Abstract
Cytomegalovirus’s (CMV’s) unique ability to drive the expansion of virus-specific T-cell populations over the course of a lifelong, persistent infection has generated interest in the virus as a potential vaccine strategy. When designing CMV-based vaccine vectors to direct immune responses against HIV or tumor antigens, it becomes important to understand how and why certain CMV-specific populations are chosen to inflate over time. To investigate this, we designed recombinant murine cytomegaloviruses (MCMV) encoding a SIINFEKL-eGFP fusion protein under the control of endogenous immediate early promoters. When mice were infected with these viruses, T cells specific for the SIINFEKL epitope inflated and profoundly dominated T cells specific for non-recombinant (i.e. MCMV-derived) antigens. Moreover, when the virus encoded SIINFEKL, T cells specific for non-recombinant antigens displayed a phenotype indicative of less frequent exposure to antigen. The immunodominance of SIINFEKL-specific T cells could not be altered by decreasing the number of SIINFEKL-specific cells available to respond, or by increasing the number of cells specific for endogenous MCMV antigens. In contrast, coinfection with viruses expressing and lacking SIINFEKL enabled co-inflation of T cells specific for both SIINFEKL and non-recombinant antigens. Because coinfection allows presentation of SIINFEKL and MCMV-derived antigens by different cells within the same animal, these data reveal that competition for, or availability of, antigen at the level of the antigen presenting cell determines the composition of the inflationary response to MCMV. SIINFEKL’s strong affinity for H2-Kb, and its early and abundant expression, may provide this epitope’s competitive advantage.
Introduction
Cytomegalovirus (CMV) establishes an asymptomatic latent or persistent infection, which is characterized by the lifelong accumulation of a large number of virus-specific T cells. This process is termed memory inflation, and has led to the exploration of CMV as a vaccine vector for HIV and for tumor antigens, with significant initial success in the SIV model (1, 2). The fact that memory inflation occurs after infection with a single-cycle CMV (3) indicates that CMV-based vaccines may be safely used even in immunosuppressed cancer patients, further increasing the appeal of this approach. The vaccine potential of this virus has elevated the importance of understanding how inflationary CMV-specific responses are selected and maintained during infection.
C57BL/6 mice mount a response to at least 20 viral antigens during acute infection with murine CMV (MCMV) (4). Most of these responses, including those to the immunodominant M45 antigen, then decline precipitously and leave small central memory (TCM) populations. In contrast, memory inflation is dominated by only three responses: those to M38, m139 and IE3, all of which are subdominant to M45 during acute infection (5). These same three epitopes display memory inflation after infection with the single cycle ΔgL-MCMV (3), which implies that non-productively infected cells harboring the viral genome can drive memory inflation.
We presume that ongoing presentation of viral epitopes must be involved in memory inflation. We have shown that memory inflation is sustained by repeated production of short-lived effectors derived from a pool of memory cells established early in infection (6). However, the reason that inflationary responses focus on just a few antigens is not well understood.
MCMV has a highly ordered sequence of lytic cycle gene expression, which starts with the transcription of Immediate Early (IE) genes and is followed by the synthesis of Early (E) and then Late (L) gene products. However, latent MCMV infection in the lungs and liver is characterized by sporadic expression of IE genes without evidence of E or L gene expression (7, 8). This is thought to be abortive reactivation, in which the virus initiates the standard lytic gene cascade, but gene expression is aborted at the IE stage (9). This scenario predicts that IE gene products would be the most abundant during latent infection and thus immunodominant, which is at least partly the case: IE3 becomes progressively more immunodominant over time in B6 mice, and pp89 (IE1)-specific responses inflate somewhat more than those specific for the E antigen m164 in BALB/c mice. Furthermore, recombinant epitopes expressed behind IE promoters provoke inflationary responses (10). However, M38 and m139, both E antigens, also provoke immunodominant inflationary responses in B6 mice, as does m164 in BALB/c mice (5). Likewise in humans, T cells target epitopes expressed with IE, E and L kinetics (11) and cells specific for the L gene product pp65 are frequently immunodominant (12–14). The viral gene expression program that drives these diverse responses is not yet clear.
Our data suggest that viral gene expression, and not productive replication, is sufficient to promote inflation of T cells specific for E gene products. This is evidenced by the ability of a single cycle ΔgL-MCMV to stimulate inflation of T cells specific for the E genes M38, m139 and m164 (3). Abortive reactivation may sometimes proceed to expression of E genes, as suggested by Simon et. al. (9). An alternate possibility is that a completely different gene expression program occurs in some infected cells. Indeed, in the rat CMV heart transplant model, expression of a subset of E genes without production of infectious virus has been described (15). It is interesting that this “persistent” pattern of gene expression involved very little IE gene expression. Similarly, expression of some viral genes in the absence of IE gene expression is reported in monocytes latently infected with human CMV (16). Hence, inflationary responses to E epitopes may be driven by different cells harboring a different program of gene expression than those that drive the IE responses.
