Abstract
Purpose
Dispositional optimism is a psychological trait associated with cardiovascular disease outcomes in adults. However, it is not known if these associations are present in adolescents. We determined if an association between optimism and 9 biomarkers of cardiometabolic risk existed during adolescence. Because cardiometabolic risk differs by race/ethnicity, we also explored if race/ethnicity moderated the optimism-cardiometabolic risk relationship.
Methods
Cross-sectional study of 529 non-Hispanic white and 421 non-Hispanic black 7th–12th graders living in greater Cincinnati in 2001–2002. Dispositional optimism was measured with the Life-Orientation Test-Revised (LOT-R) as a single continuum (OPTPESS) and as separate optimism (OPT) and pessimism (PESS) dimensions. Multivariable regression analyses tested for associations between optimism and 9 biomarkers of risk (interleukin-6, tumor necrosis factor-α, lipids, insulin, glucose, and fibrinogen) adjusting for age, gender, parent education, body mass index, smoking, and pubertal stage.
Results
OPTPESS was inversely associated with two risks (interleukin-6, β= −0.03, p=0.02; insulin, β= −0.02, p=0.01), but only among blacks. OPT and PESS were inversely related (R=−0.27, p<0.0001) and both were higher in blacks than whites (p<0.001). OPT was directly associated with high density lipoprotein among all subjects (β=0.42, p=0.03) and blacks (β=0.74, p=0.02), but not whites. Among blacks, OPT was also inversely associated with interleukin-6 (β= −0.07, p=0.0008), and triglycerides (β= −0.02, p=0.04). PESS was inversely associated with glucose, but only in whites (β= −0.38, p=0.03).
Conclusion
Associations between dispositional optimism and cardiometabolic risks are present in adolescence and vary by race/ethnicity. A better understanding of the natural history of these associations over the lifespan may help decrease disparities and prevent cardiometabolic disease.
Keywords: optimism, biomarkers, cardiometabolic risk, adolescents, disparities
INTRODUCTION
Psychological traits have been associated with cardiovascular risks and disease throughout the life course [1–5]. Dispositional optimism, hereafter referred to as “optimism”, is one such trait. Optimism is the tendency to expect positive outcomes and believe good things will happen in life and is a trait that develops early in the lifecourse [6]. Optimism has been linked to numerous aspects of adult cardiovascular health including being protective against coronary artery disease, promoting shorter recovery time from CABG, being associated with decreased risk of cardiovascular death and less progression of carotid atherosclerosis[3, 4, 7–10], and, most recently, with lower levels of inflammatory biomarkers in adults [11]. A recent review found that among all aspects of positive psychological well-being, optimism is the trait most robustly associated with reduced risk of cardiovascular events in adults [5]. In adolescents, higher optimism has been demonstrated to be protective against health risk behaviors and anthropomorphic risks, such as higher body mass index [1, 12]. However, to date, no studies of adolescents have assessed the association of optimism to the broader array of cardiometabolic risks, such as lipoproteins and inflammatory factors, which have been linked to optimism in adulthood. Though it is not known exactly when cardiovascular risk begins, numerous risk factors for cardiovascular disease (CVD), including obesity, elevated blood lipids, and diabetes, which begin during childhood have been shown to persist into adulthood. It is particularly important to study biomarkers of risk in adolescence because they are often the only signs of pathophysiology processes which lead to CVD at this stage in the life course.
This study attempts to fill this gap in the knowledge base by studying in adoloescents the relationship between optimism and an array of 9 cardiometabolic risk factors (total cholesterol, HDL-C, LDL-C, triglycerides, insulin, glucose, fibrinogen, IL-6, TNF-alpha) which have been linked to CVD risk in adulthood [13, 14]. We hypothesized that increased optimism in adolescents would be associated with decreased cardiometabolic risk. Given known racial differences in CVD risk factors and psychological traits [15–18], we further hypothesized that there would be racial/ethnic differences in the relationship of optimism to these cardiometabolic risks. Because optimism has recently been shown to play a role in obesity in adolescents [1] and inflammatory biomarkers are higher in both obese adolescents and adults [19, 20], we assessed if any demonstrated relationships between optimism and biomarkers were independent of body mass index.
METHODS
Study Sample
This cross sectional study uses data from the Princeton School District Study (PSD), a school-based study from the Princeton City School District in Greater Cincinnati, which began in the 2001–2002 school year when 1646 non-Hispanic white and non-Hispanic black 5–12th graders participated [21]. In 2008, additional funding was obtained to perform new biomarker assays using banked blood on a subsample of 1207 participants. Because inflammatory biomarkers were assessed only in this subsample of 1207, this study is restricted to that group. Further, because optimism was only assessed in participants who were in grades 7–12 in 2001–2002, we excluded 255 who were 5th–6th graders at that time, leaving a total of 952 7th–12th graders eligible for inclusion in this study. Two who had not completed the optimism scale at baseline were excluded, resulting in a final sample of 950 subjects. The Institutional Review Boards at the participating institutions approved all study procedures.
