Abstract
Dendritic cells (DC) elicit immunity to pathogens and tumors while simultaneously preserving tolerance to self. Efficacious cancer vaccines have been a challenge because they are based on tumor antigens, some of which are self-antigens and thus subject to self-tolerance. One such antigen is the tumor-associated mucin MUC1. Preclinical testing of MUC1 vaccines revealed existence of peripheral tolerance to MUC1 that compromises their efficacy. To identify mechanisms that act early post-vaccination and might predict vaccine outcome, we immunized human MUC1 transgenic mice (MUC1.Tg) i.v. with a MUC1 peptide vaccine against which they generate weak immunity, and WT mice that respond strongly to the same peptide. We analyzed differences in splenic DC phenotype and function between the two mouse strains at 24 and 72 hours post-vaccination, and also performed unbiased total gene expression analysis of the spleen. Compared to WT, MUC1.Tg spleens had significantly fewer DC and they exhibited significantly lower expression of co-stimulatory molecules, decreased motility and preferential priming of antigen-specific Foxp3+ regulatory T cells (Treg). This tolerogenic DC phenotype and function was marked by a new putative biomarker revealed by the microarray: a cohort of pancreatic enzymes (trypsin, carboxypeptidase, elastase and others) not previously reported in DC. These enzymes were strongly upregulated in the splenic DC from vaccinated WT mice and suppressed in the splenic DC of vaccinated MUC1.Tg mice. Suppression of the enzymes was dependent on Treg and on signaling through the IL-10 receptor and correlated with global down-regulation of DC immunostimulatory phenotype and function.
Keywords: Dendritic Cells, Vaccination, Tumor Immunity, Tolerance
INTRODUCTION
Dendritic cells (DC) are potent inducers of antigen-specific T cell responses and are the major cell type responsible for priming naïve T cells (1, 2). As such, they have been central to vaccination strategies aimed at inducing immunity to both pathogens and tumors (3, 4). However, DC are also important in the maintenance of homeostatic tolerance to self-antigens (Ag) (5). A large body of literature has established the ability of DC to actively induce immunological tolerance against self-Ag, and those closely related to self, thus preventing autoimmunity but also compromising effective anti-tumor immune responses (6, 7). DC utilize diverse mechanisms to mediate T cell tolerance including low expression of costimulatory molecules (8), expression of SOCS1/3 (9, 10), activation of regulatory T cells (Treg)(11), and production of immunosuppressive factors such as IL-10, TGFβ, IDO and retinoic acid (12–15). Significant effort has been devoted to manipulating the phenotype and function of in vitro cultured DC used for vaccination (16), as well as to targeting Ag in vivo to specific DC populations (17). However, modulating and evaluating the ability of a vaccine to alter the phenotype of endogenous DC populations and the type of immune response they prime is still a significant challenge. Specifically, little data exist regarding the influence of the choice of vaccine Ag on the phenotype and function of endogenous DC. It has been well established that exogenous DC used for immunization are generally short-lived in the host after transfer (18), and that transfer of Ag from vaccine DC to endogenous DC is necessary for optimal CD4+ and CD8+ T cell responses (19, 20). Therefore, understanding the impact of the choice of Ag, specifically the importance of its relative similarity to antigens against which the host is already tolerized, on endogenous DC warrants further study. Additionally, because gauging a vaccine’s efficacy often requires waiting several weeks to determine resultant antibody titers and vaccine-induced T cell function, reliable, early signatures or biomarkers of both the endogenous DC response and the ensuing immune response would be of utility.
We and others have previously shown that a long peptide (MUC1p) corresponding to five tandem repeats in the human tumor antigen MUC1 variable number of tandem repeats region is seen as a self-antigen by the human MUC1 transgenic mouse (MUC1.Tg), and that MUC1p vaccination results in hypo-responsiveness compared to a strong immune response in WT mice where MUC1p is a foreign antigen (21–23). This hypo-responsiveness results in the inability of the vaccinated mice to control growth of both transplantable and spontaneous tumors (24, 25). Variations in vaccine design have resulted in some instances in a better immune response and better tumor control (25), but they have been empirical, and without the full understanding of the underlying mechanism and early biomarkers of their efficacy, not readily predictable. Now we show that the outcome of the MUC1p vaccine that currently requires several weeks after immunization to be evaluated can be predicted as early as 24h–72h post-vaccination by the change in expression levels in DC of a group of catabolic enzymes, including trypsin, amylase, elastase, and carboxypeptidase B1, previously thought to be pancreas-restricted in expression. These enzymes are significantly up-regulated in the splenic DC of WT mice following i.v. administration of the MUC1p vaccine, but not in MUC1.Tg mice. Failure to up-regulate pancreatic enzyme expression was seen in the entire splenic DC population and was correlated with low co-stimulatory molecule expression, a decreased number of DC in the spleen, preferential priming of Foxp3+ Treg over IFNγ+ CD4+ T cells and impaired motility. Mechanistically, this DC phenotype was regulated by Treg and IL-10. The unexpected expression of pancreatic enzymes in DC and correlation with DC immunogenicity or tolerogenicity following vaccination provides a new early biomarker of vaccine efficacy.
Materials and Methods
Mice
C57BL/6, RIP.OVA, and OT-II mice were purchased from the Jackson Laboratory. MUC1.Tg mice were purchased from Dr. Sandra Gendler (Mayo Clinic) (26) and/or bred in the University of Pittsburgh animal facility. VFT mice were generated at the University of Pittsburgh Transgenic Mouse Facility. All colonies were subsequently bred and maintained at the University of Pittsburgh under specific pathogen free conditions. Experiments were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh.
Peptides
A 100mer MUC1 peptide (MUC1p) represents 5 repeats of the 20- amino-acid sequence HGVTSAPDTRPAPGSTAPPA from the MUC1 VNTR region. It was synthesized as described previously (21) by the University of Pittsburgh Genomics and Proteomics Core Laboratories. OVA323-339 peptide and ovalbumin protein were purchased from Sigma.
DC culture and vaccines
BMDC were generated according to established protocol (22). Briefly, female C57BL/6 mice (Jackson) were sacrificed and their femurs and tibiae removed. Marrow was flushed with RPMI (2% FCS, 1% Penn-Strep and 2-ME). Cells were passed through a 70μM strainer and pelleted before RBC lysis using ACK buffer. Cells were resuspended in AIM-V (Gibco), counted and plated at 1.5–2×106/mL in AIM-V containing 10–20ng/mL GM-CSF (Miltenyi). On d3 and d5 half the media was replaced with fresh AIM-V and GM-CSF. On d6 of culture, DC were harvested with 2mM EDTA, counted and (when indicated) loaded with either 30ug/mL MUC1 100mer or 100μg/mL ovalbumin and matured with 25ug/mL of Poly-ICLC (Hiltonol), a generous gift from Oncovir, overnight. On d7, cells were harvested as above. For immunizations, d7 DC were washed and resuspended in sterile PBS. Mice were immunized i.v. via the lateral tail vein with .5–1×106 DC. Soluble peptide immunizations consisted 100ug of MUC1 100mer peptide or ovalbumin and 50ug of Poly-ICLC in 100uL of PBS.
Microarray
Whole spleen from WT and MUC1.Tg mice (n=3/group) was obtained at 24h and 72h post-immunization with DC loaded with MUC1 100mer peptide. RNA extraction was performed using Trizol (Invitrogen). RNA from mice within groups was pooled followed by hybridization onto Illumina WG6 arrays. Data analysis was conducted by the University of Pittsburgh GPCL Bioinformatics Core facility using the Efficiency Analysis method of identifying differentially expressed genes (27). Microarray data have been deposited in the NCBI Gene Expression Omnibus (GEO) with the accession number GSE43503 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE43503).
