Abstract
Case reports of Burkitt lymphoma (BL) in transplant recipients suggest that the risk is markedly elevated. Therefore, we investigated the incidence of BL in 203,557 solid organ recipients in the U.S. Transplant Cancer Match Study (1987–2009) and compared it to the general population using standardized incidence ratios (SIRs). We also assessed associations with demographic and clinical characteristics, and treatments used to induce therapeutic immunosuppression. BL incidence was 10.8 per 100,000 person-years, representing 23-fold (95%CI 19–28) greater risk than in the general population, and it peaked 3–8 years after the time of transplantation. In adjusted analyses, BL incidence was higher in recipients transplanted when <18 vs. ≥35 years (incidence rate ratio [IRR] 3.49, 95% CI 2.08–5.68) and in those transplanted with a liver (IRR 2.91, 95% CI 1.68–5.09) or heart (IRR 2.39, 95% CI 1.30–4.31) compared to kidney. BL incidence was lower in females than males (IRR 0.45, 95% CI 0.28–0.71), in blacks than whites (IRR 0.33, 95% CI 0.12–0.74), in those with a baseline Epstein-Barr virus (EBV)-seropositive versus EBV-seronegative status (IRR 0.34, 95% CI 0.13–0.93), and in those treated with azathioprine (IRR 0.56, 95% CI 0.34–0.89) or corticosteroids (IRR 0.48, 95% CI 0.29–0.82). Tumors were EBV-positive in 69% of 32 cases with results. EBV positivity was 90% in those aged <18 years and 59% in those aged 18+ years. In conclusion, BL risk is markedly elevated in transplant recipients, and it is associated with certain demographic and clinical features. EBV infection was present in most but not all BL cases.
Keywords: Burkitt lymphoma, transplantation, immunosuppression, Epstein-Barr virus, non-Hodgkin lymphoma
Introduction
Burkitt lymphoma (BL) is an aggressive B-cell non-Hodgkin lymphoma (NHL) that is endemic in parts of Africa and New Guinea, [1] and occurs sporadically in Europe and North America.[2] Although endemic and sporadic BL are recognized by the World Health organization as distinct entities, [2] they are histologically indistinguishable and share in common translocation of the c-MYC proto-oncogene on chromosome 8 (8q24) near the sequences that regulate heavy or light chain immunoglobulin genes on chromosomes 14q32, 2p12, or 22q11. [3, 4] Endemic BL is linked to Epstein-Barr virus (EBV) and Plasmodium falciparum malaria infections,[5–7] but the etiology of sporadic BL has not been identified.
BL also occurs in association with immunosuppression in persons with human immunodeficiency virus (HIV) infection and in solid organ transplant recipients,[8] and EBV infection is thought to be the main driver of BL in immunosuppressed persons. [9] The risk of BL is increased 60-fold among persons with HIV as compared to the general population. [10]
Case reports of BL in transplant recipients are consistent with an elevated risk for BL,[11–13] but the incidence of BL and its risk factors have not been described. Use of solid organ transplantation has grown steadily over the past 20–30 years.[14] With improved transplant outcomes, cancer is becoming an increasing problem for transplant recipients. To gain insights into BL and obtain valuable data for clinicians and public health workers, we investigated the incidence and risk factors for BL among solid organ transplant recipients in the U.S. using data from the Transplant Cancer Match (TCM) Study for the period 1987–2009.
Methods
The U.S. TCM Study (http://transplantmatch.cancer.gov/) utilizes linked data from the Scientific Registry of Transplant Recipients (SRTR) and population-based cancer registries in 15 states and/or metropolitan areas. [15] The SRTR data come from the Organ Procurement and Tissue Network (OPTN), which coordinates transplantation in the U.S. All U.S. transplant centers routinely provide OPTN with recipient demographic, baseline clinical, 6-month post-transplant and annual follow-up data. Only transplant recipients residing in the areas with cancer registries used in the linkage were included. Population-based cancer registry data were obtained from California (1988–2008), Colorado (1988–2006), Connecticut (1973–2006), Florida (1981–2009), Georgia (1995–2008), Hawaii (1973–2007), Illinois (1986–2007), Iowa (1973–2009), Michigan (1985–2006), North Carolina (1990–2007), New Jersey (1979–2006), New York (1976–2007), the Seattle-Puget Sound area of Washington State (1974–2008), Texas (1995–2006), and Utah (1973–2008). Institutional review boards at participating sites and at the National Cancer Institute gave approval to conduct the study.
Statistical analysis
BL cases were identified from cancer registry data using morphology codes provided by the cancer registries. The morphology codes for BL used during the study period include 9750/3 in the International Classification of Diseases for Oncology (ICD-O) published in 1976 [16] for the cases registered from 1987–1991 and codes 9687/3 and 9826/3 in ICD-O-2 and ICD-O-3 published in 1990[17] and 2000 [18], respectively, for the cases registered from 1992–2009. The cancer registries re-coded the BL cases from the early period to ICD-O-3 9687/3 and 9826/3 morphology codes, [18] which were used in the current study. BL incidence in recipients was expressed as the number of cases per 100,000 person-years of follow-up. Each transplant was considered as a separate period at risk. Person-time was calculated from the time of transplantation or start of cancer registry coverage (whichever occurred later) until BL diagnosis, failure of a transplanted organ, re-transplant, death, last follow-up date, or end of cancer registry coverage (whichever occurred earliest).
Standardized incidence ratios (SIRs) were used to estimate the fold-increase in BL incidence relative to the general population. SIRs were calculated as the number of observed BL cases in the transplant recipients divided by the number of expected BL cases. The expected number of BL cases was calculated using general population rates applied to the person-time at risk in transplant recipients. These calculations were performed in strata defined by age-, race-, sex-, calendar year, and registry region and then summed to get the total expected count. Exact 95% confidence intervals (95% CIs) of the SIRs were derived.
We assessed potential BL risk factors, such as clinical and treatment characteristics, using incidence rate ratios (IRRs) calculated in univariate Poisson regression models. Sex, age at transplantation, transplanted organ, time since transplantation, race, and baseline EBV serostatus were considered a priori to be potentially important risk factors for BL, so these variables were used to construct a base multivariate model that was utilized to assess independent contribution of other covariates identified in univariate models. Calendar year of transplantation was collinear with time since transplantation, so it was not included in multivariate models. The likelihood ratio test was used to determine whether each variable was significantly associated with BL in the multivariable model. A two-sided P value of <0.05 was considered statistically significant.
We performed secondary analyses to gain insights into EBV patterns in BL tumors. BL is one type of post-transplant lymphoproliferative disease (PTLD), and some BL cases had PTLD reported to the SRTR by their transplant center. After comparing the similarity of the dates of diagnosis of the PTLD record to the date of BL recorded in the cancer registry, we concluded that a diagnosis coded as PTLD in the SRTR and as BL in the cancer registry were both referring to the same tumor episode in the recipient. Therefore, we utilized the EBV results on PTLD cases in the SRTR to infer EBV status in the tumor for the linked BL cases.
Results
We studied 203,557 transplant recipients, of whom 61% were male and 62% were non-Hispanic whites, 17% blacks and 16% Hispanics (Table I). The three most commonly transplanted organs were kidney (58%), liver (22%), and a thoracic organ (heart and/or lung, 14%). Baseline EBV serostatus was known for 34% of the recipients, among whom 83% were seropositive. Among recipients with known EBV serostatus, seropositivity did not differ by race (range was 83–84%). Baseline HIV infection status was known for 37% of the recipients, and HIV infection was rare (0.3%).
Table I.
Characteristics of the U.S. transplant recipient cohort
| Characteristic | Number of recipients | (%) |
|---|---|---|
| Sex | ||
| Male | 124,246 | (61.0) |
| Female | 79,311 | (39.0) |
| Age at transplant (years) | ||
| 0–19 | 18,149 | (8.9) |
| 20–34 | 31,202 | (15.3) |
| 35–49 | 63,831 | (31.4) |
| 50–64 | 73,423 | (36.1) |
| 65+ | 16,952 | (8.3) |
| Race/ethnicity | ||
| White, non-Hispanic | 126,051 | (61.9) |
| Black | 34,346 | (16.9) |
| Hispanic | 31,878 | (15.7) |
| Asian/Pacific Islander | 11,282 | (5.5) |
| Transplanted organ | ||
| Kidney | 117,660 | (57.8) |
| Kidney/pancreas or pancreas | 9,014 | (4.4) |
| Liver | 44,934 | (22.1) |
| Heart and/or lung | 29,142 | (14.3) |
| Other or multiple | 2,807 | (1.4) |
| Transplant number | ||
| First | 185,629 | (91.2) |
| Second | 16,397 | (8.1) |
| Third or higher | 1,531 | (0.8) |
| Calendar year of transplant | ||
| 1987–1994 | 38,818 | (19.1) |
| 1995–1999 | 52,364 | (25.7) |
| 2000–2004 | 64,762 | (31.8) |
| 2005–2009 | 47,613 | (23.4) |
| EBV serostatus at baseline | ||
| Unknown/missing | 133,878 | (65.8) |
| Negative | 11,672 | (5.7) |
| Positive | 58,007 | (28.5) |
| HIV status at baseline | ||
| Unknown/missing | 127,794 | (62.8) |
| Negative | 75,561 | (37.1) |
| Positive | 202 | (0.1) |
BL was identified in 99 recipients, and the median age at BL was 47 years. Males comprised 76% of the cases, and by race, 69% were non-Hispanic whites, 23% Hispanics, 5% blacks and 3% Asian/Pacific Islanders (Table II). The distribution of age at the time of transplantation of the BL cases was 28%, 17%, 23%, 27% and 4% for ages 0–19, 20–34, 35–49, 50–64, and 65+ years, respectively (Table II).
Table II.
Associations of Burkitt lymphoma incidence with recipient demographic characteristics
| Characteristic | BL cases | Incidence rates* | Incidence rate ratios | 95% confidence intervals |
|---|---|---|---|---|
| Gender | ||||
| Female | 24 | 6.6 | 0.48 | 0.30, 0.75 |
| Male | 75 | 13.6 | Ref. | |
| Age at transplant (years) | ||||
| 0–19 | 28 | 32.0 | 3.70 | 2.17,6.30 |
| 20–34 | 17 | 11.4 | 1.31 | 0.70,2.40 |
| 35–49 | 23 | 7.5 | 0.87 | 0.49,1.51 |
| 50–64 | 27 | 8.7 | Ref. | |
| 65+ | 4 | 6.7 | 0.78 | 0.23, 1.99 |
| Race/ethnicity | ||||
| White, non-Hispanic | 68 | 11.4 | Ref. | |
| Black | 5 | 3.7 | 0.33 | 0.11, 0.73 |
| Hispanic | 23 | 16.9 | 1.48 | 0.90, 2.34 |
| Asian/Pacific Islander | 3 | 6.0 | 0.53 | 0.13, 1.41 |
| Transplant year | ||||
| 1987–1994 | 24 | 9.2 | Ref. | |
| 1995–1999 | 48 | 15.1 | 1.64 | 1.01, 2.72 |
| 2000–2004 | 22 | 8.3 | 0.90 | 0.50, 1.61 |
| 2005–2009 | 5 | 6.9 | 0.75 | 0.25, 1.81 |
Incidence per 100,000 person-years
The anatomic site of BL was recorded as lymph nodes in 75% of the cases, and was distributed as follows: 14% in cervical, axilla region, or intra-thoracic nodes, 11% in abdominal or pelvic nodes, 32% in multiple nodal sites, and 18% in unspecified nodal sites. The anatomic sites were extranodal in 25% of the cases, and distributed as follows: 16% abdominal, including the small or large intestine and liver, 4% in bone marrow, and 5% in miscellaneous sites including the nasopharynx, mediastinum, spinal cord/meninges, and soft tissue.
A PTLD record was found in the SRTR for 53 (54%) of the 99 BL cases. The mean time from the BL diagnosis date to the PTLD diagnosis date was 0.1 month (range −9 months to +13 months). EBV was positive in 69% (22 of 32 cases) and negative in 31% (10 of 32), and unknown in 21 cases. BL was EBV positive in 90% of 10 cases age 0–17 years at BL diagnosis, 58% of 12 cases age 18–49 years, and 60% of 10 cases age 50+ years. The 22 EBV-positive PTLD/BL cases were diagnosed in four recipients who were EBV-seronegative at baseline, 4 who were EBV-seropositive, and 14 whose EBV serostatus was unknown. The 10 EBV-negative PTLD/BL cases were diagnosed in three recipients who were EBV seropositive at baseline and in seven recipients whose EBV baseline status was unknown.
The BL incidence rate in transplant recipients was 10.8 per 100,000 person-years. The incidence was low in the first two years after transplantation, but rose steeply in the third year and remained elevated for 3–8 years after transplantation (Figure 1). BL incidence was 23-fold (95%CI 19–28) greater in transplant recipients than in the general population. The incidence was 410-fold (95% CI 10–2300) higher in HIV-infected recipients than in the general population, but this result was based on only one HIV-infected case and thereby risk estimation is unstable. Among recipients, BL incidence in females was half that observed among males (P<0.001; Table II). BL incidence was also inversely related to age at transplantation (Ptrend <0.001). BL incidence among blacks was a third of that observed among whites (P=0.015), but the rate among Hispanics and Asians/Pacific Islanders was similar to that in whites. The incidence of BL among blacks compared to whites remained significantly decreased in analyses adjusting for baseline EBV serostatus (adjusted IRR 0.33, 95% CI 0.12–0.74; Table II).
Figure 1.
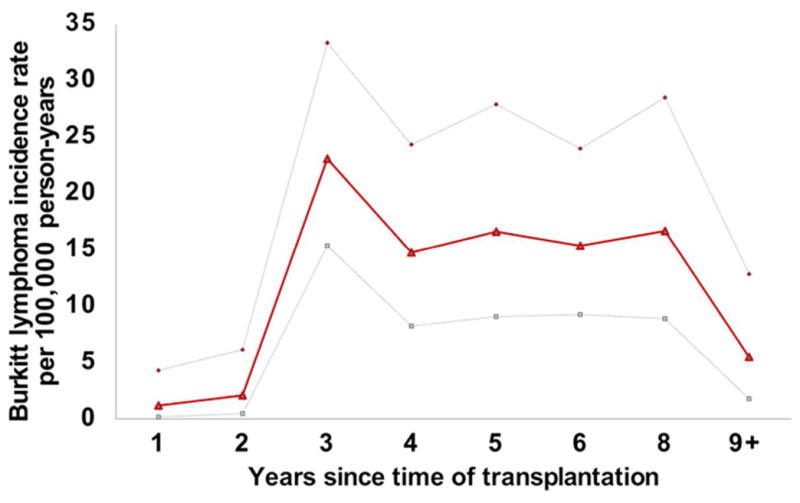
Burkitt lymphoma incidence according to time elapsed since transplant. Bold line shows actual estimated incidence, light dotted lines indicate upper and lower 95% confidence intervals of the incidence rate.
