Abstract
N6-methyl-Adenosine (m6A) is the most abundant modification in mammalian mRNA and long noncoding RNA. First discovered in 1970s, m6A modification has been proposed to function in mRNA splicing, export, stability and immune tolerance. Interest and excitement in m6A modification has recently been revived based on the discovery of a mammalian enzyme that removes m6A and the application of deep sequencing to localize modification sites. The m6A demethylase FTO controls cellular energy homeostasis and is the first enzyme discovered that reverses an RNA modification. m6A sequencing demonstrates cell type and cell state-dependent m6A patterns, indicating that m6A modifications are highly regulated. This review describes the current knowledge of mammalian m6A modifications and future perspectives on how to push the field forward.
Modifications in messenger and long non-coding RNA
Over one hundred types of RNA modifications have been identified in all three kingdoms of life (http://rna-mdb.cas.albany.edu/RNAmods/). The most chemically diverse modifications are present in ribosomal RNA (rRNA) and transfer RNA (tRNA); rRNA and tRNA modifications are commonly proposed to find-tune their structure and function 1. Most modification studies have focused on rRNA and tRNA due to their high cellular abundance, large chemical diversity and functional importance in translation.
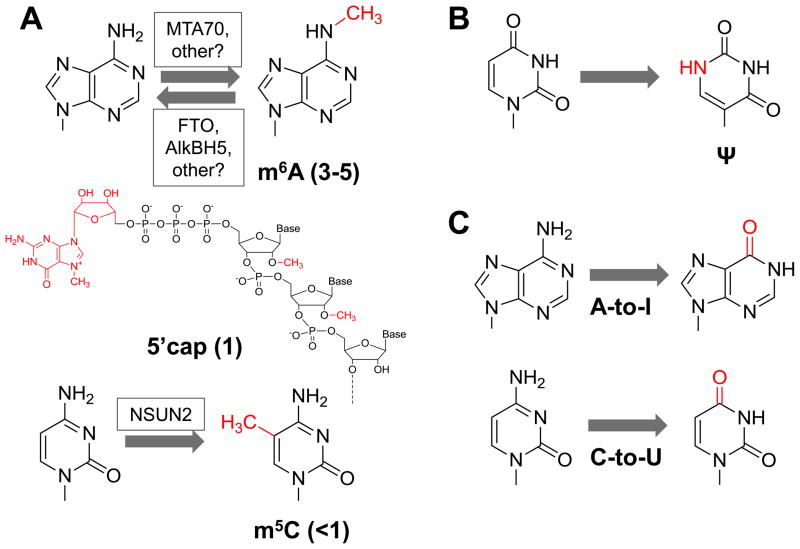
Modifications are also present in messenger RNA (mRNA) and long non-coding RNA (lncRNA) in eukaryotes (Figure 1). Additional chemical changes of specific RNA residues include adenosine to inosine (A-to-I) and cytosine to uridine (C-to-U), commonly referred to as “RNA editing” (Figure 1). The 5′ cap of mRNA, some lncRNA, and spliceosomal RNA (snRNA) is the best known example of such modifications. The 5′cap of mRNA is created by both 7-methyl-G addition to the 5′ triphosphate as well as 2′OMe modification of the first and second mRNA residues. The 5′ cap in mRNA recruits the cap-binding protein eIF4E, other initiation factors and the 40S ribosomal subunit to initiate mRNA scanning and translation at the first codon of the coding sequence. The 5′ cap is also required for mRNA stability; its removal is often a necessary step for complete mRNA degradation 2, 3. Up to three types of mRNA/lncRNA modifications are known to be present away from the 5′ cap. Two types, N6-methyl-adenosine (m6A) and 5-methyl-cytosine (m5C), have been shown to occur in significant amounts 4–7. One other type, pseudouridine (Ψ) has not been shown conclusively to be present in mRNA/lncRNA, although Ψ-modification of codons can have a profound effect on decoding 8, 9. A-to-I and C-to-U editing changes the base pairing property of the edited bases, therefore resulting in recoding of the edited codons. Biological functions of the m6A and m5C modifications in mRNA/lncRNA, however, are much less well understood. This review discusses the current knowledge and future perspectives of mRNA/lncRNA modification, focusing on m6A modification in mammals.