There is also some evidence that T cells can influence the pattern of immunodominance during memory inflation. Indeed, Holtappels et. al. (17) described a “conditional” immunodominant response specific for the viral m145 gene product in Balb/c mice, which appeared when the immunodominant m164- and IE1-derived epitopes were deleted. In line with this, Simon et. al. have suggested that T cells directly limit the cascade of viral gene expression (9). Thus, immunodominant T cell responses may restrict other epitopes from being produced. Inflationary T cell responses of particularly high avidity, either due to expression of high affinity T cell receptors (TCRs), or to abundant antigen expression, might enforce a selective advantage by suppressing expression of additional epitopes.
Here, we describe memory inflation in response to recombinant MCMVs that encode a SIINFEKL-GFP fusion protein under immediate early control. Not only did SIINFEKL promote memory inflation, it became the sole inflationary epitope during chronic infection. We used this model to explore the determinants of immunodominance in the inflationary T cell response to MCMV.
Materials and Methods
Mice
C57BL/6 mice were purchased from The Jackson Laboratory. B6.SJL-CD45.1 congenic (B6.SJL-Ptprca Pepcb/BoyJ) mice were also purchased from The Jackson Laboratory and bred to C57BL/6 mice in house to generate CD45.1/CD45.2 F1 mice as recipients for adoptive transfer experiments. OVA-Tg mice were bred from the B6.FVB-Tg(MMTV-neu/OT-I/OT-II)CBnel Tg(Trp53R172H)8512Jmr/J strain to express the Erbb2/HER-2/neu oncogene tagged with ovalbumin epitopes recognized by the OT-I and OT-II, but not the Trp53 gene (18). Breeders of this strain were obtained from The Jackson Laboratory. Mice were between the ages of 6 and 16 weeks upon infection. All studies were approved by the Institutional Biosafety Committee and the Institutional Animal Care and Use Committee at Oregon Health and Sciences University.
Virus Strains and Infections
Mice were infected i.p. with 2 × 105 PFU of virus, except in coinfection experiments, where mice were infected with 1 × 105 PFU of each virus used. Virus labeled MCMV-WT BAC was of the strain MW97.01, which is derived from a bacterial artificial chromosome of the Smith strain (19). MCMV-GFP-SL8 and MCMV-GFP-MSL8 were generated on the MW97.01 backbone. In both recombinant viruses, the SIINFEKL peptide plus 7 N-terminal amino acids from ovalbumin (SGLEQLESIINFEKL, to facilitate normal peptide excision, (20)) were fused to the C-terminal end of eGFP. In the case of MCMV-GFP-SL8, this fusion construct was targeted to replace the m128 (IE2) gene, under the control of the IE2 promoter, using established techniques (21). In the case of MCMV-GFP-MSL8, the eGFP-SL8 fusion construct was encoded with the Major Immediate Early promoter (MIEP) of HCMV and targeted to replace exon 3 of the m128 gene in MCMV. Stocks of these viruses were produced from murine embryonic fibroblasts and titered by plaque assay on Balb3T3s without centrifugal enhancement.
To produce the ΔgL viruses, an ampicillin gene fragment was inserted into the M115 (gL) gene of the MCMV-WT BAC (strain MW97.01, (22)) using homologous recombination. Stocks of this virus were produced on gL-3T3 cells, which provide gL in trans (3), and titered by plaque assay on gL-3T3s without centrifugal enhancement. The individual virus stock used in figure 3 was checked for reversion by infecting murine embryonic fibroblasts, a non complementing cell line, then passaging and monitoring these infected cells for 30 days. The growth of cells not infected by the initial inoculum confirmed the inability of the this gL-deficient virus to spread from cell to cell.
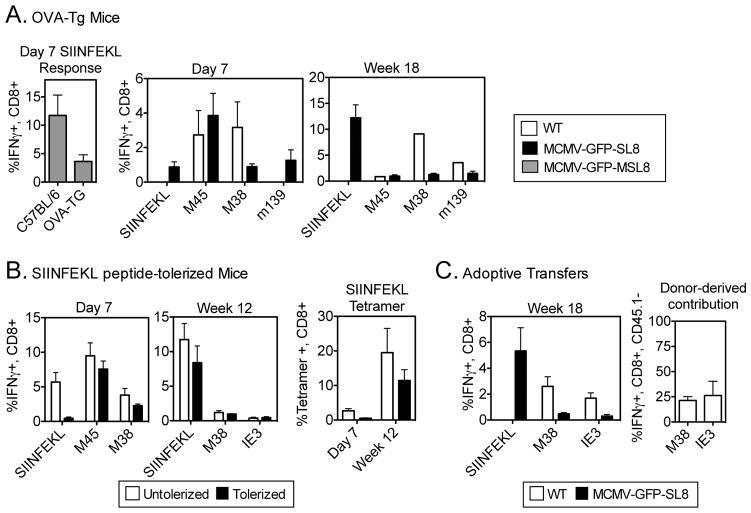
Figure 3. Precursor frequency does not contribute significantly to the immunodominance of SIINFEKL-specific CD8 T-cell responses in chronic infection.
