Abstract
Mcm10 is required for DNA replication in all eukaryotes. While the exact contribution of Mcm10 to genome replication remains heavily debated, early reports suggested that it promotes DNA unwinding and origin firing. These ideas have been solidified by recent studies that propose a role for Mcm10 in helicase activation. Although the molecular underpinnings of this activation step have yet to be revealed, structural data on Mcm10 provide further insight into a possible mechanism of action. The essential role in DNA replication initiation is not mutually exclusive with additional functions that Mcm10 may have as part of the elongation machinery. Here, we review the recent findings regarding the role of Mcm10 in DNA replication and discuss existing controversies.
Keywords: Mcm10, DNA replication, double-strand breaks, genome integrity, replication forks, OB-fold and Zn-finger domains
Replication complex assembly
Accurate replication of the genome requires an intricate coordination among multiple replicative factors even before the onset of S-phase. In budding yeast, during the G1 phase of the cell cycle head-to-head, double hexameric complexes of the minichromosome maintenance proteins 2–7 (Mcm2-7) are loaded onto replication origins by a cadre of proteins, including the origin recognition complex (ORC), Cdc6 and Cdt1 [1]. This event is often referred to as “origin licensing,” and together these factors constitute the pre-replication complex (pre-RC; Figure 1). During the G1-to-S-phase transition two kinases, the Dbf4-dependent kinase Cdc7 (DDK) and S-phase cyclin-dependent kinase (S-CDK) are required to transform the Mcm2-7 double hexamer into an active replicative helicase. DDK phosphorylates the Mcm2-7 complex, thereby allowing the recruitment of Sld3 and the helicase co-activator Cdc45. S-CDK promotes formation of the so-called pre-loading complex (pre-LC) composed of Sld2, Dpb11, DNA polymerase (Pol)-ε and a second helicase co-activator complex, GINS (go-ichi-ni-san) [2]. Sld2 and Sld3 have been shown to be the essential targets of S-CDK and their phosporylation allows the pre-LC to dock onto Sld3 via Dpb11 and deliver GINS in conjunction with Pol-ε to nascent replication complexes. GINS associates with Cdc45-Mcm2-7 to form the CMG complex (*Cdc45-Mcm2-7-GINS) [3] – the functional replicative helicase. GINS also helps to recruit Pol-ε toreplicationorigins. Pol-ε, however, is not equipped to initiate DNA synthesis. A specialized enzyme, Pol-α/primase, exists for this purpose and its association requires DNA unwinding. It is precisely this unwinding event that is so carefully timed by this complicated cascade of events, and Mcm10 appears to be crucial to execute this step [4–7]. When exactly Mcm10 associates with replication origins is still an open question and therefore we present two possible models: recruitment after Cdc45 and GINS loading [8], or alternatively, before DDK activation [4] (Figure 1). Despite this controversy, which we will discuss in more detail below, the consensus is that Mcm10 ultimately facilitates recruitment of the single stranded (ss) DNA binding protein replication factor A (RPA), and Pol-α/primase. Subsequently, Pol-α/primase proceeds with the initiation of both daughter strands. During elongation, it frequently dissociates and re-associates with the replication fork to synthesize RNA-DNA primers that are extended into Okazaki fragments by Pol-δ and PCNA (proliferating cell nuclear antigen) [1].
Figure 1.
Stepwise assembly of initiation and elongation complexes at eukaryotic replication origins. The events described here are primarily based on studies in budding yeast. Two models are shown that depict different times of Mcm10 recruitment to the origin. On the left, Mcm10 is recruited after the assembly of the CMG helicase [8]. On the right, Mcm10 is recruited before the activation of DDK [4]. (a) Formation of the pre-replication complex completes origin licensing after the minichromosome maintenance proteins 2-7 (Mcm2-7) are loaded onto DNA by a specialized loading machine comprised of ORC, Cdc6 and Cdt1 during late M/G1. The functional helicase, the Cdc45-Mcm2-7-GINS complex (CMG complex), is formed by the stepwise assembly of multiple factors. To initiate CMG formation, DDK phosphorylates Mcm2-7 subunits (red circles mark phophates) after which Sld3 and Cdc45 are recruited to the origin. S-CDK initiates formation of the pre-loading complex, comprised of Dpb11, GINS, Sld2 and Pol-ε. In addition, S-CDK phosphorylates both Sld2 and Sld3, leading to their association with Dpb11. This delivers GINS and Pol-ε to origins. GINS and Cdc45 now form a complex with Mcm2-7, resulting in the CMG helicase poised for origin unwinding. After activation of the CMG helicase, some factors such as Dpb11, Sld2, Sld3, DDK, S-CDK dissociate from the origin. (b) Mcm10 is recruited to the CMG complex and facilitates origin unwinding. Potential models describing how Mcm10 mediates origin unwinding are depicted in greater detail in Figure 2. Mcm10 either directly or indirectly recruits Pol-α to the replication fork in order to initiate DNA synthesis. (c) Proposed model of Mcm10 function during S-phase progression. Mcm10 coordinates Mcm2-7 and Pol-α during elongation through interaction with both proteins. When Mcm10 is di-ubiquitylated (yellow stars ‘Ub’), it releases Pol-α and binds to PCNA, possibly facilitating the recruitment of Pol-δ. Ctf4 cooperates with Mcm10 to load Pol-α onto DNA in higher eukaryotes, but it is not essential in budding yeast. Ctf4 is not included in steps (a) and (b) as its role during initiation remains unclear.
To gain a better understanding of the overall orchestration of eukaryotic DNA replication, this review will focus on Mcm10, which has emerged as a crucial but controversial player in the field.
The role of Mcm10 in diverse steps of DNA replication
Replication Initiation
Mcm10 was first discovered as a factor required for replicating the genome and stably maintaining minichromosomes [9, 10]. Early studies in Saccharomyces cerevisiae, Schizosaccharomyces pombe and Xenopus leavis egg extracts suggested that Mcm10 was loaded onto chromatin after origin licensing but before the initiation of DNA synthesis [4, 11, 12]. Subsequent work in multiple different model organisms revealed a potential role for Mcm10 in the recruitment of the helicase co-activator Cdc45 [4, 12, 13]. As alluded to above, this view was recently challenged by in vitro experiments with budding yeast whole cell extracts, which demonstrated that the CMG complex assembled in the absence of Mcm10 [8]. This revised model has been validated by three independent studies in budding and fission yeast [5–7]. All three groups insisted that Mcm10 is required for origin unwinding, although its exact role remains unknown. This revised model is also consistent with two other reports that noted that retention of Cdc45 on chromatin was independent of Mcm10 in vivo [11, 14]. It is worthwhile to point out that some of the observed differences (Figure 1) could be attributed to the detection methods that were utilized to examine chromatin-bound Cdc45. For example, chromatin immunoprecipitation (ChIP), which employs chemical cross-linking might enrich for loosely bound Cdc45, whereas chromatin fractionation does not. Cytological studies involving detergent washes may only visualize very tightly bound protein [14]. Furthermore, the addition of either naked DNA [8] or chromatin [4] could have affected the recruitment of Cdc45.
Whether Mcm10 is incorporated into the replication complex formally in late G1 or early S phase remains debated, perhaps also because these studies have relied heavily on depletion approaches: either through the timely destruction of heat-inducible degrons, antibody-mediated depletion or siRNA-targeted knockdown. Even if similar depletion protocols are applied, the degree to which Mcm10 is eliminated can vary significantly from experiment to experiment and this might contribute to the observed discrepancies. In the long run, recapitulating individual steps in replication complex assembly by purified proteins will certainly help to resolve some of the current controversies. It remains to be clarified if chromatin association of Mcm10 is dependent on DDK or S-CDK activities, and thus incorporated into the replication complex formally in late G1 or early S phase, respectively. Mcm10 has both ss and double stranded (ds) DNA binding activities [15–20], therefore both scenarios are theoretically possible. Despite this ongoing debate, there seems to be a strong consensus that Mcm10 binds specifically to replication origins at the G1/S transition, at approximately the same time as the two helicase co-activators Cdc45 and GINS.