Fig. 1. mRNA and lncRNA modifications.
The functional groups introduced through modifications are shown in red. (A) The three abundant modifications are m6A, 5′ cap and m5C. The 5′ cap contains both modifications at the 5′ triphosphate and 2′O-methylation of the first and sometimes the second nucleotide. The estimated number of modifications per type, per RNA molecule, is shown in parentheses 4, 6, 10. Some known human enzymes that catalyze the forward and reverse reactions are also shown 7, 10, 11, 40. MTA70: METTL3 methyltransferase like 3; FTO: fat mass and obesity associated; AlkBH5: alkB alkylation repair homolog 5; NSUN2: NOP2/Sun RNA methyltransferase family, member 2. (B) Pseudouridine modification (Ψ) may also be present, although the extent of Ψ is currently unknown. (C) RNA editing commonly refers to deamination of A to inosine and C to uridine at specific sites in mRNA.
N6-methyl-A, discovered in the 1970s 5, is the most abundant modification in eukaryotic mRNA/lncRNA. N6-methyl-A modifications in polyadenylated RNA have been found in mammals, plants and yeast 10. In mammals, m6A occurs on average in 3–5 sites per mRNA molecule, and up to 15 sites in viral RNA. Since a mammalian cell contains over 10,000 mRNA species, tens of thousands of m6A sites may exist in the mRNA population in each cell. Two recent developments have revived the interest and excitement in understanding the biological function of m6A modification. The first is the discovery in 2011 that m6A modification can be reversed by a mammalian enzyme involved in diabetes and obesity 11. The second is the transcriptome-wide mapping of tens of thousands of m6A-containing segments in mRNA and long non-coding RNA in 2012 12, 13. These developments have elevated m6A modification to a new level in understanding RNA modification in biology.
m6A studies before the advent of deep sequencing
The classical phase of m6A modification studies in mRNA and viral RNA has been comprehensively described in a 2005 review 10. In contrast to abundant rRNA and tRNA, the low abundance of mRNA presents a huge challenge in the identification of m6A sites in mRNA. By 2005, specific sites for m6A modification were determined in only two individual mRNAs. The m6A site in bovine prolactin mRNA is present in the 3′ UTR and near a consensus 3′ polyadenylation sequence. Interestingly, the extent of modification appeared to be only ~20%, suggesting that m6A modifications in mRNA may be incomplete at other sites as well. Over ten m6A sites have been found in the Rous sarcoma virus (RSV) RNA. Again, modification fractions of the RSV sites are incomplete, ranging between 20–90%. Through mutational analysis of m6A sites in vivo and in vitro, the consensus sequence of m6A modification has been defined as RRm6ACH, where R is A/G and H is A/C/U 14–17. This consensus sequence can occur once every 85 nucleotides, so that an average mRNA can have more than 30 consensus sites. Since only ~3–5 m6A sites were estimated to be present in an mRNA, the majority of the consensus sequences are either not modified, or many of them are modified but at substoichiometic amounts.
A major advance in m6A study was the identification of a human m6A methyl-transferase subunit of a nuclear-localized complex 18, 19. This methyltransferase, MTA70 (or METTL3), is a homolog of yeast IME4, which is known to introduce m6A modification in yeast mRNA during sporulation 20, 21. MTA70 is a subunit of a nearly one mega-Dalton complex. RNA interference of this methyltransferase in HeLa cells resulted in cell death through apoptosis, indicating that m6A modifications perform crucial regulatory functions. The MTA-homologs in plant and in Drosophila are essential for viability and are particularly important in certain developmental pathways 22, 23.
