Summary
Malaria parasites grow within erythrocytes, but are also free in host plasma between cycles of asexual replication. As a result, the parasite is exposed to fluctuating levels of Na+ and K+, ions assumed to serve important roles for the human pathogen, Plasmodium falciparum. We examined these assumptions and the parasite's ionic requirements by establishing continuous culture in novel sucrose-based media. With sucrose as the primary osmoticant and K+ and Cl− as the main extracellular ions, we obtained parasite growth and propagation at rates indistinguishable from those in physiological media. These conditions abolish long-known increases in intracellular Na+ via parasite-induced channels, excluding a requirement for erythrocyte cation remodeling. We also dissected Na+, K+, and Cl− requirements and found that unexpectedly low concentrations of each ion meet the parasite's demands. Surprisingly, growth was not adversely affected by up to 148 mM K+, suggesting that low extracellular K+ is not an essential trigger for erythrocyte invasion. At the same time, merozoite egress and invasion required a threshold ionic strength, suggesting critical electrostatic interactions between macromolecules at these stages. These findings provide insights into transmembrane signaling in malaria and reveal fundamental differences between host and parasite ionic requirements.
Keywords: Malaria parasites, invasion, transmembrane signaling, cation transport
Introduction
Malaria parasites are successful pathogens, infecting a broad range of vertebrates and causing disease in 200–500 million humans each year. This success depends, in part, on intracellular growth within host erythrocytes, which allows the parasite to evade host immune responses (Scherf, 2006) and access hemoglobin as a nutritive protein source (Gluzman et al., 1994). The erythrocytic cycle has been extensively studied in P. falciparum, the most virulent human pathogen, because facile in vitro propagation using donor human erythrocytes has permitted the full array of modern research approaches.
Since the development of in vitro culture more than three decades ago (Trager and Jensen, 1976), the conditions used to propagate this parasite have not changed significantly. The universally accepted culture medium is a physiological NaCl-based saline supplemented with soluble nutrients and a lipid source. This medium is designed to simulate human serum, which contains approximately 140 mM Na+, 4 mM K+, and 100 mM Cl−. Because serum and erythrocyte ionic compositions are conserved and tightly regulated in vertebrates susceptible to malaria, it has been tacitly assumed that the parasite has similar ionic needs. For example, merozoites encounters marked changes in external Na+ and K+ concentrations upon both invasion and egress from erythrocytes; the decrease in K+ at the time of egress is thought to trigger merozoite maturation (Singh et al., 2010), as also reported for other parasite stages and genera (Moudy et al., 2001; Kumar et al., 2007).
Intracellular parasite growth is also associated with erythrocyte ionic remodeling. While uninfected human erythrocytes maintain transmembrane Na+ and K+ gradients through the action of the Na+/K+ ATPase pump (Jorgensen et al., 2003), infection produces a marked increase in erythrocyte Na+ content and a parallel decrease in K+ (Overman, 1948; Ginsburg et al., 1986; Lee et al., 1988). These changes result from increased ion permeabilities at the host membrane, as mediated by the plasmodial surface anion channel (PSAC) with possible contributions from altered host transporters (Desai et al., 2000; Nguitragool et al., 2011; Staines et al., 2007). They are presumed to benefit the parasite through several mechanisms. First, increased Na+ in host cytosol creates a gradient across the parasite plasma membrane that may be used by coupled transporters to drive uptake and efflux of other solutes. A Na+/H+ exchanger, proposed for the parasite plasma membrane (Bosia et al., 1993; Bennett et al., 2007), could facilitate extrusion of metabolic acid. A Na+-phosphate cotransporter has also been proposed (Saliba et al., 2006); coupling to Na+ may allow phosphate import to levels greater than possible with uncoupled passive uptake. Second, the increasing intracellular Na+ concentration may play a role in parasite egress from the erythrocyte by promoting osmotic lysis of infected cells (Staines et al., 2001), though further modeling suggests that a lytic threshold is not reached within the required timeframe (Mauritz et al., 2009). Finally, these changes in Na+ and K+ concentrations may contribute to the membrane potential of the parasite; although a V-type H+ pump is thought to be the primary determinant of the parasite's membrane potential (Allen and Kirk, 2004), changes in Na+ and K+ may also influence the membrane potential through electrogenic transport on coupled transporters or other mechanisms. Consistent with these predictions, workers have, to date, been unable to cultivate P. falciparum in media having reduced Na+ (Brand et al., 2003), but have reported that accentuated changes in erythrocyte Na+ and K+ content do not interfere with parasite growth (Tanabe et al., 1986).
We now report continuous P. falciparum cultivation in sucrose-based media. Our studies exclude a physiological role for cation remodeling in erythrocyte cytosol after infection. They also reveal a surprising parasite tolerance to variations in extracellular Na+, K+, and Cl−, provide new insights into parasite ion utilization, and suggest targets for chemotherapeutic intervention.
Results
Continuous P. falciparum growth in sucrose-based media
Entry of Na+ into infected cells is passive and therefore requires extracellular Na+ concentrations above electrochemical equilibrium. The standard medium used for P. falciparum culture, RPMI 1640 supplemented with human serum, contains ~140 mM Na+ and fulfills this requirement (Tables S1–S3). In this medium or under in vivo conditions, the large inward Na+ gradient allows passive uptake and yields an increased erythrocyte Na+ (Overman, 1948; Ginsburg et al., 1986; Lee et al., 1988). To examine whether Na+ uptake serves an essential role for the parasite, we surveyed impermeant monovalent cations that could replace Na+ and abolish the gradient. We designed and prepared media that follow RPMI 1640, but substituted NaCl, NaHCO3, and Na2HPO4 with the corresponding salts of other cations. Replacement with either Li+ or N-methyl-d-glucamine+ salts failed to support parasite growth, but these ions may be toxic in continuous culture. Because K+ is the predominant cation in erythrocyte cytosol, we reasoned it cannot be directly toxic to the parasite. Nevertheless, equimolar substitution of Na+ with K+ also failed to support even short-term parasite growth (full-K+ medium, Tables S2–S3). This may reflect either an essential role for elevated host cytosolic [Na+] or an indirect toxic effect of high K+ concentrations. One mechanism of indirect toxicity is through osmotic lysis of infected cells in KCl-based media because K+ has a higher PSAC permeability than Na+ (Cohn et al., 2003). Consistent with this possibility, we found that trophozoite-infected cells undergo osmotic lysis in the full-K+ medium (red trace, Figure 1D; halftime of 92 ± 4 min, n = 3), as predicted by theory and previous experimental studies of Donnan equilibria in cation-permeable erythrocytes (Jacobs and Stewart, 1947; Freedman and Hoffman, 1979). This lysis was prevented by addition of 50 mM sucrose, an impermeant disaccharide that offsets the lytic effects of K+ uptake (Freedman and Hoffman, 1979).
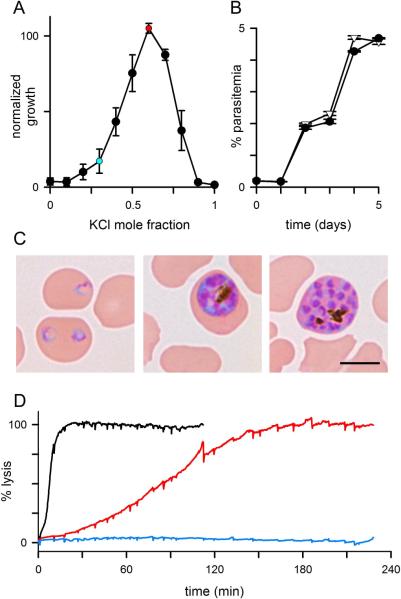
Fig. 1.
Parasite growth in low Na+-media. (A) P. falciparum growth over 5 days in media prepared with sucrose and KCl. Abscissa shows increasing mole fractions of KCl and decreasing fractions of sucrose to achieve a constant total osmolarity. Each medium was supplemented with 5% serum; growth is normalized to 100% for a control culture in RPMI 1640 medium with serum. Symbols represent mean ± S.E.M. from 4 experiments. Red and blue circles represent 4suc:6KCl and 7suc:3KCl media, respectively. (B) Identical rates of growth and expansion in 4suc:6KCl and RPMI (circles and triangles, respectively), determined by flow cytometry (n = 3 replicates each). (C) Photomicrographs of Giemsa-stained ring-, trophozoite-, and schizont-stage parasites in sucrose:KCl medium reveal unchanged parasite morphology (left to right, respectively). Scale bar, 5 μm. (D) Osmotic lysis kinetics for trophozoite-infected cells resuspended in sorbitol lysis solution (black trace), full-K+ medium with 5% serum (red), or full-K+ with 5% serum and 50 mM sucrose (blue). Infected cells undergo osmotic lysis in the full-K+ medium; lysis is prevented by addition of 50 mM sucrose. Lysis rates are inversely proportional to the PSAC permeabilities of sorbitol and K+ (black and red traces, respectively).