Is Mcm10 a helicase activator?
A bigger question in the field is whether Mcm10 has an active role in remodeling the CMG helicase to promote DNA unwinding. Mcm2-7 is loaded onto DNA as a double hexamer encircling duplex DNA [21], but appears to translocate as a single hexamer along the leading strand template [22, 23], therefore it is largely assumed that the CMG complex requires some sort of remodeling to facilitate this transition (Figure 2a). Multiple laboratories have explored the possibility that Mcm10 has a role in this process.
Figure 2.
Hypothetical models of Mcm10 promoting origin unwinding. (a) Mcm10 may facilitate the extrusion of one DNA strand from the core of the CMG helicase at the origin and aid the 3’ to 5’ ssDNA translocase activity [23]. This chain of events leads to origin unwinding and replication initiation. In this model, Mcm10 plays an active role in origin unwinding by remodeling the helicase. (b) Alternatively, Mcm10 may stabilize the formation of ssDNA via its ssDNA-binding domain after origin melting by the active CMG complex, thereby facilitating the initiation step. In this model, Mcm10 plays an indirect role in origin unwinding. Note that the two models are not mutually exclusive. After initiation, the replisome promotes DNA synthesis and the requirement for Mcm10 during this steps remains unresolved. Mcm10 may be a stable component of the replisome (top) or may associate with the elongation apparatus only transiently (bottom).
A function for Mcm10 in origin unwinding was first described in studies utilizing Xenopus egg extract to investigate in vitro replication [4]. Adding circular plasmid, a collaborative effort by the Dutta and Walter laboratories showed that highly unwound DNA structures were absent when Mcm10 was depleted from nucleoplasmic extract. As mentioned above, three recent studies in S. cerevisiae and S. pombe also suggested that Mcm10 is required to unwind DNA at the origin during replication initiation [5–7]. This conclusion was primarily based on the failure to detect RPA in close proximity of early-firing origins by ChIP. Although the lack of RPA binding is not absolute proof of failure to unwind DNA, it is a reasonable approximation and these data are consistent with the notion that extensive DNA unwinding was blocked in the absence of Mcm10. An alternative way to interpret this data is that the role of Mcm10 is more indirect, perhaps by stabilizing ssDNA through its ssDNA-binding domain (Figure 2b) [17, 24]. Masukata and colleagues attempted to substantiate this latter model by employing mutants that disrupted the conserved residues within the Zn-finger of the ssDNA-binding domain. Indeed, this mutant prevented RPA association. Curiously, despite the fact that it resulted in significantly reduced protein expression, it was more efficiently retained on chromatin than wild-type Mcm10 [5]. One interpretation would be that the mutant failed to stabilize ssDNA, diminishing the recruitment of RPA and becoming more persistently bound to dsDNA. Thus, the idea that Mcm10 might have a more indirect role in helicase activation by stabilizing unwound DNA is a very attractive model (Figure 2b) and appears, at least at this point in time, to be more compatible with the published literature. Although worth considering, there is currently no evidence to support the claim that Mcm10 directly remodels the CMG complex to alter its association with the parental DNA double strand (Figure 2a) [6]. Whether Mcm10 has a bona fide helicase activating function or remodeling activity needs to be further examined. However, in higher eukaryotes Mcm10 appears to regulate the function of the RecQL4 helicase, albeit in an inhibitory manner [25]. The N terminus of RecQL4 shows homology to yeast initiation factor Sld2 and is required for cell viability [26–28]. How the interaction between Mcm10 and RecQL4 contributes to replication initiation will require detailed biochemical analysis.
Does Mcm10 have a direct role in polymerase loading?
Although the involvement of MCM10 in the assembly or remodeling of the CMG complex remains unclear, a role for Mcm10 in the later steps of initiation or early steps of elongation – specifically, the efficient loading of lagging strand polymerases – has been confirmed by multiple independent studies [8, 11, 14, 29]. In S. cerevisiae, the recruitment of both Pol-α and Pol-δ to origin DNA is significantly reduced when Mcm10 is depleted [8]. However, this could be either a direct or indirect effect. Following the above laid-out considerations about a possible role for Mcm10 in DNA unwinding, it can also be argued that failure to proficiently unwind the DNA will result in a lack of polymerase loading. Nonetheless, many laboratories have demonstrated that Mcm10 binds the catalytic subunit of Pol-α and is required for its chromatin association [11, 14, 19, 20, 29–31]. A proteomic dissection of the budding yeast replisome identified Mcm10 together with the cohesion protein and Pol-α binding partner Ctf4 as members of a large “replisome progression complex” [22]. Similar data were obtained from co-immunoprecipitation studies in human cells and Xenopus egg extracts, which demonstrated that Mcm10, Pol-α and Ctf4 were in a common complex and postulated that Mcm10 and Ctf4 connect the lagging strand polymerase, Pol-α, with the CMG helicase [29].
It is not entirely clear if Mcm10 and Ctf4 contribute equally to Pol-α loading. Some studies are conflicting; for instance, neutralizing Ctf4-specific antibodies prevented Pol-α loading in one study [29], but had no effect in another [32]. Ctf4 might also have a different contribution in different organisms; on the one hand, CTF4 is a non-essential gene in budding yeast, therefore it is impossible that the protein is strictly required at replication forks. On the other hand, in higher eukaryotes Ctf4/And1 is required for viability, and genetic disruption of Mcm10 and knock-down of Ctf4 result in very similar cellular phenotypes in Drosophila melanogaster [33, 34]. Indeed, depletion of either Ctf4/And1 or Mcm10 has been implicated in regulating the turnover of Pol-α [29, 31].
Yet, the role of Mcm10 in Pol-α stability also remains debated. The chaperone function of ScMcm10 for Pol-α has been reproduced by three independent laboratories [11, 30, 35, 36]. However, three recent studies failed to detect any noticeable effect on the stability of the catalytic subunit of Pol-α upon loss of Mcm10 [5–7], arguing that the absence of Mcm10 is not the direct trigger of the proteasome-dependent degradation pathway targeting Pol-α in mcm10-1 mutants. It has been reported that the E2–E3 complex comprised of Ubc4 and Not4, respectively, regulates Pol-α turnover in these mutants [37]. Given the strong discrepancies that cannot be easily accounted for by differences in the experimental methods, future experiments are warranted to explore if complementation of mcm10-1 with wild-type MCM10 restores steady-state levels of the catalytic subunit of Pol-α. This would formally exclude the possibility that secondary mutations are the true cause for the observed degradation of Pol-α.
Despite the controversies outlined above, the prevailing view in the field is that Mcm10, together with Ctf4, RPA and Pol-α, contributes to replication initiation and lagging strand synthesis. Importantly, a role for Mcm10 in facilitating DNA unwinding (Figure 2) is not mutually exclusive with a function in DNA synthesis (Figure 1).
Replication elongation
Compelling evidence from multiple laboratories suggests that Mcm10 not only resides at replication origins but that it also associates with the replication fork. Mcm10 interacts with Pol-α, and Pol-α undergoes cycles of chromatin association and dissociation during Okazaki fragment synthesis, therefore Mcm10 binding to the fork is also likely transient. Indeed, to increase the chance of capturing Mcm10 on chromatin in vivo, replication can be slowed by decreasing the temperature to 20°C or adding high amounts of HU to limit nucleotide pools [11, 38]. Even without these experimental tricks, one group demonstrated that Mcm10 associated with origin-flanking regions as cells progressed through S phase, mimicking the profile of Mcm3 [39]. In addition, two independent biochemical reports support this proposal that Mcm10 is part of the replisome [22, 40]. In one, Mcm10 is readily detectable in budding yeast replisomes purified with 300 mM salt, but not 50 mM salt [22]. ScMcm10 is insoluble, therefore high salt concentrations are required to keep the protein sufficiently solubilized; DNase I treatment alone is not sufficient [11, 41]. Clear support for Mcm10 as a component of the replisome came from a second study that utilized Xenopus egg extract [40]. This study targeted multiple replication factors, including Mcm10, by ChIP of plasmid templates that contained a precisely positioned biotinylated nucleotide. The addition of streptavidin, produced a road block that caused replication fork arrest without uncoupling DNA polymerases from the helicase. Mcm10 co-localized with DNA polymerases and Mcm2-7 subunits at the biotin-streptavidin site, arguing that it is a component of the elongation machinery [40].
In addition to these biochemical studies, the identification of mcm2 mutants that suppress mcm10-1 phenotypes in budding yeast also provides evidence that Mcm10 resides at the replication fork [35]. These suppressor mutants exhibit reduced helicase activity and rescue the mcm10-1-induced replication defect by reducing ssDNA accumulation. These results strongly suggest that slowing DNA unwinding bypasses the need for Mcm10 to coordinate helicase and polymerase activities. As a result, prolonged exposure of ssDNA regions is minimized at the replication fork, preventing aberrant fork structures and fork collapse. Interestingly, mcm2 also restores the expression of Pol-α in mcm10-1 cells in a Mec1-dependent manner [35]. Mec1 is the yeast homolog of the mammalian checkpoint kinase ATR (ataxia telangiectasia mutated [ATM] and Rad3-related). It might actively promote the association of Pol-α with the replication fork in the event of fork stalling, which is consistent with a previous study [42]. In an alternative model, it was suggested that the mcm2 suppressor allele encodes a helicase subunit that confers a conformation to Mcm2-7 that resembles the “activated” state and thus bypasses the need for helicase activation through Mcm10 [6]. It was proposed that under such a scenario the Mcm2-7 complex might not work optimally. This would be compatible with the results reported by the Tye laboratory [35] and implies that further characterization of the mcm2 suppressor strain might hold the key to addressing if Mcm10 is actively remodeling the helicase as suggested in Figure 2a.
The last piece of evidence that supports a role for Mcm10 in lagging strand synthesis is its physical association with PCNA, the accessory factor for Pol-δ and Pol-ε. The interaction between Mcm10 and PCNA in budding yeast is regulated by the ubiquitylation of Mcm10, which occurs during G1 and S-phase of the cell cycle [43]. Mcm10 is mono-ubiquitylated at two distinct lysine residues. These modifications were speculated to promote a slight conformational change that facilitated access to the PCNA interacting peptide (PIP) box of Mcm10. The PIP box is buried in the core domain of Mcm10 and likely inaccessible to PCNA when the protein is unmodified. Surprisingly, a single tyrosine substitution within the PIP box of Mcm10 renders budding yeast inviable [43]. Therefore, the interaction between Mcm10 and PCNA serves an essential function. However, what this function might be remains to be determined. It is intriguing that Pol-α is required to synthesize the substrate for PCNA. Moreover, ubiquitylated Mcm10 no longer has the ability to bind Pol-α [43]. Thus, ubiquitylation of Mcm10 might serve as a switch to regulate the release of Pol-α after the completion of RNADNA primer synthesis. Subsequently, ubiquitylated Mcm10 might help with the recruitment of PCNA to extend Okazaki fragments in a timely manner (Figure 1).
In summary, strong biochemical and genetic data corroborate the idea that Mcm10 resides at replication forks and contributes to lagging strand synthesis. However, there is not complete agreement in the field about this point, as some data directly disputes this model. For example, Kanemaki and colleagues detected only very small amounts of Mcm10 in origin-flanking regions by ChIP [7]. To accurately reflect the ongoing discussion in the field, we have included an alternative version of a replication fork that associates only transiently with Mcm10 and thus the protein is not shown as a stable component (Figure 2, bottom). It is our personal opinion that there is insufficient proof to negate the evidence provided by multiple independent laboratories that speak in favor of the model that Mcm10 has an important role in DNA elongation [11, 12, 14, 15, 19, 22, 23, 29, 30, 35, 36, 38, 40, 43]. The structural features of Mcm10 that define specific interaction domains with other replisome components also reinforce this notion [24].
Structure and domains of MCM10
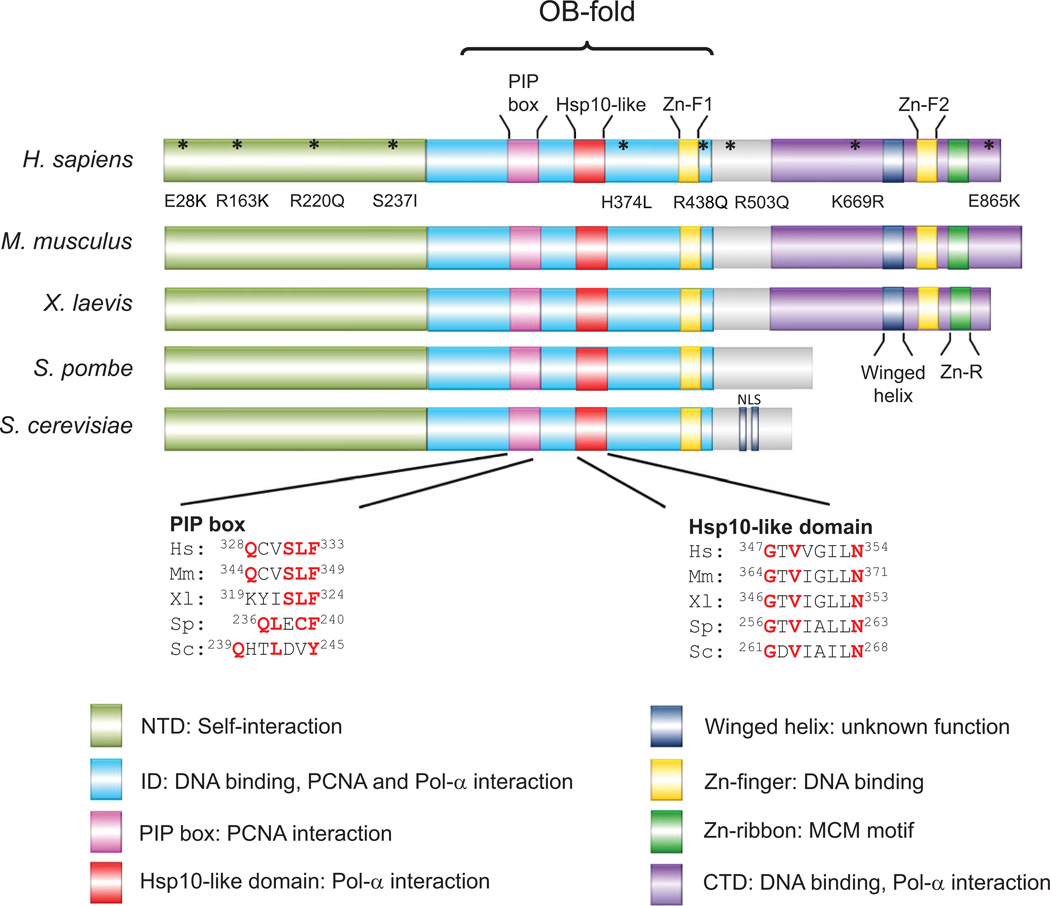
Due to a lack of overall sequence homology, it was initially assumed that Mcm10 might function differently in higher eukaryotes compared to lower eukaryotes. However, subsequent studies refuted this notion. Although human Mcm10 cannot rescue a mcm10 mutant in S. cerevisiae [44], the internal domain (ID), which is present in all eukaryotes, is highly conserved throughout evolution [16, 17]. Indeed, the ID mediates many of the important functions of Mcm10, such as its interactions with Pol-α, PCNA, ssDNA and dsDNA [11, 16–18, 43]. Systematic sequence alignment and limited protein digestion revealed two additional domains of Mcm10: an N-terminal domain (NTD) and a metazoan-specific C-terminal domain (CTD) [16].
