Abstract
B7x (B7-H4 or B7S1) is an inhibitory member of the B7 family of T cell costimulation. It is expressed in low levels in healthy peripheral tissues such as the lung epithelium, but over-expressed in a variety of human cancers with negative clinical associations including metastasis. However, the function of B7x in the cancer context, whether expressed on cancer cells or on surrounding “host” tissues, has not been elucidated in vivo. We utilized the 4T1 metastatic breast cancer model and B7x knockout (B7x−/−) mice to investigate the effect of host tissue-expressed B7x on cancer. We found that 4T1 cells were B7x negative in vitro and in vivo and B7x−/− mice had significantly fewer lung 4T1 tumor nodules than wildtype mice. Furthermore, B7x−/− mice showed significantly enhanced survival and a memory response to tumor rechallenge. Mechanistic studies revealed that the presence of B7x correlated with reduced general and tumor-specific T cell cytokine responses, as well as with an increased infiltration of immunosuppressive cells, including tumor associated neutrophils (TANs), macrophages, and regulatory T cells into tumor-bearing lungs. Importantly, the TANs strongly bound B7x protein and inhibited proliferation of both CD4 and CD8 T cells. These results suggest that host B7x may enable metastasizing cancer cells to escape local anti-tumor immune responses through interactions with the innate as well as the adaptive immune systems. Targeting the B7x pathway thus holds much promise for improving the efficacy of immunotherapy for metastatic cancer.
Keywords: B7x (B7-H4/B7S1), breast cancer, lung metastasis, neutrophils, host factor
Introduction
Metastasis is the most clinically challenging aspect of cancer. The majority of cancer-related deaths are due not to primary tumors, which are amenable to treatment, but to metastases, which are difficult to detect early and even harder to eradicate. Metastasis is an inefficient process consisting of a series of rate-limiting steps (1). Tumor cells must break away from the primary tumor, invade blood vessels, survive in the circulation, leave the bloodstream and enter tissues, and start growing into metastatic colonies. All of these steps must occur for metastasis to happen. There is currently an intense effort to understand the pathways that promote cancer metastasis so as to identify therapeutic targets and develop strategies against metastasis.
Among the many factors that are necessary for the establishment of metastases is an immunosuppressive secondary site. Once they have successfully left the primary tumor and entered and survived the circulation, metastasizing cells arrest in tissue and attempt to proliferate. These cells often develop into micrometastases which can further grow into independent tumors, but this depends largely on interactions between these cells and their new environment (1). The immune system plays an important role in these processes. In addition to infiltration by effector immune cells attempting to mount anti-tumor responses, growing metastases are inundated by immunosuppressive cells and molecules that enable tumor cells to escape destruction by the immune system (2–4). To design immunotherapies that are effective in metastatic cancers, it is necessary to understand the pathways involved in promoting tumor immunosuppression (5), particularly in the metastatic site.
B7x (B7-H4 or B7S1) is a recently-discovered protein that is overexpressed in numerous human cancers and, in many cases, correlates with negative clinical outcome (6–21). B7x expression in renal cell carcinoma and tumor vasculature is associated with cancer progression and survival (6, 14). Prostate cancer patients with strong expression of B7x by tumor cells is significantly more likely to have disease spread and are at increased risk of clinical cancer recurrence and cancer-specific death (15). In human gastric cancer, tumor expression of B7x predicts poor survival (17). Similar findings have also been reported in studies of ovarian cancer and lung cancer (10, 21). Among the negative clinical associations of B7x is increased metastasis, itself a strong predictor of mortality, as was found in lung, kidney, prostate, and gastrointestinal cancers (10, 14, 15, 17, 18, 20).
While there is a growing literature on B7x expression and associations in cancer, little is currently known regarding the particular functions of B7x in the cancer context. An inhibitory member of the B7 family of T cell costimulation, B7x binds activated T cells and inhibits proliferation and cytokine production in vitro (22–24). In contrast to classical B7 family members B7-1 and B7-2, which are expressed on antigen presenting cells (APCs) in lymphoid organs, B7x is expressed on non-immune cells in peripheral tissues (12, 25–27). We initially observe relatively high B7x mRNA expression in murine lung (22) and recently find that this extends to protein expression. Specifically, we find that normal mouse lung tissue shows expression of B7x protein on epithelial cells, but not on immune cells (25, 27). The localization of B7x and its ability to inhibit activated T cells suggest a role in modulating T cell responses on site in the periphery. Furthermore, there is some clinical evidence linking B7x expression in cancer to reduced T cell infiltration into tumors (7, 10, 19, 21, 28). These data suggest a potential pro-tumor function for B7x through the reduction of anti-tumor immune responses. B7x overexpression in cancer may be a mechanism of tumor immune evasion that results in increased survival and metastasis of cancer cells. However, this has yet to be proven in an in vivo cancer model.
In addition to B7x overexpressed in tumor cells, B7x is expressed in low levels in some normal peripheral tissues. We wondered whether the presence of B7x in particular organs, independent of that expressed on tumor cells themselves, played a role in supporting metastatic growth by biasing the immunological milieu of a metastatic site towards immunosuppression. We therefore investigated the role of B7x in a cancer model focusing specifically on B7x expressed in non-tumor, “host” tissue cells. As B7x is expressed in the lung, we used a B7x knockout (B7x−/−) mouse and 4T1, a B7x-negative lung metastasis model, and found that B7x−/− mice had reduced lung metastasis, more potent effector immune responses, and fewer regulatory T cells (Tregs), macrophages, and tumor associated neutrophils (TANs) as compared to wildtype (wt) mice. Surprisingly, we found that B7x strongly bound TANs, which could suppress proliferation of both CD4 and CD8 T cells. This study suggests that host B7x may be a factor in organs such as lung that promotes growth of metastases through immunosuppressive functions.
Material and Methods
Animals
B7x−/− mice were previously described (26) and backcrossed to BALB/c for over 10 generations. Age- and sex-matched BALB/c mice were purchased from National Cancer Institute (Fredrick, MD) and the Jackson Laboratory (Bar Harbor, ME). Mice were maintained under specific pathogen-free conditions at the Albert Einstein College of Medicine and experiments were performed according to Institutional Animal Care and Use Committee guidelines.
Cell Lines and stimulation
The 4T1 cell line was derived from a spontaneous mammary carcinoma in a BALB/c mouse (29) and cultured in DMEM containing 10% FBS and 0.4 HSP U/ml insulin. 105 4T1 cells were injected for experimental metastasis and mechanism experiments. For survival studies, 105 4T1 cells were injected initially and 2 × 105 4T1 cells were injected for re-challenge. Thy1.1/MSCV vector was used to generate retrovirus and then transfected into 4T1 cells, and Thy1.1 positive 4T1 cell transfectants, Thy1.1/4T1, were sorted out by FACS using an anti-Thy1.1 mAb. 105 Thy1.1/4T1 cells were injected into mice. Human cell lines (HL60, AP-1060, Jurkat, Raji, and HeLa) were cultured in complete RPMI, with the exception of AP-1060 cells, which were cultured in complete RPMI with 5% A5637 cell conditioned media(30). For in vitro stimulation, 4T1 cells were incubated with IFN-γ (10 and 100 ng/ml) for 24–72h. The expression of B7x and PD-L1 were then measured with FACS.