Proposed functions that m6A modification affects include mRNA splicing, nuclear export, stability and translational efficiency 10. However, it has been extremely difficult to test m6A function for an individual RNA. Unexpectedly, mutating the modified adenosine or the 3′ cytidine residue in the consensus sequence often resulted in the appearance of new m6A modification at a nearby consensus site in the same RNA 10. Further, because many sites are potentially modified in each mRNA, one may not expect a very large effect upon mutating a single modification site.
An exciting development in the studies of m6A modification function was the discovery that it can prevent recognition of the modified RNA by cellular innate immunity 24. The innate immune system recognizes self versus foreign molecules and triggers a cascade of cellular activities upon activation. Known components in the innate immune system that directly recognize RNA include toll-like receptor 3 (TLR3), which recognizes double-stranded RNA, and TLR7, which recognizes single-stranded RNA 25. The innate immune system is activated upon transfection of unmodified RNA oligonucleotides, but remains dormant upon transfection of the same RNA containing m6A modification. Interestingly, transfection of modified RNA containing m5C or Ψ also markedly reduced innate immune activation, although m6A modification was most effective in preventing activation. By contrast, compared to unmodified mRNA transcripts, complete substitution of A to m6A was highly detrimental to translation, whereas substitution of C/U to m5C/Ψ increased the level of translation 26. However, substituting ~5% of A to m6A did not affect translation levels; this low level of A-to- m6A substitution more closely resembles the m6A levels observed in natural mRNAs 26. These results suggest that one function of m6A (and potentially m5C or Ψ) modification in mRNA is to tag cellular mRNA to be “self” when presented to the innate immune system 24, 26. This insight led to the remarkable success in efficiently generating induced pluripotent stem cells (iPS) simply by transfecting m5C/Ψmodified mRNAs of the four key factors in iPS formation 27.
The discovery of two m6A demethylases
A major breakthrough was the discovery in 2011 of a mammalian enzyme that specifically removes m6A modification in polyadenylated RNA, which are primarily composed of mRNA and lncRNA 11. The fat mass and obesity associated protein (FTO) belongs to a human family of AlkB-homologues that can remove N-methylated RNA or DNA bases. Escherichia coli AlkB is a repair enzyme capable of demethylating N1-modified A or N3-modified C both in DNA and RNA 28, 29. Many AlkB-homologues or closely related proteins have been found in mammalian genomes including AlkBH1–8, FTO and Tet1–3. AlkBH8 was found to be a tRNA modification enzyme containing two catalytic domains: one catalyzes methylation of several wobble uridine modifications 30, 31 and the other catalyzes hydroxylation of the hypermodified U34 wobble base of tRNAGly(UCC) 32, 33. The Tet enzymes were found to catalyze the oxidation of m5C in DNA to 5-hydroxymethyl-C, which is a new form of DNA modification and may represent the first step in the reversal of m5C modification in chromosomal DNA 34, 35. The FTO enzyme is one of the key factors in regulating mammalian energy homeostasis; the FTO gene was identified in genome-wide association studies to be strongly linked to diabetes and obesity 36, and loss of FTO function in mice leads to a significant reduction in body mass 37. On a mechanistic level, FTO was found to selectively remove m6A in polyadenylated RNAs both in vitro and in vivo. In vivo, siRNA knockdown or overexpression of FTO increased or decreased total m6A content by ~15–20%, respectively. The FTO protein partially co-localizes with markers of nuclear speckles, which are sites of mRNA splicing. This localization of FTO activity is consistent with previous suggestions that m6A is involved in mRNA splicing. These results indicate that m6A modifications in mRNA/lncRNA play a significant role in cellular energy homeostasis and metabolism through their influences on the spliced mRNA/lncRNA populations.
FTO is the first enzyme discovered that can reverse an endogenous RNA modification. Base methylation is a dominant form of RNA modification in all cellular RNA types. Aside from m6A, which occurs in polyadenylated RNA as well as in rRNA/tRNA and snRNA, other known N-methylations in rRNA/tRNA include N1-methyl-A (m1A), N3-methyl-C (m3C), N4-methyl-C (m 4C), N1-methyl-G (m1G), N2-methyl-G (m2G), N3-methyl-U (m3U), and N3-methyl-pseudoU (m3Ψ), all of which can potentially be subjected to reversal by a similar or other mechanisms. The discovery of m6A as a cellular substrate of FTO indicates that other cellular enzymes may exist to catalyze the reversal of other base methylations 38, 39.