(A) OVA Tg mice were infected i.p. with the indicated viruses. Virus-specific CD8 T-cells were measured in the blood on day 7 and at week 18 post infection using intracellular cytokine staining. (B) C57BL/6 mice were injected with 10μg SIINFEKL peptide i.v. on days -3,-2 and -1 prior to infection with MCMV-GFP-SL8. Responses were measured in the blood on day 7 and at week 18 post infection (C) CD45.2+, CD45.1+ naive recipients received 2–5×107 unfractionated splenocytes from mice infected for 7 days with WT MCMV. Recipients were infected with WT MCMV or with MCMV-GFP-SL8 and virus -specific responses were measured in the blood at week 18 post infection. Total CD8 T-cell responses are shown on the left and percentages of CD45.2-negative donor cells contributing to either IE3 or M38 responses are shown on the right. Bars represent 4–5 mice per group. Experiments were done twice.
Intracellular Cytokine Staining and FACS Analysis
For measurement of intracellular IFN-γ, peripheral blood was collected at the indicated time points. Red blood cells were lysed with 3 ml of lysis buffer (150 mM NH4Cl, 10 mM NaHCO3) and the remaining cells were incubated for 5–6 hrs at 37°C in the presence of 10μM of the indicated peptide and brefeldin A (GolgiPlug; BD Pharmingen). Surface staining was done overnight at 4°C, and cells were fixed and permeabilized for intracellular cytokine staining with Cytofix/Cytoperm (BD Pharmingen). The following fluorescently conjugated antibodies were used (CD8α [clone 53-6.7], CD27 [clone LG.7F9], CD3 [clone 145-2C11], CD127[clone A7R34], KLRG1 [clone 2F1], IFN-γ [clone XMG1.2]), and all purchased from either BD Biosciences, eBioscience, or BioLegend. Samples were acquired on an LSR II or a FACSCalibur (both BD) and analyzed with FLowJo software (Tree Star).
Adoptive Transfers
Splenocytes from congenic mice infected for 7 days with MCMV-WT BAC were harvested, passed through a 70 μm cell strainer, washed twice with T cell media (RPMI 1640 with L-glutamine + 10% FBS + 1% penicillin/streptomycin + 5 × 10−5 M β-mercaptoethanol) and resuspended in PBS at 5 × 108 cells/ml. 100μl of this unfractionated splenocyte suspension was injected into each congenic recipient via the retro-orbital route. These mice were infected with either MCMV-GFP-SL8 or MCMV WT-BAC the following day.
RMA-S Peptide Binding and Stabilization Assays
For binding assays, TAP-deficient RMA-S cells were plated at 1×105 cells/well in 96-well plates and cultured for 16 h at 25 °C in T cell media buffered with 25mM HEPES. The cells were then washed with T cell media, incubated with different concentrations of the indicated peptides at 25 °C for 2 hours, and then incubated for an additional 2 hours at 37 °C. After this incubation, cells were washed once and stained on ice for 1 hour with PE-conjugated Y3 mAb, which binds to the class I MHC H-2Kb. The cells were then washed twice with PBS, fixed with BD Fix/Perm solution, and analyzed on a BD FACSCalibur.
Quantitative Real-time PCR
1 × 106 murine embryonic fibroblasts were infected with WT MCMV or MCMV-GFP-SL8 at a multiplicity of infection of 10. Cells were harvested at 0, 1, 2, 3, 4, 8, 18, and 24 hours post infection, and RNA was extracted using the Qiagen RNeasy Mini Kit (Qiagen). On-column DNAse treatment was performed as described in the Qiagen protocol. cDNA was generated using the Invitrogen SuperScript III First-Strand Synthesis SuperMix. A portion of each sample was treated similarly, but without the addition of reverse transcriptase to ensure that there was no DNA contamination. cDNA was then stored at −20 °C. Quantitative PCR was performed using Platinum SYBR green qPCR SuperMix UGD with ROX, using the primers at a concentration of 250nM. The samples were run on an ABI PRISM 7700 Sequence Detection System. Relative gene expression was determined by normalizing each gene to β-actin, and comparing the gene expression relative to cells at 0 h. The calculations were made following the method described in the User Bulletin Number 2: ABI Prism 7700 sequence detection system; subject, relative quanititation of gene expression (Applied Biosystems). Primer sequences follow. SL8 F: ACGTAAACGGCCACAAGTTC, SL8 R: TGAACTTCAGGGTCAGCTTG, IE3 F: GATTCAACCCGCCTGTTATG, IE3 R: GATAATTCAGGCAGCCAACC, M38 F:TCGATATTGAGCTGCTTGA, M38 R: CCCAGCCTGCAAGACTTC, m139 F: GCGCTCTGTGACAGAGTTT, m139 R: ACGAGCAACAACATGGAA.
