maxwell GL, Tian C, Carlson JW, Ozols RF, Rose PG, et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2007;25:3621–7. doi: 10.1200/JCO.2006.10.2517. [DOI] [PubMed] [Google Scholar]
- 68.Temkin SM, Lengyel E, Tergas A, Terplan M. Ovarian cancer treatment in black and white women: a comparison of clinicopathologic factors and outcome. J Clin Oncol. 2008;26(15S):16531. [Google Scholar]
- 69.Terplan M, Temkin S, Tergas A, Lengyel E. Does equal treatment yield equal out comes? The impact of race on survival in epithelial ovarian cancer. Gynecol Oncol. 2008;111(2):173–8. doi: 10.1016/j.ygyno.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Farley JH, Tian C, Rose GS, Brown CL, Birrer M, Maxwell GL. Race does not impact outcome for advanced ovarian cancer patients treated with cisplatin/paclitaxel. Cancer. 2009;115:4210–7. doi: 10.1002/cncr.24482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Farley JH, Tian C, Rose GS, Brown CL, Risinger JI, Maxwell GL. Ethnicity and clinical outcome for advanced epithelial ovarian cancer patients treated by standard cisplatin/paclitaxel chemotherapy: a combined analysis of gynecologic oncology group clinical trials. J Clin Oncol. 2008;26(15S):5573. ASCO Annual Meeting Proceedings. [Google Scholar]
- 72.O'Malley CD, Cress RD, Campleman SL, Leiserowitz GS. Survival of Californian women with epithelial ovarian cancer, 1994–1996: a population-based study. Gynecol Oncol. 2003;91:608–15. doi: 10.1016/j.ygyno.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 73.Bristow RE, Ueda S, Gerardi MA, Ajiboye OB, Ibeanu OA. Analysis of racial disparities in stage IIIC epithelial ovarian cancer care and outcomes in a tertiary gynecologic oncology referral center. Gynecol Oncol. 2011;122(2):319–23. doi: 10.1016/j.ygyno.2011.04.047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.