In light of this rescue from osmotic lysis, we partially replaced KCl in the full-K+ medium with sucrose and found that various ratios of sucrose:KCl supported parasite growth (Fig. 1A). At a 4:6 mixture by calculated osmolarity, parasite growth was quantitatively identical to that in RPMI-based medium. In this medium (4suc:6KCl, Table S2), intracellular parasites were microscopically healthy, egressed from host cells, and invaded new erythrocytes with a developmental cycle indistinguishable from that in standard medium (Fig. 1B and C). This medium supported growth of each parasite line we tested (HB3, 3D7A, W2, Indo) without detectable delay upon transfer from standard medium. Expansion of naïve cultures without delay suggests that these parasites do not require adaptive changes to grow in 4suc:6KCl. There was also no loss of parasite viability after prolonged culture in 4suc:6KCl (> 10 weeks).
Figure 1A shows a strong dependence of parasite growth on mole fractions of sucrose and KCl, with growth failure when either constituent predominates. This bimodal response suggests a balance between opposing factors. Although osmotic lysis can account for growth failure in media with high K+ mole fractions, supraphysiological K+ concentrations may also be toxic to the parasite. We therefore supplemented the full-K+ medium with 50 mM sucrose (Table S2). Although this medium is hypertonic (Table S3), parasite cultures are known to tolerate elevated osmolarities resulting from addition of sucrose (Ginsburg et al., 1986). The supplemented medium supported continuous in vitro growth and expansion of parasite cultures, in contrast to complete sterilization within 3 days in the full-K+ medium. Because sucrose is impermeant and cannot be utilized by the parasite, restored growth upon its addition strongly supports PSAC-mediated osmotic lysis as the primary mechanism of growth failure in full-K+ medium. Successful propagation also indicates that extracellular K+ levels up to 148 mM are tolerated by the parasite under these in vitro culture conditions.
Figure 1A reveals that media containing predominantly sucrose fail to support parasite growth. The possible mechanisms are examined in later sections.
Erythrocyte cation remodeling is unnecessary
The Na+ present in 4suc:6KCl (measured at 6.8 mM, Fig. 2A) was primarily contributed by serum, added as a lipid source for parasite growth. This markedly reduced value is below electrochemical equilibrium relative to the intracellular value of ~ 11 mM reported for uninfected erythrocytes (Beilin et al., 1966). This outward gradient should prevent Na+ uptake and may produce net efflux from infected cells.
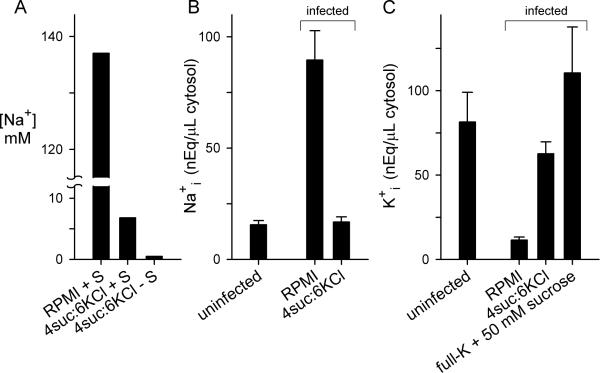
Fig. 2.
Effects on erythrocyte cytosolic ion contents. (A) Measured Na+ concentrations in indicated media with or without 5% serum (“+ S” and “− S”, respectively). (B) Erythrocyte cytosolic Na+ content in uninfected erythrocytes or in enriched trophozoite-infected cells after culturing for 24 h in indicated media with 5% serum. (C) Erythrocyte cytosolic K+ content in uninfected erythrocytes or in enriched trophozoite-infected cells after culturing for 24 h in indicated media with 5% serum.
We therefore measured Na+ and K+ contents in uninfected erythrocytes and in the host cytosol of infected cells. Host cell cytosol was selectively released with saponin, a detergent that permeabilizes the erythrocyte but not the intracellular parasite (Hsiao et al., 1991). Saponin also releases the contents of the parasitophorous vacuole surrounding the parasite; the effect on measured ion concentrations will be small because this vacuole has a negligible volume and an ionic composition similar to that of host erythrocyte cytosol due to nonselective ion channels on the vacuolar membrane (Desai et al., 1993). After correction for the parasite volume, these measurements confirmed the dramatic increase in intracellular Na+ reported for infected cells cultured in standard medium and revealed that cultivation in 4suc:6KCl abolishes these changes (Fig. 2B), consistent with elimination of the inward Na+ gradient.
Host erythrocyte K+ content was also significantly determined by extracellular availability. While growth in standard medium produced a precipitous reduction in cytosolic K+, we found that increasing extracellular K+ concentration preserved or even augmented the high erythrocyte K+ content (Fig. 2C). Notably, our measurements of erythrocyte Na+ and K+ concentrations in standard medium are in quantitative agreement with recent estimates obtained using x-ray microanalysis (Mauritz et al., 2011), allaying concerns of significant contamination by parasite contents.
These measurements establish the relationship between extracellular and cytosolic cation concentrations, as predicted by electrochemical gradient considerations. Thus, passive uptake at the host membrane accounts for the long-known changes in infected erythrocyte [Na+] and [K+] occurring under physiological conditions. Unabated growth in 4suc:6KCl reveals that this cation remodeling is not essential for parasite development.
A requirement for low external Na+
We next explored whether the parasite can tolerate further decreases in external Na+ concentration. Dialysis of human serum removed Na+, allowing preparation of a supplemented 4suc:6KCl medium with a measured [Na+]o of 0.57 mM. This medium could not support parasite growth (Fig. 3A, n > 6 attempts). Addition of NaCl restored parasite growth with an EC50 of 2.9 ± 0.5 mM [Na+]o, excluding loss of other essential factors during dialysis and implicating a parasite requirement for low millimolar Na+ concentrations.
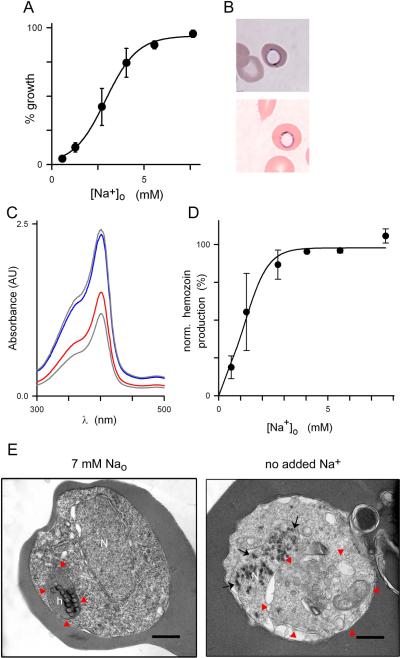
Fig. 3.
Low Na+ concentrations are required. (A) Normalized parasite growth over 72 h in 4suc:6KCl with 10% dialyzed serum and indicated Na+ concentrations, revealing a sigmoidal dose response for Na+ requirement. Mean ± S.E.M. of 9 replicates from 3 experiments. (B) Photomicrograph of Giemsa-stained trophozoite-infected cells after 24 h cultivation in 4suc:6KCl with 10% dialyzed serum without Na+ supplementation. Two parasites are shown with a rim of blue stain and central clearing. (C) Absorbance scan showing reduced β-hematin production in trophozoites cultivated in Na+-deficient medium (red trace); supplementation with 7 mM Na+ restores β-hematin production (blue trace), yielding levels similar to those observed with standard RPMI-based medium (upper gray trace). Positive control, 20 μM chloroquine (lower gray trace). AU, arbitrary units. (D) Mean ± S.E.M. β-hematin production vs. external Na+ concentration, normalized to zero for control culture with 20 μM chloroquine. (E) Transmission electron micrographs showing trophozoite-infected erythrocytes cultivated in 4suc:6KCl with 10% dialyzed serum with or without Na+ supplementation to a final 7 mM concentration (left and right panels, respectively). Red arrowheads demarcate the parasite digestive vacuole; black arrows show electron dense spots in nucleus when Na+ is not added. N, nucleus; h, normal hemozoin crystals; scale bars, 0.5 μm. Images are representative of 89 cells from two separate experiments.
In vitro culture without Na+ supplementation for only 24 h revealed reproducible morphological changes in Giemsa-stained smears (Fig. 3B). These parasites exhibited central clearing that resembled engorgement of the parasite digestive vacuole (DV) seen after exposure to protease inhibitors (Rosenthal et al., 1991). To explore a possible link between Na+ depletion and defective DV function, we examined formation of hemozoin, a byproduct of hemoglobin digestion within the DV (Hempelmann, 2007), and found a marked reduction upon culture without Na+ (Fig. 3C and D, EC50 = 1.2 mM [Na+]o).
To examine ultrastructural changes associated with Na+ depletion, we next performed transmission electron microscopy on trophozoite-stage parasites. Parasite morphology and hemozoin formation appeared normal for cells cultivated in 4suc:6KCl with dialyzed serum when the medium was supplemented with 7 mM Na+ (Fig. 3E, left panel). Cultivation without Na+ supplementation revealed abnormalities that extended the above findings. After only 24 h, these cells exhibited not only engorged digestive vacuoles and reduced hemozoin production (Fig. 3E, right panel), but also appeared to have compromised digestive vacuolar integrity. The parasite nuclei also contained numerous punctate electron densities suggestive of chromatin condensation (black arrows); these densities were never seen in the Na+-supplemented cells. This pattern of observations resembles those seen in apoptotic cells from higher organisms (Zhivotovsky and Kroemer, 2004), and now also recognized in some protozoan parasites (Reece et al., 2011).