Measures
Optimism
Optimism was measured using the Life Orientation Test-Revised (LOT-R), a six-item revision of the Life Orientation Test [22]. Although the original Life Orientation Test has been validated in youth [23], a revised version (the LOT-R) created by alternate scoring of the original test was subsequently introduced to better account for other competing predictors of disposition such as coping strategies [22], and is now the accepted measure. Because the LOT-R has not been validated for use in adolescents, we performed a sensitivity analysis comparing correlations between each biomarker with both the LOT and LOT-R (Table 1). Results supported use of the LOT-R in this age group, and is, therefore, the measure we used in these analyses. The LOT-R includes ten 4-point Likert scale items; three positively and three negatively worded items, with four filler items, (Cronbach’s alpha=0.78) [22]. There are two different ways to score the LOT-R. As originally conceived, the LOT-R is scored as a single scale where the negatively worded items are reverse coded and added to the positively worded items, with a higher score indicating greater optimism. However, there is a growing body of work which suggests that optimism may not be a single trait, but rather two distinct constructs - optimism and pessimism [24, 25]. We analyzed the LOT-R as a uni-dimensional scale to make appropriate comparisons to prior studies (OPTPESS) [11, 24, 25], and also as separate optimism (OPT) and pessimism (PESS) dimensions to take into consideration these recent advances in the field, including a recent study in adults which tested associations between optimism and inflammatory biomarkers as both uni- and bi-dimensional scales [11].
Table 1.
Pearson Correlation between LOT and LOT-R for cardiometabolic risk biomarkers
| Biomarker | LOT | LOT-R | ||
|---|---|---|---|---|
| R | p-value | R | p-value | |
| IL-6 | −0.07 | 0.03 | −0.05 | 0.10 |
| TNF-α | −0.07 | 0.05 | −0.04 | 0.24 |
| Total cholesterol | 0.02 | 0.43 | 0.003 | 0.92 |
| HDL | 0.08 | 0.003 | 0.09 | 0.001 |
| LDL | −0.0003 | 0.99 | −0.02 | 0.50 |
| Triglycerides | −0.005 | 0.86 | −0.04 | 0.11 |
| Glucose | −0.054 | 0.04 | −0.07 | 0.01 |
| Insulin | −0.07 | 0.006 | −0.08 | 0.001 |
| Fibrinogen | −0.019 | 0.47 | −0.01 | 0.59 |
Biomarkers
Fasting blood samples were collected on subjects at baseline after a minimum 10 hour fast.
Plasma insulin concentration was measured by radioimmunoassay (RIA) using an antiinsulin serum raised in guinea pigs, 125I-labeled insulin as a standard, and a double-antibody method to separate bound from free tracer. The sensitivity is 2 pM, with intra- and interassay coefficient of variation (CV) of 5% and 8%, respectively.
Glucose was measured by enzymatic method. Intra- and interassay CVs are 1.2% and 1.6%, respectively.
Fibrinogen concentration was measured with an immunoturbidimetric assay on the Hitachi 911 analyzer (Roche Diagnostics – Indianapolis, IN). The day-to-day variabilities of the assay at concentrations of 167.4, 323.6, and 554.1 mg/dL were 0.94, 1.06, and 1.50%, respectively.
Blood lipids: cholesterol was measured using the Cholesterol/HP kit from Roche (Boehringer Mannheim). The intraassay CV was 1.0% and the interassay CV was 2.2%. For high-density lipoprotein cholesterol (HDL), and HDL C-plus kit from Roche was used. This is a direct measurement (rather than a precipitation method). The intraassay CV was 1.3% and the interassay CV is 2.6%. Lipid profiles were performed on the Hitachi 704. National Cholesterol Education Program performance criteria for accuracy and precision are followed. Triglycerides were measured using a single reagent system from Roche-BMD. The CV was 4%. Low-density lipoprotein cholesterol (LDL) was calculated according to the Friedwald equation (total cholesterol-HDL-triglycerides/5) except when triglycerides were above 350 mg/dL. In those 2 cases, a direct measurement using the Roche LDL-C plus reagent was made.
Interleukin-6 (IL-6) was measured by an ultra-sensitive ELISA assay from R & D Systems. The assay employed the quantitative sandwich enzyme immunoassay technique. The assay has a sensitivity of 0.094 pg/mL, and the day-to-day variability of the assay at concentrations of 0.66, 1.97 and 8.16 pg/mL are 12.2, 7.6 and 9.9%.
Tumor Necrosis Factor-alpha (TNF-α) was measured from soluble TNF-α receptor 2 which correlates with TNF-α, remains elevated in plasma for longer periods of time, and is easy to detect. sTNFR2 was measured by an ELISA assay from R & D Systems. The assay employs the quantitative sandwich enzyme immunoassay technique. Day-to-day variabilities at concentrations of 89.9, 197, and 444 pg/mL are 5.1, 3.5, and 3.6%, respectively.
Body Mass Index
Trained staff measured the height and weight of students, who were wearing indoor clothing with shoes removed and pockets emptied. Measurements were taken in duplicate and averaged, from which body mass index (BMI) was calculated in kg/meter2, and then transformed into a standardized score (zBMI) using age- and gender-specific norms data from the Centers for Disease Control and Prevention growth charts [26].
Sociodemographics
Information on highest reported level of parental education was collected from a parent at baseline and categorized as ‘high school or less’, ‘some college or vocational degree’, ‘college’, or ‘professional’. The adolescent’s race/ethnicity, date of birth, and gender were derived from school district administrative data.
Cigarette use
In 2002–2003, cigarette use was assessed using validated items from the Youth Risk Behavior Survey, a survey that assesses lifetime use, current use (within the past 30 days), and age of initiation [27, 28]. Based on responses to these items, we classified smoking status in 2001–2002 as “never-smoked” or “ever-smoked” [29].
Pubertal Stage was determined using a validated algorithm including sex hormone levels and attainment of objective developmental milestones as described previously [30], based on serum estradiol concentration and the presence or absence of menarche for two years in females and on serum free testosterone concentration and stage of axillary hair in males.
Analyses
We assessed bivariate correlations of optimism with individual inflammatory biomarkers using Spearman Rank tests as IL-6, TNF-α, triglycerides, and insulin were not normally distributed. We then looked at the effect of optimism on each biomarker using multivariable regression to determine the independent effects after adjusting for potential confounders including age, gender, pubertal status, race/ethnicity, BMI z score, smoking, and parental education. Three separate models were run for each biomarker--one using OPTPESS, one for OPT and one for PESS. IL-6, TNF-α, triglycerides, and insulin were log transformed in multivariable analyses to reduce skewness.
To assess for effect modification by race/ethnicity, analyses were also stratified by race and we tested for an interaction between the optimism measure (either OPTPESS, OPT, or PESS) and race/ethnicity in the regression analyses. All reported probability values correspond to two-tailed tests. A significance level of 0.05 was set a priori.
RESULTS
Table 2 describes the demographic characteristics of the study sample (n=950). The mean age was 15.0 (SD=1.6) years; subjects were evenly distributed by race/ethnicity and gender. Nearly all subjects were peri- or post-pubertal. Sixty percent of participants had a normal weight and 66% had never smoked. Although no differences were observed in OPTPESS score by race, the mean OPT score was 7.7 in non-Hispanic white youth vs. 8.3 in non-Hispanic black youth (p<0.0001) and PESS score was 4.9 in non-Hispanic white youth vs. 5.3 in non-Hispanic black youth (p<0.003). Mean OPT and PESS scores were inversely correlated (rho=−0.27, p<0.0001); the correlation was stronger in non-Hispanic white youth (rho=−0.39, p<0.001) than non-Hispanic black youth (rho= −0.17, p<0.001).
Table 2.
Characteristics of the Study Sample (n=950)
| Characteristic | n | % | ||
|---|---|---|---|---|
| Sex | ||||
| Male | 465 | 49.0 | ||
| Female | 485 | 51.0 | ||
| Race | ||||
| White | 529 | 55.7 | ||
| Black | 421 | 44.3 | ||
| Parental Education | ||||
| HS or less | 203 | 21.4 | ||
| Some College/vocational | 265 | 27.9 | ||
| College | 266 | 28.0 | ||
| Post-graduate | 216 | 22.7 | ||
| Pubertal Status | ||||
| Pre-pubertal | 23 | 2.4 | ||
| Peri-pubertal | 347 | 36.5 | ||
| Post-pubertal | 580 | 61.1 | ||
| Weight Status | ||||
| Normal weight | 570 | 60.0 | ||
| Overweight | 192 | 20.2 | ||
| Obese | 188 | 19.8 | ||
| Smoking (N=917) | ||||
| Never smoked | 623 | 65.6 | ||
| Ever smoked | 294 | 30.9 | ||
| Missing | 33 | 3.5 | ||
| Mean | SD | Min | Max | |
| Age | 15.0 | 1.6 | 12.2 | 19.3 |
| OPTPESS | 14.9 | 3.1 | 0 | 24 |
| OPT (N=944) | 8.0 | 1.8 | 0 | 12 |
| PESS (N=932) | 5.1 | 2.1 | 0 | 12 |
| IL-6 (pg/ml) (N=850) | 1.3 | 1.9 | 0.2 | 16.2 |
| sTNFR2 (pg/ml) (N=827) | 2345.5 | 674.8 | 1111.2 | 8798.4 |
| Total cholesterol (mg/dL) (N=937) | 149.8 | 28.4 | 75 | 351 |
| HDL (mg/dL) (N=937) | 45.4 | 10.8 | 6 | 98 |
| LDL (mg/dL) (N=937) | 88.7 | 25.4 | 19 | 284 |
| Triglycerides (mg/dL) (N=937) | 78.6 | 44.2 | 5 | 609 |
| Glucose (mg/dL) (N=950) | 85.7 | 8.9 | 51.3, | 123.4 |
| Insulin (pM/L) (N=950) | 130.5 | 112.9 | 7.2 | 892.9 |
| Fibrinogen (mg/dL) (N=930) | 285.5 | 58.5 | 130, | 580 |
Overweight refers to a sex- and gender-specific BMI between the 85th and 95th percentile based on 2000 CDC Growth Chart standards.
Obese refers to a sex- and gender-specific BMI at or above the 95th percentile based on 2000 CDC Growth Chart standards.
Some biomarkers are assayed on a smaller sample size due to routine data collection difficulties during phlebotomy.
In bivariate analyses, we found significant associations between OPTPESS, OPT and PESS with several biomarkers (Table 3). A higher OPTPESS score was correlated with increased levels of HDL and decreased levels of insulin. For the bi-dimensional scoring, higher OPT was correlated with increased HDL levels while a higher PESS score was correlated with higher insulin levels.
Table 3.
Bivariate association between dispositional optimism and cardiometabolic risk biomarkers.
| Biomarker | OPTPESS | OPT | PESS | |||
|---|---|---|---|---|---|---|
| Spearman’s (Rho) | p-value | Spearman’s (Rho) | p-value | Spearman’s (Rho) | p-value | |
| IL-6 | −0.03 | 0.38 | 0.01 | 0.99 | 0.04 | 0.22 |
| TNF-α | −0.04 | 0.24 | −0.01 | 0.93 | 0.05 | 0.16 |
| Total cholesterol | 0.01 | 0.86 | 0.05 | 0.15 | 0.02 | 0.47 |
| HDL | 0.08 | 0.02* | 0.10 | 0.002** | −0.01 | 0.97 |
| LDL | −0.01 | 0.83 | 0.03 | 0.43 | 0.01 | 0.66 |
| Triglycerides | −0.03 | 0.37 | −0.06 | 0.06 | −0.02 | 0.47 |
| Glucose | −0.03 | 0.40 | −0.03 | 0.44 | 0.01 | 0.74 |
| Insulin | −0.08 | 0.009** | −0.04 | 0.18 | 0.07 | 0.02* |
| Fibrinogen | −0.03 | 0.35 | −0.01 | 0.69 | 0.03 | 0.35 |
p<0.05
p<0.01
OPTPESS n=950, OPT n=944, PESS n=932
Table 4 presents the multivariable regression analyses. Significant interactions between disposition and race were present for IL-6 with OPT (p=0.001), and for insulin with OPTPESS and PESS (p=0.006, p=0.02, respectively). With optimism measured as a single dimension, only non-Hispanic black adolescents had significant associations, with a one unit increase in OPTPESS corresponding to a 2.95% decrease in IL-6 (p=0.02) (graphically represented in the Figure) and a 2.22% decease in insulin (p=0.02) levels (results for IL-6, TNF-α, triglycerides, and insulin in Table 4 are log transformed; the percent change results reported in the text are calculated by multiplying the transformed values by 100%). Looking at the bi-dimensional scoring, a one unit increase in OPT was associated with a 0.42mg/dL increase in HDL level among all subjects (p=0.03) as well as a 0.74mg/dL increase among non-Hispanic black adolescents (p=0.02), while a unit increase in the OPT scale was associated with a 7.19% decrease in IL-6 (p=0.0008) and a 2.28% decrease in triglycerides (p=0.04) in non-Hispanic black adolescents. Surprisingly, glucose levels were inversely associated with pessimism among non-Hispanic white adolescents, with a one unit PESS increase associated with a 0.38mg/dL lowering of glucose levels (p=0.03). There were no other associations observed for white adolescents or the PESS dimension.
Table 4.
Multivariable regression analysis: Association between cardiometabolic risk biomarkers and dispositional optimism controlling for sociodemographic factors.
| OPTPESS | OPT | PESS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β | SE | p-value | β | SE | p-value | β | SE | p-value | |
|
Total
| |||||||||
| ln IL-6 (pg/ml) | −0.01 | 0.01 | 0.16 | −0.02 | 0.01 | 0.21† | 0.01 | 0.01 | 0.27 |
| ln TNF-α (pg/ml) | −0.01 | 0.01 | 0.21 | 0.01 | 0.01 | 0.92 | 0.01 | 0.01 | 0.05 |
| Total cholesterol (mg/dL) | −0.11 | 0.30 | 0.72 | 0.01 | 0.53 | 0.99 | 0.35 | 0.45 | 0.43 |
| HDL (mg/dL) | 0.15 | 0.11 | 0.16 | 0.42 | 0.19 | 0.03 | −0.01 | 0.16 | 0.93 |
| LDL (mg/dL) | −0.18 | 0.27 | 0.50 | −0.31 | 0.47 | 0.51 | 0.26 | 0.40 | 0.52 |
| ln Triglycerides (mg/dL) | −0.01 | 0.01 | 0.63 | −0.01 | 0.01 | 0.42 | 0.01 | 0.01 | 0.91 |
| Glucose (mg/dL) | 0.07 | 0.09 | 0.44 | −0.12 | 0.16 | 0.47 | −0.25 | 0.14 | 0.07 |
| ln Insulin (pM/L) | −0.01 | 0.01 | 0.65* | −0.01 | 0.01 | 0.92 | 0.01 | 0.01 | 0.57§ |
| Fibrinogen (mg/dL) | −0.69 | 0.59 | 0.24 | −0.88 | 1.05 | 0.40 | 0.80 | 0.89 | 0.37 |
|
| |||||||||
|
Whites
| |||||||||
| ln IL-6 (pg/ml) | 0.01 | 0.01 | 0.99 | 0.02 | 0.02 | 0.23 | 0.02 | 0.02 | 0.27 |
| ln TNF-α (pg/ml) | −0.01 | 0.01 | 0.76 | 0.01 | 0.01 | 0.26 | 0.01 | 0.01 | 0.15 |
| Total cholesterol (mg/dL) | 0.04 | 0.39 | 0.92 | 0.47 | 0.72 | 0.51 | 0.31 | 0.31 | 0.62 |
| HDL (mg/dL) | 0.14 | 0.13 | 0.27 | 0.22 | 0.24 | 0.36 | −0.18 | 0.20 | 0.38 |
| LDL (mg/dL) | −0.04 | 0.35 | 0.91 | 0.18 | 0.64 | 0.78 | 0.29 | 0.56 | 0.60 |
| ln Triglycerides (mg/dL) | −0.01 | 0.01 | 0.93 | 0.01 | 0.01 | 0.67 | 0.01 | 0.01 | 0.67 |
| Glucose (mg/dL) | 0.14 | 0.11 | 0.21 | −0.04 | 0.21 | 0.86 | −0.38 | 0.18 | 0.03 |
| ln Insulin (pM/L) | 0.01 | 0.01 | 0.13 | 0.02 | 0.01 | 0.23 | −0.02 | 0.01 | 0.17 |
| Fibrinogen (mg/dL) | −0.32 | 0.73 | 0.66 | −0.58 | 1.34 | 0.67 | 0.46 | 1.16 | 0.69 |
|
| |||||||||
|
Blacks
| |||||||||
| ln IL-6 (pg/ml) | −0.03 | 0.01 | 0.02 | −0.07 | 0.02 | 0.0008 | 0.01 | 0.02 | 0.63 |
| ln TNF-α (pg/ml) | −0.01 | 0.01 | 0.11 | −0.01 | 0.01 | 0.16 | 0.01 | 0.01 | 0.29 |
| Total cholesterol (mg/dL) | −0.36 | 0.47 | 0.44 | −0.58 | 0.79 | 0.46 | 0.52 | 0.67 | 0.43 |
| HDL (mg/dL) | 0.23 | 0.19 | 0.21 | 0.74 | 0.31 | 0.02 | 0.11 | 0.27 | 0.69 |
| LDL (mg/dL) | −0.52 | 0.41 | 0.21 | −0.95 | 0.70 | 0.18 | 0.50 | 0.59 | 0.39 |
| ln Triglycerides (mg/dL) | −0.01 | 0.01 | 0.60 | −0.02 | 0.01 | 0.04 | −0.01 | 0.01 | 0.42 |
| Glucose (mg/dL) | −0.01 | 0.15 | 0.92 | −0.13 | 0.26 | 0.61 | −0.08 | 0.22 | 0.72 |
| ln Insulin (pM/L) | −0.02 | 0.01 | 0.02 | −0.03 | 0.02 | 0.10 | 0.03 | 0.01 | 0.06 |
| Fibrinogen (mg/dL) | −1.60 | 0.98 | 0.10 | −1.35 | 1.67 | 0.42 | 1.96 | 1.40 | 0.16 |
Note: Bolded values highlight associations of p<0.05
p<0.05 for interaction between LOT-R and race
p<0.05 for interaction between optimism dimension (OPT) and race
p<0.05 for interaction between pessimism dimension (PESS) and race
IL-6, TNF-alpha, Triglycerides, and insulin are log transformed.
Each model includes a biomarker as the dependent variable, optimism (OPTPESS)/optimism (OPT)/pessimism (PESS) as the independent variable, and is adjusted for age, gender, pubertal status, race, BMI, smoking, and parental education.
Total: OPTPESS n=950, OPT n=944, PESS n=932; Whites: OPTPESS n=529, OPT n=528, PESS n=524; Blacks: OPTPESS n=421, OPT n=417, PESS n=410
Figure.
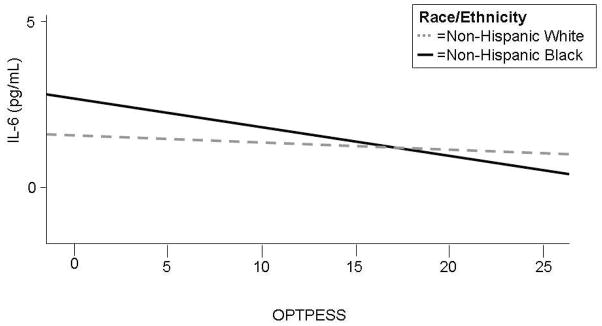
Relationship of OPTPESS to IL-6 by race/ethnicity
DISCUSSION
In this study, we demonstrate profiles of biomarkers of cardiometabolic risk by dispositional optimism which differed by race/ethnicity and trait dimension. Most of the significant associations of optimism to cardiometabolic risks were found in non-Hispanic black adolescents. Overall, we found consistent associations between higher optimism scores, measured as OPTPESS or OPT, and decreased cardiometabolic risk among non-Hispanic black adolescents as measured by several biomarker levels, in particular lower levels of IL-6 and insulin and higher HDL levels. Among non-Hispanic whites, the only significant association was of higher PESS scores with lower glucose levels. Taken together, these findings suggest that optimism measures are more consistently associated than pessimism with biomarkers of cardiometabolic risk in adolescents, and that dispositional optimism is more consistently associated with cardiometabolic risk in non-Hispanic black than non-Hispanic white adolescents, which may provide a psychological mechanism to help explain racial/ethnic disparities in cardiometabolic risk.