PCR and qRT-PCR
RNA was isolated from splenic tissue or CD11c+ bead isolated (Miltenyi) splenocytes using either an RNeasy mini kit (Qiagen) or Trizol (Invitrogen) according to manufacturer’s protocol). RT-PCR was performed using Oligo(dT) primers and SuperScript III reverse transcriptase (Invitrogen). cDNA was amplified using the following primers: trypsin (forward 1: 5′-GGCCCTTGTGGGAGCTGCTG-3′; reverse 1: 5′-GCAGGTGCACAGGAGCTGGG-3′; forward 2: 5′-GCTCTGCCCAGCTCCTGTGCACCT-3′; reverse 2: 5′-TCAGCCTGAGGCAGCAGTGGGGCAT-3′), CPB1 (forward 1: 5′-TGGTGAGTGTGGCCCTGGCT-3′; reverse 1: 5′-TCCACTTGCACGGGTGTGGC-3′ forward 2: 5′-GCCCTGGTGAAAGGTGCAGCAAAGG-3′; reverse 2: 5′-AGCCCAGTCGTCAGATCCCCCAGCA-3′), Elastase (forward: 5′-TTCCGGAAACTGACGCCCGC-3′; reverse: 5′-TGGGCCAGCTCCCCATTGGT-3′), GAPDH ( forward 1: 5′-TTGGCCGTATTGGGCGCCTG-3′; reverse 2: 5′-TCTCCAGGCGGCACGTCAGA-3′; forward 2: 5′-AGACGGCCGCATCTTCTTGTGCAGT-3′; reverse 2: 5′-TGGTGACCAGGCGCCCAATACGGC-3′), and IL-10 (forward: 5′-CTTCCCAGTCGGCCAGAGCCA-3′; reverse: 5′-CTCAGCCGCATCCTGAGGGTCT-3′). qPCR was done using a QuantiTect SYBR Green PCR kit (Qiagen) according to the manufacturer’s protocol. Reactions were run on a StepOne Plus instrument (Applied Biosystems) and data was generated using the ΔCT method.
Western blotting
Cells were lysed and run on a 10% Tris-HCL Mini-PROTEAN TGX precast gel (BioRad), followed by transfer onto a PVDF membrane. After blocking for 1hr in 5% milk, the membrane was incubated with one of the following antibodies: Rb X-CPB1 (M-134), Rb X-trypsin (M-60) (both Santa Cruz) or β-Actin (AC-74, Sigma). Blots were then incubated with the appropriate HRP-conjugated secondary antibodies (Santa Cruz) and developed using SuperSignal West chemiluminescent substrate (Pierce) before imaging on a Kodak Image Station 4000MM.
DC/T cell co-cultures
CD4 effector and regulatory T cells were isolated from C57Bl/6 mouse splenocytes using the CD4+CD25+ Regulatory T cell Isolation Kit (Miltenyi) and preactivated overnight with 1μg/ml plate-bound anti-CD3 and .5μg/ml soluble anti CD28. Bone marrow derived dendritic cells (BMDC) were generated using above described procedure used to culture vaccine DC. On day six, semi-adherent cells, which represent semi-mature dendritic cells, were removed by gentle agitation. DC were added to preexisting T cell cultures at DC:T cell ratios of 2: 5, except when both regulatory and effector T cells were added, in which case the ratio was 2:5:5. Where indicated, LPS was added to the culture along with the DC at a final concentration of 1ng/ml. At 24 hours post co-culture, DC were isolated based on plate adherence and RNA was extracted and analyzed as described.
Depleting and/or blocking antibody experiments
All antibodies were purchased from Bio-X-Cell. Mice received an i.p. injection containing 200μg of an anti CD25 antibody (clone PC-61.5.3) to deplete CD4+ regulatory T cell. 6 days following this treatment, mice were vaccinated as described and sacrificed 24 hours following vaccination. In the case of IL-10R blockade, mice were given 250μg of on an anti IL-10R antibody (clone 1B1.3A), IP. These mice were then vaccinated as described at 48–72 hours post antibody treatment along with an additional dose of 250μg of anti IL-10R antibody. Mice were sacrificed 24 hours following vaccination and second antibody dosing. An equal concentration and volume of Rat IgG1 specific for horseradish peroxidase (HRPN) was injected as a control for the depleting/blocking antibodies where indicated.
Flow cytometry
Anti-CD11c-PacificBlue, anti-CD80-FITC, anti-CD3-PerCP, anti-CD25-PE (BD Bioscience), anti-I-Ab-PeCy7, anti-CD40-APC, anti-CD86-PerCP, anti-Foxp3-PacificBlue (BioLegend), anti-IFNγ-APC, and anti-CD4-FITC (eBioscience) antibodies were used. Cells were analyzed on an LSR II (BD) and data were analyzed using FacsDiva software (BD).
Ex vivo motility assay
Pooled splenocytes were recovered from MUC1p-immunized WT and MUC1.Tg mice 48h post-immunization (n=2/group). DC were isolated with CD11c beads (Miltenyi) and plated at 2×105 cells into Poly-D-Lysine coated 35mm dishes (MatTek). Cells were labeled according to protocol with Cell Tracker Red (Invitrogen) and imaged at 10X in DIC and TRITC channels on a Nikon Eclipse live cell system at 5min intervals for 24h. Motility was analyzed using the Imaris Track algorithm in Imaris (Bitplane).
Statistics
Data show mean ± the standard error of the mean (SEM). Statistical significance between groups was defined as p≤.05 using an unpaired, 2-tailed Student’s t test (GraphPad Prism).
RESULTS
DC from MUC1p-immunized MUC1.Tg mice exhibit decreased expression of co-stimulatory molecules, preferentially induce Foxp3+ Treg cells and have reduced motility
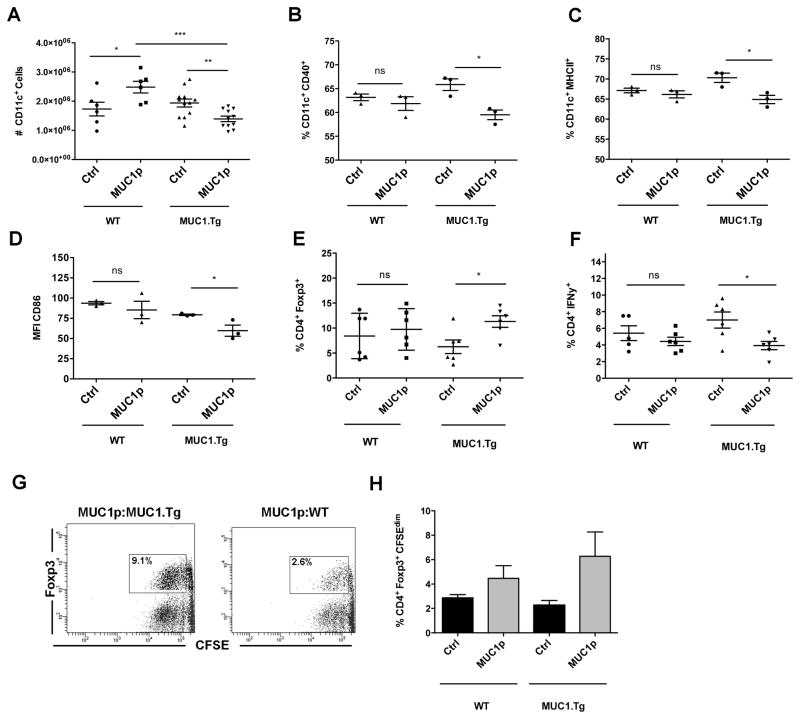
Multiple factors contribute to or limit the ability of DC to prime T cells. These include the number of antigen-loaded DC (28), expression of co-stimulatory molecules on DC and production of stimulatory or suppressive cytokines (8), and the ability of DC to move to T cell areas within lymphoid tissue (29). We found that immunization of MUC1.Tg mice with MUC1p resulted in a decrease in the absolute number of CD11c+ cells in the spleen at 24h, while the same protocol in WT mice resulted in an increase in DC number (Fig. 1A). The same immunization also resulted in differential expression of co-stimulatory molecules, with significantly fewer DC from MUC1.Tg mice expressing CD40 and MHC II (Figs. 1B and 1C), as well as a reduction in the amount of CD86 expressed by those DC (Fig. 1D), relative to immunized WT mice.