Results
SIINFEKL-specific CD8+ T cells dominate memory inflation after infection with MCMV-GFP-SL8
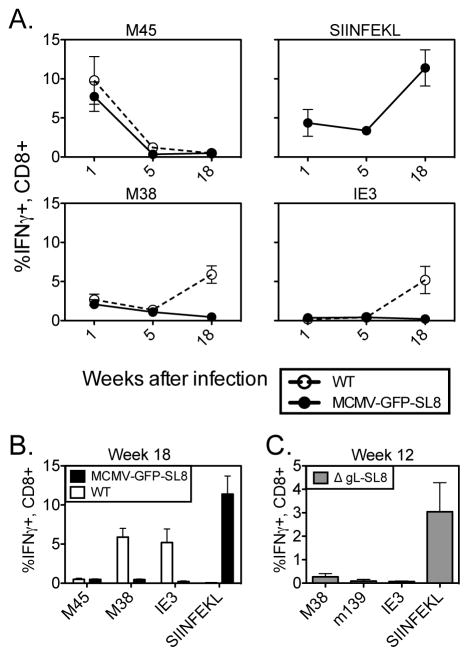
We generated a recombinant strain of MCMV expressing a GFP-SIINFEKL fusion construct under the control of the endogenous MCMV IE2 promoter (Turula et. al., manuscript submitted). After infection with this virus (MCMV-GFP-SL8), the SIINFEKL-specific CD8 T cell responses in B6 mice steadily inflated over time, becoming the dominant inflationary T cell population in these animals at chronic time points (Figures 1A and B). We also generated a virus in which the GFP-SIINFEKL fusion construct is under control of the HCMV Major Immediate Early promoter (MCMV-GFP-MSL8), resulting in approximately 10–20 fold greater GFP fluorescence after in vitro infection (not shown). SIINFEKL-specific T-cells dominated the inflationary response in mice infected with this virus as well (data not shown). Responses to IE3- and M38-derived peptides were barely detectable in these animals, whereas T cells specific for these epitopes each comprised approximately 5% of the CD8 T-cell compartment in mice infected with WT MCMV (Figure 1A and (5)). Notably, the size of M38- and IE3-specific T cell populations were similarly reduced when measured as a frequency of all cells in the blood (not shown). Moreover, the proportion of CD8+ T cells specific for M45, which are resting memory cells that do not inflate during MCMV infection, were comparable in mice infected with either virus (Figure 1 and not shown). These data indicate that proportional changes in T-cell numbers cannot explain the disappearance of IE3 and M38 inflation. Thus, the presence of the SIINFEKL epitope and the resulting T cell response suppressed inflation of IE3- and M38-specific T cells, despite evidence that M38-specific T cells were successfully primed during acute infection.
Figure 1. SL8 is profoundly immunodominant over normal responses to MCMV.
(A) C57BL/6 mice were infected i.p with the indicated MCMV viruses. Virus-specific T-cells were measured in the blood at the indicated times post infection using intracellular cytokine staining. (B) Individual responses from the two infections in part A are contrasted at week 18 post infection. (C) Mice were infected with ΔgL-MCMV and CD8 T-cell responses to the indicated epitopes were measured at Week 12 post infection using intracellular cytokine staining. Individual plot points and bars represent 4–5 mice per group. Experiments were done twice.
SIINFEKL responses dominate memory inflation when SIINFEKL is expressed in a single cycle MCMV
To determine whether the profound immunodominance of SIINFEKL would also occur in a single cycle MCMV, we produced a version of MCMV-SL8-GFP lacking gL, a glycoprotein necessary for cell entry and spread. The ΔgL-SL8 virus was confirmed to be spread-deficient as described in the methods, but still induced SIINFEKL-specific T cells to inflate and become dominant (Figure 1C), indicating that productive infection is not needed for the immunodominance of this response.
Phenotype of cells specific for inflationary epitopes after infection with MCMV-GFP-SL8
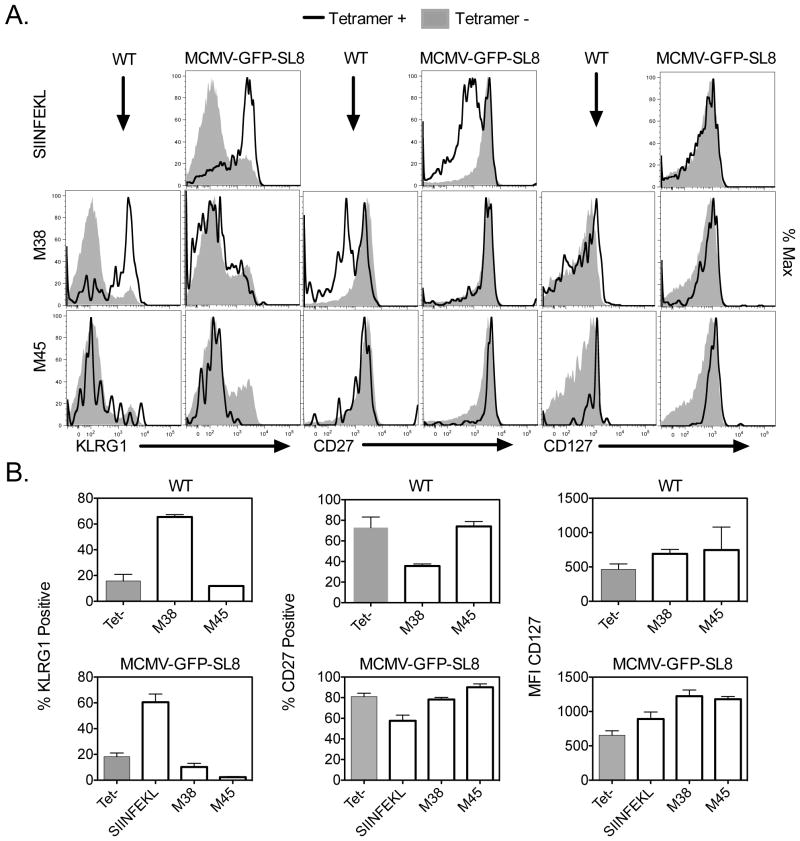
Inflationary CD8+ T cells express KLRG1, and have low levels of the IL-7 receptor (CD127) and the costimulatory molecule CD27 (6). This terminally differentiated effector phenotype is consistent with recent or repeated antigen exposure. Conversely, T cells comprising the memory response to non-inflationary epitopes M45 and M57 exhibit a memory phenotype (KLRG1−, CD27+ and CD127+), which suggests that they are rarely exposed to antigen after the acute phase of infection. Because responses to M38 and IE3 contract sharply after acute MCMV-GFP-SL8 infection, we wondered whether they would also develop a memory phenotype.
Figure 2 shows that SIINFEKL-specific CD8’s at week 18 post infection exhibit the classic phenotype of inflationary MCMV T cells, with upregulated expression of KLRG1 and downregulation of CD27 and CD127. In contrast, T cells specific for M45 mostly lacked KLRG1 and retained expression of CD127 and CD27, although some cells were KLRG1+. This is similar to their phenotype in WT infection. Strikingly, the small M38-specific population found in MCMV-GFP-SL8 infected mice had a similar phenotype to the M45-specific cells: most cells lacked KLRG1, and retained CD27 and high levels of CD127. IE3-specific cells were so infrequent that an accurate assessment of their phenotype was impossible. These results suggest that SIINFEKL-specific cells have seen antigen recently or repeatedly and that M45- and M38-specific cells encounter antigen rarely.
Figure 2. Phenotype of SIINFEKL-specific and MCMV epitope-specific responses in chronic infection.
(A) Splenocytes from mice infected for greater than 18 weeks with the indicated viruses were stained with SL8 or MCMV-specific tetramers and for the indicated surface markers. The plots shown are gated on Tetramer+ CD8+ cells (black line) or Tetramer- CD8+ cells (shaded histogram). Plots represent one mouse, which is representative of two experiments with 3–4 mice per group. (B) Averages of the percent KLRG1 positive, percent CD27 positive, or CD127 mean fluorescence intensity of tetramer positive and tetramer negative populations from the splenocytes collected in part A. Individual bars represent 3–4 mice per group. Experiment was done twice.
Altering the ratios of functional, epitope-specific cells available to respond to infection does not influence the immunodominance of SIINFEKL-specific T cells
The precursor frequency of antigen-specific T cells - either naïve or memory - is a major determinant of immunodominance during acute infections, and also affects proliferation and memory CD8 T-cell lineage decisions (23). We wondered whether we could modify the immunodominance of the SIINFEKL response during chronic infection by altering the ratios of functional, epitope-specific CD8 T-cells prior to infection. We explored this possibility in three ways.
First, we used mice that express OVA as a self-antigen behind the Mouse Mammary Tumor Virus promoter. When these mice were infected with MCMV-GFP-MSL8, the acute response to SIINFEKL was approximately one third of that in WT mice (Figure 3A), consistent with a lower number of SIINFEKL-specific precursors. Nevertheless, during chronic infection with either MCMV-GFP-MSL8 (not shown) or MCMV-GFP-SL8, the SIINFEKL response inflated at the expense of the M38 and m139 responses (Figure 3A).
Next, we reduced the number of naïve CD8+ T-cells capable of responding to SIINFEKL during acute infection by intravenous injection of SIINFEKL peptide prior to infection. Intravenous peptide provides a large amount of antigen (signal 1) in the absence of costimulation (signal 2), resulting in anergy or deletion of cognate T cells (24–26). Mice were injected i.v. with 10μg of SIINFEKL peptide on each of the 3 days prior to infection. SIINFEKL-specific T cells were not detected by ICS or tetramer staining 7 days post-infection, indicating profound suppression and probable deletion of SIINFEKL-specific cells, whereas T cells specific for MCMV epitopes were primed normally (Figure 3B). However, by week 12, SIINFEKL responses had risen to the same percentage of total CD8s as those of mice left untreated, and responses to IE3 and M38 were barely detectable (Figure 3B).
In a third experiment, we asked whether increasing the number of T cells available to respond to IE3 and M38 would enable those responses to inflate after infection with the SIINFEKL-expressing virus. Splenocytes from CD45.2+ donor mice that had been infected with WT MCMV 7 days previously were adoptively transferred into CD45.1+CD45.2+ F1 naïve recipients. These mice were then infected with MCMV-GFP-SL8. A control group received splenocytes from the same donors, but was infected with WT MCMV instead. Figure 3C shows that the SIINFEKL-specific response still dominated memory inflation at the expense of the IE3 and M38 responses. This was not because the transferred cells were unable to proliferate, as the donor cells expanded and contributed to inflation in WT-infected mice (Figure 3C). Thus, pre-expanding T cells specific for MCMV epitopes were not able to override the profound immunodominance of SIINFEKL-specific CD8 T cells in chronic infection.
Together these results suggest that the frequency of epitope-specific cells available prior to infection is not the most significant factor in determining the size of the SIINFEKL response relative to other MCMV responses during chronic infection with MCMV-GFP-SL8.
Competition for antigen shapes immunodominance during chronic MCMV infection
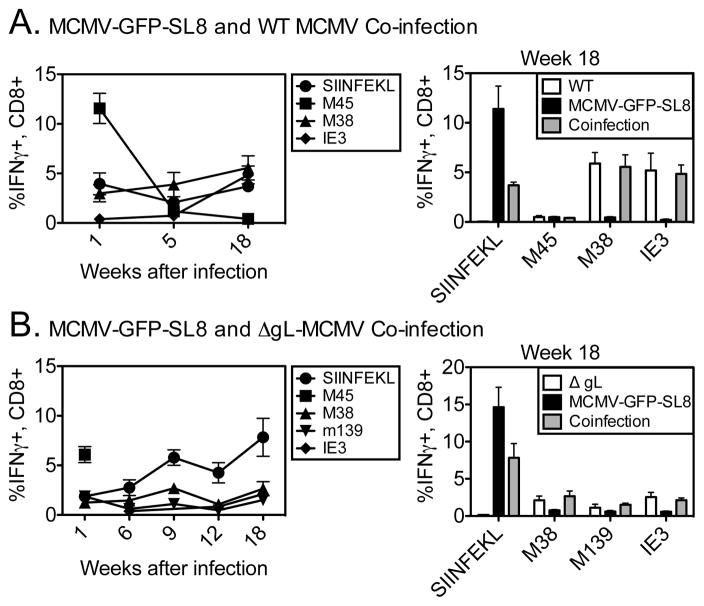
Because precursor frequency did not explain SIINFEKL’s dominance during chronic infection, we asked whether the phenomenon was the result of competition between T-cells at the level of the APC. This phenomenon has been termed immunodomination (27). To test this, we co-infected mice with both WT MCMV and MCMV-GFP-SL8. Previous work has shown that co-infection with 2 viruses yields distinct foci of infection with each individual virus (28). Thus, in our experiments, WT MCMV and MCMV-GFP-SL8 should largely infect different cells within the same host and their epitopes should be presented to T-cells by different APCs. This eliminates competition between T-cells of different specificities at the level of the APC.
In mice receiving both viruses, responses to SIINFEKL and to the MCMV epitopes IE3 and M38 were co-dominant during chronic infection (Figure 4A). We interpreted this to mean that T cells specific for endogenous MCMV gene products were able to inflate when these epitopes were not presented by APCs also presenting SIINFEKL. However, a trivial explanation for this would be that a much faster replicating WT virus would result in a greater abundance of MCMV epitopes in co-infected mice. Indeed, MCMV-SL8-GFP does grow with slightly delayed kinetics in vitro (Turula et. al., manuscript submitted).
Figure 4. Competition for antigen shapes immunodominance during chronic MCMV infection.
(A) C57BL/6 mice were infected i.p with WT MCMV and MCMV-GFP-SIINFEKL at the same time. Virus-specific T-cells were measured in the blood at the indicated times post infection using intracellular cytokine staining (B) Mice were infected i.p. with ΔgL MCMV or both ΔgL MCMV and MCMV-GFP-SIINFEKL. Virus-specific CD8 T-cell responses were measured in the blood at the indicated times post infection. The graph on the left shows the T cell responses at the indicated weeks after co-infection. The graph on the right shows the data from all groups at week 18. Individual bars represent 4–5 mice per group. Experiments were done twice.
To ensure that this was not the case, we repeated these co-infection experiments with a single-cycle virus, ΔgL-MCMV, in place of WT MCMV. Despite lacking gL, this virus can still promote memory inflation during chronic infection (Figure 4B and (3)). Nevertheless, in mice co-infected with MCMV-GFP-SL8 and ΔgL-MCMV, antigens from MCMV-GFP-SL8 would clearly be more abundant. Figure 4B shows that at 18 weeks post infection, responses to IE3, M38 and m139 were similar in co-infected mice and mice infected with ΔgL-MCMV alone. These data indicate that the results in Figure 4A are not due to differing rates of viral replication. We therefore conclude that competition at the level of the antigen presenting cell influences inflation and immunodominance during MCMV infection.
SIINFEKL is expressed earlier and has a higher MHC binding affinity than endogenous MCMV epitopes
The above data established that SIINFEKL is able to out-compete endogenous MCMV epitopes to promote T-cell inflation when presented on the same APC. The mechanisms that cause the immune system to narrowly focus T cell responses on a few immunodominant epitopes are not completely understood. That being said, some factors are obviously important: peptides that are more abundantly presented, either due to expression, processing, or binding affinity, are more likely to be the focus of these responses (29, 30).
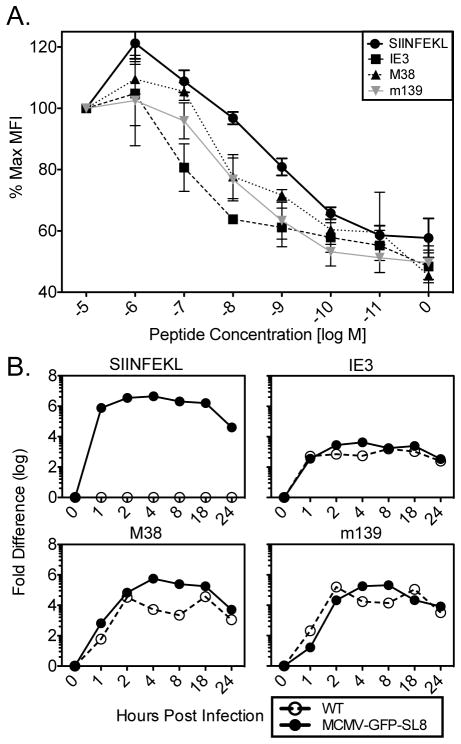
To compare the MHC binding affinity of SIINFEKL and the MCMV-derived inflationary epitopes, all of which are presented by H-2Kb, we evaluated the ability of these peptides to stabilize Kb on the surface of the TAP-deficient cell line RMA-S. Figure 5A shows that SIINFEKL bound Kb most strongly, followed by M38 and m139, with IE3 binding with the weakest affinity. Thus, a better ability to bind Kb would favor SIINFEKL presentation.
Figure 5. MHC binding affinity of MCMV epitopes and kinetics of expression.
(A) RMA-S cells were incubated with the indicated concentrations of peptide for 2hrs at 25°C and an additional 2hrs at 37°C, then washed and stained for H2-Kb expression. Experiment was done twice. Shown is the normalized mean fluorescence intensity of class I MHC on the surface of cells. (B) Murine embryonic fibroblasts were infected with the indicated viruses and RNA was harvested at the time points listed on the y-axis. cDNA was made in parallel with no reverse-transcriptase controls for each sample, and qRT-PCR was done for the indicated gene products. No signal was obtained from the no reverse transcriptase controls. Experiment was done twice.
Epitope presentation is also affected by the amount of parent protein available for degradation and presentation. Because SIINFEKL dominated memory inflation after infection with the single cycle ΔgL-SL8, we presume that cells harboring the latent viral genome, or their progeny, are responsible for the antigen presentation that drives memory inflation. Since the identity of these cells is unknown, it is not possible to definitively describe antigen synthesis and presentation at this site. However, as described above, sporadic expression of IE genes in the absence of detectable E or L genes has been described in latently infected lungs (7, 9). Preferential expression of IE genes is the likely explanation for the immunodominance of IE-encoded antigens during memory inflation. In MCMV-GFP-SL8, SIINFEKL is encoded behind the IE2 promoter and IE3 is driven by the Major Immediate Early promoter. To explore the timing of expression of SIINFEKL, IE3, M38 and m139 during lytic cycle infection in vitro, we infected murine embryonic fibroblasts with WT MCMV or MCMV-GFP-SL8, harvested RNA at various time points after infection, and performed quantitative real time PCR. SIINFEKL was expressed immediately and abundantly; IE3 was also transcribed with immediate early kinetics, but probably less abundantly, and, as expected, the E genes were expressed later (Figure 5B). These results suggest that SIINFEKL may have a quantitative and kinetic advantage over IE3 in expression during latency.
Discussion
We have shown that a GFP-SIINFEKL fusion construct, when inserted into MCMV under immediate early control, completely dominates the inflationary memory response during chronic infection with this virus. The number of SIINFEKL-specific T cells available prior to infection was not the main determinant of immunodominance since the SIINFEKL response was still dominant in mice expressing SIINFEKL as a self-antigen or after specific peptide tolerization. Conversely, adoptive transfer to increase the number of T cells specific for endogenous MCMV-derived peptides did not enable them to inflate in response to the SIINFEKL-expressing virus. However, when mice were co-infected with WT MCMV and our recombinant MCMV expressing SIINFEKL, inflationary responses developed to both SIINFEKL and endogenous MCMV epitopes. This indicated that when different cells in the same animal were infected with each of the individual viruses, and thus WT-infected APCs were able to present MCMV epitopes without the competing influence of SIINFEKL, T cells recognizing these epitopes were able to inflate alongside the SIINFEKL response. Yet, when both sets of epitopes were encoded by the same virus and presumably expressed on the same APC, T cells responding to SIINFEKL outcompeted the MCMV-specific responses. This happened either because these cells had more antigen available to them or because they were better able to access antigen. Thus, competition for - or availability of - antigen at the level of the APC plays a significant role in the selection of inflationary responses during chronic MCMV infection.
This competition may be won by the SIINFEKL response, at least in part, because patrolling SIINFEKL-specific CD8+ T cells see antigen first and go on to terminate further gene transcription. The silencing/desilencing and immune sensing hypothesis proposed by Simon et. al. suggests that T-cells specific for the IE1-derived epitope in Balb/c mice prevent further MCMV gene transcription. Consistent with this, only IE1 and IE2 transcripts have been found in latently-infected lung tissue from Balb/c mice (8). IE3 and gB were found at low levels only when the IE1 epitope was mutated such that it could no longer be presented to T-cells (9).
Indeed, work from the Cicin-Sain group shows that the context of MCMV gene expression influences whether or not an epitope generates an inflationary response (31). Dekhtiarenko et. al. infected mice with one of two recombinant viruses expressing the gB epitope from HSV-1, linked to the carboxy terminus of either IE2 or M45. Inflating gB responses were seen only when expression was controlled by the IE2 promoter. When gB was linked to M45, an E gene, gB T-cell responses dominated only during acute infection. This study lends support to the idea that ordered, temporal viral gene transcription results in immune recognition of the first viral gene products and immune silencing of downstream transcription, resulting in a bias of the T-cell response toward IE antigens.
A similar scenario is likely at play in our system, where the IE2 promoter controls SIINFEKL expression. In addition, SIINFEKL may be more abundant than other MCMV epitopes as a result of higher MHC affinity and greater transcription levels. However, in both the BALB/c model and the C57BL/6 model, inflationary memory consists of responses to E-encoded antigens as well as IE-encoded antigens. This could be explained by the idea that these responses are programmed to inflate from the time of acute infection, or by the idea that E epitopes are presented by a different cell type during latency, one that is undergoing a different program of viral gene expression. However, our data argue against both of these ideas. Inflationary responses are not programmed early during infection, as MCMV-specific T cells transferred 7 days after infection did not inflate in a host later infected with MCMV-GFP-SL8. Thus, repeated antigen exposure after priming is a necessary driver of inflationary memory. In addition, different and simultaneous gene expression programs are likely not the cause of E-gene-specific inflationary memory, as IE and E responses were equally silenced by the expression of SIINFEKL under the IE2 promoter. Thus, we favor the hypothesis that competition between T-cell clones for antigen at the level of the infected APC dictates the selection of epitopes that drive memory inflation. This hypothesis implies that, after WT MCMV infection, IE1-specific T cells (in Balb/c mice) and IE3-specific T cells (in B6 mice) fail to completely silence MCMV E-gene expression.
When considering the use of MCMV and eventually HCMV as a vaccine vector, these results emphasize the importance of gene expression kinetics and epitope availability in determining the size of inflationary memory responses to individual antigens.
Acknowledgments
This work was supported by NIH grants AIR0147206 awarded to A.B.H, K22A1081866 awarded to C.M.S., 5T32HL00778120 awarded to L.A.F, and 5T32A1078903-05 awarded to L.A.F.
The authors thank Dr. Stephen C. Jameson for supplying SIINFEKL-specific tetramer.
References
- 1.Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, Legasse AW, Axthelm MK, Oswald K, Trubey CM, Piatak MJ, Lifson JD, Nelson JA, Jarvis MA, Picker LJ. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med. 2009;15:293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, Legasse AW, Chiuchiolo MJ, Parks CL, Axthelm MK, Nelson JA, Jarvis MA, Piatak MJ, Lifson JD, Picker LJ. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473:523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Snyder CM, Cho KS, Bonnett EL, Allan JE, Hill AB. Sustained CD8+ T cell memory inflation after infection with a single-cycle cytomegalovirus. PLoS Pathog. 2011;7:e1002295. doi: 10.1371/journal.ppat.1002295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munks MW, Gold MC, Zajac AL, Doom CM, Morello CS, Spector DH, Hill AB. Genome-wide analysis reveals a highly diverse CD8 T cell response to murine cytomegalovirus. J Immunol. 2006;176:3760–3766. doi: 10.4049/jimmunol.176.6.3760. [DOI] [PubMed] [Google Scholar]
- 5.Munks MW, Cho KS, Pinto AK, Sierro S, Klenerman P, Hill AB. Four distinct patterns of memory CD8 T cell responses to chronic murine cytomegalovirus infection. J Immunol. 2006;177:450–458. doi: 10.4049/jimmunol.177.1.450. [DOI] [PubMed] [Google Scholar]
- 6.Snyder CM, