Abstract
The contributions of IRF3/7 and the type I interferons IFNα/β to the innate host defense have been extensively investigated, however, their role in thymic development is less clear. Here we show that mice lacking the type I interferon receptor IFNAR or the downstream transcription factor STAT1 harbor a significant reduction in self-antigen presenting, autoimmune-regulator AIRE+ medullary thymic epithelial cells (mTEC). Constitutive IFNAR signaling occurs in the thymic medulla in the absence of infection or inflammation. RANKL stimulation results in IFNβ-upregulation, which in turn inhibits RANK signaling and facilitates AIRE expression in mTECs. Finally, we find that IRF7 is required for thymic IFNβ–induction, maintenance of thymic architecture and mTEC differentiation. We conclude that spatially and temporally coordinated crosstalks between the RANKL/RANK and IRF7/IFNβ/IFNAR/STAT1 pathways are essential for differentiation of AIRE+ mTECs.
Introduction
Selection of antigen receptor-expressing T lymphocytes that are MHC-restricted but tolerant to self-antigens is an essential prerequisite for a functional immune system. Of vital importance to this process of central tolerance in the thymus are medullary thymic epithelial cells (mTECs) that are characterized by promiscuous gene expression of self-antigen. The autoimmune regulator (AIRE) is responsible for the expression of otherwise tissue-specific antigen (TSA) in mTECs and is necessary for T cell tolerance and prevention of autoimmunity (1, 2). Mutations in the gene encoding AIRE in humans results in development of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) (3), a multi-organ autoimmune disease characterized by multiple adrenal system defects including hypothyroidism and adrenal insufficiency as well as susceptibility to yeast infection (candidiasis) (4). Mice deficient in AIRE expression reproduce many of the defects observed in human APECED (5). The development of AIRE-expressing mTECs is governed by several TNF family members including RANK, CD40, and lymphotoxin β (LTβ) (6-9). Defects in their respective receptors or signaling components (RelB, NIK, or TRAF6) result in a reduced or absent medullary region in the thymus and development of autoimmunity in mutant mice (10-12). Thus, the proper development of AIRE-expressing mTECs is required for the maintenance of an intact thymic architecture and consequently of T cell tolerance to self-antigen.
The signal transducer and activator of transcription 1 (STAT1) is a well-defined element of the type I (IFNα/β) and type II (IFNγ) interferon-induced signaling cascades (13-15). The physiological significance of STAT1 in-vivo has been elucidated through the generation of STAT1-deficient mice by two independent laboratories (16, 17). As predicted, STAT1-deficient mice are impaired in responses to either type I or type II interferons, however, analysis of the specific T cell subpopulation in wild-type (WT) and STAT1-/- mice provided no indication for significant differences between these two strains (16-18).
Even though all IFNs utilize STAT1 as a signaling intermediary, type I and II IFNs elicit opposing effects on the progression of the demyelinating, T cell-mediated autoimmune disease multiple sclerosis (MS) (19). These contrasting effects of IFNα/β and IFNγ in MS prompt the question as to the role of STAT1 in this pathological process. To investigate this matter, we had previously used mice carrying a transgenic T cell receptor specific for myelin basic protein (TCRMBP). Upon immunization with MBP, TCRMBP-mice develop experimental autoimmune encephalomyelitis (EAE) which serves as a murine model for MS (20, 21). The dramatically increased susceptibility to EAE development of TCRMBPSTAT1-deficient mice is accounted for, at least in part, by a defect in the development and functionality of CD4+CD25+ regulatory T cells (18). However, the severity and frequency of spontaneous EAE development in the absence of STAT1 suggested that STAT1 might also be a factor in thymic events that control central tolerance. We recently reported that TCR-transgenic STAT1-/- and IFNAR-/-, but not IFNGR-/- animals displayed striking differences in T cell selection compared to WT littermates (22).
Our current studies illustrate that IRF7, IFNAR1 and STAT1, but not type II interferon, are essential for the development of mTECs and maintenance of thymic architecture, both in the presence and absence of transgenic TCR expression. Additional investigations corroborated constitutive type I interferon responses in the absence of infections that are restricted to the thymic medulla, and established a crosstalk between the RANK and interferon signaling cascades crucial for the development of AIRE-expressing mTECs.
In summary, our findings endorse the notion that the type I interferon system influences thymic structure and function, and consequently affects T cell selection, by promoting the development of mTECs, a process that involves the temporally and spatially coordinated interplay between the RANK and interferon signaling pathways.
Materials and Methods
Animals and cell culture
STAT1-/- (17), IFNAR1-/- (38), IRF3-/-(39), and IRF7-/- (36) mice have been described previously. Transgenic Mx1-Cre mice, and mT/mG mice were obtained from Jackson Laboratory (Bar Harbor, ME). Animals were between 6 and 10 weeks of age at the time of the experiments. All mice used in these experiments were housed in a pathogen-free environment and were bred and cared for in accordance with University of California, San Diego Animal Care Facility regulations. All studies involving animal have been approved by the UCSD Animal Subject Committee (Protocol S02194). The mTEC cell line TE-71 was generously provided by Dr. A. Farr, and cultured in DMEM + 10% FBS.
Flow-cytometric analysis
For immunostaining, single cell suspensions were prepared from thymi with approximately 106 cells suspended in FACS buffer (PBS pH 7.4, 1% FCS, 0.02% NaN3) and stained for 20 min in the dark on ice. FITC-anti-CD4 (GK1.5), FITC-anti-B220 (RA3-6B2), PE-anti-CD8 (53.6.7), and PE anti-CD80 were obtained from eBioscience (San Diego, CA). PE-anti-H-2Kb (AF6-88.5), biotin-anti-CD11c (HL3), biotin-anti-Rat IgG2a (RG7/1.30), and purified anti-Ep-CAM (G8.8) were obtained from BD Biosciences (San Jose, CA). APC-streptavidin was used as a secondary reagent to detect biotin-labeled mAbs. All samples were analyzed on a FACSCalibur and processed using CellQuest software (BD Biosciences) or Flow Jo (Ashland, OR).
Immunohistochemistry
Paraformaldehyde-fixed 6 μm frozen sections were blocked with 1% FBS in PBS pH 7.4 for 20 min before staining with the indicated antibodies for 2 h or overnight: biotin labeled UEA-1 (Vector labs, Burlingame, CA), anti-AIRE (Santa Cruz, Santa Cruz, CA), Keratin 5 (Covance, Emeryville, CA), and Ep-CAM (G8.8, BD Biosciences). SA-FITC or SA-APC (eBioscience) and anti-Rabbit Ig Cy3 (Jackson Immunoresearch, West Grove, PA) were used as secondary antibodies. Images were captured on a CRI Nuance Multispectral Imaging (Caliper, Hopkinton, MA) system attached to a Nikon E800 fluorescent microscope. Numbers of AIRE-positive cells were determined from 6 sections cut from the center of the thymus, discarding 20 μm between each section, from 6-8 week old WT or STAT1-/- mice.