These findings that dispositional optimism may be associated with higher or lower biomarker levels by only a fraction of the overall levels underscores the complex and multifactorial nature of these relationships and also supports the theory that psychological characteristics influence cardiometabolic risk over the life course.
Interestingly, our findings differ from adult studies of the association of cardiometabolic risk with dispositional optimism. In adults, stronger associations of cardiometabolic risk are seen with pessimism [11, 24, 25]. We found the optimism subscale to be more consistently associated with biomarker levels, in particular HDL and IL-6 levels. While Roy and colleagues found associations between biomarkers and OPTPESS and OPT scales in unadjusted analyses in adults, only fibrinogen remained significantly associated with PESS in adjusted models [11]. In contrast, we found several biomarkers - HDL cholesterol, triglycerides, insulin, and IL-6 – to be associated with dispositional optimism in adjusted analyses, but notably fibrinogen was not associated in either bivariate or adjusted analyses in these adolescents. The adult cohort was racially/ethnically diverse while our study in adolescents was limited to non-Hispanic black and white youth. These differences between studies suggest possible developmental and racial differences in the relationship of dispositional optimism to health risk over the life course.
Biomarker levels are known to vary by race/ethnicity in adolescence [17]. The different profile of biomarker associations with optimism by race/ethnicity is consistent with racial/ethnic differences in other psychological traits. For example, hostility has been shown to influence racial differences in cardiometabolic risk in adolescents and young adults [31]. Given that optimism is thought to be a stable trait over an individual’s lifetime [6, 32], these findings add to the growing body of evidence that racial/ethnic differences in risk factors during early adolescence can influence disparities in adult diseases, and have implications for when in life primary prevention can be initiated to reduce disparities in cardiovascular health.
Another noteworthy finding is that non-Hispanic black adolescents in this cohort had both higher optimism and higher pessimism scores than non-Hispanic white youth and that the correlation between the two dimensions was lower in non-Hispanic black adolescents than non-Hispanic white adolescents. The differing associations of optimism and pessimism by race/ethnicity lend further support to the bi-dimensional nature of the optimism trait; optimism and pessimism are not mutually exclusive poles of a single construct. The majority of the differing patterns of association between non-Hispanic black and white youth were seen only when this trait was assessed as a bi-dimensional construct. Studying optimism simply as a single dimension missed these nuanced associations, in particular with regards to insulin, IL-6, and HDL.
Why non-Hispanic black youth have both increased optimism and pessimism is unclear. One possible explanation is that these psychological traits develop in response to the social context of the individual during development. Socially disadvantaged children experience chronically challenging environments, which may lead to different distributions of psychological traits [33]. These differing psychological resources then lead to different adaptation responses over the life-course [34], influencing physiologic, psychological, and behavioral regulation. Our data suggest this model may work in relation to inflammatory biomarkers. The differences between our study and that of Roy et al [11] also raise the possibility that age-related differences are due to differential exposure to stressful environments over the life course.
There are several limitations to this study. The design is cross sectional so we cannot infer causality. We tested multiple biomarkers and found several significant associations, some of which may represent chance findings. We measured adiposity through body mass index rather than a direct measure of fat mass, which is known to influence these biomarkers. The PSD study did not collect physical activity data, which can influence biomarkers of cardiovascular disease, and which has been associated with optimism among elderly women [35]. Optimism may have an effect on lifestyle factors associated with cardiovascular risk including diet and exercise. Prior studies have demonstrated a link between psychological traits and CVD, separate from weight, diet, exercise, and other established CVD risk factors, suggesting that the association we found between disposition cardiometabolic biomarker profile is not fully explained through known risk factors. Our study included only non-Hispanic white and black adolescents and was limited geographically to one metropolitan complex in Midwestern United States, limiting our ability to generalize these findings to adolescents of other races or from other places. Balancing these limitations were significant strengths including a large diverse school-based cohort, and an array of well measured biomarkers.
In summary, this study demonstrates associations of dispositional optimism with biomarkers of cardiometabolic risk in healthy adolescents, and that the associations of the biomarkers to the optimism and pessimism dimensions of this trait vary by race/ethnicity. These data raise the possibility, as yet untested, that racial/ethnic differences in dispositional optimism during adolescence may contribute to altered levels of cardiometabolic risks which could, over time, lead to disparities in cardiovascular disease in adulthood. Longitudinal studies to test such a hypothesis would help us better understand when, why, and how these associations develop and may lead to improved cardiometabolic disease prevention.
Implications and Contribution.
Dispositional optimism is linked to cardiometabolic risk and disease in adults. This study demonstrates that optimism is related to biomarkers of cardiometabolic risk in adolescents but that the relationship varies by race/ethnicity, with higher optimism associated with protective cardiometabolic biomarker profiles in non-Hispanic black adolescents.