BL incidence was 3–3.5 times higher in those recipients transplanted with a liver or heart than in those transplanted with a kidney (P<0.001) (Table III). BL incidence in recipients with baseline EBV seropositive status was only one-fourth of the incidence in those with EBV seronegative status (IRR 0.24, P=0.003). A similar pattern was observed for baseline cytomegalovirus serostatus (IRR 0.4, P=0.025), based on 49% of subjects with cytomegalovirus serostatus, but the association was not independent of baseline EBV serostatus (data not shown). BL incidence was not significantly related to a history of diabetes mellitus (Table III) or another 23 specific medical conditions listed as the indication for transplant (see Supplemental Tables I-IV). The possible exception was a decreased BL incidence for non-cholestatic cirrhosis and an increased incidence with cholestatic liver disease/cirrhosis in analyses restricted to liver recipients (Table III).
Table III.
Associations of Burkitt lymphoma incidence with recipient clinical characteristics
| Characteristic | BL cases | Incidence rates* | Incidence rate ratios | 95% confidence intervals |
|---|---|---|---|---|
| Transplanted organ | ||||
| Heart | 20 | 18.1 | 2.98 | 1.68, 5.16 |
| Kidney | 32 | 6.1 | Ref. | |
| Liver | 43 | 21.3 | 3.51 | 2.23, 5.59 |
| Lung | 1 | 3.6 | 0.60 | 0.03, 2.77 |
| Other | 3 | 6.3 | 1.03 | 0.25, 2.88 |
| EBV serostatus¶ | ||||
| Negative | 8 | 22.5 | Ref. | |
| Positive | 9 | 5.4 | 0.24 | 0.09, 0.64 |
| Unknown/missing | 82 | 11.5 | 0.51 | 0.26, 1.15 |
| CMV serostatus¶ | ||||
| Negative | 14 | 13.1 | Ref. | |
| Positive | 10 | 5.2 | 0.40 | 0.17, 0.88 |
| Unknown/missing | 75 | 12.2 | 0.93 | 0.54, 1.72 |
| HIV serostatus ¶ | ||||
| Negative | 33 | 13.4 | Ref. | |
| Positive | 1 | 288 | 21.5 | 1.21, 99.7 |
| Unknown/missing | 65 | 9.7 | 0.73 | 0.48, 1.12 |
| Medical conditions | ||||
| Diabetes mellitus | ||||
| No | 57 | 13.9 | Ref. | |
| Yes | 11 | 8.9 | 0.64 | 0.32, 1.18 |
| Missing | 31 | 8.2 | 0.59 | 0.38, 0.91 |
| Non-cholestatic cirrhosis† | ||||
| No | 24 | 32.9 | Ref. | |
| Yes | 19 | 14.8 | 0.45 | 0.24, 0.82 |
| Cholestatic liver disease/cirrhosis† | ||||
| No | 31 | 18.1 | Ref. | |
| Yes | 12 | 39.4 | 2.18 | 1.07, 4.12 |
Incidence is per 100,000 person-years;
results from tests conducted at baseline;
Analysis is restricted to liver recipients
BL incidence was unrelated to HLA mismatch scores, induction immunosuppression with monoclonal antibody, or maintenance immunosuppression with cyclosporine, tacrolimus, or mammalian target of rapamycin (mTOR) inhibitors (Table IV). However, BL incidence was marginally decreased among those recipients receiving induction immunosuppression with an interleukin-2 receptor antagonist (IL2RA) (IRR 0.56, 95% CI 0.25–1.08) and significantly decreased in those receiving polyclonal antibody induction (IRR 0.41, 95% CI 0.17–0.82). BL incidence was marginally decreased among those recipients treated with azathioprine (IRR 0.69, 95% CI 0.43–1.07) and significantly decreased among those treated with mycophenolate mofetil (0.64, 95% CI 0.41–0.96) or corticosteroids (0.47, 95% CI 0.29–0.80).
Table IV.