N-terminal domain (NTD)
Existing as a structurally distinct domain, only a limited function has been assigned to the N terminus of Mcm10. In vitro assessment of the XMcm10-NTD demonstrated an ability for homodimerization [16]. Although the biological significance of Mcm10 self-interaction has not been determined, additional evidence confirms homocomplex formation of Mcm10 on DNA with an estimated 3:1 stoichiometry of ScMcm10 to ssDNA [18]. Indeed, several studies predict that more than one molecule of Mcm10 resides on chromatin to orchestrate multiple interactions with replicative factors and DNA [11, 45]. Mcm10 multimers might accommodate leading and lagging strand synthesis by stabilizing ssDNA formation and/or replication proteins. Alternatively, Mcm10 homocomplexes could be necessary to coordinate normal replication and repair in the event of replication stress.
Internal Domain
The ID (Figure 3) is the only domain displaying high sequence similarity among species. It comprises an oligosaccharide/oligonucleotide binding fold (OB-fold), first identified by sequence search [30] and later confirmed by structural analysis [17]. The OB-fold forms a typical DNA binding cleft, which also mediates an interaction with Pol-α through a conserved hydrophobic patch (also known as an Hsp10-like domain) that resides in the concave of the cleft [17, 19, 30]. It appears that Mcm10 is able to associate with DNA regardless of sequence and topology [18, 19]. A more detailed mutant analysis of XMcm10-ID further suggested that the DNA binding region spans the hydrophobic cleft and positively charged residues on the adjacent Zn-finger [17]. The high-resolution crystal structure revealed a surprising novel configuration of the Zn-finger motifs that distinguishes Mcm10 from other OB-fold/Zn finger DNA binding proteins, such as RPA or archaeal Mcm helicase [17]. Importantly, electron microscopy (EM) reconstruction studies of full-length human Mcm10 that utilized the ring-shaped archaeal Mcm helicase for molecular modeling came to the conclusion that HsMcm10 was a hexameric ring [46]. Thus, the structural differences in the assembly between the OB-fold and Zn-finger domains of XMcm10 and archaeal Mcm helicase might explain why it is not possible to dock the high-resolution structure of XMcm10-ID onto the EM reconstruction of HsMcm10 [17, 46].
Figure 3.
Functional domains of Mcm10 across species. The N-terminal domain (NTD), although not highly conserved, is responsible for self-interaction of Mcm10. The internal domain (ID) is the most highly conserved region of the protein. It comprises an oligonucleotide/oligosaccharide (OB)-fold, PCNA-interacting peptide (PIP) box and Hsp10-like domain, which mediate DNA-binding, PCNA interaction and Pol-α binding, respectively. The positions and sequence alignments of the PIP-box and Hsp10-like domain from different species are shown. The PIP box in all species (except S. cerevisiae) resembles the consensus sequence of the prokaryotic β-clamp binding site (QLsLF, s=small amino acid). The PIP box in S. cerevisiae matches the canonical PIP box (QXXM/I/LXXF/YF/Y, X=any amino acid). Both Zn-finger motifs facilitate DNA-binding: the first (Zn-F1) is conserved across species while the second (Zn-F2) is present in the C-terminal domain (CTD), a domain specific to metazoans. The asterisks in human Mcm10 indicate mutations identified in cancer cells (www.sanger.ac.uk); exact residues are shown underneath. Nuclear localization sequences (NLS) have only been identified in budding yeast. (Hs: Homo sapiens; Mm: Mus musculus; Xl: Xenopus laevis; Sp: Saccharomyces pombe; Sc: Saccharomyces cerevisiae)
In vitro, disruption of the XMcm10 Zn-finger reduces overall protein stability and binding to dsDNA, but not ssDNA [16]. It is reasonable to assume that interfering with Zn-coordination destabilizes or distorts the protein. Thus, whether the Zn-finger truly mediates DNA binding remains difficult to assess. Mutating DNA contact residues on the surface of Mcm10 without affecting protein integrity has provided a better approach to this problem. For instance, when two basic surface residues within the XMcm10 Zn-finger were mutated, ssDNA binding was significantly reduced [17]. Corresponding mutations in S. cerevisiae rendered cells sensitive to replication stress, without altering the expression of the protein [17]. Taken together, these observations implicate the Zn-finger domain in DNA binding in vivo.
Whereas ssDNA and Pol-α compete for the same binding site [19], the PIP box (the interaction motif for PCNA) of Mcm10 overlaps only partially with the DNA binding surface [17]. In budding yeast, the motif is a 3/4 match to the canonical PIP box consensus QxxM/I/LxxF/YF/Y [47]. In most other organisms, the corresponding domain more closely resembles the consensus of the prokaryotic β-clamp binding site QLsLF [47]. As mentioned earlier, the integrity of the PIP box is essential for cell viability in S. cerevisiae [43]. Because the motif is evolutionarily conserved, this might also be true for higher eukaryotes, but this awaits experimental confirmation.
C-terminal domain (CTD)
The C-terminal domain, specific to metazoa, constitutes an additional interface for DNA and protein interaction. Together, the XMcm10 ID and CTD bind DNA and the catalytic subunit of Pol-α with higher affinity than each domain alone [17]. Structural analysis by NMR revealed two additional Zn-coordinating structures within the CTD, which exist as a globular domain that is distinct from the Zn-finger in the ID [20]. Only the first Zn-finger interacts with ssDNA; the second motif bears homology to the Mcm2-7 helicase OB-fold Zn-ribbon, but has an unknown function [20]. The biological implication of an additional DNA binding domain (CTD) in metazoa is not yet clear. Nevertheless, analyzing the CTD might shed some light on how Mcm10 from higher eukaryotes functions differently from lower eukaryotes. For example, although human Ctf4/And-1 have been proposed to partner with Mcm10 to locate and stabilize Pol-α at the fork [29], this function is likely executed by Mcm10 alone in yeast, because Ctf4 is not essential.
Regulation of MCM10
A limited number of studies suggest that Mcm10 is regulated transcriptionally as well as post-translationally. E2F1 transcription factors can induce expression of Mcm10 in human cells, and this is counteracted by pRb [48]. Consistent with the role of Mcm10 in DNA replication, promoter activity can be detected during G1 and S-phase of the cell cycle [48]. Not surprisingly, Mcm10 protein expression in human cells increases as cells enter S phase and decreases late in mitosis [49]. By contrast, in budding yeast Mcm10 protein levels remain fairly constant [11]. The cell-cycle dependent reduction of human Mcm10 is thought to be proteasome dependent [49]. Interestingly, it seems that proteasomal degradation is not only important for normal cell-cycle dependent protein oscillation, but becomes activated during DNA damage. Upon UV irradiation Mcm10 is rapidly degraded, which is believed to block replication as cells initiate repair processes [50, 51]. As alluded to earlier, non-proteolytic ubiquitylation of Mcm10 has also been observed, and this modification regulates the interaction with PCNA [43].
Interestingly, SpMcm10 is phosphorylated in vitro by DDK. However, if this occurs in vivo is unclear [52]. Phosphorylation of Mcm10 by the ATM and ATR checkpoint kinases has also been observed in a large-scale proteomics study that sought novel ATM/ATR substrates [53]. Although extensive analysis is required to validate these findings, they implicate post-translational regulation of Mcm10 during normal replication as well as checkpoint activation.