Thymocyte stimulation
Splenic T cells or thymocytes were stimulated with the indicated concentrations of IFNβ (Biogen Idec, Cambridge, MA) for 30 minutes and cells were lysed in and subjected to SDS-PAGE and western blot. Blots were probed for phospho-STAT1 and STAT1 (Cell Signaling). Thymocytes from TCROTII or TCROTII STAT1-/- mice were stimulated for 24 hrs by addition to wells containing thymic stromal cells as APCs that were loaded with Ova323-339 peptide. Cells were then stained for RANKL expression using anti-mRANKL PE (eBioscience) and analyzed by flow cytometry.
mTEC isolation and stimulation
Thymic stromal cells from WT or STAT1-/- mice were purified as described (1). Briefly, thymi were cut into ~1 mm pieces and digested with collagenase/dispase (Sigma-Aldrich, St. Louis, Mo) to generate single-cell suspensions, and stromal cells were enriched over a Percoll gradient (GE healthcare, Piscataway, NJ). CD45- stromal cells were analyzed by flow cytometry for mTEC markers or stimulated with 500 ng/mL RANKL and/or 1000 U/mL recombinant, CHO cell-expressed, murine IFNβ (Biogen Idec) for the indicated times. Quantitative RT-PCR was carried out on Trizol (Sigma) purified mRNAs from stimulated stromal cells and analyzed for the expression of IFNβ using the following primers 5′-CTG AAT GGA AAG ATC AAC CTC AC-3′ 5′-TAC CTT TGC ACC CTC CAG TAA TA-3′. Taqman Gene Expression Assays were used for AIRE, INS2, and CRP (Applied Biosystems, Carlsbad, CA). Analysis was carried out on an ABI Prism 7000.
Results
Reduced medullary region in thymi of IFNAR1- or STAT1-deficient mice
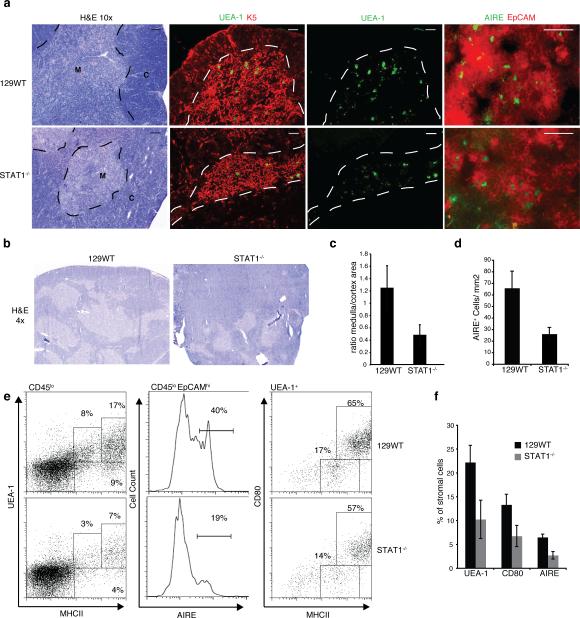
We previously reported that mice deficient for STAT1 and IFNAR1, but not IFNGR, display defective deletion of autoreactive thymocytes. As mTECs are a crucial element in this process, we contemplate IFNα/β might be playing a role in mTEC development. Abnormal thymic structure has been described previously in mice that contain mutations in particular signaling pathways such as LTβR, RANK, and CD40 (7, 8, 23). Taking into consideration that STAT1-deficient animals had shown similar susceptibility to autoimmune disease as the above mice we decided to analyze the thymic architecture in STAT1-deficient non-TCR transgenic mice. H&E staining of thymic sections from STAT1-/- mice revealed a reduced medullary region in the thymic lobe as compared to WT mice (Fig. 1a, left). This was further confirmed by immunofluorescence staining of thymic sections for keratin 5, a cell surface marker highly expressed in immature mTECs, and to a significantly lesser extent in mature mTECs (24) (Fig. 1a, center left). A reduced ratio of medullary to cortical area in thymi of STAT1-/- mice was revealed in several sections from multiple mice (Fig. 1b, quantitated in 1c). In addition, UEA-1 staining of tissue sections corroborated the concept of a reduced number of mTECs in STAT1-deficient thymi when compared to their WT counterparts (Fig.1a, center right). To further scrutinize the apparent reduction in mature mTECs, we determined the number of AIRE+ cells in the areas of the thymus characterized by high expression levels of EpCAM, a cell surface marker upregulated during mTEC maturation (25). Thymi from STAT1-deficient mice exhibited clearly reduced numbers of AIRE+ cells compared to those from WT mice (Fig. 1a, right, quantitated in 1d). In addition to UEA-1hi, mature mTECs are also characterized by high expression levels of CD80 and MHC II. Stromal cells derived from the thymi of WT and STAT1-/- mice were purified and stained for the indicated markers (Fig. 1e). As anticipated, we noted significantly reduced numbers of UEA-1hiMHCIIhi cells as well as AIRE+EPCAM+ cells (Fig. 1e, left and center panels; quantitated in Fig. 1f) and CD80+MHCIIhi cells (not shown) in STAT1-deficient animals. However, when gated specifically on UEA-1hi cells, no difference in CD80+MHCIIhi cells was seen between wild-type and STAT1-/- mice (Fig. 1e, right). Similar alterations were seen in the mTEC populations of IFNAR1-/- mice, but not IFNGR-/- animals (data not shown). In summary, these findings illustrate that STAT1- or IFNAR1-deficiency diminishes the number of mature AIRE-expressing mTECs, and suggest a novel function for type I interferons in thymic development.
Figure 1. Impaired medullary thymic architecture in IFNAR-/- and STAT1-/- mice.
a) Thymic architecture in WT129 and STAT1-/- mice as revealed by H&E staining of thymic sections (left panels); Keratin 5 (red) and UEA-1 (green) staining (middle panels), bar = 100μm; EpCAM (red) and AIRE (green) staining (right panels), bar = 50μm; M, medulla; C, cortex. b) Low magnification (4x) of H&E stains. c) Ratio of medullary to cortical cellularity in thymic sections from WT and STAT1-/- mice (data collected using ImageJ (NIH) and represent avg +/-SD of 3 sections each from 3 mice (p<0.05). d) AIRE+ cells per unit area (quantified from thymus sections shown in Fig.1a, lower panels) e) mTEC populations in WT and STAT1-/- thymi as determined by flow cytometric expression analysis of MHCII and UEA-1 (left), as well as AIRE and EpCAM (middle) on CD45lo stromal cells; MHCII and CD80 expression on UEA-1hi gated stromal cells. f) mTEC populations in WT and STAT1-/- thymi (n=4) using the indicated markers.
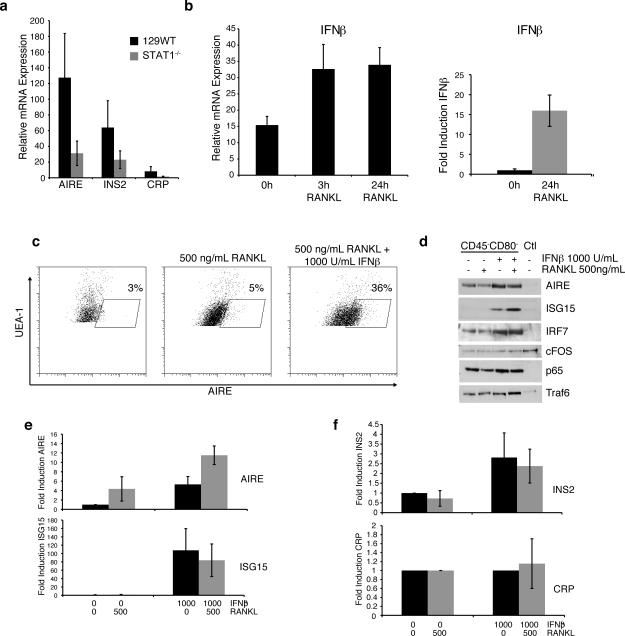
mTECs are recognized for the promiscuous, AIRE-dependent expression of tissue-restricted self-antigen encoding genes. Since the thymi of STAT1- or IFNAR1-deficient mice contain fewer AIRE-expressing cells, we evaluated the relative levels of two mTEC specific self-antigens, where expression of one depends on AIRE (Insulin 2), but the other is AIRE-independent (C-reactive protein) (1). Analysis of mRNAs from WT or STAT1-/- thymic stromal cells uncovered decreased transcripts for both INS and CRP in STAT1-deficient stromal cells (Fig. 2a). Importantly, we also observed concomitantly reduced AIRE mRNA levels in STAT1-/- epithelial cells, confirming our previous flow-cytometric and histological studies (Fig. 2a). These data support the concept that diminished mTEC numbers rather than a reduction of cellular AIRE expression are responsible for the attenuated self-antigen expression in the absence of STAT1. Together, these findings demonstrate a vital contribution of the IFNα/β-STAT1 axis in the functional development of self-antigen expressing mTECs.
Figure 2. IFNβ promotes AIRE expression following RANKL stimulation.
a) AIRE, INS2, and CRP mRNA levels in thymic stromal cells from WT and STAT1-/- mice (avg+/-sd; n=4) b) Thymic epithelial cells from WT mice (left) or TE-71 cells (right) were stimulated with 500 ng/mL RANKL for the indicated times and IFNβ mRNA levels were determined by qPCR (n = 4). c) Thymic epithelial cells were left untreated or pretreated with 1000 U/mL IFNβ prior to stimulation with 500 ng/mL RANKL for 24 hrs. UEA-1+ cells were analyzed for intracellular AIRE expression by flow cytometry. d) Thymic epithelial cells were depleted of CD80+/hi cells and then stimulated with 500 ng/mL RANKL, 1000 U/mL IFNβ or both for 24hrs. Lysates were probed for AIRE, ISG15, IRF7, cFOS, p65, and Traf6 by Western blotting (representative of three experiments). e) TE-71 cells were treated as indicated, and mRNA levels for AIRE and ISG15 were determined by qPCR (avg+/-sd; n=3). f) Thymic epithelial cells were treated as indicated and mRNA levels for CRP and INS2 were determined by qPCR (avg+/-sd; n=4).
IFNβ control over RANK signaling is required to maintain AIRE levels in thymic stromal cells
The TNF family members LTβ, CD40, and RANK all contribute the development of mTECs, and deletion of any of the genes encoding these cell surface molecules or their downstream effectors results in defects of medullary development. As a crosstalk between the RANK ligand (RANKL) and interferon signaling pathways had been demonstrated previously in the maturation process of osteoclasts (26), we elected to investigate RANK signaling in thymic stromal cells. Similar to osteoclasts, treatment of freshly isolated thymic stromal cells or the mTEC cell line TE-71 with RANKL resulted in the induction of IFNβ mRNA expression (Fig. 2b; see also Fig. 4b). In accordance with previous reports, mTECs rapidly lose AIRE expression in culture (Fig. 2c, left panel) as determined by intracellular staining for AIRE and flow cytometric analysis. Although RANKL treatment alone of thymic stromal cells slightly increased the number of UEA-1hi cells (Fig. 2c middle), co-stimulation with both IFNβ and RANKL resulted in a pronounced increase in AIRE+ cells (Fig. 2c, right panel). IFNβ by itself was capable of inducing AIRE expression in both thymic stromal cells (Fig. 2d) as well as in the TE-71 cells (Fig. 2e). In addition, co-stimulation with IFNβ and RANKL did not result in a reduction of either c-fos, p65, or Traf6 (Fig. 2d), indicating that interferon is not causing the degradation of components of the RANK signal transduction pathway in mTECs. This presents a contrast to the mechanism of RANKL and IFNβ opposition during osteoclast differentiation where IFNβ treatment causes the loss of c-fos induction by RANKL (26). Finally, analysis of self-antigen expression following stromal cell stimulation indicates that interferon alone can induce expression of the AIRE-dependent self-antigen INS2 (Fig. 2f, top), but had little effect on the expression of the AIRE-independent self-antigen CRP (Fig. 2f, bottom). These findings not only demonstrate that IFNβ is induced by RANK signaling on thymic stromal cells, but that a crosstalk exists between the RANK- and IFNAR-initiated signaling pathways that governs AIRE expression in the thymus.
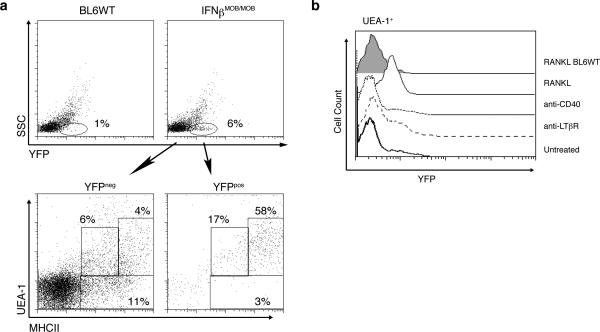
Figure 4. IFNβ production by UEA-1hi medullary thymic epithelial cells.
a) Flow cytometric analysis of YFP expression in total cells from thymus of WT (top left panel) or IFNβMOB/MOB mice (top right panel). Lower panels show UEA-1 and MHCII expression in YFP- (left panel) and YFP+ (right panel) cells in thymi of IFNβMOB/MOB mice. b) Stromal cells were purified from thymi of IFNβMOB/MOB mice and treated with anti-CD40 (1:100 dilution of supernatant), anti-LTβR (500ng/ml) or RANK ligand (500ng/ml) for 24 hours. Histogram illustrates IFNβ:YFP expression in UEA-1+ cells following the indicated treatments. Data is representative of three separate experiments.
Previously selected CD4+ T cells are required to promote mTEC development through RANKL expression (6). As STAT1-/- T cells were unable to restore impaired mTEC development in RAG-/- mice in complementation chimera assays (data not shown), we reasoned that RANKL expression on activated thymocytes might be influenced by STAT1 and that consequently a T-cell intrinsic defect might contribute to the impaired development of the medullary compartment in the thymi of IFNAR1-/- and STAT1-/- mice. Utilizing OTII-transgenic mice with antigen-specificity toward ovalbumin-derived peptides, we exposed WT thymic stromal cells to Ova peptide and co-cultured them with either WT or STAT1-/- OTII+ thymocytes. Twenty-four hours following culture initiation, RANKL expression on thymocytes was analyzed by flow cytometry. As illustrated in Suppl. Fig. 1, activation of both WT and STAT1-deficient thymocytes resulted in increased RANKL expression on their cell surface, however, high levels of RANKL were only observed in WT thymocytes (Suppl. Fig. 1, lower right panel). Consequently, the failure of mature STAT1-/- T cells to support the development of mTECs in RAG-/- chimeras is likely due to their inability to express sufficient levels of RANKL upon activation. In summary, these observations imply both a T cell-intrinsic and extrinsic role for STAT1 in mTEC development and function.
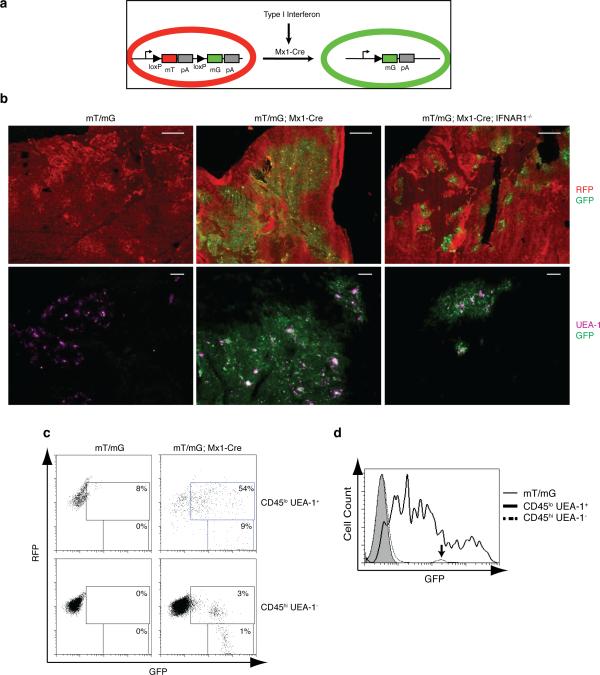
Constitutive Interferon signaling in the thymic medulla
The phenotype observed in STAT1-/- and IFNAR-/- animals is highly indicative of tonic IFNα/β signaling in the thymus even in the absence of infectious events. Indeed, IFNβ expression in the thymus had been reported, and suggested that type I interferon signaling might be taking place in the thymus. To determine the exact compartment in which interferon signaling occurs in the thymi of uninfected, non-immunized animals, we devised a transgenic reporter mouse strain. There, a tandem red fluorescence protein (RFP) called tdTomato is followed by a stop codon and a subsequent EGFP-coding region (27). LoxP sites flank the tdTomato gene including the stop codon such that expression of Cre recombinase results in the excision of the tdTomato-coding sequence and the stop codon, releasing expression of the downstream GFP. We crossed these animals to mice harboring the Cre transgene under the control of the type I interferon-inducible Mx-1 promoter, which have been widely utilized to inducibly delete LoxP-flanked alleles by treating mice with either IFNβ or the interferon-inducing TLR3 ligand polyI:C (28). Conceptually, all cells in these animals display red fluorescence until exposure to type I interferon leads to their transition into green fluorescence (Fig. 3a). Analysis of these double-transgenic mice (mT/mG; Mx1-Cre) revealed the abundant, Mx1-Cre transgene-dependent emergence of GFP-positive cells in the thymic medulla, but not in the cortical region (Fig. 3b, top middle panel), indicating that constitutive interferon signaling in the thymus is highly restricted to the thymic medulla. As anticipated, further immunohistochemical analysis detected UEA-1-positive mTECs only among the GFP-positive cell population (Fig. 3b, lower panels and 3c). Fig. 3c illustrates that CD45loUEA-1hi cells express a wider range of GFP as compared to CD45hi cells that display one uniform peak (indicated by arrow) on histograms for GFP expression (Fig. 3d). Unexpectedly, only a very small fraction of thymic T cells expressed GFP in double-transgenic mice (Suppl. Fig. 2, middle panels), prompting us to analyze the expression levels of the interferon receptor during thymocyte development. Intriguingly, we observed that both double and single positive thymocytes expressed reduced levels of IFNAR1 on their surface (Suppl. Fig. 3a) as compared to CD4 or CD8 single positive splenocytes. This finding offers an explanation for the previously reported reduced ability of thymocytes to respond to type I interferons (29). Accordingly, thymocytes do not exhibit the same responsiveness to IFNβ stimulation as splenic T cells, as revealed by reduced phosphorylation of STAT1 (Suppl. Fig. 3b). Thus, although apparently significant amounts of interferon are being produced in the thymic medulla, only the thymic stromal cells but not thymocytes are capable of responding to it. mTECs exhibit promiscuous gene expression through AIRE which could be an alternative explanation to interferon signaling for the expression of the Mx1-Cre transgene in the medulla. Indeed, Mx-1 Cre was suggested to exhibit “leaky” expression (28, 30). To verify that tdTomato excision and subsequent GFP expression in double-transgenic mice was dependent on interferon signaling rather than promiscuous, AIRE-mediated Mx1-Cre expression, we bred the double-transgenic mice onto an IFNAR1-/- background. Elimination of IFNAR1 in mT/mG; Mx1-Cre mice resulted in a dramatic reduction in the number of GFP-positive mTECs (Fig. 3b, right panels). In accordance, fewer UEA-1 positive cells were detected in the absence of IFNAR1 (compare Fig. 3b, lower middle and lower right panels). The fact that a few residual GFP-expressing cells can be identified in IFNAR1-/- thymi could possibly be attributed to signaling via the IFNγ receptor, which is expressed in cells of the epithelial lineage and shares signaling mediators such as STAT1 and 2 with IFNAR1. Thus, even though type I interferons are predominantly responsible for thymic Mx1-Cre expression, the possibility remains that mTEC-specific Mx1-Cre expression might also occur as a consequence of minute IFNγ- or AIRE-initiated events, albeit in a significantly less competent manner. Importantly, no GFP+ cells were detected among the thymocyte population or in the periphery of mT/mG; Mx1-Cre; IFNAR1-/- mice (Suppl. Fig. 2, bottom row, and data not shown), demonstrating that Mx1-Cre expression in lymphocytes was dependent on type I IFN signaling.
Figure 3. Constitutive interferon signaling in the thymus is restricted to the medullary region.
a) Schematic diagram of the interferon response reporter mice. Interferon-dependent Cre expression results in the excision of the mT sequence coding RFP and expression of mG encoding GFP (Muzumdar, et al. (27)). b) RFP and GFP expression in sections of thymi from WT and IFNAR1-/- mice carrying mT/mG and Mx1-Cre transgenes (upper panels, bar = 500μm). Sections were also stained for UEA-1 (lower panels, pseudo-colored purple, bar = 100μm). c) Flow cytometric analysis of CD45loUEA-1+ mTEC cells (upper panels) and CD45hiUEA-1- hematopoietic cells (lower panels) from thymi of either Mx1-Cre- or Mx1-Cre+ mT/mG mice. d) Histogram comparing GFP expression levels in CD45loUEA-1+ mTECs of Mx1-Cre- or Mx1-Cre+ mT/mG mice; arrow indicates GFP levels in CD45hi hematopoietic cells.
RANKL induces IFNβ expression in UEA-1+ mTECs
To identify the cellular sources of IFNβ, the type I interferon commonly produced first, we utilized knock-in mice which express yellow fluorescent protein (YFP) from a bicistronic IFNβ:YFP mRNA (31). Flow cytometric analysis of YFP expression in total cells from thymus of naïve IFNβMOB/MOB mice revealed that ~6% of thymocytes constitutively express IFNβ (Fig. 4, top panel). The vast majority of the YFP+ cells were characterized as UEA-1+ and MHC IIhigh cells (Fig. 4a, lower panels), consistent with our hypothesis that AIRE- immature mTECs are the major source of type I interferon in the thymus. As previously indicated, IFNβ is typically produced by pDCs and other cell types after recognition of pathogen-associated molecular patterns (PAMPs) via pattern recognition receptors (PRRs). However, IFNβ production by UEA-1+ mTECs occurs independent of such stimulation, thus raising the question as to the identity of the factor(s) that control type I interferon release in the thymus. To this end, thymic stromal cells were purified from thymus of IFNβMOB/MOB mice and treated with RANKL for 24 hours. As shown in Fig. 4b, a clear upregulation of YFP expression in response to RANKL could be observed in UEA-1+ mTECs, consistent with our earlier findings illustrated in Fig. 2b. In contrast, no induction of YFP:IFNβ was noticeable when the cells were stimulated through CD40 or the LTβ–receptor (Fig. 4b), indicating that RANKL is indeed responsible for the presence of IFNβ in the thymic medulla.
IRF7-deficient mice display defects in mTEC development and IFNβ expression after RANK stimulation
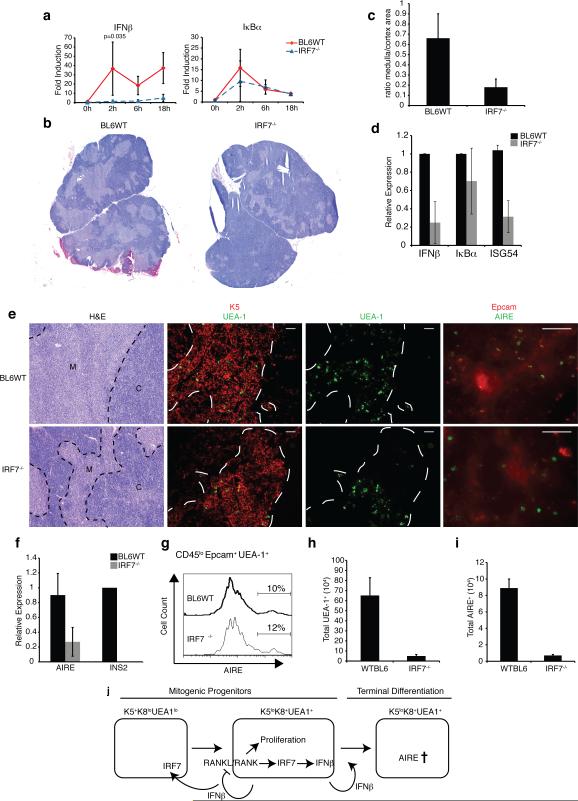
Our results demonstrated a need for tonic IFNα/β signaling in promoting the development and maturation of AIRE+ mTECs, and revealed that UEA-1+AIRE- immature mTECs are the primary source of IFNβ in the thymus. Previous work with osteoclasts demonstrated that IFNβ production following RANK signaling was dependent on c-Fos and independent of the expression of Interferon Regulatory Factors (IRFs) (26). However, no IRF7-/- cells were employed in that study, and in light of the fact that IRFs 3 and 7 are required for induction of IFNβ in response to pathogens, we decided to investigate interferon production following RANKL stimulation in mTECs derived from IRF3- and IRF7-deficient mice. As shown in Fig. 5a, IRF7-deficient thymic stromal cells were incapable of inducing IFNβ message following RANKL stimulation, whereas significant IFNβ message could be induced in wild-type cells (Fig. 5a, left graph, p<0.05), or IRF3-deficient stroma (not shown). Furthermore, no difference in the RANK signaling pathway leading up to induction of IkBα was observed (Fig. 5a, right graph, p>0.05). Thus, IRF7 appears necessary for interferon production in response to RANKL stimulation in the thymus.
Figure 5. IRF7 is necessary for thymic IFNβ expression and mTEC development.
a) Thymic stromal cells from WT or IRF7-/- mice were purified and stimulated with 500 ng/mL RANKL for the indicated time points. Expression of IFNβ and IκBα mRNAs was determined by qPCR. b) Thymic architecture in WT and IRF7-/- mice revealed by H&E staining of thymic sections (bar = 500 μm) c) Ratio of medullary to cortical cellularity in thymic sections from WT and IRF7-/- mice (data collected using ImageJ (NIH) and represent avg +/-sd of 3 sections each from 3 mice (p<0.05). d) mRNA levels of IFNβ, Iκbα, and ISG54 in thymic stromal cells from WT or IRF7-/- mice as determined by qPCR. e) Thymic architecture in WT and IRF7-/- mice as revealed by H&E staining of thymic sections (left panels), Keratin 5 and UEA-1 expression (middle panels, bar = 100 μm), UEA-1 only, as well as EpCAM and AIRE expression (right panels, bar = 50 μm) f) Relative mRNA levels of AIRE and INS2 in purified stromal cells from WT and IRF7-/-thymi measured by qPCR (n = 3). g) Histogram shows percentage of AIRE+ cells in the CD45loEpcam+UEA-1hi gate of thymic stromal cells from WT and IRF7-/- mice. h) Total number of UEA-1hi cells as determined by flow cytometric analysis of CD45loEpCAM+UEA-1hi mTEC populations from WT and IRF7-/- thymi. i) Total number of AIRE+ cells based on flow cytometric analysis of CD45loEpCAM+UEA-1hiAIRE+ mTEC populations in WT and IRF7-/- thymi (n = 4). j) Hypothetical model of IFNβ production and function in the thymus.
Based on the above findings it seemed reasonable to expect that the loss of IRF7 would have a detrimental impact on the mTEC population. Indeed, H&E staining revealed a clear reduction in the thymic medullary region in IRF7-deficient mice as compared to wild-type animals (Fig. 5b), which is highlighted by a significant alteration of the mTEC:cTEC ratio (Fig. 5c). Accordingly, we noted a reduction in the ‘interferon-signature’ in freshly isolated thymic stroma (Fig. 5d). Immunostaining for keratin 5 indicated the presence of immature mTECs in the IRF7-deficient thymus, however, there was a drastic reduction in the number of UEA-1+ cells (Fig. 5e, middle panels), with a concomitant diminution of mature AIRE+ cells (Fig. 5e, right panels). Accordingly, total stromal cells isolated from IRF7-/- thymi contained lower levels of AIRE mRNA, accompanied by a severe reduction in the expression of the AIRE-dependent self-antigen INS2 (Fig. 5f). Flow-cytometric analysis of the few remaining mature mTECs revealed that the amount of AIRE protein in the IRF7-/- cells is identical to that found in their wild-type counterparts (Fig. 5g). However, the total numbers of UEA-1+ and AIRE+ cells were dramatically reduced in the thymi of IRF7-/- mice compared to those of wild-type animals (Figs. 5h and i). Thus, we conclude that the lack of IFNβ production by IRF7-/- stromal cells illustrated in Fig. 5a is the result of severely diminished numbers of the IFNβ-producing cells.
In summary, our findings indicate that mTECs experience a hindrance in the progression from the immature K14+K5hiUEA-1- population to mature UEA-1+ and subsequently AIRE expressing cells in the absence of IRF7. We find that the presence of IRF7 is required in order for RANKL to facilitate the production of IFNβ, which in turn is crucial for the differentiation of immature mTECs into mature AIRE+ cells capable of promiscuous self-antigen expression.
Discussion
Our previous work demonstrated that STAT1-deficiency dramatically increases the incidence of autoimmune disease (18). In addition to decreased CD4+CD25+ regulatory T cell function, it seemed conceivable that the absence of STAT1 also leads to impaired deletion of auto-reactive T cells in the thymus. Indeed, we found that both STAT1 and the type I IFN receptor chain IFNAR, but not the IFNGR, are crucial for the processes governing the deletion of autoreactive CD8+ cells in the TCRHY model system (22). Importantly, we discovered that the coexistence of WT thymocytes restored the impaired elimination of TCRHYSTAT1-/- thymocytes in male BM chimeric mice, whereas BM of TCR-/- mice which lack mature T cells failed to support the purging of the autoreactive TCRHYSTAT1-/- T cells, thus strongly supporting the notion that mature T cells contribute to the efficiency of the selection process in a STAT1-dependent manner. It is of interest to note that a similar effect was described in mice lacking CD40 (32). Specifically, it was shown that CD40L plays a non-cell intrinsic role in deletion of self-reactive thymocytes. Although the molecular mechanism behind this effect was not known at the time, we now know that CD40 plays a role in mTEC development (23). As development of mTECs, which exhibit a short half-life (33) and are indispensible for negative selection of self-reactive T cells, is dependent on their T cell interactions, we decided to analyze the architecture of STAT1-/- thymi. Indeed, STAT1-/- mice display a reduced medullary compartment as well as a reduction in the number of mature mTECs expressing CD80, UEA-1, and AIRE (Fig. 1). The defects in mTEC development in STAT1-/- mice were strikingly similar to defects found in the absence of the TNF family members RANK, CD40, and LTβ (7, 8, 23). The importance of crosstalk between the RANK and interferon signaling cascades had previously been described in osteoclast differentiation and maturation (26). There, RANKL presented by mature T cells stimulates RANK on osteoclast precursors, resulting in their proliferation and simultaneous production of IFNβ. Autocrine stimulation via IFNAR subsequently leads to the termination of RANK signaling, causing the terminal differentiation of the precursors into mature, non-proliferating osteoclasts. We reasoned that a similar interplay might govern the development of thymic epithelial cells. Indeed, we noted that IFNβ is induced by treatment of thymic stromal cells with soluble RANKL. However, addition of exogenous IFNβ was able to promote the maintenance of AIRE expression during RANKL stimulation, consistent with the notion that RANKL promotes expansion of the mTEC population whereas IFNβ mediates their differentiation and/or survival. In accordance with this concept we noticed elevated keratin 5 expression (characteristic of UEA-1 negative, immature mTECs) in STAT1-deficient thymi with a parallel reduction in the expression of cell surface markers associated with mature mTECs (Fig. 1e). We therefore conclude that interferon production following RANK signaling provides a feed-back loop that facilitates the terminal differentiation of mTECs, which is impaired in the absence of IFNAR or STAT1. These striking observations offer a new model for the development of medullary thymic epithelia that involves crosstalk between the RANK and type I interferon signaling pathways.
Under the above outlined hypothesis one has to assume the continuous production of type I interferons in the thymic medulla independent of infectious processes or other immune-response provoking events. In contrast to IFNγ, the role of which in T cell development has been studied extensively, comparatively little is known about the contributions of IFNα/β to this process. We therefore designed an animal model that allows for the identification of cells that have been exposed to type I interferon. By means of this mT/mG; Mx1-Cre system we observe spatially tightly restricted interferon responses in the thymus that are almost exclusively limited to the medulla (Fig. 3). Our studies using mT/mG; Mx1-Cre mice lacking either IFNAR or STAT1 revealed a dramatic decline of GFP-positive mTECs compared to thymi from mice with an intact IFNα/β signaling cascade, corroborating the vital contributions of this cytokine to the development of the thymic medullary compartment. Intriguingly, IFNAR-/- reporter mice still exhibited some GFP expression in the remaining UEA-1+ cells. One possible explanation might be redundancy to type I interferon signaling in mTECs as epithelial cells in general are responsive to IL-29 (IFNγ). Although IFNγ signals through a receptor distinct from IFNAR1/2, it also employs STAT1/2 heterodimers and stimulates an overlapping gene profile (34). Nevertheless, such IFNγ expression in the thymus is apparently insufficient to promote mTEC maturation in IFNAR-/- mice (Fig. 1 and 3). Alternatively, an interferon regulatory factor family member could be mediating residual expression of Mx1-Cre in IFNAR-/- mice. In support of our model, a search of the public GEO profiles database indicates a strong interferon signature in mTECs as compared to cTECs. In fact many of the markers used to identify mature mTEC such as CD80, CD86 and MHCII are interferon-stimulated genes. Interestingly, microarray profiles of purified mTEC revealed that CD80hi mTEC exhibit a reduction in both components of interferon signaling as well as a reduction in interferon stimulated genes as compared to CD80lo mTEC, which may be an indication of chronic interferon exposure of mature differentiated CD80hi mTEC.
Consistent with our findings, the production of interferon in the thymus has recently also been described through the use of a luciferase transgene inserted into the IFNβ gene locus (35). In these animals, luciferase activity was linked to AIRE expression (35). Although it was possible that IFNβ is a direct target of AIRE-controlled transcription, the absence of AIRE results in a misdirection of mTEC development as noted in AIRE-deficient animals (24). Our studies indicate that the main IFNβ-producing cells in the thymus are UEA-1+ cells, regardless of AIRE expression (Fig. 4a).
In an effort to identify the factors controlling IFNβ production in the thymus, we discovered that IRF7 is not only eliciting interferon expression downstream of RANK, but also that IRF7-deficient mice harbor severe defects in mTEC development (Fig. 5). As IRF3-/- mice were found to have normal thymic compartments and IFNβ production, this represents only the second instance of an IRF7-dependent/IRF3-independent induction of IFNβ, the other involving PAMP-stimulated plasmacytoid dendritic cells (36). Further studies will be aimed to elucidate the pathways involved in IRF7 activation downstream of RANK as well as the consequences of the loss of IRF7 in terms of T cell tolerance and autoimmunity.
Taken together, the data presented here illustrate a complex interplay between mature and developing T lymphocytes and mTECs that involves RANK and type I interferon signaling. There are persistent controversies on the exact stages mTECs progress through during their development and maturation. A common model holds that K5+K8+UEA-1- common precursors develop into K5-K8+UEA-1- cTECs and the major mTEC population characterized as K5+K8-UEA-1-. A small population progresses into medullary K5-K8+ cells that start to express UEA-1, and eventually AIRE (37). Our data is consistent with the notion that IFNβ produced by UAE-1+ cells acts on K5+K8+UEA-1- precursors to promote their upregulation of IRF7 and progression into UAE-1+ cells, thus explaining the diminution of UAE-1+ cells in mice lacking IRF7, IFNAR or STAT1. In addition, IFNβ likely also functions in an autocrine feedback loop, terminating the RANK signaling and facilitating the upregulation of AIRE in terminally differentiated UAE-1+ cells (Fig. 5j).
As IFNAR-, STAT1 and IRF7-deficient mice have been frequently used to assess the role of the type I interferon system in antiviral defense, our new findings might also merit a re-evaluation of the conclusions drawn from these studies, as failure to clear distinct infections might be due to altered T cell populations at the onset of the experiment rather than a sole function of IRF7/IFN/STAT1 in the innate immune response. Lastly, our findings might have additional implications due to the fact that many viruses are capable of inhibiting interferon signaling and thus evading the immune response. Therefore, viruses that infect thymic cell populations might evoke immune disorders by repressing interferon signaling in medullary thymic stromal cells, consequently breaking tolerance of self-reactive thymocytes or upsetting Treg cell development.
Supplementary Material
Acknowledgements
We would like to thank Drs. A. Farr, A. Goldrath, S. Hedrick, E. Zuniga and R. Rickert for many helpful discussions and advice. 1
Abbreviations
- AIRE
autoimmune regulator
- EpCAM
epithelial cell adhesions molecule
- IFNα/β
interferon alpha/beta
- IFNAR
interferon alpha/beta receptor
- IFNGR
interferon gamma receptor
- IRF7
interferon regulatory factor 7
- K5
keratin 5
- K8
keratin 8
- mTEC
medullary thymic epithelial cell
- RANK(L)
receptor activator of nuclear factor kappa-B (ligand)
- STAT1
signal transducer and activator of transcription
- TCR
T cell receptor
- UEA-1
ulex europaeus agglutinin-1
- YFP
yellow fluorescent protein
Footnotes
This work was supported by NIH/NIAID grant AI71223 and funding from The National Multiple Sclerosis Society (54109A) to M.D.
References
- 1.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 2.Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC. Aire regulates negative selection of organ-specific T cells. Nat Immunol. 2003;4:350–354. doi: 10.1038/ni906. [DOI] [PubMed] [Google Scholar]
- 3.Finnish-German AC. An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat Genet. 1997;17:399–403. doi: 10.1038/ng1297-399. [DOI] [PubMed] [Google Scholar]
- 4.Peterson P, Nagamine K, Scott H, Heino M, Kudoh J, Shimizu N, Antonarakis SE, Krohn KJ. APECED: a monogenic autoimmune disease providing new clues to self-tolerance. Immunol Today. 1998;19:384–386. doi: 10.1016/s0167-5699(98)01293-6. [DOI] [PubMed] [Google Scholar]
- 5.Ramsey C, Winqvist O, Puhakka L, Halonen M, Moro A, Kampe O, Eskelin P, Pelto-Huikko M, Peltonen L. Aire deficient mice develop multiple features of APECED phenotype and show altered immune response. Hum Mol Genet. 2002;11:397–409. doi: 10.1093/hmg/11.4.397. [DOI] [PubMed] [Google Scholar]
- 6.Hikosaka Y, Nitta T, Ohigashi I, Yano K, Ishimaru N, Hayashi Y, Matsumoto M, Matsuo K, Penninger JM, Takayanagi H, Yokota Y, Yamada H, Yoshikai Y, Inoue J, Akiyama T, Takahama Y. The cytokine RANKL produced by positively selected thymocytes fosters medullary thymic epithelial cells that express autoimmune regulator. Immunity. 2008;29:438–450. doi: 10.1016/j.immuni.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 7.Dunn RJ, Luedecker CJ, Haugen HS, Clegg CH, Farr AG. Thymic overexpression of CD40 ligand disrupts normal thymic epithelial organization. J Histochem Cytochem. 1997;45:129–141. doi: 10.1177/002215549704500116. [DOI] [PubMed] [Google Scholar]
- 8.Boehm T, Scheu S, Pfeffer K, Bleul CC. Thymic medullary epithelial cell differentiation, thymocyte emigration, and the control of autoimmunity require lympho-epithelial cross talk via LTbetaR. J Exp Med. 2003;198:757–769. doi: 10.1084/jem.20030794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chin RK, Lo JC, Kim O, Blink SE, Christiansen PA, Peterson P, Wang Y, Ware C, Fu YX. Lymphotoxin pathway directs thymic Aire expression. Nat Immunol. 2003;4:1121–1127. doi: 10.1038/ni982. [DOI] [PubMed] [Google Scholar]
- 10.Burkly L, Hession C, Ogata L, Reilly C, Marconi LA, Olson D, Tizard R, Cate R, Lo D. Expression of relB is required for the development of thymic medulla and dendritic cells. Nature. 1995;373:531–536. doi: 10.1038/373531a0. [DOI] [PubMed] [Google Scholar]
- 11.Kajiura F, Sun S, Nomura T, Izumi K, Ueno T, Bando Y, Kuroda N, Han H, Li Y, Matsushima A, Takahama Y, Sakaguchi S, Mitani T, Matsumoto M. NF-kappa B-inducing kinase establishes self-tolerance in a thymic stroma-dependent manner. J Immunol. 2004;172:2067–2075. doi: 10.4049/jimmunol.172.4.2067. [DOI] [PubMed] [Google Scholar]
- 12.Akiyama T, Maeda S, Yamane S, Ogino K, Kasai M, Kajiura F, Matsumoto M, Inoue J. Dependence of self-tolerance on TRAF6-directed development of thymic stroma. Science. 2005;308:248–251. doi: 10.1126/science.1105677. [DOI] [PubMed] [Google Scholar]
- 13.David M. Signal transduction by type I interferons. Biotechniques. 2002;(Suppl):58–65. [PubMed] [Google Scholar]
- 14.Muller M, Laxton C, Briscoe J, Schindler C, Improta T, Darnell JE, Jr., Stark GR, Kerr IM. Complementation of a mutant cell line: central role of the 91 kDa polypeptide of ISGF3 in the interferon-alpha and - gamma signal transduction pathways. Embo J. 1993;12:4221–4228. doi: 10.1002/j.1460-2075.1993.tb06106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schindler C, Darnell JE., Jr. Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Annu Rev Biochem. 1995;64:621–651. doi: 10.1146/annurev.bi.64.070195.003201. [DOI] [PubMed] [Google Scholar]