Physiological increases in erythrocyte [Na+] do not facilitate phosphate acquisition and utilization
The increased erythrocyte [Na+]i observed under physiological conditions may benefit the parasite by creating an inward Na+ gradient at the parasite plasma membrane. Such a gradient could be used to transport required solutes on Na+-coupled transporters. We tested the proposal of facilitated uptake of inorganic phosphate (Pi) on a 2:1 Na+:Pi cotransporter known as PfPiT (Saliba et al., 2006). With such a coupled transporter, cultivation in 4suc:6KCl would reduce parasite Pi uptake because the Na+ gradient at the parasite plasma membrane has been abolished. Based on the measured reduction in host cytosolic [Na+] (Fig. 2B) and requirements for coupled transport (Turner, 1985), steady-state Pi accumulation would be reduced by 28-fold if the proposed cotransporter is the primary uptake mechanism for this nutrient. This reduction is based on thermodynamic considerations and does not depend on the relative rates of uptake and subsequent Pi utilization. Reduced Pi accumulation may adequately sustain parasite growth in media containing high Pi concentrations (5.6 mM, Table S1). However, when the external Pi concentration is reduced to threshold levels required for parasite survival, its acquisition via a Na+-coupled cotransporter would depend critically on an inward Na+ gradient at the parasite plasma membrane. We therefore compared the dose response for extracellular Pi requirement in 4suc:6KCl medium and in RPMI (Fig. 4). These experiments confirmed a need for external phosphate and identified limiting concentrations. In contrast to the prediction of an increased requirement for external Pi when erythrocyte [Na+]i is reduced, the dose responses were identical in these two media (EC50 = 0.6 ± 0.2 mM, P = 0.3 for no measurable difference). Because the intracellular sites and rates of Pi utilization are unknown and may be affected by changes in Pi availability, our studies cannot exclude the proposed cotransporter. However, they do indicate that the parasite's demands for Pi are fulfilled equally well without increases in erythrocyte [Na+]i.
Fig. 4.
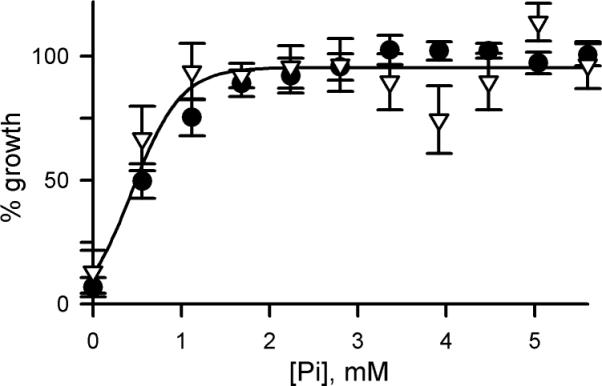
Increased cytosolic Na+ does not promote phosphate acquisition. Phosphate dose responses for parasite growth over 5 days in RPMI vs. 4:6 sucrose:KCl with 5% serum (circles and triangles, respectively; mean of 3 measurements each). In contrast to the prediction for Na+-coupled uptake, the phosphate EC50 is not increased when erythrocyte Na+i is reduced.
External K+ is also required
Having determined that the parasite tolerates both reduced Na+ and increased K+, we next examined in vitro cultivation with reduced extracellular [K+]. Because the erythrocyte contains K+ levels greater than those in either human plasma or standard medium (Fig. 2B, Tables S2–S3), we initially predicted parasites could obtain required K+ from erythrocyte stores and be propagated in K+-free medium. Nevertheless, an NaCl-based medium prepared without use of K+ salts and supplemented with dialyzed serum was unable to support parasite propagation (Fig. 5A; zeroK medium, Tables S2–S3; measured extracellular [K+] < 60 μM). Addition of 5 mM KCl restored growth, excluding loss of other factors upon dialysis of serum. Dose response experiments revealed an EC50 of 0.17 mM external K+ for parasite growth over 72 h (Fig. 5B). This estimate was, however, confounded by the large K+ stores within erythrocytes: extracellular K+ concentrations may be raised by either efflux from cells or by low-level hemolysis in cultures.
Fig. 5.
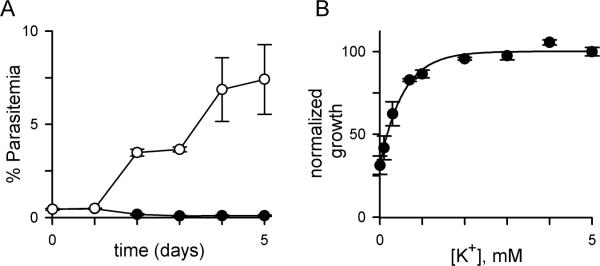
A requirement for low levels of external K+. (A) Parasite growth in zeroK medium with 5% dialyzed serum with or without 5 mM KCl supplementation (white and black symbols, respectively; mean ± S.E.M of 4 trials), evaluated by microscopy of Giemsa-stained smears. (B) 72 h growth rate vs. external [K+] in the same medium, measured using SYBR Green I. Mean ± S.E.M. of replicates from 4 experiments.
A nontoxic organic anion quantifies external Cl− requirement
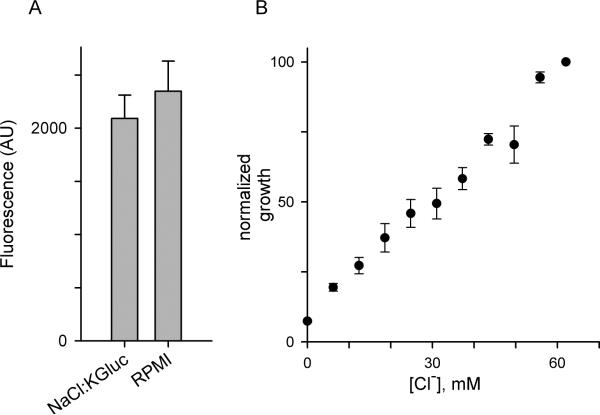
In light of the malaria parasite's remarkable tolerance to changes in monovalent cation concentrations, we wondered whether Cl−, the predominant physiological anion, serves an essential role in parasite biology. Unabated growth in the 4suc:6KCl medium indicates that significant reductions in extracellular Cl− concentration are tolerated (measured [Cl–] after serum supplementation, 73 mM), but compromised growth with KCl mole fractions less than 0.5 may reflect a Cl– requirement (Fig. 1A). To obtain more direct evidence, we sought to reduce the Cl− concentration in 4suc:6KCl without changing the K+ or sucrose concentrations. This can be achieved by substitution of Cl− with a nontoxic, nonphysiological anion. We therefore surveyed toxicity of candidate anions through partial replacement of NaCl in standard media with salts of candidate anions. Because these media provide sufficient Na+, K+, and Cl− to support continuous in vitro propagation, growth in these substitution experiments would indicate that the candidate anion is nontoxic. These surveys revealed that gluconate− is nontoxic at concentrations up to 65 mM (NaCl:KGluc in Table S2, Fig. 6A; P = 0.16 for a growth difference relative to standard media over 5 days, paired Student's t test using 4 trials).
Fig. 6.
Gluconate− substitution and a Cl− requirement. (A) Matched parasite growth over 5 days in NaCl:KGluc in comparison to standard RPMI medium, each supplemented with 10% serum. Bars represent mean ± S.E.M. SYBR Green I fluorescence in arbitrary units. (B) 72 h parasite growth as a function of external [Cl−] in 4suc:6KCl with 10% dialyzed serum. Reduced external Cl− concentrations were achieved through isomolar substitution of KCl with K-gluconate. Mean ± S.E.M. of SYBR Green I fluorescence normalized to 100% for 4suc:6KCl (n = 6 trials).
We then examined parasite growth at a range of Cl− concentrations below those in the 4suc:6KCl medium using isomolar substitution of KCl with K-gluconate; this approach maintains constant solution osmolarity, fixed concentrations of all other constituents, and is not confounded by toxicity. These experiments revealed a parasite requirement for external Cl− with an EC50 of 31 ± 3.3 mM (Fig. 6B, n = 6). The graded nearly linear dependence we observed suggests Cl− serves multiple roles in parasite propagation. Consistent with this, studies using synchronous ring- or trophozoite-stage cultures in Cl− deficient media suggested detrimental effects at each stage (not shown).