A second human m6A-demethylase has just been described 40. Like FTO, AlkBH5 is another member of the AlkB-homologous gene family of dioxygenases. Perturbation of AlkBH5 significantly affects nuclear mRNA export and RNA metabolism. Furthermore, AlkBH5 appears to be involved in the assembly of mRNA processing factors in nuclear speckles. The physiological effect of AlkBH5 deficient mice includes impaired male fertility. The discovery of a second m6A-demethylase strongly indicates that reversible m6A modification likely has broad functions in mammalian biology.
Both mRNA m6A methyltransferase (MTA70) and mRNA m6A demethylase (FTO and AlkBH5) are catalytic subunits of much larger complexes in the cell that presumably confer specificity of m6A addition and removal. These accessory factors need to be identified using modern proteomic techniques to further advance studies of m6A function.
Transcriptome-wide profiling of m6A in mRNA and lncRNA
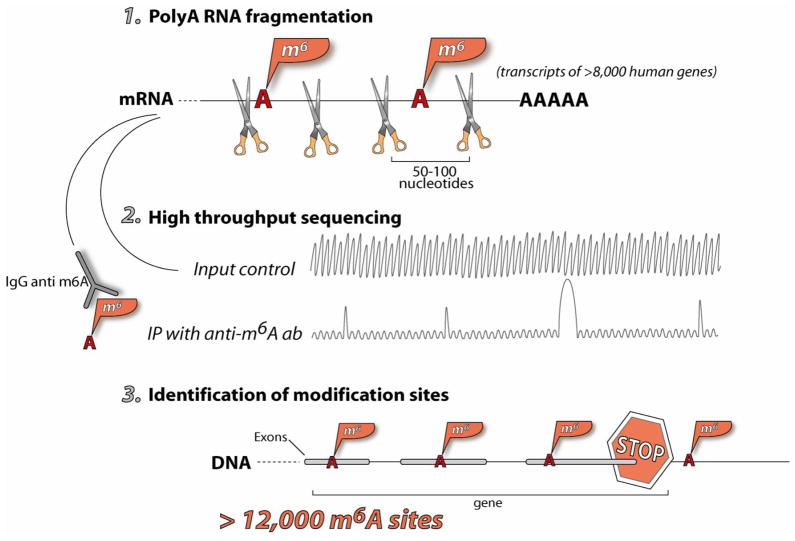
A new wave of breakthroughs in 2012 took advantage of recent advances in high throughput sequencing 12, 13. Reverse transcriptase does not discriminate m6A from A in bulk cDNA synthesis, therefore, m6A cannot be currently detected by standard high throughput sequencing methods. Using m6A-specific antibodies originally generated for m6A modification studies of spliceosomal RNA, two independent studies developed similar methods that enabled the identification of ten thousands of m6A-containing mRNA/lncRNA segments in different tissues and stress conditions (Figure 2). In these studies, polyadenylated RNAs are first fragmented to ~50–100 nucleotides then pulled down with an m6A-antibody, followed by sequencing of the immunoprecipitated RNA fragments. Analysis of the high throughput sequences reveals ~200-residue-wide peaks; many, although not all contain the known m6A consensus sequences. These m6A profiling studies showed that m6A modifications are enriched around the stop codon of the coding regions of mRNA, although the function of this enrichment is currently unknown. Further, m6A modifications have distinct distributions among tissue types and stress conditions, thus indicating that each cell type and cell state likely have unique m6A modification patterns. Another very exciting finding from these studies was the discovery of m6A modification in many long non-coding RNAs, indicating that m6A modifications are not confined to mRNA. The transcriptome-wide m6A profiles are similar to the profiles for m5C modifications 7. Both modifications are non-randomly distributed in mRNA with enrichment in and near the 3′UTR, and both are also found in non-coding RNAs.