RT-PCR
RNA was isolated from tissue using the Trizol system (Sigma) and converted to cDNA using the Im-Prom-II system (Promega). Primers for B7x cDNA or beta-actin were used in PCR to detect B7x expression.
Experimental lung metastasis quantification
Mice were sacrificed and injected intratracheally with 15% India ink (Sigma). Lungs were excised and fixed/destained overnight in Fekete’s solution (31) and surface nodules were enumerated under a dissecting microscope.
Cell isolation and flow cytometry
Following mouse anesthetization and perfusion with PBS, lungs and spleens were digested using GentleMACS (Miltenyi Biotec) or frosted glass. After RBC lysis, filtered cell suspensions were resuspended in FACS buffer (0.5% BSA in PBS) or for co-culture experiments, in sterilized MACS buffer (0.5% BSA, 2 mM EDTA in PBS). In co-culture experiments, T cells were purified from lung using Thy1.2 MACS beads (Miltenyi Biotec) in sterile MACS buffer. Cells were incubated with anti-mouse CD16/32 (eBioscience) to block Fc receptors and then stained with the following biotin- or fluorophore-conjugated anti-mouse monoclonal antibodies: From eBioscience, anti-B220, B7-H4 (clone 9), PD-L1, CD11b, CD11c, CD4, CD45, CD49b (clone Dx5), CD8α, F4/80, FoxP3 (clone FJK-16s), GR1 (clone RB6-8C5), IFN-γ, Streptavidin, TNF-α; From BD Biosciences, anti-CD4, CD11b, Ly6G (clone 1A8), and Ly6C; From Abd Serotec, F4/80-alexa fluor 647. For intracellular staining, cells were permeablized using the FoxP3 staining buffer set (eBioscience). Samples were acquired on a FACSCalibur, LSRII or LSRII yellow (BD Biosciences), and analyzed with FlowJo (Treestar). Cells were gated on live cells using forward scatter and Live/dead marker (Molecular Probes), and then on CD45-positive cells, followed by additional lineage markers.
Intracellular cytokine staining
Cells were stimulated in 37°C for around 6 h with 0.5 or 1 ug/ml PMA (Enxo Life Science International) and 1.87 ug/ml ionomycin (Sigma) in the presence of Golgistop (BD Biosciences) and monensin (Sigma). Cells were washed, Fc blocked, and stained with CD4, CD8, and Live/dead marker, then permeablized and stained with anti-IFN-γ, TNF-α, or isotype controls in permeablization buffer for 30 min.
Tumor-specific cytokine stimulation
Bone marrow cells were isolated from the femur and tibia of naïve mice and cultured in complete RPMI containing GM-CSF and IL-4 (20 ng/ml each) (BD Biosciences), replaced every other day for 6 d, to generate dendritic cells (DCs). Cells were incubated for an additional day with 200 ng/ml LPS and 4T1 lysate, generated through 3 freeze/thaw cycles. Loaded DCs were incubated for 18 h with cell suspensions from lungs of day 18 4T1 intravenous (i.v.) injected mice. Golgiplug (BD Biosciences) was added during the last 4 hours, and then cells were stained extracellularly and for cytokine production as above.
T cells and neutrophils co-culture assays
T cells were purified from naïve spleen using Thy1.2 MACS beads (Miltenyi Biotec) and labeled with CFSE (Invitrogen) at a final concentration of 2.5 μM. Neutrophils were isolated from bone marrow or lung by labeling single cell suspensions with FITC-conjugated Ly6G antibody (BD Biosciences) followed by positive selection using FITC MACS beads. Purity was generally > 90%. Neutrophils and T cells were co-cultured in complete RPMI in 96 well round bottom plates coated with 5 μg/ml anti-CD3 (145-2C11) (eBioscience). After 4 days, cells were labeled and acquired by FACS. Proliferation index was calculated by FlowJo (32).
Cytospin
Ly6Ghi cells were sorted using a FACSAria (BD Biosciences), resuspended in PBS with 10% FBS, centrifuged in a Cytospin 2 (Shandon), stained with harris hematoxylin (Fisher), and photographed by Coolscope Digital camera (Nikon).
Histology
Lungs were fixed in zinc fixative (BD Pharmingen) and embedded in paraffin. Five μm sections were stained with harris hematoxylin and eosin (Polyscientific) and photographed by CoolScope.
Ig fusion proteins
B7x-Ig and control Hu-IgG were generated as previously described (22). MACS-purified neutrophils or total cell suspensions were incubated with 1 μg/100ul Ig protein for 45 min on ice. Cells were washed, incubated with fluorophore-conjugated anti-human IgG Fcγ (Jackson Immunoresearch) for 30 min, and acquired by FACS.
Statistics
Statistical analysis was performed with Prism software (Graphpad) using the unpaired Student t-test or the log-rank test (survival studies), with results typically displayed as mean ± SEM. P values of < 0.05 were considered statistically significant.
Results
B7x deficiency protects mice from experimental lung metastasis
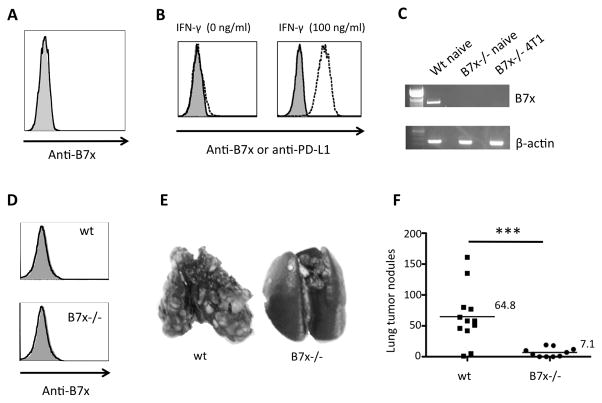
We recently show that B7x protein is detected on the bronchial epithelium of the lung (25), but not on any cells of hematopoietic origin or lymphoid tissues (25–27). Therefore, we utilized B7x−/− mice on a BALB/c background and a syngeneic pulmonary metastasis model, 4T1, to explore the effect of tissue-expressed B7x on cancer metastasis. Like naïve B7x−/− mice on 129 background (26), naïve BALB/c B7x−/− mice had no apparent phenotype and had normal numbers and distribution of immune cells. 4T1 is an aggressive, highly metastatic mammary carcinoma cell line that spontaneously metastasizes to lung (29). We first ascertained that 4T1 cells did not express B7x so as to preclude recognition of B7x as a foreign antigen in B7x−/− mice. 4T1 cells did not express B7x in vitro (Fig. 1A). This did not change upon stimulation with IFN- γ. Whereas IFN-γ stimulation was able to up-regulate PD-L1 expression on 4T1 cells, it had no effect on B7x expression (Fig. 1B). Furthermore, 4T1 tumors did not express B7x in vivo. RT-PCR of mRNA isolated from 4T1 metastatic lung of B7x−/− mice did not detect B7x expression (Fig. 1C). Finally, we i.v. injected Thy1.1/4T1 cells into mice and found that Thy1.1 positive cells (4T1) from metastatic lung of wt and B7x−/− mice were B7x negative (Fig.1D). 4T1-induced pulmonary metastasis in B7x−/− and wt mice was thus a suitable model for our study of the effects of B7x expressed in the tissue of the metastatic site.