- 16.Durbin JE, Hackenmiller R, Simon MC, Levy DE. Targeted Disruption of the Mouse Stat1 Gene Results in Compromised Innate Immunity to Viral Disease. Cell. 1996;84:443–450. doi: 10.1016/s0092-8674(00)81289-1. [DOI] [PubMed] [Google Scholar]
- 17.Meraz MA, White JM, Sheehan KC, Bach EA, Rodig SJ, Dighe AS, Kaplan DH, Riley JK, Greenlund AC, Campbell D, Carver-Moore K, DuBois RN, Clark R, Aguet M, Schreiber RD. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell. 1996;84:431–442. doi: 10.1016/s0092-8674(00)81288-x. [DOI] [PubMed] [Google Scholar]
- 18.Nishibori T, Tanabe Y, Su L, David M. Impaired Development of CD4+ CD25+ Regulatory T Cells in the Absence of STAT1: Increased Susceptibility to Autoimmune Disease. J Exp Med. 2004;199:25–34. doi: 10.1084/jem.20020509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karp CL, Biron CA, Irani DN. Interferon beta in multiple sclerosis: is IL-12 suppression the key? Immunol Today. 2000;21:24–28. doi: 10.1016/s0167-5699(99)01541-8. [DOI] [PubMed] [Google Scholar]
- 20.Goverman J, Woods A, Larson L, Weiner LP, Hood L, Zaller DM. Transgenic mice that express a myelin basic protein-specific T cell receptor develop spontaneous autoimmunity. Cell. 1993;72:551–560. doi: 10.1016/0092-8674(93)90074-z. [DOI] [PubMed] [Google Scholar]
- 21.Lafaille JJ, Nagashima K, Katsuki M, Tonegawa S. High incidence of spontaneous autoimmune encephalomyelitis in immunodeficient anti-myelin basic protein T cell receptor transgenic mice. Cell. 1994;78:399–408. doi: 10.1016/0092-8674(94)90419-7. [DOI] [PubMed] [Google Scholar]
- 22.Moro H, Otero DC, Tanabe Y, David M. T cell-intrinsic and - extrinsic contributions of the IFNAR/STAT1-axis to thymocyte survival. PLoS One. 2011;6:e24972. doi: 10.1371/journal.pone.0024972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akiyama T, Shimo Y, Yanai H, Qin J, Ohshima D, Maruyama Y, Asaumi Y, Kitazawa J, Takayanagi H, Penninger JM, Matsumoto M, Nitta T, Takahama Y, Inoue J. The tumor necrosis factor family receptors RANK and CD40 cooperatively establish the thymic medullary microenvironment and self-tolerance. Immunity. 2008;29:423–437. doi: 10.1016/j.immuni.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 24.Dooley J, Erickson M, Farr AG. Alterations of the medullary epithelial compartment in the Aire-deficient thymus: implications for programs of thymic epithelial differentiation. J Immunol. 2008;181:5225–5232. doi: 10.4049/jimmunol.181.8.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farr A, Nelson A, Truex J, Hosier S. Epithelial heterogeneity in the murine thymus: a cell surface glycoprotein expressed by subcapsular and medullary epithelium. J Histochem Cytochem. 1991;39:645–653. doi: 10.1177/39.5.2016514. [DOI] [PubMed] [Google Scholar]
- 26.Takayanagi H, Kim S, Matsuo K, Suzuki H, Suzuki T, Sato K, Yokochi T, Oda H, Nakamura K, Ida N, Wagner EF, Taniguchi T. RANKL maintains bone homeostasis through c-Fos-dependent induction of interferon-beta. Nature. 2002;416:744–749. doi: 10.1038/416744a. [DOI] [PubMed] [Google Scholar]
- 27.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 28.Kuhn R, Schwenk F, Aguet M, Rajewsky K. Inducible gene targeting in mice. Science. 1995;269:1427–1429. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- 29.Marino JH, Van De Wiele CJ, Everhart JM, Masengale R, Naukam RJ, Schniederjan MJ, Vo S, Teague TK. Attenuation of cytokine responsiveness during T cell development and differentiation. J Interferon Cytokine Res. 2006;26:748–759. doi: 10.1089/jir.2006.26.748. [DOI] [PubMed] [Google Scholar]
- 30.Chan IT, Kutok JL, Williams IR, Cohen S, Kelly L, Shigematsu H, Johnson L, Akashi K, Tuveson DA, Jacks T, Gilliland DG. Conditional expression of oncogenic K-ras from its endogenous promoter induces a myeloproliferative disease. J Clin Invest. 2004;113:528–538. doi: 10.1172/JCI20476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scheu S, Dresing P, Locksley RM. Visualization of IFNbeta production by plasmacytoid versus conventional dendritic cells under specific stimulation conditions in vivo. Proc Natl Acad Sci U S A. 2008;105:20416–20421. doi: 10.1073/pnas.0808537105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams JA, Sharrow SO, Adams AJ, Hodes RJ. CD40 ligand functions non-cell autonomously to promote deletion of self-reactive thymocytes. J Immunol. 2002;168:2759–2765. doi: 10.4049/jimmunol.168.6.2759. [DOI] [PubMed] [Google Scholar]
- 33.Gray D, Abramson J, Benoist C, Mathis D. Proliferative arrest and rapid turnover of thymic epithelial cells expressing Aire. J Exp Med. 2007;204:2521–2528. doi: 10.1084/jem.20070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sommereyns C, Paul S, Staeheli P, Michiels T. IFN-lambda (IFN-lambda) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. PLoS Pathog. 2008;4:e1000017. doi: 10.1371/journal.ppat.1000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lienenklaus S, Cornitescu M, Zietara N, Lyszkiewicz M, Gekara N, Jablonska J, Edenhofer F, Rajewsky K, Bruder D, Hafner M, Staeheli P, Weiss S. Novel reporter mouse reveals constitutive and inflammatory expression of IFN-beta in vivo. J Immunol. 2009;183:3229–3236. doi: 10.4049/jimmunol.0804277. [DOI] [PubMed] [Google Scholar]
- 36.Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, Shimada N, Ohba Y, Takaoka A, Yoshida N, Taniguchi T. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- 37.Klug DB, Carter C, Crouch E, Roop D, Conti CJ, Richie ER. Interdependence of cortical thymic epithelial cell differentiation and T- lineage commitment. Proc Natl Acad Sci U S A. 1998;95:11822–11827. doi: 10.1073/pnas.95.20.11822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muller U, Steinhoff U, Reis LFL, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional Role of Type I and Type II Interferons in Antiviral Defense. Science. 1994;264 doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 39.Sakaguchi S, Negishi H, Asagiri M, Nakajima C, Mizutani T, Takaoka A, Honda K, Taniguchi T. Essential role of IRF-3 in lipopolysaccharide-induced interferon-beta gene expression and endotoxin shock. Biochem Biophys Res Commun. 2003;306:860–866. doi: 10.1016/s0006-291x(03)01049-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.