Associations of Burkitt lymphoma incidence with HLA mismatch and treatment characteristics
| Characteristic | BL cases | Incidence rates* | Incidence rate ratios | 95% confidence Intervals |
|---|---|---|---|---|
| HLA mismatch score¶ | ||||
| 0 | 3 | 4.2 | 0.37 | 0.09, 1.06 |
| 1 | 5 | 16.7 | 1.44 | 0.48, 3.53 |
| 2 | 10 | 12.0 | 1.04 | 0.47, 2.14 |
| 3 | 13 | 8.2 | 0.70 | 0.34, 1.39 |
| 4 | 14 | 8.0 | 0.69 | 0.34, 1.35 |
| 5 | 21 | 11.6 | Ref. | |
| 6 | 6 | 6.6 | 0.57 | 0.21, 1.34 |
| Missing | 27 | 21.9 | 1.89 | 1.07, 3.38 |
| Induction medications | ||||
| Polyclonal antibody | ||||
| No | 92 | 11.9 | Ref. | |
| Yes | 7 | 4.9 | 0.41 | 0.17, 0.82 |
| Monoclonal antibody | ||||
| No | 90 | 10.6 | Ref. | |
| Yes | 9 | 13.2 | 1.24 | 0.58, 2.33 |
| IL2RA | ||||
| No | 91 | 11.5 | Ref. | |
| Yes | 8 | 6.4 | 0.56 | 0.25, 1.08 |
| Maintenance medications | ||||
| Cyclosporine | ||||
| No | 46 | 11.2 | Ref. | |
| Yes | 53 | 10.5 | 0.93 | 0.63, 1.39 |
| Tacrolimus | ||||
| No | 62 | 10.7 | Ref. | |
| Yes | 37 | 11.0 | 1.03 | 0.68, 1.53 |
| mTOR inhibitors | ||||
| No | 97 | 11.2 | Ref. | |
| Yes | 2 | 4.7 | 0.43 | 0.07, 1.34 |
| Azathioprine | ||||
| No | 74 | 12.0 | Ref. | |
| Yes | 25 | 8.3 | 0.69 | 0.43, 1.07 |
| MMF | ||||
| No | 67 | 12.8 | Ref. | |
| Yes | 32 | 8.2 | 0.64 | 0.41, 0.96 |
| Corticosteroids | ||||
| No | 18 | 20.9 | Ref. | |
| Yes | 81 | 9.8 | 0.47 | 0.29, 0.80 |
IL2RA= interleukin-2 receptor antagonist; mTOR= mammalian target of rapamycin; MMF=mycophenolate mofetil ;
Incidence per 100,000 person-years ;
HLA mismatch score is the sum of mismatches at A, B, and DR loci.
In a multivariate analysis adjusting for age, transplanted organ, and follow-up time, BL incidence was significantly decreased in female transplant recipients compared to males and in those who were EBV-seropositive at baseline compared to EBV-seronegative (Table V). BL risk was significantly lower in blacks compared to whites, but no difference in incidence was noted when race/ethnicity was categorized as white versus non-white. After adjustment, receipt of azathioprine and corticosteroids remained significantly associated with decreased BL incidence, but associations with IL2RA, polyclonal antibodies, and mycophenolate mofetil were no longer significant. In analyses restricted to liver recipients, and after adjustment for variables in the base model, BL incidence remained decreased for non-cholestatic cirrhosis (IRR 0.46, 95% CI 0.22–0.96) and increased for cholestatic liver disease/cirrhosis (IRR 3.14, 95% CI 1.51–6.18).
Table V.
Multivariate associations of Burkitt lymphoma incidence with demographic, clinical, and treatment characteristics
| Characteristic | Incidence rate ratios* | 95% Confidence intervals |
|---|---|---|
| Base model | ||
| Gender | ||
| Female | 0.45 | 0.28, 0.71 |
| Male | Ref. | |
| Age at transplant | ||
| 0–19 | 3.49 | 2.08, 5.68 |
| 20–34 | 2.36 | 1.28, 4.10 |
| 35+ | Ref. | |
| Race/ethnicity | ||
| White, Non- Hispanic | Ref. | |
| All others ¶ | 0.93 | 0.59, 1.42 |
| Transplanted organ | ||
| Heart | 2.39 | 1.30, 4.31 |
| Kidney | Ref. | |
| Liver | 2.91 | 1.68, 5.09 |
| Other | 1.01 | 0.30, 2.59 |
| Baseline EBV serostatus | ||
| Negative | Ref. | |
| Positive | 0.34 | 0.13, 0.93 |
| Unknown/missing | 0.61 | 0.29, 1.44 |
| Time since transplant | ||
| <2 years | 0.12 | 0.04, 0.27 |
| 2–<3 years | 1.73 | 1.09, 2.70 |
| 3+ years | Ref. | |
| Additional variables, adjusted results | ||
| Polyclonal antibody | ||
| No | Ref. | |
| Yes | 0.64 | 0.27, 1.32 |
| IL2RA | ||
| No | Ref. | |
| Yes | 0.92 | 0.39, 1.87 |
| Azathioprine | ||
| No | Ref. | |
| Yes | 0.56 | 0.34, 0.89 |
| MMF | ||
| No | Ref. | |
| Yes | 1.08 | 0.67, 1.69 |
| Corticosteroids | ||
| No | Ref. | |
| Yes | 0.48 | 0.29, 0.82 |
The base model includes gender, age at transplant, race, transplanted organ, and EBV status. Estimates for each medication were adjusted for the variables in the base model, but not for each other.
Other includes blacks, Hispanics, and Asians/Pacific Islanders.
Discussion
Utilizing data from a large registry linkage study of transplant recipients, we show that the risk of BL in transplant recipients in the U.S. is elevated 23-fold compared to the general population. The increase might even be higher (410-fold) among HIV-infected transplant recipients, but this result is based on one case and should be interpreted cautiously. In multivariate models, BL was positively associated with male sex, transplantation at age <18 years, and receipt of a liver or heart. BL was negatively associated with having a baseline EBV seropositive status and treatment with azathioprine or corticosteroids.
The higher BL incidence in children and lower incidence in females, independent of EBV, has been reported in endemic, [19, 20] sporadic, [21] and HIV-related BL. [22] The higher BL incidence in whites than in blacks has been reported in sporadic BL [21, 23, 24] and AIDS-related BL. [22] These patterns, common to all subtypes of BL, indicate that age, gender, and race are markers of intrinsic susceptibility to BL, although the nature of this susceptibility is unknown. Our finding that BL risk was lower in recipients receiving certain medications is intriguing. Corticosteroids modulate lymphocyte function and comprise one component of some therapeutic regimens for BL. [25] We also noted decreased risk of BL in recipients treated with mTOR inhibitors (IRR= 0.43), but the result was not statistically significant, perhaps because mTOR inhibitors have only been recently introduced in this setting.
We found an elevated risk of BL among recipients of heart or liver transplants, which possibly reflects differences in immunosuppression protocols or immunologic properties unique to those organs. BL risk was decreased with non-cholestatic cirrhosis and increased with cholestatic liver disease/cirrhosis as the indications for liver transplant but, because we examined many medical conditions, these associations may be chance findings.
Our data confirm that EBV is implicated in most (69%) BL cases in transplant recipients. [11, 13] Our results also indicate that 31% of cases with EBV data were EBV-negative [9]. EBV-negative cases were diagnosed in transplant recipients who were EBV-seropositive at baseline, suggesting EBV-negativity is not necessarily an indication of lack of EBV infection at the time of transplantation. Similar results have been described in case reports as well. [11, 13] EBV positivity in the tumor varied with age. EBV was detected in 90% of cases of <18 years, but in only 59% of cases diagnosed at or after 18 years. Interestingly, the high EBV positivity (82%) reported by Picarsic et al. [11] was based on BL cases diagnosed in recipients aged <18 years. In other settings, EBV positivity is lower in BL adult cases. For example, EBV positivity is 40–60% in HIV/AIDS-related BL [2], which consists mostly of adults. Similarly, EBV is detected in at most 20% of cases of sporadic BL, [2, 26] but these cases also include a substantial proportion of adult cases. Thus, EBV-positive and EBV-negative BL may be distinct entities, whose distribution might also vary with age. [27] If so, EBV-positive and EBV-negative BL may originate from distinct progenitor B cells, [28], carry distinct mutations in host genes,[29–31] and may contribute to multimodal patterns of BL.[22, 27]
EBV-seronegative transplant recipients experienced a four-fold higher incidence for BL than those who were EBV-seropositive. This result indicates that the sequential relationship between EBV infection and onset of immunosuppression influences the risk for BL. Nonetheless, baseline EBV serostatus were missing for 66% of recipients, so our inferences should be interpreted cautiously. The BL latency period was about four years on average, which contrasts with the steep increase in risk in the first year after transplantation of both diffuse large B-cell lymphoma (the most common lymphoma in transplant recipients) [32] and Kaposi sarcoma, [33] although both are also virus-associated malignancies. This longer latency suggests that progression to BL is due to chronic EBV infection. Alternatively, the lower incidence of BL in the first few years after transplantation may reflect the intensity of immunosuppression during that period. We previously noted a paradoxical decline in BL risk in HIV/AIDS patients with very low CD4 lymphocyte counts, [22] which may be a clue to the requirement for some functional T cells in BL progression. [22]
The strengths of our study include its large size, representativeness of the U.S. transplant population, and use of cancer registry linkage for comprehensive and standard identification of BL cases. Because transplant recipients were classified according to their residence at the time of transplantation, but overtime, some could have moved away. The impact of this includes inflating the denominator because these recipients were assumed to still under follow-up or under estimating the outcomes because BL occurring in those recipients who moved away would be missed by the cancer registries. However, we have previously reported that outmigration was quite minimal (approximately 6% at 10 years after transplantation), [15] thus, it is unlikely to have substantially distorted our estimate of BL incidence. The lack of central review by expert hematopathologists is a limitation, which could have introduced misclassification. Incompleteness of data, especially of baseline EBV, CMV, and HIV serostatus and EBV status of the tumors, are also limitations. In addition, because we examined many potential BL risk factors, some associations could have been due to chance.
In conclusion, BL risk is markedly elevated in transplant recipients, and risk is associated with male sex, young age, baseline EBV status, allograft type, and certain treatments used to induce therapeutic immunosuppression. BL cases in transplant recipients included both EBV positive and negative tumors. This suggests the presence of distinct BL entities, which may differ in etiology.
Acknowledgments
The authors gratefully acknowledge the support and assistance provided by individuals at the Health Resources and Services Administration (including Monica Lin), the SRTR (Ajay Israni, Bertram Kasiske, Paul Newkirk, Jon Snyder), and the following cancer registries: the states of California, Colorado (Jack Finch), Connecticut (Lou Gonsalves), Florida (Brad Wohler), Georgia (Rana Bayakly), Hawaii, Iowa, Illinois (Lori Koch), Michigan (Glenn Copeland), New Jersey (Xiaoling Niu), New York (Amy Kahn), North Carolina (Chandrika Rao), Texas (Melanie Williams), and Utah (Janna Harrell), and the Seattle-Puget Sound area of Washington (Margaret Madeleine). We also thank analysts at Information Management Services for programming support (David Castenson, Ruth Parsons).
The views expressed in this paper are those of the authors and should not be interpreted to reflect the views or policies of the National Cancer Institute, Health Resources and Services Administration, SRTR, cancer registries, or their contractors. This research was supported in part by the Intramural Research Program of the National Cancer Institute and by training grant number T32CA126607, Clinical and Laboratory Research Training for Surgical Oncologists.
During the initial period when registry linkages were performed, the SRTR was managed by Arbor Research Collaborative for Health in Ann Arbor, MI (contract HHSH234200537009C); beginning in September 2010, the SRTR was managed by Minneapolis Medical Research Foundation in Minneapolis, MN (HHSH250201000018C). The following cancer registries were supported by the National Program of Cancer Registries of the Centers for Disease Control and Prevention: California (agreement 1U58 DP000807-01), Colorado (U58 DP000848-04), Georgia (5U58DP000817-05), Illinois (5658DP000805-04), Michigan (5U58DP000812-03), New Jersey (5U58/DP000808-05), New York (15-0351), North Carolina (U58DP000832), and Texas (5U58DP000824-04). The following cancer registries were supported by the SEER Program of the National Cancer Institute: California (contracts HHSN261201000036C, HHSN261201000035C, and HHSN261201000034C), Connecticut (HHSN261201000024C), Hawaii (HHSN261201000037C, N01-PC-35137, and N01-PC-35139), Iowa (N01-PC-35143), New Jersey (HHSN261201000027C N01-PC-54405), Seattle-Puget Sound (N01-PC-35142), and Utah (HHSN261201000026C). Additional support was provided by the states of California, Colorado, Connecticut, Illinois, Iowa, New Jersey, New York (Cancer Surveillance Improvement Initiative 14-2491), Texas, and Washington, as well as the Fred Hutchinson Cancer Research Center in Seattle, WA.
References
- 1.Burkitt D. A sarcoma involving the jaws in African children. Br J Surg. 1958;46:218–223. doi: 10.1002/bjs.18004619704. [DOI] [PubMed] [Google Scholar]
- 2.Leoncini L, Raphael M, Stein H, et al., editors. Burkitt lymphoma. Lyon: International Agency for Research on Cancer (IARC); 2008. pp. 262–264. [Google Scholar]
- 3.Dalla-Favera R, Bregni M, Erikson J, et al. Human c-myc onc gene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma cells. Proc Natl Acad Sci U S A. 1982;79:7824–7827. doi: 10.1073/pnas.79.24.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein G. Specific chromosomal translocations and the genesis of B-cell-derived tumors in mice and men. Cell. 1983;32:311–315. doi: 10.1016/0092-8674(83)90449-x. [DOI] [PubMed] [Google Scholar]
- 5.Carpenter LM, Newton R, Casabonne D, et al. Antibodies against malaria and Epstein-Barr virus in childhood Burkitt lymphoma: a case-control study in Uganda. Int J Cancer. 2008;122:1319–1323. doi: 10.1002/ijc.23254. [DOI] [PubMed] [Google Scholar]
- 6.Mutalima N, Molyneux E, Jaffe H, et al. Associations between Burkitt lymphoma among children in Malawi and infection with HIV, EBV and malaria: results from a case-control study. PLoS ONE. 2008;3:e2505. doi: 10.1371/journal.pone.0002505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burkitt D. A children’s cancer dependent on climatic factors. Nature. 1962;194:232–234. doi: 10.1038/194232a0. [DOI] [PubMed] [Google Scholar]
- 8.Ziegler JL, Beckstead JA, Volberding PA, et al. Non-Hodgkin’s lymphoma in 90 homosexual men. Relation to generalized lymphadenopathy and the acquired immunodeficiency syndrome. N Engl J Med. 1984;311:565–570. doi: 10.1056/NEJM198408303110904. [DOI] [PubMed] [Google Scholar]
- 9.Bornkamm GW. Epstein-Barr virus and its role in the pathogenesis of Burkitt’s lymphoma: an unresolved issue. Semin Cancer Biol. 2009;19:351–365. doi: 10.1016/j.semcancer.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123:187–194. doi: 10.1002/ijc.23487. [DOI] [PubMed] [Google Scholar]
- 11.Picarsic J, Jaffe R, Mazariegos G, et al. Post-transplant Burkitt lymphoma is a more aggressive and distinct form of post-transplant lymphoproliferative disorder. Cancer. 2011;117:4540–4550. doi: 10.1002/cncr.26001. [DOI] [PubMed] [Google Scholar]