Figure 1.
4T1 cells are B7x negative and B7x knockout mice have reduced lung metastasis of 4T1. (A) 4T1 cells were stained with anti-B7x (open line) or isotype control (shaded line). (B) 4T1 cells were stimulated with IFN-γ (100 ng/ml) for three days and then stained with anti-B7x (open line), anti-PD-L1 (dash line) or isotype control (shaded line). (C) RT-PCR of mRNA isolated from naïve or 4T1 metastatic lung. (D) Thy1.1 positive cells (4T1) from tail-vein Thy1.1/4T1 injected lungs of wt and B7x−/− mice were stained with anti-B7x (open line) or isotype control (shaded line). (E, F) Lung metastases resulting from tail-vein 4T1 injection; representative lungs after India ink injection (E) and lung tumor nodule counts (F) pooled from 2 independent experiments, n=10–12. ***P<0.001.
B7x−/− and wt mice were i.v. injected in the tail-vein with 4T1 in an experimental metastasis study, where tumor cells deposited in the circulation are transported to the heart and frequently arrest in the lungs. By day 17 or 18 post i.v.-injection, wt mice appeared sick and all mice were sacrificed. We evaluated the lungs of these mice for metastatic nodules and found that numerous tumor nodules were visible on the surface of the lungs of wt mice, whereas B7x−/− mice had significantly fewer lung nodules (Fig. 1E–F). The average number of lung tumor nodules in wt mice was approximately 9 times higher than that in B7x−/− mice (64.8 in the wt group vs. 7.1 in the B7x−/− group). As the only difference in these two groups of mice was tissue expression of B7x, such as in the lung, these results suggest that local B7x expression was sufficient to support and promote the growth of metastatic colonies arising from cancer cells reaching the lungs.
B7x knockout mice can survive 4T1 challenge and generate memory response
Survival studies were also conducted using the 4T1 tail-vein model. In agreement with the lung tumor nodule results, B7x−/− mice had a significantly lower death rate than wt mice. By day 33 post-injection, 100% of wt mice were dead, whereas only 26% of B7x−/− mice had died. At the end of ten weeks, one third of the B7x−/− mice were still alive and appeared healthy (Fig. 2A). We investigated whether the surviving B7x−/− had generated a memory response to 4T1. These B7x−/− mice were re-challenged on day 71 with double the number of 4T1 cells and all of them remained alive and appeared healthy. Upon sacrifice at day 140, the lungs were found free of visible surface nodules. Furthermore, H&E staining was performed on lung sections of these mice, and the lung tissue appeared metastasis-free (Fig. 2B). The 100% survival rate and tumor-free status in B7x−/− mice after 4T1 re-challenge confirmed a memory response against 4T1.
Figure 2.
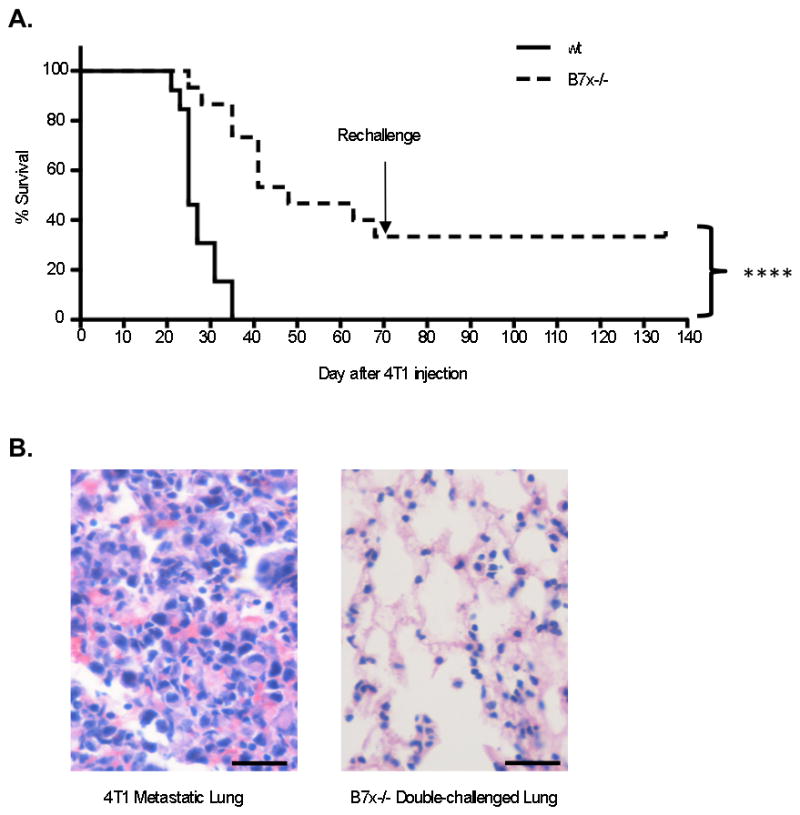
B7x knockout mice can survive 4T1 and 4T1 re-challenge. (A) Survival of mice injected with 4T1 cells in the tail-vein, n=13–15, representative of 2 separate experiments. At 71 d post-injection, remaining mice were re-challenged with double the number of 4T1 cells. (B) H&E staining of lung sections of the double-challenged mice at day 140 and other 4T1-injected mice. Left image: lung tissue bearing 4T1 metastasis. Right image: representative lung tissue from the double-challenged B7x−/− mice. ****P<0.0001. Scale bar = 25 μm, 40x.
B7x knockout mice generate higher T cell cytokine responses
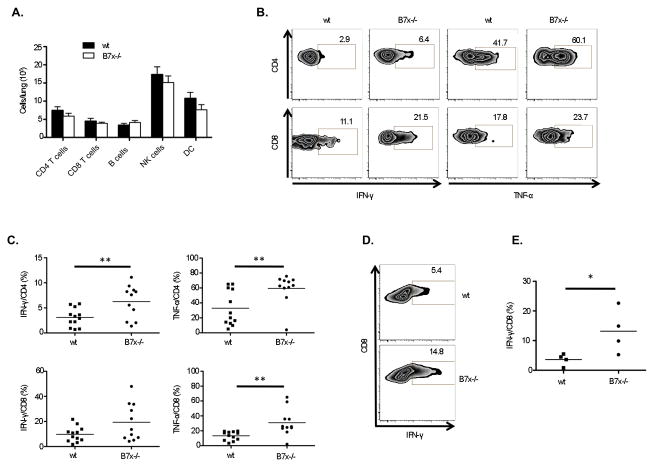
The 4T1 metastasis and survival results revealed that tissue B7x deficiency resulted in protection from malignancy with the ability to generate immune memory. To investigate the protective mechanism, we isolated the immune cell infiltrate from the lungs of i.v. 4T1-injected wt and B7x−/− mice and analyzed the cell populations by flow cytometry. Surprisingly, we did not find a significant difference in the total number of T cells, B cells, NK cells, or dendritic cells (DCs) (Fig. 3A). However, a functional difference between T cells in the lungs of B7x−/− and wt mice was observed in the enhanced ability of T cells derived from the lungs of B7x−/− mice to produce inflammatory cytokines IFN-γ and TNF-α in response to PMA/ionomycin stimulation (Fig. 3B–C). We further tested whether T cells from lungs of B7x−/− mice had an increased ability to generate a tumor-specific response. DCs were generated from wt bone marrow and loaded with 4T1 lysate, and then incubated with total immune cells isolated from lungs of 4T1-injected wt and B7x−/− mice. We found that the CD8 T cells from B7x−/− mice produced significantly more IFN-γ in response to 4T1 lysate-loaded DCs than those from wt mice (Fig. 3D–E). T cells from 4T1-injected B7x−/− mice thus appeared more functionally competent and tumor-responsive than those from 4T1-injected wt mice, suggesting that T cells from wt but not B7x−/− lungs may have been in a suppressed state.