Concluding remarks
In the past decade, biochemical and in vivo studies have remarkably advanced our understanding of Mcm10. Despite some controversies, the emerging model is that Mcm10 is essential for replication initiation – at a defined step that promotes DNA unwinding. Moreover, Mcm10 also contributes to replication fork progression, maybe by coordinating DNA helicase and polymerization activities during lagging strand synthesis. A better understanding of these aspects of Mcm10 function will not only answer fundamental questions about DNA replication but will also potentially impact our understanding of human health and diseases that are characterized by aberrant replication. In addition to its role in normal replication, Mcm10 might participate in other pathways of DNA metabolism, such as DNA repair or S phase checkpoint activation. None of these areas has been rigorously investigated. Exploring these potential roles of Mcm10 in the future might reveal novel functions of Mcm10 in preserving genome integrity.
Figure I.
Synthetic genetic interactions of mcm10. (a) Summary of genes that are synthetically lethal with mcm10 or cdc23 (the fission yeast mcm10 homolog), including various replication genes, double stranded break repair genes and checkpoint genes [35, 66–69]. Red and blue colors indicate essential and nonessential genes, respectively. Underlined genes indicate physical interactions between the corresponding gene products and Mcm10 [6, 10, 22, 29, 33, 35, 39, 44, 67, 69, 77]. (b) Synthetic genetic array analysis identified genes that are synthetically sick (red line) or synthetically healthier (green line) with mcm10-1. The analysis was performed at 30°C with an array of non-essential single gene deletion mutants. The thickness of the lines indicates the relative intensity of the interaction.
Box 1: Mcm10 participates in the genome integrity network and is dysregulated in cancer.
Emerging evidence points to a role of Mcm10 in protecting genome integrity. Mcm10 and RPA, together with Claspin, Timeless (human homologs of the S. cerevisiae Mrc1 and Tof1 proteins, respectively), RFC subunits (replication factor C, the loading apparatus of PCNA) and the Dbf4 subunit of DDK were all identified as strong suppressors of spontaneous chromosome breakage [54]. Notably, however, Ctf4 was not uncovered in this genome-wide siRNA screen that quantitatively assessed γH2AX foci formation in HeLa cells. This suggests that Ctf4 and Mcm10 are not completely redundant. Mcm10, among other replication proteins, was also identified in a second, independent screen for regulators of DSB repair, in which nuclear bodies of the repair protein 53BP1 in U2OS cells were used as a readout for DSBs [55]. These results confirmed preceding studies from two different laboratories, which performed targeted knockdown of Mcm10 in HeLa and U2OS cells to demonstrate that the loss of Mcm10 compromises the ability to complete DNA replication, causes a dramatic increase of DSBs, and triggers checkpoint activation or apoptosis in large parts of the population [31, 56, 57]. Altogether, these data are compatible with the idea that Mcm10 controls multiple processes during DNA replication and actively contributes to the stability of the eukaryotic replication fork. In addition, the finding that Mcm10 indirectly interacts with Nbs1, a subunit of the MRN (Mre11, Rad50, Nbs1) complex, suggests that it may be involved in replication fork restart or DSB repair [32].
Based on its role in DNA replication and genome maintenance, including largely unexplored interactions with silencing [58–60] and centromere binding proteins [61], one might expect Mcm10 to contribute to diseases with aberrant proliferation, such as cancer. Indeed, several cancer-associated mutations have been identified in Mcm10 (www.sanger.ac.uk; Figure 3). However, the biological significance of these mutations is unknown. Elevated transcript levels of Mcm10 are frequently associated with certain types of cancers (www.oncomine.org) [62], raising the possibility that Mcm10 is the target of specific oncogenes. In fact, Mcm10 is regulated by the N-MYC oncogene in neuroblastoma [63] and the Ewing’s sarcoma (EWS)-derived oncogenes in Ewing’s tumors [64]. Lastly, Mcm10 interacts directly with RecQL4, which is mutated in individuals afflicted with Rothmund Thomson syndrome (RTS) [25]. This pre-mature aging syndrome predisposes patients to cancer, most frequently osteosarcoma. It is possible that Mcm10 plays a role in RTS.
Box 2: Understanding the function of Mcm10 through genetic interactions.
Studying the genetic interactions of MCM10 not only identifies the general cellular networks Mcm10 participates in, but also helps to elucidate what role it plays at the replication fork. Highlights of mcm10 genetic interactions reported in literature and from a recent synthetic genetic array screen (for a complete list, refer to Data Repository of Yeast Genetic Interactions: http://drygin.ccbr.utoronto.ca/ [65]) are summarized in Figure I. mcm10-1 mutants are synthetically lethal with mutations in genes encoding various replication factors such as Cdc45, Orc2, Orc5, Dpb11, Dna2, and genes encoding subunits of Pol-ε and Pol-δ [66–69]. In addition, mcm10 displays synthetic lethality with mcm2, 4, 5, 6 and 7 [67–69] and strong interactions with mutants defective in repair and checkpoint activation (mms22, mms1, mre11, rad50, sgs1, exo1, srs2, mec1, rad53) [35, 66, 68]. This suggests that the loss of Mcm10 induces replication stress during DNA synthesis, leading to aberrant DNA structures and S-phase checkpoint activation [35, 41]. These data imply that Mcm10 indeed plays a role in maintaining the integrity of the replication fork.
The strong negative interaction with slx5/8, mutants of a SUMO-targeted ubiquitin ligase complex, supports the notion that Mcm10 is a guardian of genome integrity during replication and confirms siRNA screen results in human cells (Text Box 1) [54, 55]. Slx5/8 has been implicated in a novel repair mechanism of persistent DSBs at the nuclear periphery [70]. Intriguingly, mutation of genes encoding components of the Rpd3 complex, sin3 and sds3, improves the viability of mcm10 mutants. Rpd3 has a role in suppressing late origin firing through activation of the S-phase checkpoint [71, 72]. It is possible that the firing of additional origins helps mcm10 mutants to complete S phase and thus improves growth. Other noteworthy strong mcm10-1 genetic interactors are ctf18, ctf8 and dcc1, all three of which encode subunits of the replication factor C-like alternative clamp loader complex, which is required for sister chromatid cohesion [73]. Moreover, the Rfc-like complex has been implicated in S phase checkpoint regulation [74], post-replicative repair pathway [75], and loading of PCNA [76]. A more careful analysis of the genetic interaction network of MCM10 will hopefully provide further insight into how Mcm10 protects genome integrity during replication.
Acknowledgements
The authors wish to acknowledge funding from the NIH (GM074917) and the Leukemia & Lymphoma Society to A-K Bielinsky and thank Dr. Eric Hendrickson for proofreading of the manuscript.
Glossary
- γH2AX
phosphorylated H2AX on Ser139; forms foci in response to DSBs and mediates DNA repair through interaction with various repair proteins.
- 53BP1
p53 binding protein 1; forms foci in response to DSBs; relays ATM-mediated checkpoint signaling.
- ATM
ataxia telangiectasia mutated; a checkpoint kinase crucial for DSB repair; human homolog of yeast Tel1.
- ATR
ATM and Rad3-related kinase; plays a role in checkpoint signaling in response to replication fork stalling; human homolog of yeast Mec1.
- Cdc45
cell division cycle 45; recruited to the origin during initiation; part of the CMG complex.
- Cdc6
cell division cycle 6; subunit of the pre-RC.
- CDK
cyclin dependent kinase; S-CDK is essential for S-phase initiation; phosphorylates Sld2 and Sld3, which help to recruit GINS and Cdc45 to the origin.
- Cdt1
chromatin licensing and DNA replication factor 1; subunit of the pre-RC.
- Chk1
checkpoint kinase 1; downstream target of ATR; plays a role in signaling in response to replication stress.