Figure 1. Immunization of MUC1.Tg mice with MUC1p results in decreased splenic DC number and costimulatory molecule expression, and preferential priming of Foxp3+ Treg.
(A) WT and MUC1.Tg mice were immunized with unloaded DC (ctrl) or DC loaded with MUC1p. 24h post-immunization total splenic DC numbers were analyzed. Each symbol represents one mouse with bars showing mean ± SEM from three pooled independent experiments, with each experiment including 2–4 mice per group. (B–D) WT and MUC1.Tg mice were immunized as in (A). 48h post immunization bulk splenocytes were stained for FACS analysis. Data represent percentage of positive cells within the CD11c+ gate (B–C) or the MFI of cells within the CD11c+ gate (D). Symbols represent individual mice with bars showing mean ± SEM and are representative of 2 independent experiments. (E–H) WT and MUC1.Tg mice were immunized as in (A). 24h later, splenic DC were bead isolated, loaded with OVA and co-cultured with OT-II CD4 T cells for 7 days. On day 7, OT-II cells were treated with PMA/Ionomycin and analyzed by FACS. Each symbol represents an individual mouse with bars depicting mean ± SEM. Data are pooled from two independent experiments. (G) OT-II CD4+ T cells were labeled with CFSE and cultured as in (E–H). On day 7, CFSE dilution was assessed in CD4+Foxp3+ cells. Representative dot plots from MUC1p vaccinated WT and MUC1.Tg mice are shown (G). (H) Bars represent mean percentage proliferation ± SEM of OT-II CD4+Foxp3+ cells. Data are representative of two independent experiments.
To examine the ability of DC that have been exposed to a self Ag induced environment to prime naïve CD4+ T cells, we again immunized WT and MUC1.Tg mice with MUC1p and isolated total splenic DC 24h post-immunization. The DC were immediately loaded with OVA and co-cultured with naïve, CFSE-labeled OT-II CD4+ T cells that recognize an I-Ab-restricted OVA peptide. After 7 days, T cells from those co-cultures were analyzed by flow cytometry. DC recovered from immunized MUC1.Tg mice primed a significantly higher percentage of Foxp3+ (Fig. 1E) and fewer IFNγ producing OT-II T cells compared to DC recovered from immunized WT mice (Fig. 1F). DC can induce antigen-specific Treg proliferation (30) so we examined the relative proliferation of CD4+Foxp3+ Tregs. DC recovered from MUC1p vaccinated MUC1.Tg mice induced higher OT-II Treg proliferation compared to DC from MUC1p vaccinated WT animals (Figs. 1G and 1H).
While costimulatory molecule expression was decreased in DC recovered from mice that received immunization with self peptide, we found that immunization of MUC1.Tg mice with MUC1p surprisingly resulted in increased expression of CD74 (the MHC II invariant chain) in DC at 72h, compared to DC from MUC1p immunized WT mice (Fig. 2A). Previous studies have shown that expression of CD74 is inversely correlated to in vivo motility of DC (31). We purified splenic CD11c+ cells from WT and MUC1.Tg mice 72h post-MUC1p immunization and analyzed them immediately ex vivo using live cell microscopy. DC isolated from MUC1.Tg mice traveled shorter distances (Fig. 2B) and had smaller net displacements (Fig. 2C) than DC from WT mice.
Figure 2. Immunization of MUC1.Tg mice with MUC1p results in decreased DC motility.
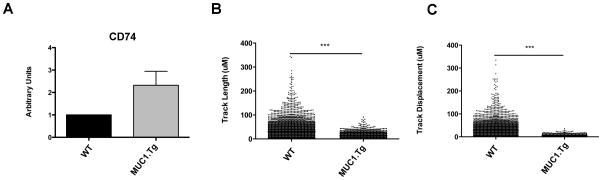
(A) WT and MUC1.Tg mice were vaccinated i.v. with DC loaded with MUC1p. RNA was extracted from pooled splenic DC 72h post vaccination for qRT-PCR. Bars represent mean ± SEM. Data are representative of three independent experiments. (B) and (C) WT and MUC1.Tg mice were vaccinated as in (A). 72h post immunization, splenic DC were bead isolated for live cell imaging. The track length (B) and displacement (C) were analyzed after 20h in culture. Each dot represents a single DC and bars depict mean ± SEM. Data are from two mice comparing 6×103 DC per group.
Differential expression in vivo of pancreatic enzymes in DC in response to vaccination with a foreign versus a self-antigen
We were interested in comparing early (24h–72h) post-immunization events in the spleens of WT versus MUC1.Tg mice that might reveal one or more new mechanisms induced by the presence of a self-antigen that could mediate antigen-specific peripheral tolerance. Accordingly, we immunized i.v. WT and MUC1.Tg mice with DC loaded with MUC1p as previously and conducted whole transcriptome analysis of total splenic RNA at 24h and 72h post-immunization.
We identified 189 genes differentially expressed at both time points, with the most unexpected being a group of seven pancreatic catabolic enzymes and several of their isoforms that had not previously been reported to be expressed in lymphoid tissue (Table I). Significantly lower levels (between 10–80 fold) of transcripts for these enzymes were found in the total splenic RNA from MUC1p-vaccinated MUC1.Tg mice relative to WT mice
Table I.
Pancreatic enzymes are expressed at a significantly lower level in the spleens of MUC1p-vaccinated MUC1.Tg mice compared to MUC1p-vaccinated WT mice1
| Gene | Accession Number | Fold Change (24h) | Fold Change (72h) |
|---|---|---|---|
| Trypsin 1 | XM_001477976.1 | −9.736 | −20.824 |
| Elastase 1 | NM_033612.1 | −11.531 | −27.754 |
| Carboxypeptidase B1 | NM_029706.1 | −14.302 | −30.478 |
| Trypsin 10 | NM_001038996.1 | −24.006 | −36.193 |
| Trypsin 4 | NM_011646.5 | −32.199 | −48.856 |
| Elastase 2A | NM_007919.2 | −44.824 | −81.952 |
| Amylase 2 | NM_001042711.2 | −85.541 | −88.073 |
WT and MUC1.Tg mice (n=3/group) were immunized i.v. with DC loaded with MUC1p. At 24h and 72h, spleens were harvested, total splenic RNA isolated, pooled within groups, and whole transcriptome analysis was conducted. A cohort of catabolic enzymes with previously characterized pancreatic expression were significantly under-expressed in the spleens of immunized MUC1.Tg mice, relative to immunized WT mice. Data reflect expression of genes in immunized MUC1.Tg mice relative to immunized WT mice.