- 12.Penn I. Secondary neoplasms as a consequence of transplantation and cancer therapy. Cancer Detection and Prevention. 1988;12:39–57. [PubMed] [Google Scholar]
- 13.Zimmermann H, Reinke P, Neuhaus R, et al. Burkitt post-transplantation lymphoma in adult solid organ transplant recipients: Sequential immunochemotherapy with rituximab (R) followed by cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or R-CHOP is safe and effective in an analysis of 8 patients. Cancer. 2012;118:4715–4724. doi: 10.1002/cncr.27482. [DOI] [PubMed] [Google Scholar]
- 14.KDIGO clinical practice guideline for the care of kidney transplant recipients. American Journal of Transplantation. 2009;9 (Suppl 3):S1–155. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 15.Engels EA, Pfeiffer RM, Fraumeni JF, Jr, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306:1891–1901. doi: 10.1001/jama.2011.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.International Classification of Diseases for Oncology. Geneva: World Health Organization; 1976. [Google Scholar]
- 17.International Classification of Diseases for Oncology. Geneva: World Health Organization; 1990. [Google Scholar]
- 18.Fritz A, Percy C, Jack A, et al., editors. International Classification of Diseases for Oncology (ICD-O-3) Geneva (Switzerland): World Health Organization; 2000. [Google Scholar]
- 19.Ogwang MD, Bhatia K, Biggar RJ, et al. Incidence and geographic distribution of endemic Burkitt lymphoma in northern Uganda revisited. Int J Cancer. 2008;123:2658–2663. doi: 10.1002/ijc.23800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emmanuel B, Kawira E, Ogwang MD, et al. African Burkitt lymphoma: age-specific risk and correlations with malaria biomarkers. Amer J Trop Med & Hyg. 2011;84:397–401. doi: 10.4269/ajtmh.2011.10-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mbulaiteye SM, Biggar RJ, Bhatia K, et al. Sporadic childhood Burkitt lymphoma incidence in the United States during 1992–2005. Pediatr Blood Cancer. 2009;53:366–370. doi: 10.1002/pbc.22047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guech-Ongey M, Simard EP, Anderson WF, et al. AIDS-related Burkitt lymphoma in the United States: what do age and CD4 lymphocyte patterns tell us about etiology and/or biology? Blood. 2010;116:5600–5604. doi: 10.1182/blood-2010-03-275917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Thompson TD, Miller JW, et al. Cancer incidence among children and adolescents in the United States, 2001–2003. Pediatrics. 2008;121:e1470–1477. doi: 10.1542/peds.2007-2964. [DOI] [PubMed] [Google Scholar]
- 24.Levine PH, Kamaraju LS, Connelly RR, et al. The American Burkitt’s Lymphoma Registry: eight years’ experience. Cancer. 1982;49:1016–1022. doi: 10.1002/1097-0142(19820301)49:5<1016::aid-cncr2820490527>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 25.Patte C, Auperin A, Michon J, et al. The Societe Francaise d’Oncologie Pediatrique LMB89 protocol: highly effective multiagent chemotherapy tailored to the tumor burden and initial response in 561 unselected children with B-cell lymphomas and L3 leukemia. Blood. 2001;97:3370–3379. doi: 10.1182/blood.v97.11.3370. [DOI] [PubMed] [Google Scholar]
- 26.Klein G. Burkitt lymphoma--a stalking horse for cancer research? Semin Cancer Biol. 2009;19:347–350. doi: 10.1016/j.semcancer.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Mbulaiteye SM, Anderson WF, Bhatia K, et al. Trimodal age-specific incidence patterns for Burkitt lymphoma in the United States, 1973–2005. Int J Cancer. 2010;126:1732–1739. doi: 10.1002/ijc.24934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bellan C, Lazzi S, Hummel M, et al. Immunoglobulin gene analysis reveals 2 distinct cells of origin for EBV-positive and EBV-negative Burkitt lymphomas. Blood. 2005;106:1031–1036. doi: 10.1182/blood-2005-01-0168. [DOI] [PubMed] [Google Scholar]
- 29.Schmitz R, Young RM, Ceribelli M, et al. Burkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomics. Nature. 2012;490:116–120. doi: 10.1038/nature11378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sander S, Calado DP, Srinivasan L, et al. Synergy between PI3K Signaling and MYC in Burkitt Lymphomagenesis. Cancer cell. 2012;22:167–179. doi: 10.1016/j.ccr.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giulino-Roth L, Wang K, Macdonald TY, et al. Blood. 2012. Targeted genomic sequencing of pediatric Burkitt lymphoma identifies recurrent alterations in anti-apoptotic and chromatin-remodeling genes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quinlan SC, Morton LM, Pfeiffer RM, et al. Increased risk for lymphoid and myeloid neoplasms in elderly solid-organ transplant recipients. CEBP. 2010;19:1229–1237. doi: 10.1158/1055-9965.EPI-09-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mbulaiteye SM, Engels EA. Kaposi’s sarcoma risk among transplant recipients in the United States (1993–2003) Int J Cancer. 2006;119:2685–2691. doi: 10.1002/ijc.22233. [DOI] [PubMed] [Google Scholar]