Acknowledgments
This research was supported by the National Institutes of Health grant R01HD041527, R01DK59183, and K23HL103841.
Abreviations
- CVD
cardiovascular disease
- IL-6
interleukin-6
- TNF-α
tumor necrosis factor alpha
- sTNFR2
soluble tumor necrosis factor receptor type 2
- HDL
high-density lipoprotein cholesterol
- LDL
low-density lipoprotein cholesterol
- PSD
Princeton School District Study
- LOT
Life Orientation Test
- LOT-R
Life Orientation Test-Revised
- OPTPESS
dispositional optimism, single dimension
- OPT
optimism subscale, PESS, pessimism subscale
- BMI
body mass index
- RIA
radioimmunoassay
- CV
coefficient of variation
- SD
standard deviation
Footnotes
The authors have no financial disclosures or potential conflicts of interest to report.
The corresponding author affirms that everyone who has contributed significantly to the paper is listed as an author or in the Acknowledgements.
This work was presented in part as a poster at the Pediatric Academic Societies meeting, May 2011; Denver, Colorado.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Khullar D, Oreskovic NM, Perrin JM, et al. Optimism and the socioeconomic status gradient in adolescent adiposity. J Adolesc Health. 2011;49:553–555. doi: 10.1016/j.jadohealth.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fontaine KR, Cheskin LJ. Optimism and obesity treatment outcomes. J Clin Psychol. 1999;55:141–143. doi: 10.1002/(sici)1097-4679(199901)55:1<141::aid-jclp15>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 3.Giltay EJ, Kamphuis MH, Kalmijn S, et al. Dispositional optimism and the risk of cardiovascular death: the Zutphen Elderly Study. Arch Intern Med. 2006;166:431–436. doi: 10.1001/archinte.166.4.431. [DOI] [PubMed] [Google Scholar]
- 4.Kubzansky LD, Sparrow D, Vokonas P, et al. Is the glass half empty or half full? A prospective study of optimism and coronary heart disease in the normative aging study. Psychosom Med. 2001;63:910–916. doi: 10.1097/00006842-200111000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Boehm JK, Kubzansky LD. The Heart’s Content: The Association Between Positive Psychological Well-Being and Cardiovascular Health. Psychol Bull. 2012 doi: 10.1037/a0027448. [DOI] [PubMed] [Google Scholar]
- 6.Scheier MF, Carver CS. Optimism, coping, and health: assessment and implications of generalized outcome expectancies. Health Psychol. 1985;4:219–247. doi: 10.1037//0278-6133.4.3.219. [DOI] [PubMed] [Google Scholar]
- 7.Hemingway H, Marmot M. Clinical Evidence: Psychosocial factors in the etiology and prognosis of coronary heart disease: systematic review of prospective cohort studies. West J Med. 1999;171:342–350. [PMC free article] [PubMed] [Google Scholar]
- 8.Rosengren A, Hawken S, Ounpuu S, et al. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:953–962. doi: 10.1016/S0140-6736(04)17019-0. [DOI] [PubMed] [Google Scholar]
- 9.Scheier MF, Matthews KA, Owens JF, et al. Dispositional optimism and recovery from coronary artery bypass surgery: the beneficial effects on physical and psychological well-being. J Pers Soc Psychol. 1989;57:1024–1040. doi: 10.1037//0022-3514.57.6.1024. [DOI] [PubMed] [Google Scholar]
- 10.Matthews KA, Raikkonen K, Sutton-Tyrrell K, et al. Optimistic attitudes protect against progression of carotid atherosclerosis in healthy middle-aged women. Psychosom Med. 2004;66:640–644. doi: 10.1097/01.psy.0000139999.99756.a5. [DOI] [PubMed] [Google Scholar]
- 11.Roy B, Diez-Roux AV, Seeman T, et al. Association of optimism and pessimism with inflammation and hemostasis in the Multi-Ethnic Study of Atherosclerosis (MESA) Psychosom Med. 2010;72:134–140. doi: 10.1097/PSY.0b013e3181cb981b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patton GC, Tollit MM, Romaniuk H, et al. A prospective study of the effects of optimism on adolescent health risks. Pediatrics. 2011;127:308–316. doi: 10.1542/peds.2010-0748. [DOI] [PubMed] [Google Scholar]
- 13.Zakai NA, Katz R, Jenny NS, et al. Inflammation and hemostasis biomarkers and cardiovascular risk in the elderly: the Cardiovascular Health Study. J Thromb Haemost. 2007;5:1128–1135. doi: 10.1111/j.1538-7836.2007.02528.x. [DOI] [PubMed] [Google Scholar]
- 14.Ridker PM, Rifai N, Cook NR, et al. Non-HDL cholesterol, apolipoproteins A-I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. JAMA. 2005;294:326–333. doi: 10.1001/jama.294.3.326. [DOI] [PubMed] [Google Scholar]