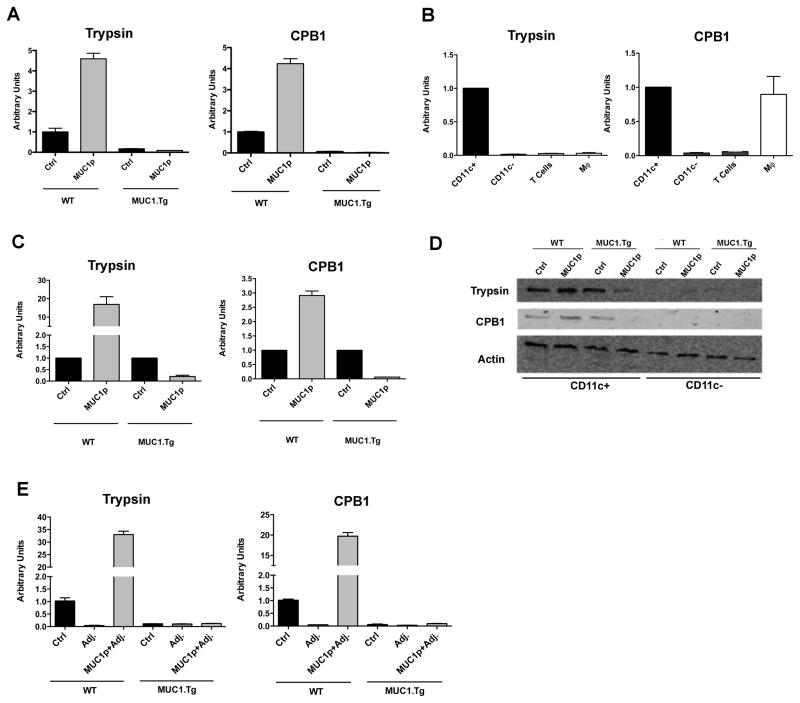
Since the expression of each of these enzymes mimicked the entire cohort, we used trypsin 1 and carboxypeptidase B1 (CPB1) as representatives for more detailed analysis. qPCR analysis of total splenic RNA recapitulated the microarray data, showing a lack of up-regulation of trypsin and CPB1 transcript in spleens from MUC1.Tg mice post immunization with MUC1p relative to significant up-regulation in WT mice (Fig. 3A). Because there was little information about pancreatic enzymes in hematopoietic cells, we analyzed their baseline expression in different WT spleen cell populations: purified CD11c+ DC, T cells, bone marrow-derived macrophages (BMDM) and CD11c-depleted bulk splenocytes which included, among other cell types, B cells. CD11c+ DC expressed trypsin and CPB1 (Fig. 3B) as well as all the other enzymes identified in the gene array (not shown). BMDM expressed CPB1 but not trypsin, while purified T cells and CD11c depleted spleen cells were negative for both. Further dissection of the DC compartment into plasmacytoid DC, CD8α+ DC and CD8α− DC revealed that all DC subpopulations express these enzymes post vaccination while CD11c− cells do not (Fig. S1). Furthermore we show that these same pancreatic enzymes found in murine DC are also found in human monocyte-derived DC (Fig. S2).
Figure 3. Immunization of WT but not MUC1.Tg mice with MUC1p results in up-regulation of pancreatic enzymes in splenic DC.
(A) WT and MUC1.Tg mice were injected i.v with unloaded BMDC (ctrl) or BMDC loaded with MUC1 peptide (MUC1p). 24h later spleens were harvested, pooled according to group, and RNA extracted for qRT-PCR. Arbitrary Units were normalized to WT mice given the ctrl vaccine. Bars represent mean ± SEM. Data are representative of two independent experiments. (B) Splenic DC from unvaccinated mice were isolated with CD11c+ beads (n=3), total splenic T cells were isolated using negative selection via MACS depletion of CD3− cells, and BMDM (MΦ) were cultured for 8 days in the presence of L-cell supernatant as a source of M-CSF. RNA was isolated from all populations for qRT-PCR analysis. Units were normalized to expression levels in CD11c+ cells.Bars represent mean ± SEM. Data representative of two independent experiments. (C) WT and MUC1.Tg mice were immunized as in (A). At 24h, splenic DC were isolated using CD11c+ beads for analysis by qRT-PCR or Western blotting for trypsin and CPB1 (D). Bars represent mean ± SEM after normalization to control vaccination. Data are representative of two (C) and three (D) independent experiments. (E) Mice were immunized i.v. with PBS (ctrl), Poly-ICLC (adj), or soluble MUC1p admixed with Poly-ICLC (MUC1p + Adj). 24h later spleens were harvested for qRT-PCR analysis. Bars represent mean ± SEM normalized to PBS control and are representative of four independent experiments.
To confirm that the enzyme’s expression profiles observed in the whole spleen after immunization of WT and MUC1.Tg mice with MUC1p reflected primarily what was occurring in CD11c+ DC, we repeated the immunizations and 24 hours later examined changes in trypsin and CPB1 expression in purified DC. The failure to up-regulate expression post-immunization was recapitulated in DC recovered from MUC1.Tg mice, while DC isolated from immunized WT mice dramatically increased these transcripts (Fig. 3C). This was also observed at the protein level by Western blotting of whole cell lysates from DC purified from MUC1p-immunized WT and MUC1.Tg mice. CD11c-depleted splenocytes were negative confirming that DC are the main cell population that expresses these enzymes (Fig. 3D).
Finally, we show that immunization with soluble MUC1p admixed with Poly-ICLC adjuvant (a TLR3 agonist) also led to up-regulation of trypsin and CPB1 in WT mice but not in MUC1.Tg mice (Fig. 3E). Adjuvant alone had no effect on these enzymes in either mouse strain. Thus the process is antigen dependent rather than delivery system or adjuvant dependent and it is regulated in all DC rather than only in the exogenous DC delivering the antigen.
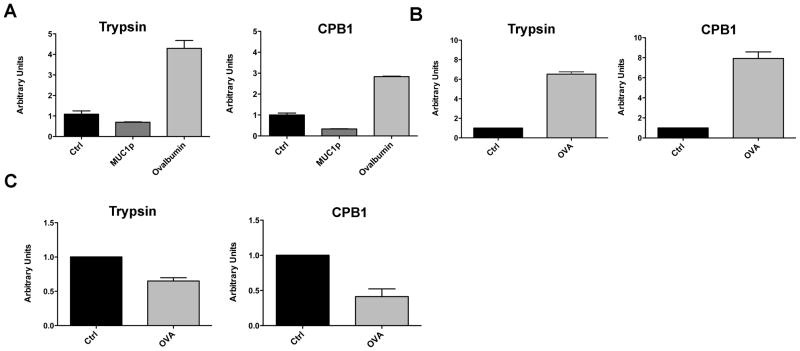
To show that differential regulation of these enzymes in WT and MUC1.Tg mice was driven by exposure to foreign versus self Ag rather than by a physiologic difference between WT and MUC1.Tg mice, we immunized MUC1.Tg mice with OVA, a foreign Ag in that mouse strain, and examined total and DC-specific splenic RNA 24h later. In contrast to MUC1p and control immunized mice, we found up-regulation of enzymes in the total splenic RNA and DC RNA of OVA immunized MUC1.Tg mice (Figs. 4A and 4B).
Figure 4. Failure of DC to up-regulate pancreatic enzymes following immunization with MUC1p as a self-antigen is recapitulated in the OVA model of self-tolerance.
(A) MUC1.Tg mice were immunized i.v. with PBS (ctrl), soluble MUC1p or ovalbumin (OVA) admixed with Poly-ICLC. Spleens were harvested at 24h post immunization and pooled for qRT-PCR analysis. Bars represent mean ± SEM normalized to PBS control. Data are representative of three independent experiments. (B) MUC1.Tg mice were immunized i.v. with unloaded DC (ctrl) or DC loaded with OVA (OVA). 24h post-immunization splenic DC were MACS purified for qRT-PCR analysis. Bars represent mean ± SEM normalized to ctrl. Data are representative of three independent experiments. (C) RIP.OVA mice were immunized and processed as in (B). Bars represent mean ± SEM normalized to ctrl vaccination. Data are representative of two independent experiments.
We also wanted to show this regulation by a self Ag in another model of self-tolerance to be certain that it was not unique to the MUC1.Tg strain or MUC1p as Ag. We immunized RIP.OVA mice, which express the ovalbumin gene under transcriptional control of the rat insulin promoter and are tolerant to OVA protein (32), with DC loaded with OVA. The DC recovered from these mice also failed to up-regulate trypsin and CPB1 (Fig. 4C).