Fig. 2. m6A sequencing method and results 12, 13.
Starting from total polyadenylated RNA, step 1 is to chemically fragment RNA to ~50–100 nucleotides. This sample is split in two; one is used as input control, and the other immunoprecipitated with anti-m6A antibody to isolate RNA fragments containing m6A modification. Both samples are deep sequenced (step 2), and the sequencing results compared to identify m6A-containing RNA segments (step 3). The m6A modification is markedly enriched around the stop codon of mRNA, although the reason for this enrichment is unclear.
The demonstration that m6A modification in mRNA/lncRNA has distinct patterns in response to developmental or environmental cues is akin to m5C modification in chromosomal DNA where distinct patterns are present depending on cell type and cell state in order to establish epigenetic maintenance and regulation. It is likely that m6A modification patterns also follow a well defined but distinct pattern depending on cell type and cell state. The identification of m6A patterns in mRNA/lncRNA that correlate to cell type and states could therefore establish the concept of “RNA epigenetics” as another layer of epigenetic regulation 38, 39.
A major challenge will be the development of a high throughput technique that identifies m6A modification in mRNA/lncRNA at single nucleotide resolution. The current technique of using an m6A antibody generates m6A candidate sites within ~200 nucleotide segments, but there is no simple method to actually validate the presence of m6A modification there. Furthermore, the known m6A consensus sequence cannot explain many sequencing peaks, suggesting that more than one m6A modification enzyme is present in cells, and each enzyme may have distinct requirements for m6A modification. The development of an m6A sequencing technique with single nucleotide resolution may involve chemical reactions that are specific for m6A, but not for unmodified A. Chemically reacted m6A-containing RNA fragments can then be enriched by affinity pull-down followed by sequencing. Another potential method involves single molecule sequencing which has been shown to be capable of detecting m6A modification in DNA 41. Another highly desirable feature of new m6A sequencing technologies would be the determination of the m6A modification fraction at each site. All m6A modifications in cellular mRNA or viral RNA that are mapped to single nucleotide resolution have 20–90% methylation for each site. Why cells maintain fractional m6A modification at these sites is unclear; it is difficult to derive functional hypotheses given the paucity of such experimental data. Fractional modification can be anywhere from 0–100%, so multiple regulatory states may be available depending on the extent of modification at each site.
Several proteins that seem to prefer binding to m6A modified RNA have also been identified from cell lysates using a known viral m6A-containing RNA oligonucleotide as in vitro bait 12. Some of these m6A binding proteins have been shown previously to bind RNA, although a few had no previously known association with RNA. At this time, it remains to be determined whether these proteins actually bind m6A-containing RNA in vivo and whether m6A binding of these proteins is functional. In any case, identification of such proteins strongly suggests that an important function of m6A modification is to recruit specific proteins to modified mRNA or lncRNA. Thus, m6A modification has potential to introduce a new layer of biological regulation through m6A binding proteins and beyond.