An ionic strength requirement for merozoite egress and invasion
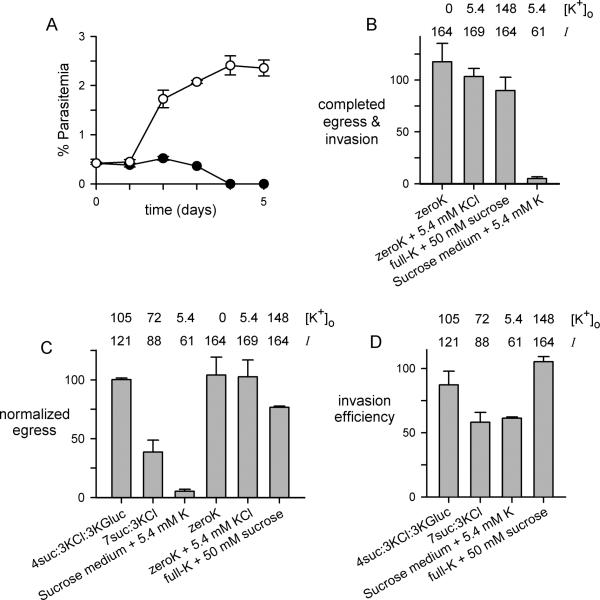
Although an inadequate supply of Cl− partially accounts for growth failure in sucrose:KCl mixtures with high sucrose mole fractions (Fig. 1A), the relatively low Cl− EC50 value suggested that there may be additional determinants of parasite survival and replication. For example, a medium containing a KCl mole fraction of 0.3 provides Cl− at a level near the EC50 (7suc:3KCl, 32.6 mM in Table S3), but does not support parasite growth (Fig. 1A, blue circle). We therefore compared expansion of cultures in this medium to one with sucrose partially replaced with the nonphysiological salt, K-gluconate (4suc:3KCl:3Kgluc, Table S2). These two media have identical Na+ and Cl− concentrations, physiological osmolarities, K+ at levels within parasite tolerances, and the full complement of essential organic solutes (Table S3). Nevertheless, we found that 4suc:3KCl:3Kgluc allowed expansion of parasite cultures whereas 7suc:3KCl could not support growth (Fig. 7A).
Fig. 7.
An ionic strength requirement for parasite cultivation. (A) Parasite growth in 4suc:3KCl:3Kgluc and 7suc:3KCl media (white and black symbols, respectively; mean ± S.E.M. of 5 replicates from two trials). Both media were supplemented with 7% human serum, which provides sufficient Na+ for cultivation. (B) Progression from late-stage schizonts to ring-stage infected erythrocytes in indicated media. (C) Number of merozoites released from late stage-schizont cultures over a 4 h incubation in each medium. (D) Erythrocyte invasion by mechanically freed merozoites resuspended in indicated media. In panels (B), (C), and (D), each bar represents the mean ± S.E.M. of up to 8 trials after normalization to 100% in matched controls using RPMI 1640 medium. The nominal K+ concentration and calculated ionic strength (I) of each medium prior to addition of serum is listed above the bars. Two media, zeroK with or without added K+, were supplemented with dialyzed serum to minimize contaminating K+, but all other media used undialyzed serum to provide Na+ at required levels.
This result suggests that, in addition to the defined requirements for individual ions, in vitro parasite growth also depends on a minimum ionic strength. Ionic strength, calculated as the weighted sum of the concentrations of ions in a solution, is important in biology because the functions of many proteins and other macromolecules depend on solvation and electrostatic interactions with other cellular components (Spitzer and Poolman, 2009). Notably, 4suc:3KCl:3Kgluc has an ionic strength identical to that of 4suc:6KCl, but the value for 7suc:3KCl medium is significantly lower (Table S3).
We therefore examined ionic strength effects on growth of synchronous parasite cultures with the 4suc:3KCl:3Kgluc and 7suc:3KCl media. Ring-stage infected erythrocytes matured to schizonts equally well in these media when examined over a single parasite cycle (not shown), consistent with the expectation that reduced ionic strength in the extracellular space alone should not affect electrostatic interactions between macromolecules within parasite compartments. Instead, developmental events that require direct exposure to the external medium, such as merozoite egress and host cell invasion, may account for better propagation of cultures in 4suc:3KCl:3Kgluc. Similar reasoning has motivated studies showing that direct parasite exposure to ions such as K+ influence merozoite activation in plasmodia and toxoplasma (Singh et al., 2010; Moudy et al., 2001).
To explore possible effects of K+ and solution ionic strength on extraerythrocytic parasite forms, we first harvested late-stage schizont infected cells from standard medium and examined progression into the next host cell cycle after transfer to engineered media (Fig. 7B). After an 18 h incubation, measured ring-stage parasitemias revealed that cycle progression is not significantly affected by changes in external [K+] varying over the range of undetectably low to 148 mM (zeroK and full-K + 50 mM sucrose media, respectively; P = 0.27). Progression was however abolished in an isotonic sucrose-based medium with a physiological K+ concentration and the lowest achievable ionic strength without compromising HCO3− and other nutritive ions (sucrose medium + 5.4 mM K, Tables S2 and S3; P = 10−4). Because reduced progression may result from effects on either merozoite egress and activation or the subsequent invasion of a new erythrocyte, we next examined these individual steps. Merozoite egress decreased monotonically with ionic strength (Fig. 7C). In contrast, changes in external K+ concentrations over the range achievable with our media did not adversely affect egress, as long as a physiological ionic strength was preserved (Fig. 7C, zeroK media and full-K + 50 mM sucrose). Erythrocyte invasion, examined using mechanically released invasive merozoites (Srinivasan et al., 2011), was also not affected by supraphysiological K+ concentrations, but decreased as ionic strength was reduced (Fig. 7D). Confocal video microscopy revealed aborted interactions between merozoites and erythrocytes in the 7suc:3KCl medium (Videos S1 and S2, showing comparison to standard RPMI 1640 medium), further supporting reduced invasion efficiency. Reduced ionic strength therefore interferes with parasite growth via compounded effects on merozoite egress and invasion of new erythrocytes.
Discussion
Although Na+ and K+ share many physicochemical properties, most cells readily distinguish these cations. Animals and some other eukaryotes use a conserved Na+/K+ ATPase pump to establish opposing transmembrane gradients for these ions (Jorgensen et al., 2003); these gradients store energy that can be used to generate transmembrane potentials, drive co- and countertransport of solutes, and fine-tune enzymatic activities in controlled ionic environments. Although the P. falciparum genome lacks a clear ortholog of known Na+/K+ ATPases, it has many genes for P-type ATPases that could function as Na+ and/or K+ pumps (Thever and Saier, Jr., 2009; Martin et al., 2009). Unfortunately, the precise function of these putative transporters cannot be determined through computational analysis alone because the determinants of solute selectivity within this superfamily are poorly understood (Sanchez and Blanco, 2004). For this and other reasons, it has been tacitly assumed that malaria parasites recognize and use these ions in ways resembling those of their vertebrate hosts (Ginsburg et al., 1986; Lee et al., 1988; Staines et al., 2001; Brand et al., 2003; Singh et al., 2010). In contrast to these expectations, our studies reveal that the parasite tolerates a surprisingly broad range of Na+, K+, and Cl− concentrations. Broad parasite tolerance to changes in extracellular H+ has also been recently demonstrated (Lyko et al., 2012).
Malaria parasite infection is associated with increased Na+ and decreased K+ concentrations in erythrocyte cytosol (Fig. 2; Overman, 1948; Ginsburg et al., 1986; Lee et al., 1988). These changes result primarily from passive cation flux through PSAC (Desai et al., 2000), but there may also be contributions from activated host channels and reduced Na+/K+ pump activity (Staines et al., 2001; Staines et al., 2007). Stable PSAC mutants, linkage analysis, and DNA transfection studies support an unusual ion channel determined by parasite clag3 genes (Hill et al., 2007; Lisk et al., 2008; Nguitragool et al., 2011). Parasite cultivation studies using reduced concentrations of key nutrients have also implicated the clag3 genes and determined that PSAC serves an essential role in parasite nutrient acquisition (Pillai et al., 2012).
Increased Na+ and decreased K+ in erythrocyte cytosol, though well-established, are dispensable because in vitro propagation is undeterred by conditions that abolish both of these changes. These findings conflict with the conclusions reached by another study that also used modified culture conditions (Brand et al., 2003). There, the effects of 8–16 hour exposures to buffered salt and sugar solutions on short-term parasite growth were examined. Because replacement of Na+ with either N-methyl-D-glucamine (NMDG+) or K+ adversely affected expansion of cultures at the 48 h timepoint, the authors concluded that Na+ uptake at the host membrane is required for parasite survival. Comparison to our conditions for successful cultivation reveals several differences that may account for decreased viability in their study. Maybe most importantly, osmotic lysis of infected cells in K+ solutions, as occurs with media lacking impermeant solutes such as Na+ or sucrose (Fig. 1D), was apparently not considered; such lysis would contribute to overestimation of parasite Na+ requirement. Na+ replacement with NMDG+ may also produce osmotic lysis of infected cells, depending on this substitute cation's PSAC permeability. Another experimental difference—their use of buffered solutions prepared without amino acids, vitamins, or a lipid source (Table S1 and dialyzed human serum in our media)—may also have contributed to decreased viability because these constituents are essential for parasite cultivation (Divo et al., 1985; Istvan et al., 2011). Finally, removal of HCO3−, also absent from their solutions, may have yielded acute pH changes in parasite compartments due to CO2 redistribution across membranes.