- Chk2
checkpoint kinase 2; downstream target of ATM; plays a role in checkpoint signaling in response to DSBs.
- CMG
Cdc45-Mcm2-7-GINS complex; required for initiation and elongation.
- Ctf4/And-1
chromosome transmission fidelity 4; required for sister chromatid cohesion; interacts with pol-α; proposed role in pol-α loading onto chromatin together with Mcm10.
- DAX1
Dosage-sensitive sex reversal adrenal hypoplasia congenita critical region on the X chromosome, gene 1; a nuclear receptor which contains a DNA-binding domain; a direct target of the EWS/FLI1 oncogene.
- DDK
Dbf4 dependent kinase; a kinase essential for S-phase initiation; facilitates Mcm2-7 helicase activation by phosphorylation.
- Dna2
DNA synthesis defective 2; a helicase/endonuclease involved in Okazaki fragment maturation and DNA repair.
- Dpb11
DNA polymerase B II 11; recruited to the origin during initiation; involved in checkpoint signaling.
- E2F1
E2F transcription factor 1; a member of E2F transcription factor family; regulates transcription of various replication proteins.
- ETS
E-twenty six; a family of transcription factors.
- EWS/FLI1
a fusion oncoprotein between EWS (Ewing sarcoma 1) and FLI1 (friend leukemia integration 1); a high percentage of Ewing's sarcoma family of tumors exhibit this fusion protein.
- Exo1
exonuclease 1; 5’ to 3’ exonuclease and flap endonuclease important for recombination and DSB repair.
- GINS
go-ichi-ni-san; a heterotetramer composed of Psf1-3 and Sld5; recruited to the origin during initiation; part of the CMG complex.
- Hsp10-like domain
a domain present in Hsp10 which mediates Hsp60 binding; Hsp10 and Hsp60 function in protein folding; mediates Mcm10 interaction with Pol α.
- Mcm2-7 helicase
a heterohexamer complex composed of Mcm2-7; replication helicase for unwinding DNA.
- Mms1
methyl methanesulfonate sensitivity 1; a subunit of an E3 ubiquitin ligase complex; important for preventing fork collapse and recovery during replication stress; function together with Mms22.
- Mms22
methyl methanesulfonate sensitivity 22; a subunit of an E3 ubiquitin ligase complex; important for preventing fork collapse and recovery during replication stress; function together with Mms1.
- MRN complex
heterotrimeric complex composed of Mre11 (meiotic recombination 11)-radiation sensitive 50 (Rad50) and Nbs1 (Nijimegen breakage syndrome 1); promotes ATM-mediated checkpoint activation and resection at DSBs.
- Nbs1
Nijimegen breakage syndrome 1; a subunit of MRN complex.
- Not4
ubiquitin-protein ligase; regulates stability of the pol-α catalytic subunit.
- OB-fold
oligosaccharide/oligonucleotide binding fold; a β-barrel protein structure which mediates DNA binding.
- Orc
Origin recognition complex; a complex which recognizes the origin of replication; facilitates loading of Mcm2-7 helicase together with Cdc6 and Cdt1.
- PCNA
proliferating cell nuclear antigen; a DNA sliding clamp and processivity factor required for elongation.
- PIP box
PCNA-interacting protein box; a motif, which mediates PCNA-interaction of various proteins.
- Pol-α
part of the DNA polymerase-α/primase complex, which required for de novo DNA synthesis.
- Pol-δ
lagging-strand DNA polymerase.
- Pol-ε
leading-strand DNA polymerase.
- pRb
retinoblastoma protein; a tumor suppressor; regulates cell cycle; binds E2F1 transcription factor and inhibits its function.
- Pre-RC
pre-replication complex; composed of Mcm2-7 helicase, Orc, Cdc6 and Cdt1.
- Rad53
radiation sensitive 53; downstream kinase of Mec1 crucial for checkpoint activation.
- RecQL4
RecQ helicase; unwinds dsDNA in a 3’ to 5’ direction; involved in DNA repair and genome maintenance; human ortholog of yeast Sgs1.
- RFC-like alternative clamp loader complex
a clamp loader complex composed of Ctf18, Ctf8 and Dcc1 (defective in sister chromatid cohesion 1); required for sister chromatid cohesion; loads and unloads PCNA; involved in DNA replication checkpoint.
- RPA
replication protein A; heterotrimeric protein complex which binds to ssDNA during replication.
- Rpd3
reduced potassium dependency 3; a histone deacetylase; regulates transcription and chromatin remodeling; suppresses late-origin firing.
- Sds3
suppressor of defective silencing 3; part of Sin3-Rpd3 histone deacetylase complex; regulates transcription and chromatin remodeling; suppresses late-origin firing.
- Sgs1
slow growth suppressor 1; RecQ helicase; involved in DNA repair and genome maintenance.
- Sin3
switch independent 3; part of Sin3-Rpd3 histone deacetylase complex; regulates transcription and chromatin remodeling; suppresses late-origin firing.
- Sld2
synthetically lethal with dpb11-1; loaded onto the origin during initiation; binds to the C-terminus of Dpb11.
- Sld3
synthetically lethal with dpb11-1; loaded onto the origin during initiation; binds to the N-terminus of Dpb11.
- Slx5/8
synthetic lethal with sgs1 of unknown function X; SUMO-targeted ubiquitin ligase heterodimer; important for genome maintenance and repair.
- Srs2
suppressor of rad six; DNA helicase involved in homologous recombination.
- Ubc4
Ubiquitin conjugating; ubiquitin-conjugating enzyme E2; regulates stability of pol-α catalytic subunit.