Concluding remarks
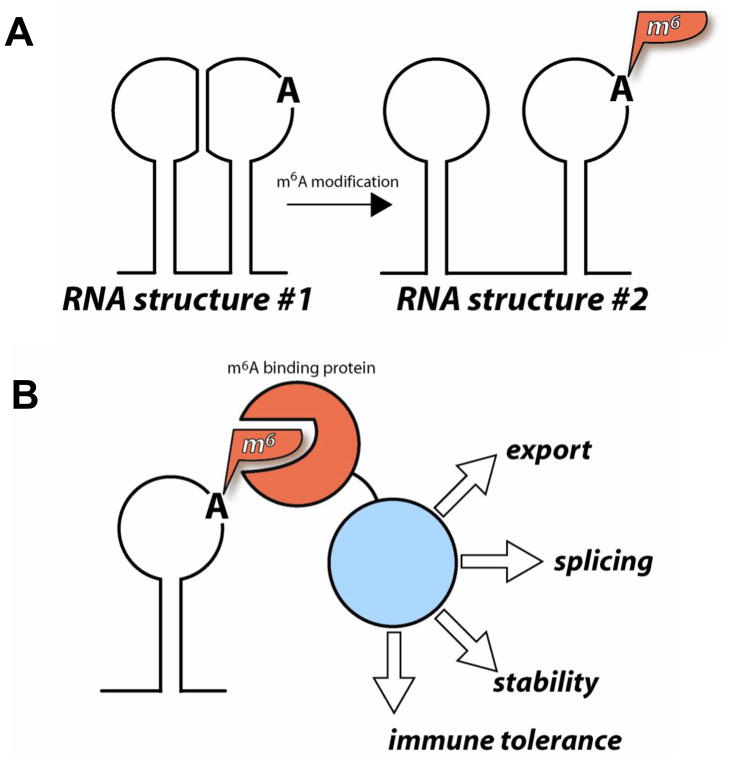
N6-methyl-A modification can exert its function in at least two ways: alteration of RNA structure or recruitment of specific m6A binding proteins (Figure 3). The addition of a single methyl-group to the N6-position of adenosine does not perturb Watson-Crick base pairing. However, m6A modification is known to weaken RNA secondary structure 42, and can affect RNA tertiary structure, in particular tertiary interactions involving base triples or Hoogsteen pairs that rely on the N6 proton as a Hydrogen bond donor. N6-methyl-A modification may also prevent RNA folding into alternate structures, as demonstrated for certain base methylations in tRNA 43. That N6-methylation might create new binding sites for specific proteins is an attractive and readily identifiable function for specific m6A modification sites. Recruitment of m6A binding proteins to specific sites in the RNA would be a way to concurrently recruit additional complexes that bind m6A, such as the spliceosome, the mRNA export machinery, or chromatin remodeling enzymes.
Fig. 3. Functional effects of m6A modification.
(A) m6A modification could alter RNA structure either through weakening of base pairing or through the loss of one of the two hydrogen bond donors at the N6-position of A. This H-bond donor could be involved in RNA tertiary structure. (B) m6A modification could generate a new binding site for proteins that recognize m6A modified RNA. The m6A binding proteins can recruit additional complexes involved in cellular processes such as mRNA splicing, export, stability and immune tolerance.
In my opinion, the current biggest challenge for the field is to develop methods that can perturb m6A modification at specific sites in order to directly assess m6A function in specific genes. RNA interference or over-expression of an mRNA may simply decrease or increase both modified and unmodified RNA alike. In a few cases (summarized in 10), mutation of a known m6A site in an mRNA resulted in additional modification at a nearby consensus site, so that one cannot simply assume that mutation of a known site would not lead to the modification of cryptic sites nearby that may perform the same function. Further, functional understanding of a specific site should also take into account that all currently known m6A sites in mRNA and viral RNA are incompletely modified, so that one may need to explain why cells simultaneously maintain two RNA species that differ only at the site of m6A modification.
In summary, since the discovery of m6A modification in mRNA in 1970s, the field has come a long way to establish this modification as a wide spread biological process in many branches of eukaryotes. The m6A modifications have been proposed to play significant roles in cellular RNA functions including mRNA splicing, nuclear export, stability, and immune tolerance. The presence of appreciable amounts of m6A in lncRNAs suggests that m6A can also be important in cellular processes other than protein synthesis. Despite these advances, many technical, conceptual and biological challenges remain, and the functional significance of m6A modification in specific RNAs remains to be determined. The field is wide open for exploration.
Acknowledgments
RNA modification studies in my lab are supported by a EUREKA grant from the NIH (GM88599). I thank Dr. Chuan He for insightful discussions and comments on the manuscript, Dr. Renaud Geslain and Nian Liu for figure preparations.