Parasite growth without erythrocyte cation remodeling challenges presumed roles for coupled transporters at the parasite plasma membrane. Increased host erythrocyte Na+, as observed under physiological in vitro and in vivo conditions, creates an inward Na+ gradient at the parasite plasma membrane because the intervening parasitophorous vacuolar membrane is freely permeable to ions (Desai et al., 1993; Desai and Rosenberg, 1997). The Na+ gradient at the parasite plasma membrane can, in principle, be used to facilitate transport of other solutes on coupled transporters. In addition to the proposed Na+-phosphate cotransporter we considered (Fig. 4), a Na+/H+ exchanger has been proposed to utilize the increased erythrocyte [Na+]i for metabolic acid extrusion (Bosia et al., 1993; Wunsch et al., 1998; Bennett et al., 2007). Because 4suc:6KCl aborts formation of a large Na+ gradient at the parasite plasma membrane, our studies provide evidence against such facilitated acid extrusion. Instead, an H+ ATPase pump seems to be more likely (Mikkelsen et al., 1986; Spillman et al., 2008).
Our findings also have implications for other actively-studied aspects of host-parasite interactions, especially those related to mechanisms for parasite egress and invasion of erythrocytes. It has been unclear whether parasite egress depends on an osmotic component of host erythrocyte rupture. The ongoing Na+ uptake by infected cells under physiological conditions creates an osmotic load, producing cell swelling and eventual host cell lysis. Mathematical modeling has suggested that this osmotic lysis may be the final trigger for host cell rupture and parasite egress (Staines et al., 2001). However, a more advanced model that incorporates the effects of hemoglobin digestion predicts that a lytic threshold would not be reached by the end of the intracellular cycle (Mauritz et al., 2009). Our study resolves this uncertainty with direct experimental evidence. Normal progression of the parasite cycle and unaltered growth rates in the 4suc:6KCl medium excludes an osmotic trigger for host cell rupture: this medium abolishes the osmotic load by preventing Na+ uptake and further prevents cell swelling through inclusion of sucrose, which remains impermeant at the host membrane (Ginsburg et al., 1985). An explosive membrane rupture event driven by specific parasite enzymes seems to be more likely (Glushakova et al., 2005; Yeoh et al., 2007; Arastu-Kapur et al., 2008; Dvorin et al., 2010).
Shortly after rupture, the released merozoites must expose specific protein ligands that then interact with erythrocyte surface receptors to mediate invasion of new host cells. This ligand exposure is thought to be triggered by a decrease in external K+ concentration at the merozoite surface upon host cell rupture (Singh et al., 2010). Merozoites subjected to decreased external K+ exhibit both phospholipase C activation and Ca2+ mobilization, consistent with the proposed trigger. One caveat is that the threshold K+ concentration for merozoite activation would need to be below the reduced K+ concentration in infected erythrocyte cytosol (Fig. 2C, estimated at < 30 mM in Mauritz et al., 2011; Lee et al., 1988) to avoid merozoite activation prior to egress; it would also need to be higher than normal human plasma K+ concentrations to ensure faithful triggering. The threshold K+ concentration estimated for activation of Toxoplasma gondii merozoites, 80 mM (Moudy et al., 2001), is too high to be suitable for plasmodia. The precise contribution of K+ in plasmodial merozoite maturation and invasion should be reevaluated in light of our studies, which demonstrate continuous cultivation and the requisite completion of merozoite invasion with a broad range of external K+ concentrations (1 to 148 mM). One possibility is that a drop in external K+ may be only one of several triggers for merozoite activation, a scenario that would parallel known redundancies in invasion ligands (Jiang et al., 2011). Abscisic acid may be one such alternative trigger because it has been shown to mobilize Ca++ in T. gondii (Nagamune et al., 2008). Use of multiple triggers and ligands during invasion is appealing because it may maximize the likelihood of successful parasite replication.
Our studies reveal previously unknown effects of extracellular ionic strength on both merozoite egress and host cell invasion. Ionic strength is a derived parameter that quantifies electrostatic contributions to intermolecular interactions. Because egress and invasion were adversely affected by reductions in ionic strength (7suc:3KCl and sucrose medium + 5.4 mM K, Fig. 7; Table S3), we propose charged domains on proteins or lipids that are critical for these events under physiological conditions. Intermolecular interactions that occur in direct contact with the extracellular medium will be most susceptible. Multiple protein-protein interactions are required for invasion, including RON2-AMA1 (Lamarque et al., 2011; Srinivasan et al., 2011), the MSP1 complex and its processing by subtilisin proteases (Holder, 2009), and basigin-PfRH5 (Crosnier et al., 2011). Compromised interactions between these or other macromolecular complexes may conservatively account for the observed ionic strength effect; a better understanding of these interactions may lead to the development of merozoite-specific therapies.
We propose that Na+ uptake at the host membrane is a nonessential by-product of channels required for nutrient acquisition and possibly other purposes (Pillai et al., 2012). Then, PSAC's broad selectivity profile may have been acquired through strong pressure for uptake of nutrients of varying size and charge. There must also have been selection for sufficiently low Na+ permeability to prevent osmotic lysis of infected cells in host plasma. When combined with possible salutary effects of excess hemoglobin digestion (Mauritz et al., 2009), this requirement is adequately met by the observed Na+ permeability, estimated at 10−3.5–10−5 relative to that of Cl−. Consistent with these unique evolutionary pressures, we are unaware of another well-characterized ion channel that combines broad solute selectivity with similarly stringent exclusion for a single small monovalent ion. PSAC apparently achieves its unparalleled selectivity profile through complex, incompletely understood mechanisms that recognize and distinguish between permeating solutes (Cohn et al., 2003). Greater exclusion of Na+ would prevent changes in erythrocyte ionic composition under physiological conditions, but our studies indicate that the resulting benefit to the parasite would be negligible.
Parasite growth was steeply dependent on the relative amounts of sucrose and KCl (Fig. 1A), with only intermediate ratios capable of supporting continuous growth. Growth failure at KCl mole fractions greater than 0.7 resulted from osmotic lysis of infected cells in these media (Fig. 1D); consistent with a primary effect of osmotic lysis, continuous parasite growth was restored in the full-K+ medium upon isolated addition of sucrose at concentrations known to prevent lysis due to the Donnan effect (Freedman and Hoffman, 1979; full-K+ medium with 50 mM sucrose, Table S2). Under physiological conditions, infected erythrocytes avoid this lytic effect by maintaining a lower PSAC permeability to Na+ than K+ (Cohn et al., 2003). Whereas increasing Na+ permeability accelerates osmotic lysis, a higher K+ permeability would yield infected cell shrinkage in host plasma due to an outward K+ gradient at the erythrocyte membrane. Channel design considerations therefore favor greater K+ permeability to avoid premature cell lysis, but also avoid excessive cell shrinkage by optimizing the relative permeabilities of these two cations. The multiple design constraints on PSAC may be partially relaxed by the parasite's tolerance to large changes in Na+ and K+ concentrations.
Although parasite cultures tolerate marked changes in external Na+ and K+ concentrations, we also found that P. falciparum requires both ions, albeit at levels well below those present in normal human plasma. Our experiments implicate distinct sites of requirement for these two ions, but additional studies will be needed to reveal the molecular targets and the corresponding roles of the ions in parasite physiology. Because uninfected erythrocytes have a high intracellular [K+], we were surprised to find that parasite propagation requires extracellular K+ (Fig. 5). Our attempts to examine the precise role and stage-specificity were complicated by the erythrocyte K+ stores. Growth failure without external K+ may therefore reflect a gradual depletion of erythrocyte and parasite stores, a K+ requirement for extracellular merozoite forms that we could not discern with short-term egress and invasion studies, and possibly other mechanisms.
Growth failure in sucrose-based media with KCl mole fractions below 0.5 (Fig. 1A) resulted from a combination of at least two factors: reduced [Cl−] and an effect of low ionic strength on merozoite egress and invasion (Figs. 6 and 7). By identifying gluconate− as a nontoxic substitute anion, we were able to isolate and quantify growth inhibition resulting from reduced [Cl−]. In addition to a specific parasite need for Cl−, reducing Cl− may lead to indirect detrimental effects on in vitro cultures. One such mechanism may involve reversal of Cl−/HCO3− exchange on the erythrocyte membrane anion exchanger. Cl− efflux, resulting from the imposed outward gradient, may significantly raise erythrocyte cytosolic pH via coupled HCO3− uptake (Alper, 2009). Removal of external HCO3− and cultivation in a CO2-free atmosphere should alleviate this pH change and determine if this mechanism contributes to parasite killing in reduced [Cl−]. Unfortunately, our attempts to adapt parasite cultures to HCO3−-free media have been unsuccessful.
The broad range of Na+, K+, Cl−, and H+ concentrations tolerated by malaria parasites is consistent with their close phylogenetic relationship to dinoflagellates (Keeling et al., 2005). These free-living eukaryotes tolerate drastic changes in external ion composition, growing well in both fresh and sea water environments (Pistocchi et al., 2011). Retaining their ancestors' tolerance to variations in ion concentrations is clearly beneficial to malaria parasites as they encounter large shifts in Na+ and K+ during their life cycle within vertebrate hosts. This tolerance should also assist the parasite in navigating diverse environments within the mosquito vector, where changes in fluid osmolarity, pH, and ion concentration are well-known (Kang'ethe et al., 2007).