Regulation of expression of pancreatic enzymes in DC is dependent on CD4+ regulatory T cells
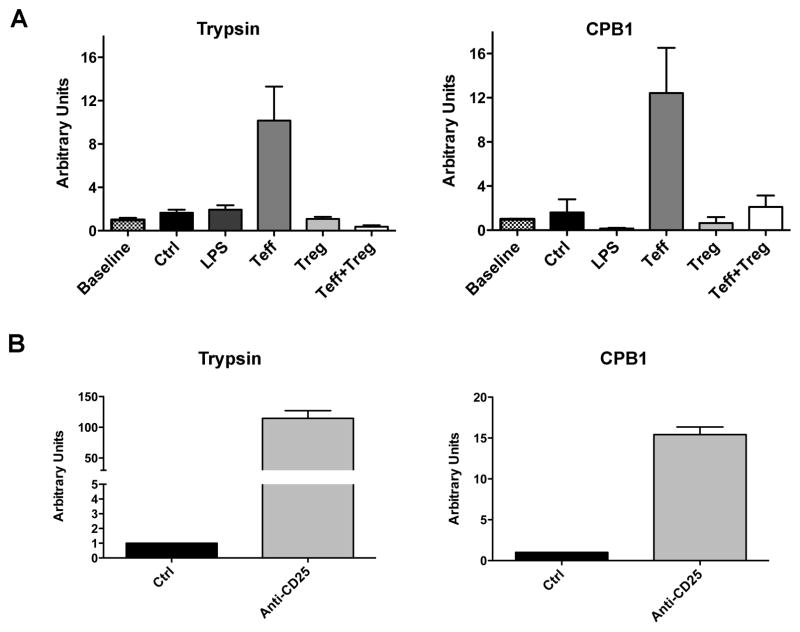
Given the antigen specificity of Treg and their ability to modulate DC phenotype and function (33, 34), we hypothesized that the differential expression of pancreatic enzymes in DC might mark DC that had been acted upon by Treg. We cultured BMDC with bead isolated CD4+ Teff and/or Treg, polyclonally activated with anti-CD3 and anti-CD28 antibodies. After 24 hours of co-culture, we found that DC up-regulated trypsin and CPB1 in the presence of activated Teff, but not in the presence of Treg. Importantly, simultaneous culture of DC with Teff and Treg also resulted in low levels of trypsin and CPB1 in DC, demonstrating that Treg actively suppress the ability of Teff to induce enzyme up-regulation. LPS alone had no effect on enzyme levels. (Fig. 5A)
Figure 5. Interactions between DC and CD4 T cells regulate expression levels of pancreatic enzymes in DC.
(A) DC were cultured alone (ctrl), with LPS, or with polyclonally activated CD25-CD4+ T cells (Teff) and/or CD25+CD4+ T cells (Treg). After 24h of co-culture DC were separated and mRNA was extracted for qRT-PCR analysis. Units were standardized against levels pre-culture (baseline). Bars represent mean ± SEM. Data are representative of two independent experiments. (B) MUC1.Tg mice were treated with an antibody against CD25 to deplete regulatory CD4 T cells (Anti-CD25) or with an isotype control (ctrl). 2 days following depletion, mice were vaccinated with soluble MUC1p plus Poly-ICLC i.v. Splenic RNA was extracted 24h post vaccination for qRT-PCR analysis. Units were standardized against isotype control treated mice. Bars represent mean ± SEM respectively. Data are representative of 3 independent experiments.
To determine if Treg played a similar role in vivo, MUC1.Tg mice were depleted of Treg by injection of anti-CD25 antibody and subsequently vaccinated with soluble MUC1p admixed with Poly-ICLC adjuvant. In control Treg competent mice, we observed the anticipated DC phenotype with suppressed enzyme expression, while DC from immunized Treg-depleted MUC1.Tg mice up-regulated the enzymes (Fig. 5B).
IL-10 is required in vivo for suppression of pancreatic enzyme expression in DC
One of the few transcripts in the gene array data that was higher at 24 hours post vaccination in MUC1.Tg mice compared to WT mice was IL-10 (not shown). To confirm, we vaccinated mice with soluble MUC1p admixed with Poly-ICLC and saw a dramatic increase in IL-10 transcript levels (Fig. 6A). Given the known ability of IL-10 to modulate DC phenotype and function in the direction of tolerance versus immunogenicity (35), we hypothesized that it might also be participating in the suppression of DC pancreatic enzyme levels. Accordingly, we treated MUC1.Tg and WT mice with an antibody against the IL-10 receptor (IL-10R) prior to vaccination with MUC1p (Fig. 6B). Blockade of the IL-10R in vivo resulted in DCs that had equal levels of pancreatic enzymes in both WT and MUC1.Tg mice in response to MUC1p vaccination.
Figure 6. IL-10 is required in vivo for regulation of pancreatic enzymes expression in DC.
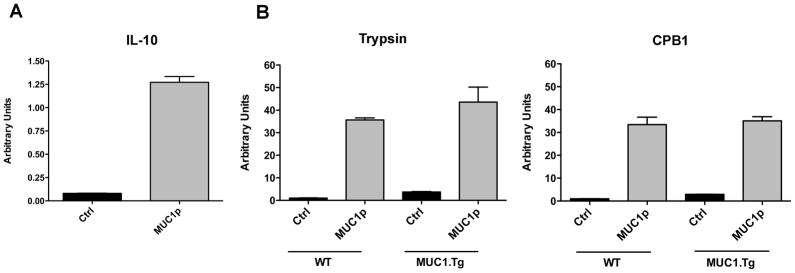
(A) MUC1.Tg mice were immunized with PBS (ctrl) or a soluble MUC1p admixed with Poly-LCIC (MUC1p). IL-10 expression was measured by qRT-PCR on total splenic mRNA 24h post vaccination. Bars represent mean ± SEM. Data are representative of at least 4 independent experiments. (B) WT and MUC1.tg mice were treated with an IL-10R blocking antibody followed by i.v. immunization with PBS (ctrl) or MUC1p as in (A). 24h post vaccination splenic RNA was extracted for qRT-PCR analysis. Units were normalized to WT ctrl. Bars represent mean ± SEM. Data are representative of two independent experiments.
DISCUSSION
Our data reveal the presence of a new pancreatic enzyme signature in DC that may be predictive very early post -vaccination (24–72h) of downstream antigen-specific T cell responses. The enzymes comprising this signature (e.g. trypsin, CPB1, elastase) have well-characterized functions in the pancreas but have not been previously reported in DC. Differential expression of these enzymes in DC following immunization with a self or a foreign Ag was associated with dramatic changes in the immunogenicity of the endogenous splenic DC compartment. A number of other peptidases utilized by DC, especially in the context of antigen processing and presentation, have been characterized (36) and an expanding repertoire of enzymes involved in generating MHCI-restricted peptides is beginning to be elucidated. None of them, however, fall into the category of pancreatic enzymes. Our interest in these enzymes was generated by the observation that their expression levels seen in total spleen gene array were differentially regulated in response to immunization with a self versus a foreign antigen. They are up-regulated following exposure to a foreign antigen (e.g. MUC1p in WT mice) and suppressed following exposure to a self-antigen (e.g. MUC1p in MUC1.Tg mice). Importantly, the signature of pancreatic enzyme expression by DC is not dependent on whether the antigen is also a tumor antigen. Our data show that immunization of RIP.OVA mice with OVA results in a similar failure to up-regulate DC enzymes while vaccination of MUC1.Tg mice induces up-regulation, suggesting that this is a general marker corresponding to the maintenance of self-tolerance rather than a unique characteristic of MUC1-specific immunity. As early as 24h post vaccination and until at least 72h, the differential expression pattern of these enzymes was observed in the total CD11c+ splenic compartment. This was independent of whether antigen was presented on exogenous DC that had taken up and processed the peptide prior to immunization, or as soluble antigen plus adjuvant. This illustrates the fact that both the initial DC presenting the antigen as well as all other DC in the spleen that either gained access to the antigen or were subject to microenvironmental changes, such as increased IL-10 initiated by the antigen, were suppressed presumably in order to not propagate anti-self responses.