Abbreviations
- m6A
N6-methyl-adenosine
- m5C
5-methyl-cytosine
- Ψ
pseudouridine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grosjean H, editor. Fine-tuning of RNA functions by modification and editing. Springer-Verlag; 2005. [Google Scholar]
- 2.Mamane Y, et al. eIF4E--from translation to transformation. Oncogene. 2004;23:3172–3179. doi: 10.1038/sj.onc.1207549. [DOI] [PubMed] [Google Scholar]
- 3.Topisirovic I, et al. Cap and cap-binding proteins in the control of gene expression. Wiley Interdiscip Rev RNA. 2011;2:277–298. doi: 10.1002/wrna.52. [DOI] [PubMed] [Google Scholar]
- 4.Dubin DT, Taylor RH. The methylation state of poly A-containing messenger RNA from cultured hamster cells. Nucleic Acids Res. 1975;2:1653–1668. doi: 10.1093/nar/2.10.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desrosiers R, et al. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc Natl Acad Sci U S A. 1974;71:3971–3975. doi: 10.1073/pnas.71.10.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rottman FM, et al. N6-adenosine methylation in mRNA: substrate specificity and enzyme complexity. Biochimie. 1994;76:1109–1114. doi: 10.1016/0300-9084(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 7.Squires JE, et al. Widespread occurrence of 5-methylcytosine in human coding and non-coding RNA. Nucleic Acids Res. 2012;40:5023–5033. doi: 10.1093/nar/gks144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karijolich J, Yu YT. Converting nonsense codons into sense codons by targeted pseudouridylation. Nature. 2011;474:395–398. doi: 10.1038/nature10165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parisien M, et al. Rationalization and prediction of selective decoding of pseudouridine-modified nonsense and sense codons. RNA. 2012;18:355–367. doi: 10.1261/rna.031351.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bokar JA. The biosynthesis and functional roles of methylated nucleosides in eukaryotic mRNA. In: Grosjean H, editor. Fine-tuning of RNA functions by modification and editing. Springer-Verlag; 2005. pp. 141–178. [Google Scholar]
- 11.Jia G, et al. N6-Methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7:885–887. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dominissini D, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–206. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- 13.Meyer KD, et al. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell. 2012;149:1635–1646. doi: 10.1016/j.cell.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schibler U, et al. Comparison of methylated sequences in messenger RNA and heterogeneous nuclear RNA from mouse L cells. J Mol Biol. 1977;115:695–714. doi: 10.1016/0022-2836(77)90110-3. [DOI] [PubMed] [Google Scholar]
- 15.Kane SE, Beemon K. Precise localization of m6A in Rous sarcoma virus RNA reveals clustering of methylation sites: implications for RNA processing. Mol Cell Biol. 1985;5:2298–2306. doi: 10.1128/mcb.5.9.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Csepany T, et al. Sequence specificity of mRNA N6-adenosine methyltransferase. J Biol Chem. 1990;265:20117–20122. [PubMed] [Google Scholar]
- 17.Harper JE, et al. Sequence specificity of the human mRNA N6-adenosine methylase in vitro. Nucleic Acids Res. 1990;18:5735–5741. doi: 10.1093/nar/18.19.5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bokar JA, et al. Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. RNA. 1997;3:1233–1247. [PMC free article] [PubMed] [Google Scholar]
- 19.Bokar JA, et al. Characterization and partial purification of mRNA N6-adenosine methyltransferase from HeLa cell nuclei. Internal mRNA methylation requires a multisubunit complex. J Biol Chem. 1994;269:17697–17704. [PubMed] [Google Scholar]