- Zn-finger
zinc finger: a protein structure with Zn-coordinating residues which mediate DNA binding.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bell SP, Dutta A. DNA replication in eukaryotic cells. Annu Rev Biochem. 2002;71:333–374. doi: 10.1146/annurev.biochem.71.110601.135425. [DOI] [PubMed] [Google Scholar]
- 2.Masai H, et al. Eukaryotic chromosome DNA replication: where, when, and how? Annu Rev Biochem. 2011;79:89–130. doi: 10.1146/annurev.biochem.052308.103205. [DOI] [PubMed] [Google Scholar]
- 3.Moyer SE, et al. Isolation of the Cdc45/Mcm2-7/GINS (CMG) complex, a candidate for the eukaryotic DNA replication fork helicase. Proc Natl Acad Sci U S A. 2006;103:10236–10241. doi: 10.1073/pnas.0602400103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wohlschlegel JA, et al. Xenopus Mcm10 binds to origins of DNA replication after Mcm2-7 and stimulates origin binding of Cdc45. Mol Cell. 2002;9:233–240. doi: 10.1016/s1097-2765(02)00456-2. [DOI] [PubMed] [Google Scholar]
- 5.Kanke M, et al. Mcm10 plays an essential role in origin DNA unwinding after loading of the CMG components. EMBO J. 2012;31:2182–2194. doi: 10.1038/emboj.2012.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Deursen F, et al. Mcm10 associates with the loaded DNA helicase at replication origins and defines a novel step in its activation. EMBO J. 2012;31:2195–2206. doi: 10.1038/emboj.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watase G, et al. Mcm10 plays a role in functioning of the eukaryotic replicative DNA helicase, Cdc45-Mcm-GINS. Curr Biol. 2012;22:343–349. doi: 10.1016/j.cub.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 8.Heller RC, et al. Eukaryotic origin-dependent DNA replication in vitro reveals sequential action of DDK and S-CDK kinases. Cell. 2011;146:80–91. doi: 10.1016/j.cell.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solomon NA, et al. Genetic and molecular analysis of DNA43 and DNA52: two new cell-cycle genes in Saccharomyces cerevisiae. Yeast. 1992;8:273–289. doi: 10.1002/yea.320080405. [DOI] [PubMed] [Google Scholar]
- 10.Merchant AM, et al. A lesion in the DNA replication initiation factor Mcm10 induces pausing of elongation forks through chromosomal replication origins in Saccharomyces cerevisiae. Mol Cell Biol. 1997;17:3261–3271. doi: 10.1128/mcb.17.6.3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ricke RM, Bielinsky AK. Mcm10 regulates the stability and chromatin association of DNA polymerase-alpha. Mol Cell. 2004;16:173–185. doi: 10.1016/j.molcel.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 12.Gregan J, et al. Fission yeast Cdc23/Mcm10 functions after pre-replicative complex formation to promote Cdc45 chromatin binding. Mol Biol Cell. 2003;14:3876–3887. doi: 10.1091/mbc.E03-02-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sawyer SL, et al. Mcm10 and Cdc45 cooperate in origin activation in Saccharomyces cerevisiae. J Mol Biol. 2004;340:195–202. doi: 10.1016/j.jmb.2004.04.066. [DOI] [PubMed] [Google Scholar]
- 14.Yang X, et al. Nuclear distribution and chromatin association of DNA polymerase alpha-primase is affected by TEV protease cleavage of Cdc23 (Mcm10) in fission yeast. BMC Mol Biol. 2005;6:13. doi: 10.1186/1471-2199-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fien K, et al. Primer utilization by DNA polymerase alpha-primase is influenced by its interaction with Mcm10p. J Biol Chem. 2004;279:16144–16153. doi: 10.1074/jbc.M400142200. [DOI] [PubMed] [Google Scholar]
- 16.Robertson PD, et al. Domain architecture and biochemical characterization of vertebrate Mcm10. J Biol Chem. 2008;283:3338–3348. doi: 10.1074/jbc.M706267200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warren EM, et al. Structural basis for DNA binding by replication initiator Mcm10. Structure. 2008;16:1892–1901. doi: 10.1016/j.str.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eisenberg S, et al. Novel DNA binding properties of the Mcm10 protein from Saccharomyces cerevisiae. J Biol Chem. 2009;284:25412–25420. doi: 10.1074/jbc.M109.033175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warren EM, et al. Physical interactions between Mcm10 DNA DNA polymerase alpha. J Biol Chem. 2009;284:24662–24672. doi: 10.1074/jbc.M109.020438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robertson PD, et al. Solution NMR structure of the C-terminal DNA binding domain of Mcm10 reveals a conserved MCM motif. J Biol Chem. 2010;285:22942–22949. doi: 10.1074/jbc.M110.131276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Remus D, et al. Concerted loading of Mcm2-7 double hexamers around DNA during DNA replication origin licensing. Cell. 2009;139:719–730. doi: 10.1016/j.cell.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gambus A, et al. GINS maintains association of Cdc45 with MCM in replisome progression complexes at eukaryotic DNA replication forks. Nat Cell Biol. 2006;8:358–366. doi: 10.1038/ncb1382. [DOI] [PubMed] [Google Scholar]
- 23.Fu YV, et al. Selective bypass of a lagging strand roadblock by the eukaryotic replicative DNA helicase. Cell. 2011;146:931–941. doi: 10.1016/j.cell.2011.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du W, et al. Structural biology of replication initiation factor Mcm10. Subcell Biochem. 2012;62:197–216. doi: 10.1007/978-94-007-4572-8_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu X, et al. MCM10 mediates RECQ4 association with MCM2-7 helicase complex during DNA replication. EMBO J. 2009;28:3005–3014. doi: 10.1038/emboj.2009.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sangrithi MN, et al. Initiation of DNA replication requires the RECQL4 protein mutated in Rothmund-Thomson syndrome. Cell. 2005;121:887–898. doi: 10.1016/j.cell.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 27.Matsuno K, et al. The N-terminal noncatalytic region of Xenopus RecQ4 is required for chromatin binding of DNA polymerase alpha in the initiation of DNA replication. Mol Cell Biol. 2006;26:4843–4852. doi: 10.1128/MCB.02267-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohlenschlager O, et al. The N-terminus of the human RecQL4 helicase is a homeodomain-like DNA interaction motif. Nucleic Acids Res. 2012;40:8309–8324. doi: 10.1093/nar/gks591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu W, et al. Mcm10 and And-1/CTF4 recruit DNA polymerase alpha to chromatin for initiation of DNA replication. Genes Dev. 2007;21:2288–2299. doi: 10.1101/gad.1585607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ricke RM, Bielinsky AK. A conserved Hsp10-like domain in Mcm10 is required to stabilize the catalytic subunit of DNA polymerase-alpha in budding yeast. J Biol Chem. 2006;281:18414–18425. doi: 10.1074/jbc.M513551200. [DOI] [PubMed] [Google Scholar]
- 31.Chattopadhyay S, Bielinsky AK. Human Mcm10 regulates the catalytic subunit of DNA polymerase-alpha and prevents DNA damage during replication. Mol Biol Cell. 2007;18:4085–4095. doi: 10.1091/mbc.E06-12-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wawrousek KE, et al. Xenopus DNA2 is a helicase/nuclease that is found in complexes with replication proteins And-1/Ctf4 and Mcm10 and DSB response proteins Nbs1 and ATM. Cell Cycle. 2010;9:1156–1166. doi: 10.4161/cc.9.6.11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Apger J, et al. Multiple functions for Drosophila Mcm10 suggested through analysis of two Mcm10 mutant alleles. Genetics. 2010;185:1151–1165. doi: 10.1534/genetics.110.117234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gosnell JA, Christensen TW. Drosophila Ctf4 is essential for efficient DNA replication and normal cell cycle progression. BMC Mol Biol. 2011;12:13. doi: 10.1186/1471-2199-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee C, et al. Alternative mechanisms for coordinating polymerase alpha and MCM helicase. Mol Cell Biol. 2010;30:423–435. doi: 10.1128/MCB.01240-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang J, et al. Ctf4p facilitates Mcm10p to promote DNA replication in budding yeast. Biochem Biophys Res Commun. 2010;395:336–341. doi: 10.1016/j.bbrc.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Haworth J, et al. Ubc4 and Not4 regulate steady-state levels of DNA polymerase-alpha to promote efficient and accurate DNA replication. Mol Biol Cell. 2010;21:3205–3219. doi: 10.1091/mbc.E09-06-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raveendranathan M, et al. Genome-wide replication profiles of S-phase checkpoint mutants reveal fragile sites in yeast. EMBO J. 2006;25:3627–3639. doi: 10.1038/sj.emboj.7601251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor M, et al. Mcm10 interacts with Rad4/Cut5(TopBP1) and its association with origins of DNA replication is dependent on Rad4/Cut5(TopBP1) DNA Repair (Amst) 2011;10:1154–1163. doi: 10.1016/j.dnarep.