Our data suggest that a DC presenting a self-antigen is rapidly affected by interactions with pre-existing Treg specific for that antigen, as depletion of Treg restores antigen-specific up-regulation of pancreatic enzymes. A large number and repertoire of MUC1p-specific Tregs could arise from thymic expression of MUC1 in MUC1.Tg mice (37), or through prior exposure to antigen in a sub-immunogenic setting. We also show that IL-10 is an important soluble regulatory mediator that is likely elicited either directly or indirectly by Treg upon encounter with self-antigen on DC and is involved in the suppression of pancreatic enzyme expression in addition to its well-characterized effects on DC stimulatory capacity and CD80/86 and MHCII expression (38–40)
The most stimulating question is how are the vast majority of splenic DC (and potentially all) simultaneously either prevented from or stimulated to induce an immune response, the surrogate marker of which is up-regulation or lack of expression of pancreatic enzymes. At least two possibilities in addition to diffusion of IL-10 and/or other cytokines exist: 1) highly efficient Ag distribution throughout the spleen such that many DC are presenting self Ag and are therefore individually affected by the action of Treg or T effector cells, and/or 2) highly effective signal transduction to all other DC in the organ from a rare DC that is presenting the antigen and has been affected by Treg or T effector cell. There is support in the literature for both mechanisms (41, 42).
The term “infectious tolerance” has been applied to the process by which one population of leukocytes transfers tolerance to another. In most instances, this involves Treg suppression of T effector cell generation either through a direct contact or through elaboration of regulatory factors (43). Tolerogenic DC have also been implicated because of their ability to promote the generation of iTreg (44, 45). Most of the studies showing these interactions have been performed in vitro and although similar regulation has been postulated in vivo, most data in support of it have been generated by pharmacologic manipulations of the system (46). We suggest that our results provide evidence that infectious tolerance occurs in vivo. We propose a two-step model of infectious tolerance. The first step is a signal to all DC in the lymphoid organ, and presumably other tissues where self-antigens can be processed and presented by DC, to prevent the up-regulation of pancreatic enzymes. This step is immediate and is initiated by the first encounter of a self-antigen-presenting DC and a Treg. The earliest time point we studied was 24h post-vaccination when expression of the enzyme cohort was already suppressed. However, we suspect that the signal is sent much earlier depending on the route of antigen delivery. With an exogenous DC-based vaccine, the antigen is already processed when the DC enters a lymphoid organ such as spleen, and the suppression signal from Treg may be very quickly generated and propagated. In the case of a soluble antigen entering a lymphoid organ, there is likely a minor delay in suppression due to the time it takes for resident DC to take up, process and present the antigen. The second step is delayed and involves the conversion of the DC into a phenotypically and functionally tolerogenic cell that primarily supports generation of Treg. We show that DC recovered from spleens exposed to self Ag through vaccination expressed low levels of costimulatory molecules and had reduced motility, likely resulting in less efficient traffic into T cell zones, and primed the expansion of more Treg than Teff cells when cultured with antigen specific T cells. In vivo, this would assure that self-antigen specific Treg continue to be primed for the duration of antigen exposure, which would likely protect the host from autoimmunity in non-pathologic conditions, but may also be responsible for preventing effective anti-tumor immunity.
We are reporting a new observation that will require further studies to fully elucidate the exact mechanism involved, especially at the level of the regulated DC. We do not know the exact role of pancreatic enzymes in DC, whether they are involved in antigen processing or other DC-intrinsic functions. Nor can we yet postulate how their expression is coordinately regulated. However, the expression levels of trypsin and CPB1 provide an early readout of the effects of self or foreign Ag on the phenotype and function of endogenous splenic DC. The microarray data did not reveal any candidate transcription factors that are differentially expressed in the regulated DC that could be responsible for this enzyme cohort’s transcriptional control. We expect that the 24-hour time point may have been too late for identifying such factor(s). Now that our attention is focused specifically on these enzymes and DC, we will look at much earlier time points. We also have not yet fully explored the role of IL-10 and the precise signals it provides to the DC and how those signals relate to enzyme suppression, or other effects on DC phenotype.
We expect that immune hypo-responsiveness reported to vaccines based on many other tumor associated antigens (47, 48) could also be explained by how similar or different they are from those same antigens expressed on normal cells. The ability of immunization with self-antigens other than MUC1p to tolerize endogenous splenic DC remains to be tested. However, the conservation of enzyme expression patterns between immunizations using different self-antigens leads us to envision a similar conservation in the resultant DC phenotype. Therefore, pancreatic enzyme expression in DC represents a new finding and suggests an easily accessible signature that can be used to assess almost immediately the suitability of a particular antigen and the effect of a particular immune manipulation designed to either induce tolerance or immunity. This can be particularly helpful in animal models where various immunotherapeutic approaches are being tested and multiple approaches compared. Time could be saved and animals spared if the final outcome (e.g. tissue graft acceptance or a tumor rejection) were not the primary, and to date the main endpoints by which the success of the immune manipulation could be evaluated.
Our specific interest is the response to a tumor antigen vaccine and determining how best to evaluate and compare efficacy early after vaccination, rather than waiting for the results of a tumor challenge in an animal or tumor recurrence in a patient. Previous work has shown that various MUC1p-based vaccines can fail to eradicate or slow the growth of MUC1+ tumors in MUC1.Tg mice while remaining effective in WT mice where MUC1p is a foreign antigen (24, 25). Vaccine-induced control of tumor growth is depended on CD4+ T cells which are not fully functional in immunized MUC1.Tg mice compared to WT (23) (21). This study provides evidence that the defect in anti-tumor immunity in MUC1.Tg mice is attributable in part to splenic DC preferentially priming CD4+ T cells into Foxp3+ Treg versus IFNγ+ cells likely via low costimulatory molecule expression and impaired motility.
Our previous studies have emphasized the importance of antigen selection, especially in the case of non-viral tumor associated antigens (49). This study confirms the importance of proper antigen selection that in some cases may outweigh the importance of adjuvants or delivery systems. Among the many tumor associated antigens that have been fully characterized (50), it should be possible to focus on those that are less self and more foreign due to many differences in their post-translational modifications between normal and tumor cells. As we have shown previously, a tumor-specific sugar added to MUC1p to create MUC1.Tn results in strong immunogenicity rather than tolerance in immunized MUC1.Tg mice (51). The wrong antigen or the wrong epitope, on the other hand, leads to DC suppression, infectious tolerance, and further promotion of Treg generation that not only fails to achieve an effective antitumor immune response, but may actually promote tumor growth by selectively expanding tumor-antigen-specific Treg (52). Depletion of Treg with anti-CD25 antibodies or diphtheria toxin have shown a good deal of promise in preclinical models of cancer immunotherapy (53–57). IL-10R blockade has also been shown to improve overall vaccine responses in several models, while IL-10 production, specifically by CD4+CD25+ Treg is negatively correlated with vaccine success (58, 59). We propose that these treatments work because they prevent DC-propagated infectious tolerance.
Supplementary Material
Acknowledgments
We wish to thank Dr. Sean Ryan for early contributions to this project, Ms. Jia Xue for expert technical assistance and Dr. Simon Watkins at the University of Pittsburgh Center for Biologic Imaging for expert advice on live cell imaging.
Footnotes
This work was supported by NIH grants RO1 CA168392 (O.J.F) and T32 CA082084 (A.M.F. and D.M.M.) and the University of Pittsburgh Cancer Center Core Support Grant 2P30 CA47904.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finn OJ. Cancer immunology. N Engl J Med. 2008;358:2704–2715. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- 5.Ohnmacht C, Pullner A, King SBS, Drexler I, Meier S, Brocker T, Voehringer D. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J Exp Med. 2009;206:549–559. doi: 10.1084/jem.20082394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hadeiba H, Lahl K, Edalati A, Oderup C, Habtezion A, Pachynski R, Nguyen L, Ghodsi A, Adler S, Butcher EC. Plasmacytoid dendritic cells transport peripheral antigens to the thymus to promote central tolerance. Immunity. 2012;36:438–450. doi: 10.1016/j.immuni.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 8.Manicassamy S, Pulendran B. Dendritic cell control of tolerogenic responses. Immunological reviews. 2011;241:206–227. doi: 10.1111/j.1600-065X.2011.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evel-Kabler K, Song XT, Aldrich M, Huang XF, Chen SY. SOCS1 restricts dendritic cells’ ability to break self tolerance and induce antitumor immunity by regulating IL-12 production and signaling. J Clin Invest. 2006;116:90–100. doi: 10.1172/JCI26169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Chu N, Rostami A, Zhang GX. Dendritic cells transduced with SOCS-3 exhibit a tolerogenic/DC2 phenotype that directs type 2 Th cell differentiation in vitro and in vivo. J Immunol. 2006;177:1679–1688. doi: 10.4049/jimmunol.177.3.1679. [DOI] [PubMed] [Google Scholar]
- 11.Jonuleit H, Schmitt E, Steinbrink K, Enk AH. Dendritic cells as a tool to induce anergic and regulatory T cells. Trends in Immunology. 2001;22:394–400. doi: 10.1016/s1471-4906(01)01952-4. [DOI] [PubMed] [Google Scholar]
- 12.Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol. 2001;2:725–731. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- 13.Perruche S, Zhang P, Liu Y, Saas P, Bluestone JA, Chen W. CD3-specific antibody-induced immune tolerance involves transforming growth factor-beta from phagocytes digesting apoptotic T cells. Nat Med. 2008;14:528–535. doi: 10.1038/nm1749. [DOI] [PubMed] [Google Scholar]
- 14.Munn DH, Sharma MD, Hou D, Baban B, Lee JR, Antonia SJ, Messina JL, Chandler P, Koni PA, Mellor AL. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest. 2004;114:280–290. doi: 10.1172/JCI21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalinski P. Dendritic cells in immunotherapy of established cancer: Roles of signals 1, 2, 3 and 4. Curr Opin Investig Drugs. 2009;10:526–535. [PMC free article] [PubMed] [Google Scholar]
- 17.Nchinda G, Kuroiwa J, Oks M, Trumpfheller C, Park CG, Huang Y, Hannaman D, Schlesinger SJ, Mizenina O, Nussenzweig MC, Uberla K, Steinman RM. The efficacy of DNA vaccination is enhanced in mice by targeting the encoded protein to dendritic cells. J Clin Invest. 2008;118:1427–1436. doi: 10.1172/JCI34224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mullins DW, Sheasley SL, Ream RM, Bullock TN, Fu YX, Engelhard VH. Route of immunization with peptide-pulsed dendritic cells controls the distribution of memory and effector T cells in lymphoid tissues and determines the pattern of regional tumor control. J Exp Med. 2003;198:1023–1034. doi: 10.1084/jem.20021348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kleindienst P, Brocker T. Endogenous dendritic cells are required for amplification of T cell responses induced by dendritic cell vaccines in vivo. J Immunol. 2003;170:2817–2823. doi: 10.4049/jimmunol.170.6.2817. [DOI] [PubMed] [Google Scholar]
- 20.Yewdall AW, Drutman SB, Jinwala F, Bahjat KS, Bhardwaj N. CD8+ T cell priming by dendritic cell vaccines requires antigen transfer to endogenous antigen presenting cells. PLoS ONE. 2010;5:e11144. doi: 10.1371/journal.pone.0011144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turner MS, Cohen PA, Finn OJ. Lack of effective MUC1 tumor antigen-specific immunity in MUC1-transgenic mice results from a Th/T regulatory cell imbalance that can be corrected by adoptive transfer of wild-type Th cells. J Immunol. 2007;178:2787–2793. doi: 10.4049/jimmunol.178.5.2787. [DOI] [PubMed] [Google Scholar]
- 22.Ryan SO, Turner MS, Gariépy J, Finn OJ. Tumor antigen epitopes interpreted by the immune system as self or abnormal-self differentially affect cancer vaccine responses. Cancer Res. 2010;70:5788–5796. doi: 10.1158/0008-5472.CAN-09-4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tempero RM, VanLith ML, Morikane K, Rowse GJ, Gendler SJ, Hollingsworth MA. CD4+ Lymphocytes Provide MUC1-Specific Tumor Immunity In Vivo That Is Undetectable In Vitro and Is Absent in MUC1 Transgenic Mice. The Journal of Immunology. 1998;161:5500–5506. [PubMed] [Google Scholar]
- 24.Mukherjee P, Madsen CS, Ginardi AR, Tinder TL, Jacobs F, Parker J, Agrawal B, Longenecker BM, Gendler SJ. Mucin 1-specific immunotherapy in a mouse model of spontaneous breast cancer. J Immunother. 2003;26:47–62. doi: 10.1097/00002371-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Soares MM, Mehta V, Finn OJ. Three different vaccines based on the 140-amino acid MUC1 peptide with seven tandemly repeated tumor-specific epitopes elicit distinct immune effector mechanisms in wild-type versus MUC1-transgenic mice with different potential for tumor rejection. J Immunol. 2001;166:6555–6563. doi: 10.4049/jimmunol.166.11.6555. [DOI] [PubMed] [Google Scholar]
- 26.Peat N, Gendler SJ, Lalani N, Duhig T, Taylor-Papadimitriou J. Tissue-specific expression of a human polymorphic epithelial mucin (MUC1) in transgenic mice. Cancer Res. 1992;52:1954–1960. [PubMed] [Google Scholar]
- 27.Jordan R, Patel S, Hu H, Lyons-Weiler J. Efficiency analysis of competing tests for finding differentially expressed genes in lung adenocarcinoma. Cancer Inform. 2008;6:389–421. doi: 10.4137/cin.s791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kingston D, Schmid MA, Onai N, Obata-Onai A, Baumjohann D, Manz MG. The concerted action of GM-CSF and Flt3-ligand on in vivo dendritic cell homeostasis. Blood. 2009;114:835–843. doi: 10.1182/blood-2009-02-206318. [DOI] [PubMed] [Google Scholar]
- 29.Miller MJ, Hejazi AS, Wei SH, Cahalan MD, Parker I. T cell repertoire scanning is promoted by dynamic dendritic cell behavior and random T cell motility in the lymph node. Proc Natl Acad Sci U S A. 2004;101:998–1003. doi: 10.1073/pnas.0306407101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zou T, Caton AJ, Koretzky GA, Kambayashi T. Dendritic cells induce regulatory T cell proliferation through antigen-dependent and -independent interactions. J Immunol. 2010;185:2790–2799. doi: 10.4049/jimmunol.0903740. [DOI] [PubMed] [Google Scholar]
- 31.Faure-André G, Vargas P, Yuseff MI, Heuzé M, Diaz J, Lankar D, Steri V, Manry J, Hugues S, Vascotto F, Boulanger J, Raposo G, Bono MR, Rosemblatt M, Piel M, Lennon-Duménil AM. Regulation of dendritic cell migration by CD74, the MHC class II-associated invariant chain. Science. 2008;322:1705–1710. doi: 10.1126/science.1159894. [DOI] [PubMed] [Google Scholar]
- 32.de Witte MA, Coccoris M, Wolkers MC, van den Boom MD, Mesman EM, Song JY, van der Valk M, Haanen JBAG, Schumacher TNM. Targeting self-antigens through allogeneic TCR gene transfer. Blood. 2006;108:870–877. doi: 10.1182/blood-2005-08-009357. [DOI] [PubMed] [Google Scholar]
- 33.Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T. Regulatory T cells: how do they suppress immune responses? Int Immunol. 2009;21:1105–1111. doi: 10.1093/intimm/dxp095. [DOI] [PubMed] [Google Scholar]
- 34.Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, Baker J, Jeffery LE, Kaur S, Briggs Z, Hou TZ, Futter CE, Anderson G, Walker LS, Sansom DM. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Smedt T, Van Mechelen M, De Becker G, Urbain J, Leo O, Moser M. Effect of interleukin-10 on dendritic cell maturation and function. Eur J Immunol. 1997;27:1229–1235. doi: 10.1002/eji.1830270526. [DOI] [PubMed] [Google Scholar]
- 36.Jensen PE. Recent advances in antigen processing and presentation. Nat Immunol. 2007;8:1041–1048. doi: 10.1038/ni1516. [DOI] [PubMed] [Google Scholar]
- 37.Cloosen S, Arnold J, Thio M, Bos GM, Kyewski B, Germeraad WT. Expression of tumor-associated differentiation antigens, MUC1 glycoforms and CEA, in human thymic epithelial cells: implications for self-tolerance and tumor therapy. Cancer Res. 2007;67:3919–3926. doi: 10.1158/0008-5472.CAN-06-2112. [DOI] [PubMed] [Google Scholar]
- 38.Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997;159:4772–4780. [PubMed] [Google Scholar]
- 39.De Smedt T, Van Mechelen M, De Becker G, Urbain J, Leo O, Moser M. Effect of interleukin-10 on dendritic cell maturation and function. European Journal of Immunology. 1997;27:1229–1235. doi: 10.1002/eji.1830270526. [DOI] [PubMed] [Google Scholar]
- 40.Brossart P, Zobywalski A, Grünebach F, Behnke L, Stuhler G, Reichardt VL, Kanz L, Brugger W. Tumor Necrosis Factor α and CD40 Ligand Antagonize the Inhibitory Effects of Interleukin 10 on T-Cell Stimulatory Capacity of Dendritic Cells. Cancer Research. 2000;60:4485–4492. [PubMed] [Google Scholar]
- 41.Lindquist RL, Shakhar G, Dudziak D, Wardemann H, Eisenreich T, Dustin ML, Nussenzweig MC. Visualizing dendritic cell networks in vivo. Nat Immunol. 2004;5:1243–1250. doi: 10.1038/ni1139. [DOI] [PubMed] [Google Scholar]
- 42.Watkins SC, Salter RD. Functional connectivity between immune cells mediated by tunneling nanotubules. Immunity. 2005;23:309–318. doi: 10.1016/j.immuni.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 43.Kendal AR, Waldmann H. Infectious tolerance: therapeutic potential. Curr Opin Immunol. 2010;22:560–565. doi: 10.1016/j.coi.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 44.Tarbell KV, Yamazaki S, Olson K, Toy P, Steinman RM. CD25+ CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J Exp Med. 2004;199:1467–1477. doi: 10.1084/jem.20040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turley DM, Miller SD. Peripheral tolerance induction using ethylenecarbodiimide-fixed APCs uses both direct and indirect mechanisms of antigen presentation for prevention of experimental autoimmune encephalomyelitis. J Immunol. 2007;178:2212–2220. doi: 10.4049/jimmunol.178.4.2212. [DOI] [PubMed] [Google Scholar]
- 46.Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7:610–621. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- 47.Degl’Innocenti E, Grioni M, Capuano G, Jachetti E, Freschi M, Bertilaccio MT, Hess-Michelini R, Doglioni C, Bellone M. Peripheral T-cell tolerance associated with prostate cancer is independent from CD4+CD25+ regulatory T cells. Cancer Res. 2008;68:292–300. doi: 10.1158/0008-5472.CAN-07-2429. [DOI] [PubMed] [Google Scholar]
- 48.Gritzapis AD, I, Voutsas F, Lekka E, Papamichail M, Baxevanis CN. Peptide vaccination breaks tolerance to HER-2/neu by generating vaccine-specific FasL(+) CD4(+) T cells: first evidence for intratumor apoptotic regulatory T cells. Cancer Res. 2010;70:2686–2696. doi: 10.1158/0008-5472.CAN-09-2517. [DOI] [PubMed] [Google Scholar]
- 49.Farkas AM, Finn OJ. Vaccines based on abnormal self-antigens as tumor-associated antigens: immune regulation. Semin Immunol. 2010;22:125–131. doi: 10.1016/j.smim.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 50.Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, Matrisian LM. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ryan SO, Vlad AM, Islam K, Gariépy J, Finn OJ. Tumor-associated MUC1 glycopeptide epitopes are not subject to self-tolerance and improve responses to MUC1 peptide epitopes in MUC1 transgenic mice. Biol Chem. 2009;390:611–618. doi: 10.1515/BC.2009.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beyer M, Schultze JL. Regulatory T cells in cancer. Blood. 2006;108:804–811. doi: 10.1182/blood-2006-02-002774. [DOI] [PubMed] [Google Scholar]
- 53.Klages K, Mayer CT, Lahl K, Loddenkemper C, Teng MWL, Ngiow SF, Smyth MJ, Hamann A, Huehn J, Sparwasser T. Selective Depletion of Foxp3+ Regulatory T Cells Improves Effective Therapeutic Vaccination against Established Melanoma. Cancer Research. 2010;70:7788–7799. doi: 10.1158/0008-5472.CAN-10-1736. [DOI] [PubMed] [Google Scholar]
- 54.Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor Rejection by in Vivo Administration of Anti-CD25 (Interleukin-2 Receptor α) Monoclonal Antibody. Cancer Research. 1999;59:3128–3133. [PubMed] [Google Scholar]
- 55.Sutmuller RPM, van Duivenvoorde LM, van Elsas A, Schumacher TNM, Wildenberg ME, Allison JP, Toes REM, Offringa R, Melief CJM. Synergism of Cytotoxic T Lymphocyte–Associated Antigen 4 Blockade and Depletion of Cd25+ Regulatory T Cells in Antitumor Therapy Reveals Alternative Pathways for Suppression of Autoreactive Cytotoxic T Lymphocyte Responses. The Journal of Experimental Medicine. 2001;194:823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Viehl C, Moore T, Liyanage U, Frey D, Ehlers J, Eberlein T, Goedegebuure P, Linehan D. Depletion of CD4+CD25+ Regulatory T Cells Promotes a Tumor-Specific Immune Response in Pancreas Cancer–Bearing Mice. Annals of Surgical Oncology. 2006;13:1252–1258. doi: 10.1245/s10434-006-9015-y. [DOI] [PubMed] [Google Scholar]
- 57.Casares N, Arribillaga L, Sarobe P, Dotor J, Lopez-Diaz de Cerio A, Melero I, Prieto J, Borrás-Cuesta F, Lasarte JJ. CD4+/CD25+ Regulatory Cells Inhibit Activation of Tumor-Primed CD4+ T Cells with IFN-γ-Dependent Antiangiogenic Activity, as well as Long-Lasting Tumor Immunity Elicited by Peptide Vaccination. The Journal of Immunology. 2003;171:5931–5939. doi: 10.4049/jimmunol.171.11.5931. [DOI] [PubMed] [Google Scholar]
- 58.Yang H, Guimaraes-Walker A, Hibbs S, Dong T, Stacey A, Borrow P, Hanke T, Davenport MP, McMichael A, Dorrell L. Interleukin-10 responses to therapeutic vaccination during highly active antiretroviral therapy and after analytical therapy interruption. AIDS. 2009;23:2226–2230. doi: 10.1097/QAD.0b013e328331a424. [DOI] [PubMed] [Google Scholar]
- 59.Brooks DG, Lee AM, Elsaesser H, McGavern DB, Oldstone MB. IL-10 blockade facilitates DNA vaccine-induced T cell responses and enhances clearance of persistent virus infection. J Exp Med. 2008;205:533–541. doi: 10.1084/jem.20071948. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.