- 20.Clancy MJ, et al. Induction of sporulation in Saccharomyces cerevisiae leads to the formation of N6-methyladenosine in mRNA: a potential mechanism for the activity of the IME4 gene. Nucleic Acids Res. 2002;30:4509–4518. doi: 10.1093/nar/gkf573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bodi Z, et al. Yeast targets for mRNA methylation. Nucleic Acids Res. 2010;38:5327–5335. doi: 10.1093/nar/gkq266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhong S, et al. MTA is an Arabidopsis messenger RNA adenosine methylase and interacts with a homolog of a sex-specific splicing factor. Plant Cell. 2008;20:1278–1288. doi: 10.1105/tpc.108.058883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hongay CF, Orr-Weaver TL. Drosophila Inducer of MEiosis 4 (IME4) is required for Notch signaling during oogenesis. Proc Natl Acad Sci U S A. 2011;108:14855–14860. doi: 10.1073/pnas.1111577108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kariko K, et al. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23:165–175. doi: 10.1016/j.immuni.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Kawai T, Akira S. Toll-like receptor and RIG-I-like receptor signaling. Ann N Y Acad Sci. 2008;1143:1–20. doi: 10.1196/annals.1443.020. [DOI] [PubMed] [Google Scholar]
- 26.Kariko K, et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol Ther. 2008;16:1833–1840. doi: 10.1038/mt.2008.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warren L, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aas PA, et al. Human and bacterial oxidative demethylases repair alkylation damage in both RNA and DNA. Nature. 2003;421:859–863. doi: 10.1038/nature01363. [DOI] [PubMed] [Google Scholar]
- 29.Ougland R, et al. AlkB restores the biological function of mRNA and tRNA inactivated by chemical methylation. Mol Cell. 2004;16:107–116. doi: 10.1016/j.molcel.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Fu D, et al. Human AlkB homolog ABH8 Is a tRNA methyltransferase required for wobble uridine modification and DNA damage survival. Mol Cell Biol. 2010;30:2449–2459. doi: 10.1128/MCB.01604-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Songe-Moller L, et al. Mammalian ALKBH8 possesses tRNA methyltransferase activity required for the biogenesis of multiple wobble uridine modifications implicated in translational decoding. Mol Cell Biol. 2010;30:1814–1827. doi: 10.1128/MCB.01602-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu Y, et al. The AlkB domain of mammalian ABH8 catalyzes hydroxylation of 5-methoxycarbonylmethyluridine at the wobble position of tRNA. Angew Chem Int Ed Engl. 2010;49:8885–8888. doi: 10.1002/anie.201001242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van den Born E, et al. ALKBH8–mediated formation of a novel diastereomeric pair of wobble nucleosides in mammalian tRNA. Nat Commun. 2011;2:172. doi: 10.1038/ncomms1173. [DOI] [PubMed] [Google Scholar]
- 34.Tahiliani M, et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Branco MR, et al. Uncovering the role of 5-hydroxymethylcytosine in the epigenome. Nat Rev Genet. 2012;13:7–13. doi: 10.1038/nrg3080. [DOI] [PubMed] [Google Scholar]
- 36.Gerken T, et al. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science. 2007;318:1469–1472. doi: 10.1126/science.1151710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fischer J, et al. Inactivation of the Fto gene protects from obesity. Nature. 2009;458:894–898. doi: 10.1038/nature07848. [DOI] [PubMed] [Google Scholar]
- 38.He C. Grand challenge commentary: RNA epigenetics? Nat Chem Biol. 2010;6:863–865. doi: 10.1038/nchembio.482. [DOI] [PubMed] [Google Scholar]
- 39.Yi C, Pan T. Cellular dynamics of RNA modification. Acc Chem Res. 2011;44:1380–1388. doi: 10.1021/ar200057m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng G, et al. ALKBH5 Is a Mammalian RNA Demethylase that Impacts RNA Metabolism and Mouse Fertility. Mol Cell. 2012 doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flusberg BA, et al. Direct detection of DNA methylation during single-molecule, real-time sequencing. Nat Methods. 2010;7:461–465. doi: 10.1038/nmeth.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kierzek E, Kierzek R. The thermodynamic stability of RNA duplexes and hairpins containing N6-alkyladenosines and 2-methylthio-N6-alkyladenosines. Nucleic Acids Res. 2003;31:4472–4480. doi: 10.1093/nar/gkg633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.El Yacoubi B, et al. Biosynthesis and Function of Posttranscriptional Modifications of Transfer RNAs. Annu Rev Genet. 2012;46:69–95. doi: 10.1146/annurev-genet-110711-155641. [DOI] [PubMed] [Google Scholar]