Despite this broad tolerance to changes in external composition, several new directions for therapeutic intervention against malaria are suggested by our findings. Rapid killing of the parasite when Na+ is removed suggests mechanisms to fine-tune this ion's concentration with the intracellular parasite, possibly via regulated transporters on its plasma membrane. K+ and Cl−, also required at low concentrations, may be similarly controlled in intracellular and extracellular stages of bloodstream parasites. The novel media reported here may assist in identifying and characterizing specific inhibitors of these regulatory mechanisms.
Experimental Procedures
Parasite cultivation and design of new media
P. falciparum laboratory lines were cultivated by standard methods and maintained under 5% O2, 5% CO2, 90% N2 at 37 °C. Transfer to engineered media utilized naïve parasite cultures after washing to prevent carryover.
To prepare low Na+ media, we followed RPMI 1640 composition (Tables S1–S2), but replaced NaHCO3 and Na2HPO4 with corresponding K+ salts and substituted NaCl with identical concentrations of LiCl, NMDG-Cl, or indicated mixtures of KCl and sucrose. The pH of each medium was adjusted to 7.4 with KOH prior to addition of 5% human serum, which contributed nearly all of the measured Na+. Exhaustive dialysis of serum, where used, was performed against 4suc:6KCl or distilled water as required; the molecular weight cutoff of the dialysis tubing was 3500 Da. Successful removal of cations after dialysis was confirmed with ion sensitive electrodes. Albumax II, a lipid-rich bovine serum albumin formulation, was found to have a significant residual Na+ content and was therefore not used.
Phosphate dose responses were performed using media prepared with HPO −4 salts at indicated concentrations. The K+-free medium, zeroK, was prepared by replacement of KCl in RPMI 1640 with NaCl. Parasite cultivation with reduced extracellular Cl− required a non-toxic replacement anion. To find a suitable anion, we surveyed acetate−, citrate−, glutamate−, gluconate−, F−, SO4−2, SCN− and NO3− by equimolar substitution of 65 mM NaCl with K+ or Na+ salts; this concentration was chosen because it approximates the Cl− concentration of 4suc:6KCl medium. Because 4suc:6KCl medium produces unabated parasite growth, lack of measurable toxicity at this concentration is a minimum requirement for anion substitution experiments designed to examine parasite Cl− requirement. By this criterion, gluconate− was the only nontoxic anion; it was therefore also used in ionic strength experiments.
Growth assays
Expansion of synchronous parasite cultures was evaluated with Giemsa stained smears and SYBR Green I detection of parasite DNA; both methods producing similar results in all experiments. SYBR Green I measurements were carried out as described previously (Lisk et al., 2008) with modifications. Sorbitol synchronized ring-stage parasites were seeded in 96-well plates at 0.5–1.0% parasitemia and 2% hematocrit in indicated media. Cultures were maintained at 37 °C for 72 h prior to addition of lysis buffer (20 mM Tris, 10 mM EDTA, 0.016% saponin, and 1.6% triton X100, pH 7.5) and SYBR Green I nucleic acid gel stain at a 5,000× dilution (Invitrogen). After incubation for 45 min in the dark, parasite nucleic acid production was quantified with fluorescence measurements (excitation/emission wavelengths of 485/528 nm).
Flow cytometry was used for quantifying merozoite invasion efficiency and daily changes in parasitemia on cultivation using engineered media; results were consistent with counts obtained by examination of smears. The method used incubation of cultures simultaneously with SYBR Green I nucleic acid stain (8000× dilution, Invitrogen; excitation/emission of 497/520 nm) and MitoTracker Deep Red (84 nM, Molecular Probes; excitation/emission of 644/665 nm) to identify viable intracellular parasites, as described previously (Ekland et al., 2011). After a 30 min incubation at 37 °C in 96-well flat bottom plates at a 0.2% hematocrit, the cells were washed to remove unbound dye and resuspended in PBS before counting on an Accuri C6 flow cytometer (BD Biosciences).
Osmotic lysis experiments
Trophozoite-infected cells were harvested from cultures in standard media by the percoll/sorbitol method, washed, and resuspended at 0.1% hematocrit in sorbitol lysis solution (280 mM sorbitol, 20 mM Na-HEPES, 0.1 mg/mL BSA, pH 7.4) or in indicated parasite culture media. Transmittance of 700 nm light through these cell suspensions was then continuously recorded to track cell lysis, as described (Wagner et al., 2003).
Ion content measurements
Na+, K+, and Cl− sensitive electrodes (Orion) were used to measure final ion concentrations in engineered media, exclude contaminants in commercial reagents, and estimate erythrocyte cytosolic ion composition. Synchronous ring-stage cultures were cultivated in indicated media for 24 h, enriched by percoll-sorbitol, washed and returned to culture for 2 h. Cells were then counted with a hemocytometer and washed in 50 mM sucrose with either 150 mM NaCl or 150 mM KCl to remove extracellular K+ or Na+, respectively. Erythrocyte cytosol was selectively released into the same buffer with 0.3% saponin (Sigma Aldrich), a detergent that does not lyse the intracellular parasite. Na+ and K+ contaminants in saponin, as detected in control ion sensitive electrode measurements, were removed by dialysis against distilled water (MWCO of 3500 Da); the lowest saponin concentration required for host membrane permeabilization with minimal leakage from parasite compartments was determined using the dialyzed detergent. After a brief incubation (15–30 s), erythrocyte cytosol was harvested by centrifugation to remove the intracellular parasite (2300 × g, 4 min); ionic strength adjustor was added to the cytosol as recommended by the manufacturer. Standards were also prepared using ionic strength adjustor and revealed Nernstian responses for each electrode (52–56 mV/decade), indicating excellent sensitivity and specificity.
Ion content measurements were converted to concentrations based on the independently measured volume of erythrocyte cytosol within trophozoite-infected cells. Cultures were harvested and percoll-enriched 24 h after sorbitol synchronization to match conditions used in the above ion-sensitive electrode experiments. Hemoglobin released by saponin treatment was quantified using oxidation of 3, 3', 5, 5'-tetramethylbenzidine and used to estimate the volume of the host compartment of infected cells as described previously (Wagner et al., 2003). The average volume of erythrocyte cytosol, 41 ± 3 fL/cell, was used for the uniform conversion of all content measurements to concentrations.
Hemozoin measurements
Insoluble hemozoin, a byproduct of parasite hemoglobin digestion, was measured as described previously (Lisk et al., 2008). Briefly, sorbitol synchronized parasites were cultivated in indicated media for 30 h, harvested, lysed in ice-cold 5 mM Na2HPO4, pH 7.6. Matched cultures in RPMI 1640 medium without or with 20 μM chloroquine served as positive and negative controls for hemozoin production. Hemozoin was pelleted by centrifugation (27,000 × g, 30min), washed, and digested for 16 h at room temperature in 2 ml of 2.5% sodium dodecyl sulfate, 25 mM Tris, pH 7.8. The insoluble pellet was incubated in the same solution after addition of 0.1 N NaOH to release the ferriprotoporphyrin IX incorporated in beta-hematin. The pigment released was detected with absorbance wavelength scans and was quantified at 405 nm.
Transmission electron microscopy
Sorbitol synchronized cultures were cultivated in 4suc:6KCl medium supplemented with 10% dialyzed serum and either 0 or 7 mM NaCl for 30 h. The cells were washed in PBS with 0.1 g/L BSA prior to fixation at 4 °C for 1 h in the same medium with 2% paraformaldehyde and 2.5% glutaraldehyde. The cells were then postfixed for 1 h with 0.5% osmium tetroxide-0.8% potassium ferricyanide, stained overnight with 1% uranyl acetate at 4°C, dehydrated with a graded ethanol series, and embedded in Spurr's resin. Thin sections were cut with an RMC MT-7000 ultramicrotome (Ventana), stained with 1% uranyl acetate and Reynold's lead citrate, and visualized at 80 kV on a Philips CM-10 transmission electron microscope (FEI Company). Images were acquired with a digital camera system (Advanced Microscopy Techniques).
Merozoite egress and erythrocyte invasion studies
Mature schizont-infected cells were grown in standard RPMI 1640 medium, purified by percollsorbitol centrifugation, and allowed to recover in RPMI 1640 for 30 min at 37 °C. To examine the effects of medium composition or ionic strength on merozoite egress, the harvested infected cells were suspended in engineered media for 4 h at 37 °C; free merozoites were then counted using a hemocytometer. RPMI 1640 supplemented with serum at the same concentration was used a positive control in each experiment. Control experiments revealed that 2 μM E64 abolished egress in the engineered media, confirming active egress of merozoites (not shown).
Invasive merozoites for erythrocyte invasion studies were prepared using a parasite line selected for prolonged merozoite viability, as previously described (Srinivasan et al., 2011). Free merozoites were centrifuged, washed once in the respective media and resuspended. A matched control of freed merozoites that were identically washed with standard medium was included in each experiment. Pre-warmed human erythrocytes were added to these merozoites prior to incubation at 37 °C for 4 h. The efficiency of invasion was measured by flow cytometry of SYBR Green I-stained parasites and is presented after normalization relative to the control RPMI 1640 medium.