Figure 3.
Analysis of lung immune cell infiltrate reveals higher cytokine responses to general and tumor-specific stimulation by T cells from knockout mice. (A) Numbers of immune effector cells infiltrating lungs at 17–18 d after 4T1 injection, pooled from 2–5 experiments, n=8–22. (B, C) PMA/ionomycin-stimulated T cells from lungs of 4T1-injected mice were analyzed for cytokine production. Percentages of IFN-γ+ or TNF-α+ cells are shown in representative FACS plots (B) and pooled from 3 independent experiments, each dot representing an individual mouse, n=11–12 (C). (D, E) CD8 T cell cytokine response after stimulation with 4T1 lysate-loaded DCs shown as representative FACS plots (D) and percentages of IFN-γ+ cells (E), n=4. *P<0.05; **P<0.01.
B7x knockout mice have a lower immunosuppressive cell infiltrate
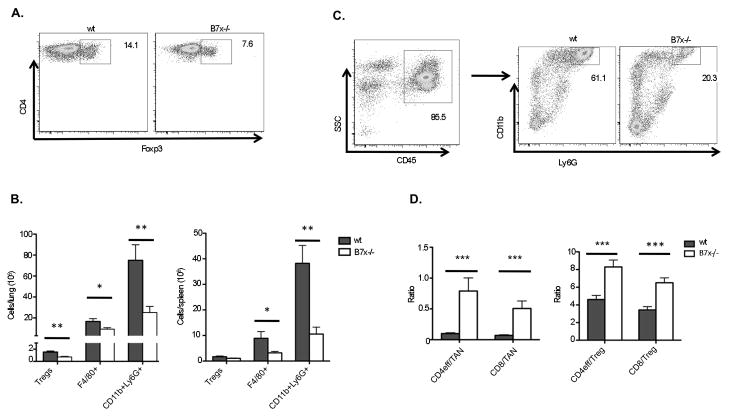
Suppression of T cells in the context of cancer often results from tumor recruitment of immunosuppressive cells and factors. We therefore investigated the immunosuppressive cell infiltrates in the lungs of wt and B7x−/− mice. We found that wt mice had a significantly higher percentage and total number of Foxp3+CD4+ Tregs in the lungs than B7x−/− mice (Fig. 4A–B). F4/80+ macrophages were also increased in number in lung as well as spleen of wt mice (Fig. 4B). Most striking, however, was the huge population of CD11b+Ly6G+ cells found infiltrating both lung and spleen of metastatic mice (Fig. 4B–C). It has been previously reported that implanted 4T1 tumors induce a leukemoid reaction resulting in myeloid cell over-production in bone marrow and high levels of granulocytic cells in peripheral blood and spleen (33). In the tail-vein 4T1 model, we too observed a large increase in percentage and total number of these myeloid cells in spleen as well as in lung of wt and B7x−/− mice. However, the percentage and total number were significantly reduced in B7x−/− mice. Tumor-associated cells double-positive for CD11b and Ly6G are often referred to as myeloid derived suppressor cells (MDSCs) (34). Both Tregs and MDSCs are known to suppress T cell responses. The reduction of both of these cell populations in B7x−/− mice afforded them a significantly higher ratio of effector CD4 and CD8 T cells to the Tregs and presumably suppressive MDSCs (Fig. 4D). These results suggest that the presence of B7x may have resulted in indirect inhibitory effects on T cells through suppressor cell populations.
Figure 4.
B7x knockout mice have fewer suppressive cells and a higher ratio of effector to suppressor cells. Cell suspensions from lungs and spleens of day 17–18 4T1-injected wt and B7x−/− mice were stained with lineage markers. (A) Representative FACS plots of permeablized CD4 cells stained for Foxp3. (B) Cell type numbers determined by flow cytometry, pooled from 4–5 independent experiments, n=16–22 (lungs, left) and 2–4 experiments, n=8–10 (spleens, right). (C) Representative FACS plots of CD11b+Ly6G+ cells among total CD45+ cells in wt and B7x−/− lungs. (D) The ratios of Foxp3-CD4+ (CD4eff) and CD8 T cells to CD11b+Ly6G+ (TAN) cells (left) and CD4eff and CD8 T cells to Foxp3+ CD4+ (Treg) cells (right) were calculated within each lung sample. Results are pooled from 4 independent experiments, n=16–18. *P<0.05; **P<0.01; ***P<0.001.
Metastatic lung-infiltrating CD11b+Ly6G+ cells are GR1hi tumor-associated neutrophils
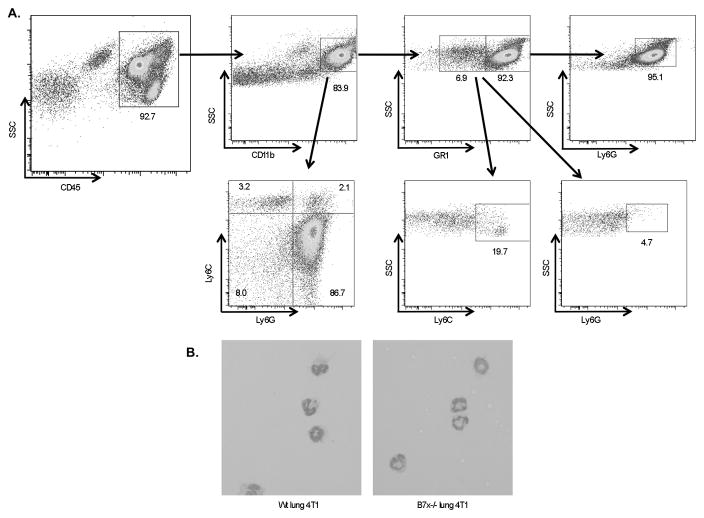
Because of the overwhelming CD11b+Ly6G+ MDSC infiltrate into the lung and spleen of 4T1-injected mice and significant reduction in B7x−/− mice, we took a closer look at the phenotype and function of these cells. MDSCs have been reported as a heterogeneous group of cells that can include monocytic and/or granulocytic cells at various stages of maturity. The Ly6G marker on MDSCs can help to identify the MDSC subtype, depending on the antibody clone. The Ly6G antibody clone RB6-8C5, also called GR1, recognizes the epitopes Ly6C on monocytic cells as well as a granulocytic epitope also called Ly6G (35). The Ly6G antibody clone 1A8, on the other hand, recognizes only the granulocyte-specific Ly6G epitope (36). We used the 1A8 clone in the experiments evaluating the percentage and number of CD11b+Ly6G+ cells infiltrating the lung and spleen as well as in all subsequent experiments. We found by flow cytometry that the cells expressing CD11b and Ly6G clone 1A8 were GR1hi and Ly6Clo/int and that the overwhelming majority of myeloid cells (CD11b+) and CD11b+GR1+ cells in this model were Ly6Ghi granulocytes (Fig. 5A).