- 15.Riolo SA, Nguyen TA, Greden JF, et al. Prevalence of depression by race/ethnicity: findings from the National Health and Nutrition Examination Survey III. Am J Public Health. 2005;95:998–1000. doi: 10.2105/AJPH.2004.047225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Twenge JM, Crocker J. Race and self-esteem: meta-analyses comparing whites, blacks, Hispanics, Asians, and American Indians and comment on Gray-Little and Hafdahl (2000) Psychol Bull. 2002;128:371–408. doi: 10.1037/0033-2909.128.3.371. discussion 409-20. [DOI] [PubMed] [Google Scholar]
- 17.Goodman E, McEwen BS, Huang B, et al. Social inequalities in biomarkers of cardiovascular risk in adolescence. Psychosom Med. 2005;67:9–15. doi: 10.1097/01.psy.0000149254.36133.1a. [DOI] [PubMed] [Google Scholar]
- 18.Chyu L, Upchurch DM. Racial and ethnic patterns of allostatic load among adult women in the United States: findings from the National Health and Nutrition Examination Survey 1999–2004. J Womens Health (Larchmt) 2011;20:575–583. doi: 10.1089/jwh.2010.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Field AE, Cook NR, Gillman MW. Weight status in childhood as a predictor of becoming overweight or hypertensive in early adulthood. Obes Res. 2005;13:163–169. doi: 10.1038/oby.2005.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen XM, Lane J, Smith BR, et al. Changes in inflammatory biomarkers across weight classes in a representative US population: a link between obesity and inflammation. J Gastrointest Surg. 2009;13:1205–1212. doi: 10.1007/s11605-009-0904-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodman E, Adler NE, Daniels SR, et al. Impact of objective and subjective social status on obesity in a biracial cohort of adolescents. Obes Res. 2003;11:1018–1026. doi: 10.1038/oby.2003.140. [DOI] [PubMed] [Google Scholar]
- 22.Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the Life Orientation Test. J Pers Soc Psychol. 1994;67:1063–1078. doi: 10.1037//0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
- 23.Goodman E, Knight JR, DuRant RH. Use of the Life Optimism Test among adolescents in a clinical setting: a report of reliability testing. J Adolesc Health. 1997;21:218–220. doi: 10.1016/S1054-139X(97)00123-7. [DOI] [PubMed] [Google Scholar]
- 24.Kubzansky LD, Kubzansky PE, Maselko J. Optimism and pessimism in the context of health: bipolar opposites or separate constructs? Pers Soc Psychol Bull. 2004;30:943–956. doi: 10.1177/0146167203262086. [DOI] [PubMed] [Google Scholar]
- 25.Herzberg PY, Glaesmer H, Hoyer J. Separating optimism and pessimism: a robust psychometric analysis of the revised Life Orientation Test (LOT-R) Psychol Assess. 2006;18:433–438. doi: 10.1037/1040-3590.18.4.433. [DOI] [PubMed] [Google Scholar]
- 26.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002;11(246):1–190. [PubMed] [Google Scholar]
- 27.Brener ND, Kann L, McManus T, et al. Reliability of the 1999 youth risk behavior survey questionnaire. J Adolesc Health. 2002;31:336–342. doi: 10.1016/s1054-139x(02)00339-7. [DOI] [PubMed] [Google Scholar]
- 28.Wills TA, Cleary SD. The validity of self-reports of smoking: analyses by race/ethnicity in a school sample of urban adolescents. Am J Public Health. 1997;87:56–61. doi: 10.2105/ajph.87.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finkelstein DM, Kubzansky LD, Goodman E. Social status, stress, and adolescent smoking. J Adolesc Health. 2006;39:678–685. doi: 10.1016/j.jadohealth.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 30.Dolan LM, Bean J, D’Alessio D, et al. Frequency of abnormal carbohydrate metabolism and diabetes in a population-based screening of adolescents. J Pediatr. 2005;146:751–758. doi: 10.1016/j.jpeds.2005.01.045. [DOI] [PubMed] [Google Scholar]
- 31.Goodman E, Must A, Daniels SR, et al. Hostility and adiposity mediate disparities in insulin resistance among adolescents and young adults. J Pediatr. 2010;157:572–7. 577.e1. doi: 10.1016/j.jpeds.2010.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park CL, Folkman S. Stability and change in psychosocial resources during caregiving and bereavement in partners of men with AIDS. J Pers. 1997;65:421–447. doi: 10.1111/j.1467-6494.1997.tb00960.x. [DOI] [PubMed] [Google Scholar]
- 33.Gallo LC, Matthews KA. Understanding the association between socioeconomic status and physical health: do negative emotions play a role? Psychol Bull. 2003;129:10–51. doi: 10.1037/0033-2909.129.1.10. [DOI] [PubMed] [Google Scholar]
- 34.McEwen BS. From molecules to mind. Stress, individual differences, and the social environment. Ann N Y Acad Sci. 2001;935:42–49. [PubMed] [Google Scholar]
- 35.Steptoe A, Wright C, Kunz-Ebrecht SR, et al. Dispositional optimism and health behaviour in community-dwelling older people: associations with healthy ageing. Br J Health Psychol. 2006;11:71–84. doi: 10.1348/135910705X42850. [DOI] [PubMed] [Google Scholar]