Experiments measuring the combined effects on egress and invasion used enriched schizont-infected cells incubated with prewarmed erythrocytes at 1% hematocrit and 2% parasitemia for 18 h in indicated media. Efficiency of rupture and invasion was measurement by counting the number of ring-stage infected cells by flow cytometry, as described above.
Supplementary Material
Acknowledgements
We thank Wandy Beatty for assistance with electron microscopy.
This work was supported by the Intramural Research Programs of the National Institutes of Health, NIAID and the Medicines for Malaria Venture (MMV).
Footnotes
The authors have no conflicts of interest to declare.
References
- Allen RJ, Kirk K. The membrane potential of the intraerythrocytic malaria parasite Plasmodium falciparum. J Biol Chem. 2004;279:11264–11272. doi: 10.1074/jbc.M311110200. [DOI] [PubMed] [Google Scholar]
- Alper SL. Molecular physiology and genetics of Na+-independent SLC4 anion exchangers. J Exp Biol. 2009;212:1672–1683. doi: 10.1242/jeb.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arastu-Kapur S, Ponder EL, Fonovic UP, Yeoh S, Yuan F, Fonovic M, et al. Identification of proteases that regulate erythrocyte rupture by the malaria parasite Plasmodium falciparum. Nat Chem Biol. 2008;4:203–213. doi: 10.1038/nchembio.70. [DOI] [PubMed] [Google Scholar]
- Beilin LJ, Knight GJ, Munro-Faure AD, Anderson J. The sodium, potassium, and water contents of red blood cells of healthy human adults. J Clin Invest. 1966;45:1817–1825. doi: 10.1172/JCI105485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett TN, Patel J, Ferdig MT, Roepe PD. Plasmodium falciparum Na+/H+ exchanger activity and quinine resistance. Mol Biochem Parasitol. 2007;153:48–58. doi: 10.1016/j.molbiopara.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosia A, Ghigo D, Turrini F, Nissani E, Pescarmona GP, Ginsburg H. Kinetic characterization of Na+/H+ antiport of Plasmodium falciparum membrane. J Cell Physiol. 1993;154:527–534. doi: 10.1002/jcp.1041540311. [DOI] [PubMed] [Google Scholar]
- Brand VB, Sandu CD, Duranton C, Tanneur V, Lang KS, Huber SM, Lang F. Dependence of Plasmodium falciparum in vitro growth on the cation permeability of the human host erythrocyte. Cell Physiol Biochem. 2003;13:347–356. doi: 10.1159/000075122. [DOI] [PubMed] [Google Scholar]
- Cohn JV, Alkhalil A, Wagner MA, Rajapandi T, Desai SA. Extracellular lysines on the plasmodial surface anion channel involved in Na+ exclusion. Mol Biochem Parasitol. 2003;132:27–34. doi: 10.1016/j.molbiopara.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Crosnier C, Bustamante LY, Bartholdson SJ, Bei AK, Theron M, Uchikawa M, et al. Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature. 2011;480:534–537. doi: 10.1038/nature10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai SA, Bezrukov SM, Zimmerberg J. A voltage-dependent channel involved in nutrient uptake by red blood cells infected with the malaria parasite. Nature. 2000;406:1001–1005. doi: 10.1038/35023000. [DOI] [PubMed] [Google Scholar]
- Desai SA, Krogstad DJ, McCleskey EW. A nutrient-permeable channel on the intraerythrocytic malaria parasite. Nature. 1993;362:643–646. doi: 10.1038/362643a0. [DOI] [PubMed] [Google Scholar]
- Desai SA, Rosenberg RL. Pore size of the malaria parasite's nutrient channel. Proc Natl Acad Sci USA. 1997;94:2045–2049. doi: 10.1073/pnas.94.5.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divo AA, Geary TG, Davis NL, Jensen JB. Nutritional requirements of Plasmodium falciparum in culture. I. Exogenously supplied dialyzable components necessary for continuous growth. J Protozool. 1985;32:59–64. doi: 10.1111/j.1550-7408.1985.tb03013.x. [DOI] [PubMed] [Google Scholar]
- Dvorin JD, Martyn DC, Patel SD, Grimley JS, Collins CR, Hopp CS, et al. A plant-like kinase in Plasmodium falciparum regulates parasite egress from erythrocytes. Science. 2010;328:910–912. doi: 10.1126/science.1188191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekland EH, Schneider J, Fidock DA. Identifying apicoplast-targeting antimalarials using high-throughput compatible approaches. FASEB J. 2011;25:3583–3593. doi: 10.1096/fj.11-187401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman JC, Hoffman JF. Ionic and osmotic equilibria of human red blood cells treated with nystatin. J Gen Physiol. 1979;74:157–185. doi: 10.1085/jgp.74.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg H, Handeli S, Friedman S, Gorodetsky R, Krugliak M. Effects of red blood cell potassium and hypertonicity on the growth of Plasmodium falciparum in culture. Z Parasitenkd. 1986;72:185–199. doi: 10.1007/BF00931146. [DOI] [PubMed] [Google Scholar]
- Ginsburg H, Kutner S, Krugliak M, Cabantchik ZI. Characterization of permeation pathways appearing in the host membrane of Plasmodium falciparum infected red blood cells. Mol Biochem Parasitol. 1985;14:313–322. doi: 10.1016/0166-6851(85)90059-3. [DOI] [PubMed] [Google Scholar]
- Glushakova S, Yin D, Li T, Zimmerberg J. Membrane transformation during malaria parasite release from human red blood cells. Curr Biol. 2005;15:1645–1650. doi: 10.1016/j.cub.2005.07.067. [DOI] [PubMed] [Google Scholar]
- Gluzman IY, Francis SE, Oksman A, Smith CE, Duffin KL, Goldberg DE. Order and specificity of the Plasmodium falciparum hemoglobin degradation pathway. J Clin Invest. 1994;93:1602–1608. doi: 10.1172/JCI117140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempelmann E. Hemozoin biocrystallization in Plasmodium falciparum and the antimalarial activity of crystallization inhibitors. Parasitol Res. 2007;100:671–676. doi: 10.1007/s00436-006-0313-x. [DOI] [PubMed] [Google Scholar]
- Hill DA, Pillai AD, Nawaz F, Hayton K, Doan L, Lisk G, Desai SA. A blasticidin S-resistant Plasmodium falciparum mutant with a defective plasmodial surface anion channel. Proc Natl Acad Sci USA. 2007;104:1063–1068. doi: 10.1073/pnas.0610353104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder AA. The carboxy-terminus of merozoite surface protein 1: structure, specific antibodies and immunity to malaria. Parasitology. 2009;136:1445–1456. doi: 10.1017/S0031182009990515. [DOI] [PubMed] [Google Scholar]
- Hsiao LL, Howard RJ, Aikawa M, Taraschi TF. Modification of host cell membrane lipid composition by the intra- erythrocytic human malaria parasite Plasmodium falciparum. Biochem J. 1991;274:121–132. doi: 10.1042/bj2740121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Istvan ES, Dharia NV, Bopp SE, Gluzman I, Winzeler EA, Goldberg DE. Validation of isoleucine utilization targets in Plasmodium falciparum. Proc Natl Acad Sci US. 2011;108:1627–1632. doi: 10.1073/pnas.1011560108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs MH, Stewart DR. Osmotic properties of the erythrocyte; ionic and osmotic equilibria with a complex external solution. J Cell Physiol. 1947;30:79–103. doi: 10.1002/jcp.1030300106. [DOI] [PubMed] [Google Scholar]
- Jiang L, Gaur D, Mu J, Zhou H, Long CA, Miller LH. Evidence for erythrocyte-binding antigen 175 as a component of a ligand-blocking blood-stage malaria vaccine. Proc Natl Acad Sci USA. 2011;108:7553–7558. doi: 10.1073/pnas.1104050108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen PL, Hakansson KO, Karlish SJ. Structure and mechanism of Na,K-ATPase: functional sites and their interactions. Annu Rev Physiol. 2003;65:817–849. doi: 10.1146/annurev.physiol.65.092101.142558. [DOI] [PubMed] [Google Scholar]
- Kang'ethe W, Aimanova KG, Pullikuth AK, Gill SS. NHE8 mediates amiloride-sensitive Na+/H+ exchange across mosquito Malpighian tubules and catalyzes Na+ and K+ transport in reconstituted proteoliposomes. Am J Physiol Renal Physiol. 2007;292:F1501–F1512. doi: 10.1152/ajprenal.00487.2005. [DOI] [PubMed] [Google Scholar]
- Keeling PJ, Burger G, Durnford DG, Lang BF, Lee RW, Pearlman RE, et al. The tree of eukaryotes. Trends Ecol Evol. 2005;20:670–676. doi: 10.1016/j.tree.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Kumar KA, Garcia CR, Chandran VR, Van RN, Zhou Y, Winzeler E, Nussenzweig V. Exposure of Plasmodium sporozoites to the intracellular concentration of potassium enhances infectivity and reduces cell passage activity. Mol Biochem Parasitol. 2007;156:32–40. doi: 10.1016/j.molbiopara.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Lamarque M, Besteiro S, Papoin J, Roques M, Vulliez-Le NB, Morlon-Guyot J, et al. The RON2-AMA1 interaction is a critical step in moving junction-dependent invasion by apicomplexan parasites. PLoS Pathog. 2011;7:e1001276. doi: 10.1371/journal.ppat.1001276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P, Ye Z, Van Dyke K, Kirk RG. X-ray microanalysis of Plasmodium falciparum and infected red blood cells: effects of qinghaosu and chloroquine on potassium, sodium, and phosphorus composition. Am J Trop Med Hyg. 1988;39:157–165. doi: 10.4269/ajtmh.1988.39.157. [DOI] [PubMed] [Google Scholar]
- Lisk G, Pain M, Gluzman IY, Kambhampati S, Furuya T, Su XZ, et al. Changes in the plasmodial surface anion channel reduce leupeptin uptake and can confer drug resistance in P. falciparum-infected erythrocytes. Antimicrob Agents Chemother. 2008;52:2346–2354. doi: 10.1128/AAC.00057-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyko B, Hammershaimb EA, Nguitragool W, Wellems TE, Desai SA. A high-throughput method to detect Plasmodium falciparum clones in limiting dilution microplates. Malar J. 2012;11:124. doi: 10.1186/1475-2875-11-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RE, Ginsburg H, Kirk K. Membrane transport proteins of the malaria parasite. Mol Microbiol. 2009;74:519–528. doi: 10.1111/j.1365-2958.2009.06863.x. [DOI] [PubMed] [Google Scholar]
- Mauritz JM, Esposito A, Ginsburg H, Kaminski CF, Tiffert T, Lew VL. The homeostasis of Plasmodium falciparum-infected red blood cells. PLoS Comput Biol. 2009;5:e1000339. doi: 10.1371/journal.pcbi.1000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauritz JM, Seear R, Esposito A, Kaminski CF, Skepper JN, Warley A, et al. X-ray microanalysis investigation of the changes in Na, K, and hemoglobin concentration in Plasmodium falciparum-infected red blood cells. Biophys J. 2011;100:1438–1445. doi: 10.1016/j.bpj.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen RB, Wallach DF, Van DE, Nillni EA. Membrane potential of erythrocytic stages of Plasmodium chabaudi free of the host cell membrane. Mol Biochem Parasitol. 1986;21:83–92. doi: 10.1016/0166-6851(86)90082-4. [DOI] [PubMed] [Google Scholar]
- Moudy R, Manning TJ, Beckers CJ. The loss of cytoplasmic potassium upon host cell breakdown triggers egress of Toxoplasma gondii. J Biol Chem. 2001;276:41492–41501. doi: 10.1074/jbc.M106154200. [DOI] [PubMed] [Google Scholar]
- Nagamune K, Hicks LM, Fux B, Brossier F, Chini EN, Sibley LD. Abscisic acid controls calcium-dependent egress and development in Toxoplasma gondii. Nature. 2008;451:207–210. doi: 10.1038/nature06478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguitragool W, Bokhari AA, Pillai AD, Rayavara K, Sharma P, Turpin B, et al. Malaria parasite clag3 genes determine channel-mediated nutrient uptake by infected red blood cells. Cell. 2011;145:665–677. doi: 10.1016/j.cell.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overman RR. Reversible cellular permeability alterations in disease. In vivo studies on sodium, potassium and chloride concentrations in erythrocytes of the malarious monkey. Am J Physiol. 1948;152:113–121. doi: 10.1152/ajplegacy.1947.152.1.113. [DOI] [PubMed] [Google Scholar]
- Pillai AD, Nguitragool W, Lyko B, Dolinta K, Butler MM, Nguyen ST, et al. Solute restriction reveals an essential role for clag3-associated channels in malaria parasite nutrient acquisition. Mol Pharmacol. 2012;82:1104–1114. doi: 10.1124/mol.112.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistocchi R, Pezzolesi L, Guerrini F, Vanucci S, Dell'aversano C, Fattorusso E. A review on the effects of environmental conditions on growth and toxin production of Ostreopsis ovata. Toxicon. 2011;57:421–428. doi: 10.1016/j.toxicon.2010.09.013. [DOI] [PubMed] [Google Scholar]
- Reece SE, Pollitt LC, Colegrave N, Gardner A. The meaning of death: evolution and ecology of apoptosis in protozoan parasites. PLoS Pathog. 2011;7:e1002320. doi: 10.1371/journal.ppat.1002320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal PJ, Wollish WS, Palmer JT, Rasnick D. Antimalarial effects of peptide inhibitors of a Plasmodium falciparum cysteine proteinase. J Clin Invest. 1991;88:1467–1472. doi: 10.1172/JCI115456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliba KJ, Martin RE, Broer A, Henry RI, McCarthy CS, Downie MJ, et al. Sodium-dependent uptake of inorganic phosphate by the intracellular malaria parasite. Nature. 2006;443:582–585. doi: 10.1038/nature05149. [DOI] [PubMed] [Google Scholar]
- Sanchez G, Blanco G. Residues within transmembrane domains 4 and 6 of the Na,K-ATPase alpha subunit are important for Na+ selectivity. Biochemistry. 2004;43:9061–9074. doi: 10.1021/bi049484s. [DOI] [PubMed] [Google Scholar]
- Scherf A. A greedy promoter controls malarial var-iations. Cell. 2006;124:251–253. doi: 10.1016/j.cell.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Singh S, Alam MM, Pal-Bhowmick I, Brzostowski JA, Chitnis CE. Distinct external signals trigger sequential release of apical organelles during erythrocyte invasion by malaria parasites. PLoS Pathog. 2010;6:e1000746. doi: 10.1371/journal.ppat.1000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillman NJ, Allen RJ, Kirk K. Acid extrusion from the intraerythrocytic malaria parasite is not via a Na+/H+ exchanger. Mol Biochem Parasitol. 2008;162:96–99. doi: 10.1016/j.molbiopara.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Spitzer J, Poolman B. The role of biomacromolecular crowding, ionic strength, and physicochemical gradients in the complexities of life's emergence. Microbiol Mol Biol Rev. 2009;73:371–388. doi: 10.1128/MMBR.00010-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan P, Beatty WL, Diouf A, Herrera R, Ambroggio X, Moch JK, et al. Binding of Plasmodium merozoite proteins RON2 and AMA1 triggers commitment to invasion. Proc Natl Acad Sci USA. 2011;108:13275–13280. doi: 10.1073/pnas.1110303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staines HM, Alkhalil A, Allen RJ, De Jonge HR, Derbyshire E, Egee S, et al. Electrophysiological studies of malaria parasite-infected erythrocytes: current status. Int J Parasitol. 2007;37:475–482. doi: 10.1016/j.ijpara.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staines HM, Ellory JC, Kirk K. Perturbation of the pump-leak balance for Na+ and K+ in malaria- infected erythrocytes. Am J Physiol Cell Physiol. 2001;280:C1576–C1587. doi: 10.1152/ajpcell.2001.280.6.C1576. [DOI] [PubMed] [Google Scholar]
- Tanabe K, Izumo A, Kageyama K. Growth of Plasmodium falciparum in sodium-enriched human erythrocytes. Am J Trop Med Hyg. 1986;35:476–478. doi: 10.4269/ajtmh.1986.35.476. [DOI] [PubMed] [Google Scholar]
- Thever MD, Saier MH., Jr. Bioinformatic characterization of p-type ATPases encoded within the fully sequenced genomes of 26 eukaryotes. J Membr Biol. 2009;229:115–130. doi: 10.1007/s00232-009-9176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Turner RJ. Stoichiometry of cotransport systems. Ann N Y Acad Sci. 1985;456:10–25. doi: 10.1111/j.1749-6632.1985.tb14839.x. [DOI] [PubMed] [Google Scholar]
- Wagner MA, Andemariam B, Desai SA. A two-compartment model of osmotic lysis in Plasmodium falciparum-infected erythrocytes. Biophys J. 2003;84:116–123. doi: 10.1016/S0006-3495(03)74836-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunsch S, Sanchez CP, Gekle M, Grosse-Wortmann L, Wiesner J, Lanzer M. Differential stimulation of the Na+/H+ exchanger determines chloroquine uptake in Plasmodium falciparum. J Cell Biol. 1998;140:335–345. doi: 10.1083/jcb.140.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeoh S, O'Donnell RA, Koussis K, Dluzewski AR, Ansell KH, Osborne SA, et al. Subcellular discharge of a serine protease mediates release of invasive malaria parasites from host erythrocytes. Cell. 2007;131:1072–1083. doi: 10.1016/j.cell.2007.10.049. [DOI] [PubMed] [Google Scholar]
- Zhivotovsky B, Kroemer G. Apoptosis and genomic instability. Nat Rev Mol Cell Biol. 2004;5:752–762. doi: 10.1038/nrm1443. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.