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Pacek M, et al. Localization of MCM2-7, Cdc45, and GINS to the site of DNA unwinding during eukaryotic DNA replication. Mol Cell. 2006;21:581–587. doi: 10.1016/j.molcel.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 41.Liang DT, Forsburg SL. Characterization of Schizosaccharomyces pombe mcm7(+) and cdc23(+) (MCM10) and interactions with replication checkpoints. Genetics. 2001;159:471–486. doi: 10.1093/genetics/159.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cobb JA, et al. Replisome instability, fork collapse, and gross chromosomal rearrangements arise synergistically from Mec1 kinase and RecQ helicase mutations. Genes Dev. 2005;19:3055–3069. doi: 10.1101/gad.361805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Das-Bradoo S, et al. Interaction between PCNA and diubiquitinated Mcm10 is essential for cell growth in budding yeast. Mol Cell Biol. 2006;26:4806–4817. doi: 10.1128/MCB.02062-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Izumi M, et al. The human homolog of Saccharomyces cerevisiae Mcm10 interacts with replication factors and dissociates from nuclease-resistant nuclear structures in G(2) phase. Nucleic Acids Res. 2000;28:4769–4777. doi: 10.1093/nar/28.23.4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cook CR, et al. A novel zinc finger is required for Mcm10 homocomplex assembly. J Biol Chem. 2003;278:36051–36058. doi: 10.1074/jbc.M306049200. [DOI] [PubMed] [Google Scholar]
- 46.Okorokov AL, et al. Hexameric ring structure of human MCM10 DNA replication factor. EMBO Rep. 2007;8:925–930. doi: 10.1038/sj.embor.7401064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dalrymple BP, et al. A universal protein-protein interaction motif in the eubacterial DNA replication and repair systems. Proc Natl Acad Sci USA. 2001;98:11627–11632. doi: 10.1073/pnas.191384398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoshida K, Inoue I. Expression of MCM10 and TopBP1 is regulated by cell proliferation and UV irradiation via the E2F transcription factor. Oncogene. 2004;23:6250–6260. doi: 10.1038/sj.onc.1207829. [DOI] [PubMed] [Google Scholar]
- 49.Izumi M, et al. Cell cycle-dependent proteolysis and phosphorylation of human Mcm10. J Biol Chem. 2001;276:48526–48531. doi: 10.1074/jbc.M107190200. [DOI] [PubMed] [Google Scholar]
- 50.Sharma A, et al. Ultraviolet radiation stress triggers the downregulation of essential replication factor Mcm10. J Biol Chem. 2010;285:8352–8362. doi: 10.1074/jbc.M109.041129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaur M, et al. CRL4-DDB1-VPRBP ubiquitin ligase mediates the stress triggered proteolysis of Mcm10. Nucleic Acids Res. 2012 doi: 10.1093/nar/gks366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee JK, et al. The Cdc23 (Mcm10) protein is required for the phosphorylation of minichromosome maintenance complex by the Dfp1-Hsk1 kinase. Proc Natl Acad Sci U S A. 2003;100:2334–2339. doi: 10.1073/pnas.0237384100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matsuoka S, et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316:1160–1166. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- 54.Paulsen RD, et al. A genome-wide siRNA screen reveals diverse cellular processes and pathways that mediate genome stability. Mol Cell. 2009;35:228–239. doi: 10.1016/j.molcel.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lukas C, et al. 53BP1 nuclear bodies form around DNA lesions generated by mitotic transmission of chromosomes under replication stress. Nat Cell Biol. 2011;13:243–253. doi: 10.1038/ncb2201. [DOI] [PubMed] [Google Scholar]
- 56.Park JH, et al. Knockdown of human MCM10 exhibits delayed and incomplete chromosome replication. Biochem Biophys Res Commun. 2008;365:575–582. doi: 10.1016/j.bbrc.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 57.Park JH, et al. Knockdown of human MCM10 activates G2 checkpoint pathway. Biochem Biophys Res Commun. 2008;365:490–495. doi: 10.1016/j.bbrc.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 58.Douglas NL, et al. Dual roles for Mcm10 in DNA replication initiation and silencing at the mating-type loci. Mol Biol Rep. 2005;32:197–204. doi: 10.1007/s11033-005-2312-x. [DOI] [PubMed] [Google Scholar]
- 59.Liachko I, Tye BK. Mcm10 is required for the maintenance of transcriptional silencing in Saccharomyces cerevisiae. Genetics. 2005;171:503–515. doi: 10.1534/genetics.105.042333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liachko I, Tye BK. Mcm10 mediates the interaction between DNA replication and silencing machineries. Genetics. 2009;181:379–391. doi: 10.1534/genetics.108.099101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Locovei AM, et al. The CENP-B homolog, Abp1, interacts with the initiation protein Cdc23 (MCM10) and is required for efficient DNA replication in fission yeast. Cell Div. 2006;1:27. doi: 10.1186/1747-1028-1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu C, et al. Integrating gene expression and protein-protein interaction network to prioritize cancer-associated genes. BMC Bioinformatics. 2012;13:1471–2105. doi: 10.1186/1471-2105-13-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koppen A, et al. Direct regulation of the minichromosome maintenance complex by MYCN in neuroblastoma. Eur J Cancer. 2007;43:2413–2422. doi: 10.1016/j.ejca.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 64.Garcia-Aragoncillo E, et al. DAX1, a direct target of EWS/FLI1 oncoprotein, is a principal regulator of cell-cycle progression in Ewing's tumor cells. Oncogene. 2008;27:6034–6043. doi: 10.1038/onc.2008.203. [DOI] [PubMed] [Google Scholar]
- 65.Koh JL, et al. DRYGIN: a database of quantitative genetic interaction networks in yeast. Nucleic Acids Res. 2010;38:502–507. doi: 10.1093/nar/gkp820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kawasaki Y, et al. Interactions between Mcm10p and other replication factors are required for proper initiation and elongation of chromosomal DNA replication in Saccharomyces cerevisiae. Genes Cells. 2000;5:975–989. doi: 10.1046/j.1365-2443.2000.00387.x. [DOI] [PubMed] [Google Scholar]
- 67.Homesley L, et al. Mcm10 and the MCM2-7 complex interact to initiate DNA synthesis and to release replication factors from origins. Genes Dev. 2000;14:913–926. [PMC free article] [PubMed] [Google Scholar]
- 68.Araki Y, et al. Budding yeast mcm10/dna43 mutant requires a novel repair pathway for viability. Genes Cells. 2003;8:465–480. doi: 10.1046/j.1365-2443.2003.00648.x. [DOI] [PubMed] [Google Scholar]
- 69.Hart EA, et al. Fission yeast Cdc23 interactions with DNA replication initiation proteins. Curr Genet. 2002;41:342–348. doi: 10.1007/s00294-002-0316-9. [DOI] [PubMed] [Google Scholar]
- 70.Nagai S, et al. Functional targeting of DNA damage to a nuclear pore-associated SUMO-dependent ubiquitin ligase. Science. 2008;322:597–602. doi: 10.1126/science.1162790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aparicio JG, et al. The Rpd3-Sin3 histone deacetylase regulates replication timing and enables intra-S origin control in Saccharomyces cerevisiae. Mol Cell Biol. 2004;24:4769–4780. doi: 10.1128/MCB.24.11.4769-4780.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Knott SR, et al. Genome-wide replication profiles indicate an expansive role for Rpd3L in regulating replication initiation timing or efficiency, and reveal genomic loci of Rpd3 function in Saccharomyces cerevisiae. Genes Dev. 2009;23:1077–1090. doi: 10.1101/gad.1784309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mayer ML, et al. Identification of RFC(Ctf18p, Ctf8p, Dcc1p): an alternative RFC complex required for sister chromatid cohesion in S cerevisiae. Mol Cell. 2001;7:959–970. doi: 10.1016/s1097-2765(01)00254-4. [DOI] [PubMed] [Google Scholar]
- 74.Crabbe L, et al. Analysis of replication profiles reveals key role of RFC-Ctf18 in yeast replication stress response. Nat Struct Mol Biol. 2010;17:1391–1397. doi: 10.1038/nsmb.1932. [DOI] [PubMed] [Google Scholar]
- 75.Gellon L, et al. New functions of Ctf18-RFC in preserving genome stability outside its role in sister chromatid cohesion. PLoS Genet. 2011;7 doi: 10.1371/journal.pgen.1001298. e1001298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bermudez VP, et al. The alternative Ctf18-Dcc1-Ctf8-replication factor C complex required for sister chromatid cohesion loads proliferating cell nuclear antigen onto DNA. Proc Natl Acad Sci USA. 2003;100:10237–10242. doi: 10.1073/pnas.1434308100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ramachandran N, et al. Self-assembling protein microarrays. Science. 2004;305:86–90. doi: 10.1126/science.1097639. [DOI] [PubMed] [Google Scholar]