Figure 5.
CD11b+Ly6G+ cells infiltrating metastatic wt and B7x−/− lungs are GR1hi morphologically mature neutrophils. (A) Metastatic lung cell suspensions were stained with lineage markers. Shown is a representative wt lung sample gated on CD45 and analyzed for CD11b, GR1, Ly6G, and Ly6C expression. (B) Ly6Ghi cells sorted from lungs of 4T1-injected wt and B7x−/− mice were stained with hematoxylin following Cytospin centrifugation. Representative images are shown, 40x.
To further identify these cells morphologically, we FACS-sorted Ly6Ghi cells from wt and B7x−/− 4T1-metastatic lungs, adhered them to slides using Cytospin, and stained with hematoxylin. We found that the sorted cells showed the characteristic morphology of neutrophils, with ring-shaped and/or segmented nuclei (Fig. 5B), confirming that the myeloid cell influx in this model was predominantly neutrophilic. Therefore, our results suggest that host B7x affected tumor-associated neutrophils in pulmonary metastasis.
Neutrophils isolated from tumor-bearing lungs suppress T cell proliferation
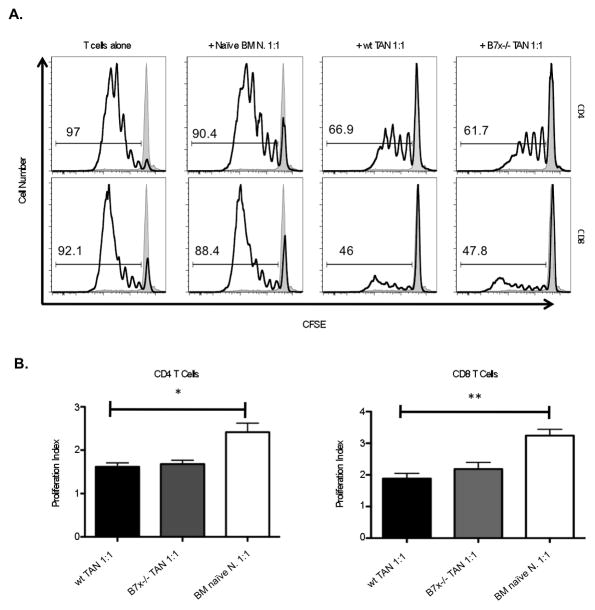
MDSCs have been reported to inhibit T cell responses in various models (37–39). We therefore tested whether the neutrophilic MDSCs, or TANs, in the 4T1-metastatic lungs were indeed capable of suppressing T cell responses. We designed an in vitro co-culture system, stimulating CFSE-labeled T cells from naïve wt spleen with plate-bound anti-CD3 in the presence of neutrophils purified from either naïve bone marrow (control) or metastatic lung (TANs) from wt or B7x−/− mice. We found that TANs from metastatic lungs, but not naïve bone marrow neutrophils, suppressed T cell proliferation as observed by CFSE dilution, when plated in a ratio of at least 1:1 neutrophils to T cells (Fig. 6A). After 4 days in culture with stimulation, CFSE dilution revealed that around 90% or more of T cells plated alone or with bone marrow naïve neutrophils were in divisions 1–7, whereas only 30–70% of T cells co-cultured with TANs were in division; in many cases the majority of T cells co-cultured with TANs had not divided at all. Correspondingly, the proliferation index of T cells cultured with lung TANs was significantly lower in both CD4 and CD8 T cells than those cultured with control neutrophils (Fig. 6B). Surprisingly, TANs from B7x−/− mice as well as wt mice could suppress T cell proliferation, suggesting that the TANs infiltrating both wt and B7x−/− mice were suppressive and differed in number (Fig. 4C) but not in function (Fig 6B).
Figure 6.
Neutrophils isolated from tumor-bearing lungs suppress T cell proliferation. 105 CFSE-labeled T cells from naïve spleen were stimulated with plate-bound anti-CD3 alone or in the presence of 105 neutrophils purified from naïve bone marrow or from 4T1-metastatic wt or B7x−/− lungs. After 4 d, cells were stained with CD4, CD8, and Live/dead marker, and acquired by flow cytometry. (A) Representative FACS plots show CFSE dilution among live CD4-gated and CD8-gated stimulated (open line) compared to unstimulated control (shaded line) T cells. (B) Proliferative index of CD4 and CD8 T cells co-cultured with control or wt tumor associated neutrophils was calculated by FlowJo. Results are pooled from 2–3 independent experiments, n=3–10. *P<0.05; **P<0.01.
B7x-Ig strongly binds tumor associated neutrophils
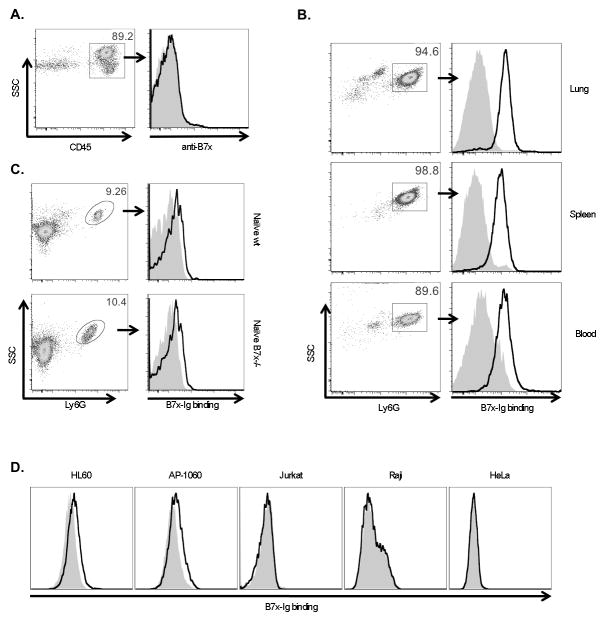
The ability of neutrophils from 4T1-metastatic lungs to suppress the proliferation of T cells suggested a mechanism by which T cell responses against growing metastases in the lung were kept limited. We next investigated the role of B7x expression in lung tissue in this process. We asked whether B7x was directly involved in regulating immune cell responses through expression on the surface of immune cells or through interactions with these cells. B7x protein expression has been reported inconsistently, with some reports showing B7x is induced on some immune cells (23, 24), whereas others reporting immune cells are B7x negative (26, 27, 40). We found that all CD45+ immune cells infiltrating 4T1-metastatic lungs of wt mice including APCs and neutrophils, were negative for B7x expression (Fig. 7A). We therefore turned our attention to immune cells that could bind B7x. As the B7x receptor is currently unknown, we looked for cells that might express the B7x receptor using an engineered fusion protein consisting of the extracellular domain of B7x and the Fc portion of human IgG1 (22), which could be detected with an anti-human IgG fluorophore-conjugated antibody. To our surprise, we found that this B7x-Ig fusion protein bound very strongly to neutrophils isolated from 4T1 metastatic lungs (Fig. 7B), suggesting that neutrophils express a receptor for B7x. The B7x-Ig could also slightly bind naïve neutrophils, from lungs of wt and B7x−/− mice (Fig. 7C) and two human cell lines of neutrophilic lineage HL60 and AP-60, but not of other human cell lines such as Jurkat, Raji, and HeLa cells (Fig. 7D). The strongest binding, however, was observed in neutrophils from the lungs, spleen, and blood of mice whose lungs were highly metastatic, suggesting that the neutrophils in these mice upregulate their putative B7x receptor in response to growing 4T1 metastases or may be a different type of neutrophil that have a receptor with a stronger affinity for B7x. These findings suggest an unexpected mechanism of immunosuppression in metastatic mice, whereby B7x on local lung tissue binds TANs, which, together with Tregs, suppress T cells. Perhaps together with inhibition of T cells by direct binding of lung B7x, TAN-induced suppression results in reduced antitumor immunity and increased metastasis.
Figure 7.
Tumor-associated neutrophils strongly bind B7x. (A) Single cell suspensions from lungs of wt 4T1-injected mice were stained with CD45 and anti-B7x (open line) or isotype control (shaded line). (B, C) Ly6G+ cells isolated from lung and spleen and RBC-depleted blood cells from 4T1-injected wt mice (B) and naïve wt and B7x−/− lung cell suspensions (C) were incubated with B7x-Ig (open line) or control Hu-IgG (shaded line) followed by staining with Ly6G and antibody recognizing the Ig portion of both fusion proteins (anti-HuIgG). (D) Cells were incubated with B7x-Ig (open line) or Hu-IgG (shaded line) and stained with anti-HuIgG. Histograms in A-C are representative of 2–5 separate experiments. Staining of HL-60 was repeated three times with similar results.
Discussion
This study reveals that host B7x promotes metastatic tumor growth in an in vivo cancer model. While many clinical studies have shown B7x overexpression in human cancers and a strong correlation to metastasis, cancer recurrence, and/or cancer related-death (6–17), it was unclear whether B7x was causative or merely correlative. In this study, B7x deficiency in mice resulted in significant protection from lung metastases and increased survival in the 4T1 tumor model.
Using 4T1 in an experimental metastasis model highlighted the effect of lung B7x specifically on the growth and establishment of metastasized cells. Tumor cells were directly deposited into the circulation, bypassing primary tumor and the early stages of metastasis. As lung is the first organ receiving blood supply from the circulation, tumor cells in the circulation preferentially extravasate there and, if the conditions are right, establish “metastases” (41). This model therefore did not allow for exploration of the effect of B7x on most of the steps of the metastatic cascade, but did directly evaluate the connection between presence of host B7x and growth of metastatic colonies from cells that have successfully metastasized to lung tissue. As B7x is present in the lungs of wt mice, but not in B7x−/− mice, the highly increased growth of lung metastases in wt mice supported the notion that B7x was a factor in the lungs promoting metastatic growth.
It has been previously hypothesized that B7x upregulated on cancer cells protects them from tumor-infiltrating CTLs by binding and inhibiting them, thus enabling tumor progression (22, 42, 43). We found that the T cells in B7x−/− lungs appeared to be more functional and tumor-responsive than those in wt mice, as they produced more inflammatory cytokines in response to general and tumor-specific stimulation. One explanation for this is that B7x expressed on lung tissue cells in the wt mice acted directly on T cells by binding them and inhibiting their function. Because T cells were less functional in wt mice, tumors grew and recruited more Tregs and TANs, which had further suppressive effects on T cells. This cycle was reduced in B7x−/− mice as their T cells remained functional and were more capable of controlling tumor growth.
A second explanation is that the TANs recruited into tumor-bearing lungs had a more dominant role in inhibiting T cell function and this was enhanced by the presence of B7x. An association between B7x expression and neutrophils has been reported in the context of infection. In one study, B7x−/− mice infected intraperitoneally with L. monocytogenes had an increased neutrophil response as compared to wt mice (44). On the other hand, B7x−/− mice intranasally infected with S. pneumonia had a reduced neutrophil response (25). While neither study describes the nature of link between B7x and neutrophils, a connection between B7x and neutrophil density is nonetheless highlighted. In the present study, we found that TANs were highly increased in the lungs of wt mice, corresponding to the extent of lung metastasis. We found that these TANs were capable of suppressing T cell proliferation in vitro, suggesting that they functioned to reduce anti-tumor immune responses in the lungs, thereby supporting metastatic growth. These results are consistent with other studies demonstrating various pro-tumor effects of TANs (45), including a recent study in which depletion of TANS with anti-Ly6G increased antitumor immunity in experimental lung metastasis model (46). Furthermore, an important, unexpected finding in this study was that TANs could bind B7x. To our knowledge, this is the first study revealing B7x binding to cells other than activated T cells. We found that B7x-Ig could slightly bind naïve neutrophils from lung, and strongly bind TANs from highly metastatic lungs. This suggested that TANs upregulated their putative B7x receptor in response to growing metastases. Furthermore, it suggested that B7x had an important effect on TANs, which in turn could suppress T cell responses against tumor. Therefore, it is possible that the binding of B7x expressed on lung or other tissue cells in wt mice to TANs led to enhanced TAN density and/or function and thereby resulted in increased metastatic growth. Future work is necessary to distinguish whether host tissue-expressed B7x primarily causes immunosuppression through direct inhibition of T cells or through effects on TANs resulting in indirect inhibition of T cells, or through a combination of both mechanisms.
We identified the CD11b+Ly6G+ cells infiltrating 4T1-metastatic lungs as TANs on the basis of their expression of the specific granulocytic Ly6G marker, neutrophil morphology, and infiltration into tumor. If and how TANs are distinct from granulocytic MDSCs is currently under investigation (47, 48). We, however, did not distinguish between these terms and consider our findings a contribution to MDSC research in general and the granulocytic MDSC and TAN fields in particular. While there are extensive reports showing suppression of T cells by monocytic MDSCs or MDSCs consisting of mixed monocytic and granulocytic subsets, there is less definitive evidence regarding the suppressive capabilities of granulocytic MDSCs or TANs (48). Furthermore, most published reports on MDSCs focus on MDSC suppression only of CD8 T cells or only under antigen-specific conditions (34, 37, 39, 49, 50). By contrast, in the tail-vein 4T1 model, we revealed TAN suppression of proliferation of both CD4 and CD8 T cells in response to general anti-CD3 stimulation. This unusual result may be due to the fact that we utilized CD11b+Ly6G+ cells from tumor-bearing lungs, as opposed to splenic MDSCs, which are used in many studies. Tumor MDSCs have been reported to be more suppressive than splenic MDSCs (51).
While the binding of B7x-Ig to TANs provided an important link between TAN accumulation and the presence of B7x, the functional relationship between B7x and TANs was difficult to decipher. Using in vitro systems, we failed to detect any effect of B7x-Ig on TAN survival or production of suppressive factors, such as ROS, arginase, or nitric oxide (data not shown). Furthermore, TANs from B7x−/− mice could also suppress T cells in vitro if the TANs were isolated from mice with metastatic lungs, which happened in a percentage of B7x−/− mice. Therefore, the most salient difference between TANs in wt and B7x−/− mice was their number. The reduction of both TANs and Tregs in B7x−/− mice provided them with a far more favorable effector:suppressor ratio than wt mice, which could explain the enhanced T cell responses to ex vivo stimulation as well as the reduced 4T1 metastasis and improved survival in B7x−/− mice. Further study is necessary to clarify the exact functional relationship between B7x expressed in non-tumor tissue, MDSCs, effector T cells, and Tregs as well as to identify the receptor for B7x both on T cells and myeloid cells.
Acknowledgments
This work was supported in part by DOD PC094137 and NIH DP2DK083076. Y.M.A., K.C.O., K.G., and K.A.H. were supported by NIH training grant T32GM007491, GM007288, T32DK007218, and T32DK007513, respectively. The Albert Einstein Cancer Center is supported by NIH P30CA013330.
We thank Fred Miller (Wayne State University School of Medicine) for providing 4T1 cells, Robert Gallagher (Montefiore Medical Center) for providing HL-60 and AP-1060 cell lines and protocols, and James Allison (Memorial Sloan-Kettering Cancer Center) for support at the start of this project. We thank the Albert Einstein College of Medicine Flow Cytometry Core for assistance.
References
- 1.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 2.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Souza AP, Bonorino C. Tumor immunosuppressive environment: effects on tumor-specific and nontumor antigen immune responses. Expert Rev Anticancer Ther. 2009;9:1317–1332. doi: 10.1586/era.09.88. [DOI] [PubMed] [Google Scholar]
- 4.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg SA. Overcoming obstacles to the effective immunotherapy of human cancer. Proc Natl Acad Sci U S A. 2008;105:12643–12644. doi: 10.1073/pnas.0806877105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krambeck AE, Thompson RH, Dong H, Lohse CM, Park ES, Kuntz SM, Leibovich BC, Blute ML, Cheville JC, Kwon ED. B7-H4 expression in renal cell carcinoma and tumor vasculature: associations with cancer progression and survival. Proc Natl Acad Sci U S A. 2006;103:10391–10396. doi: 10.1073/pnas.0600937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mugler KC, Singh M, Tringler B, Torkko KC, Liu W, Papkoff J, Shroyer KR. B7-h4 expression in a range of breast pathology: correlation with tumor T-cell infiltration. Appl Immunohistochem Mol Morphol. 2007;15:363–370. doi: 10.1097/01.pai.0000213159.79557.71. [DOI] [PubMed] [Google Scholar]
- 8.Salceda S, Tang T, Kmet M, Munteanu A, Ghosh M, Macina R, Liu W, Pilkington G, Papkoff J. The immunomodulatory protein B7-H4 is overexpressed in breast and ovarian cancers and promotes epithelial cell transformation. Exp Cell Res. 2005;306:128–141. doi: 10.1016/j.yexcr.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 9.Simon I, Zhuo S, Corral L, Diamandis EP, Sarno MJ, Wolfert RL, Kim NW. B7-h4 is a novel membrane-bound protein and a candidate serum and tissue biomarker for ovarian cancer. Cancer Res. 2006;66:1570–1575. doi: 10.1158/0008-5472.CAN-04-3550. [DOI] [PubMed] [Google Scholar]
- 10.Sun Y, Wang Y, Zhao J, Gu M, Giscombe R, Lefvert AK, Wang X. B7-H3 and B7-H4 expression in non-small-cell lung cancer. Lung Cancer. 2006;53:143–151. doi: 10.1016/j.lungcan.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Tringler B, Liu W, Corral L, Torkko KC, Enomoto T, Davidson S, Lucia MS, Heinz DE, Papkoff J, Shroyer KR. B7-H4 overexpression in ovarian tumors. Gynecol Oncol. 2006;100:44–52. doi: 10.1016/j.ygyno.2005.08.060. [DOI] [PubMed] [Google Scholar]
- 12.Tringler B, Zhuo S, Pilkington G, Torkko KC, Singh M, Lucia MS, Heinz DE, Papkoff J, Shroyer KR. B7-h4 is highly expressed in ductal and lobular breast cancer. Clin Cancer Res. 2005;11:1842–1848. doi: 10.1158/1078-0432.CCR-04-1658. [DOI] [PubMed] [Google Scholar]
- 13.Yao Y, Wang X, Jin K, Zhu J, Wang Y, Xiong S, Mao Y, Zhou L. B7-H4 is preferentially expressed in non-dividing brain tumor cells and in a subset of brain tumor stem-like cells. J Neurooncol. 2008;89:121–129. doi: 10.1007/s11060-008-9601-x. [DOI] [PubMed] [Google Scholar]
- 14.Thompson RH, Zang X, Lohse CM, Leibovich BC, Slovin SF, Reuter VE, Cheville JC, Blute ML, Russo P, Kwon ED, Allison JP. Serum-soluble B7x is elevated in renal cell carcinoma patients and is associated with advanced stage. Cancer Res. 2008;68:6054–6058. doi: 10.1158/0008-5472.CAN-08-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zang X, Thompson RH, Al-Ahmadie HA, Serio AM, Reuter VE, Eastham JA, Scardino PT, Sharma P, Allison JP. B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc Natl Acad Sci U S A. 2007;104:19458–19463. doi: 10.1073/pnas.0709802104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zang X, Sullivan PS, Soslow RA, Waitz R, Reuter VE, Wilton A, Thaler HT, Arul M, Slovin SF, Wei J, Spriggs DR, Dupont J, Allison JP. Tumor associated endothelial expression of B7-H3 predicts survival in ovarian carcinomas. Mod Pathol. 2010;23:1104–1112. doi: 10.1038/modpathol.2010.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang J, Zhu Y, Wu C, Shen Y, Wei W, Chen L, Zheng X, Sun J, Lu B, Zhang X. Tumor expression of B7-H4 predicts poor survival of patients suffering from gastric cancer. Cancer Immunol Immunother. 2010;59:1707–1714. doi: 10.1007/s00262-010-0900-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen LJ, Sun J, Wu HY, Zhou SM, Tan Y, Tan M, Shan BE, Lu BF, Zhang XG. B7-H4 expression associates with cancer progression and predicts patient’s survival in human esophageal squamous cell carcinoma. Cancer Immunol Immunother. 2011;60:1047–1055. doi: 10.1007/s00262-011-1017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arigami T, Uenosono Y, Hirata M, Hagihara T, Yanagita S, Ishigami S, Natsugoe S. Expression of B7-H4 in blood of patients with gastric cancer predicts tumor progression and prognosis. J Surg Oncol. 2010;102:748–752. doi: 10.1002/jso.21722. [DOI] [PubMed] [Google Scholar]
- 20.Arigami T, Uenosono Y, Ishigami S, Hagihara T, Haraguchi N, Natsugoe S. Clinical significance of the B7-H4 coregulatory molecule as a novel prognostic marker in gastric cancer. World J Surg. 2011;35:2051–2057. doi: 10.1007/s00268-011-1186-4. [DOI] [PubMed] [Google Scholar]
- 21.Miyatake T, Tringler B, Liu W, Liu SH, Papkoff J, Enomoto T, Torkko KC, Dehn DL, Swisher A, Shroyer KR. B7-H4 (DD-O110) is overexpressed in high risk uterine endometrioid adenocarcinomas and inversely correlated with tumor T-cell infiltration. Gynecol Oncol. 2007;106:119–127. doi: 10.1016/j.ygyno.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 22.Zang X, Loke P, Kim J, Murphy K, Waitz R, Allison JP. B7x: a widely expressed B7 family member that inhibits T cell activation. Proc Natl Acad Sci U S A. 2003;100:10388–10392. doi: 10.1073/pnas.1434299100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sica GL, I, Choi H, Zhu G, Tamada K, Wang SD, Tamura H, Chapoval AI, Flies DB, Bajorath J, Chen L. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. 2003;18:849–861. doi: 10.1016/s1074-7613(03)00152-3. [DOI] [PubMed] [Google Scholar]
- 24.Prasad DV, Richards S, Mai XM, Dong C. B7S1, a novel B7 family member that negatively regulates T cell activation. Immunity. 2003;18:863–873. doi: 10.1016/s1074-7613(03)00147-x. [DOI] [PubMed] [Google Scholar]
- 25.Hofmeyer KA, Scandiuzzi L, Ghosh K, Pirofski LA, Zang X. Tissue-expressed B7x affects the immune response to and outcome of lethal pulmonary infection. J Immunol. 2012;189:3054–3063. doi: 10.4049/jimmunol.1200701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei J, Loke P, Zang X, Allison JP. Tissue-specific expression of B7x protects from CD4 T cell-mediated autoimmunity. J Exp Med. 2011;208:1683–1694. doi: 10.1084/jem.20100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JS, Scandiuzzi L, Ray A, Wei J, Hofmeyer KA, Abadi YM, Loke P, Lin J, Yuan J, Serreze DV, Allison JP, Zang X. B7x in the periphery abrogates pancreas-specific damage mediated by self-reactive CD8 T cells. J Immunol. 2012;189:4165–4174. doi: 10.4049/jimmunol.1201241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y, Yang C, Xie Z, Zou L, Ruan Z, Zhang X, Tang Y, Fei L, Jia Z, Wu Y. Expression of the novel co-stimulatory molecule B7-H4 by renal tubular epithelial cells. Kidney Int. 2006;70:2092–2099. doi: 10.1038/sj.ki.5001867. [DOI] [PubMed] [Google Scholar]
- 29.Aslakson CJ, Miller FR. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 1992;52:1399–1405. [PubMed] [Google Scholar]
- 30.Sun Y, Kim SH, Zhou DC, Ding W, Paietta E, Guidez F, Zelent A, Ramesh KH, Cannizzaro L, Warrell RP, Gallagher RE. Acute promyelocytic leukemia cell line AP-1060 established as a cytokine-dependent culture from a patient clinically resistant to all-trans retinoic acid and arsenic trioxide. Leukemia. 2004;18:1258–1269. doi: 10.1038/sj.leu.2403372. [DOI] [PubMed] [Google Scholar]
- 31.Fan TM. Murine and Canine Models of Appendicular Osteosarcoma. Current Protocols in Pharmacology. Current Protocols in Pharmacology. 2007:14.11.11–14.11.16. doi: 10.1002/0471141755.ph1401s37. [DOI] [PubMed] [Google Scholar]
- 32.Roederer M. Interpretation of cellular proliferation data: Avoid the panglossian. Cytometry A. 2011 doi: 10.1002/cyto.a.21010. [DOI] [PubMed] [Google Scholar]
- 33.DuPre SA, Hunter KW., Jr Murine mammary carcinoma 4T1 induces a leukemoid reaction with splenomegaly: association with tumor-derived growth factors. Exp Mol Pathol. 2007;82:12–24. doi: 10.1016/j.yexmp.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 34.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peranzoni E, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, Bronte V. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol. 2010;22:238–244. doi: 10.1016/j.coi.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 36.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 37.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corzo CA, Cotter MJ, Cheng P, Cheng F, Kusmartsev S, Sotomayor E, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol. 2009;182:5693–5701. doi: 10.4049/jimmunol.0900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 40.Kamimura Y, Kobori H, Piao J, Hashiguchi M, Matsumoto K, Hirose S, Azuma M. Possible involvement of soluble B7-H4 in T cell-mediated inflammatory immune responses. Biochem Biophys Res Commun. 2009;389:349–353. doi: 10.1016/j.bbrc.2009.08.144. [DOI] [PubMed] [Google Scholar]
- 41.Elkin M, Vlodavsky I. Tail vein assay of cancer metastasis. Curr Protoc Cell Biol. 2001;Chapter 19(Unit 19):12. doi: 10.1002/0471143030.cb1902s12. [DOI] [PubMed] [Google Scholar]
- 42.Zang X, Allison JP. The B7 family and cancer therapy: costimulation and coinhibition. Clin Cancer Res. 2007;13:5271–5279. doi: 10.1158/1078-0432.CCR-07-1030. [DOI] [PubMed] [Google Scholar]
- 43.Barach YS, Lee JS, Zang X. T cell coinhibition in prostate cancer: new immune evasion pathways and emerging therapeutics. Trends Mol Med. 2011;17:47–55. doi: 10.1016/j.molmed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu G, Augustine MM, Azuma T, Luo L, Yao S, Anand S, Rietz AC, Huang J, Xu H, Flies AS, Flies SJ, Tamada K, Colonna M, van Deursen JM, Chen L. B7-H4-deficient mice display augmented neutrophil-mediated innate immunity. Blood. 2009;113:1759–1767. doi: 10.1182/blood-2008-01-133223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Srivastava MK, Zhu L, Harris-White M, Kar U, Huang M, Johnson MF, Lee JM, Elashoff D, Strieter R, Dubinett S, Sharma S. Myeloid suppressor cell depletion augments antitumor activity in lung cancer. PLoS One. 2012;7:e40677. doi: 10.1371/journal.pone.0040677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Youn JI, Collazo M, Shalova IN, Biswas SK, Gabrilovich DI. Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. J Leukoc Biol. 2012;91:167–181. doi: 10.1189/jlb.0311177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fridlender ZG, Sun J, Mishalian I, Singhal S, Cheng G, Kapoor V, Horng W, Fridlender G, Bayuh R, Worthen GS, Albelda SM. Transcriptomic analysis comparing tumor-associated neutrophils with granulocytic myeloid-derived suppressor cells and normal neutrophils. PLoS One. 2012;7:e31524. doi: 10.1371/journal.pone.0031524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gabrilovich DI, Velders MP, Sotomayor EM, Kast WM. Mechanism of immune dysfunction in cancer mediated by immature Gr-1+ myeloid cells. J Immunol. 2001;166:5398–5406. doi: 10.4049/jimmunol.166.9.5398. [DOI] [PubMed] [Google Scholar]
- 50.Dolcetti L, Peranzoni E, Ugel S, Marigo I, Fernandez Gomez A, Mesa C, Geilich M, Winkels G, Traggiai E, Casati A, Grassi F, Bronte V. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur J Immunol. 2010;40:22–35. doi: 10.1002/eji.200939903. [DOI] [PubMed] [Google Scholar]
- 51.Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, Cho HI, Celis E, Quiceno DG, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207:2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]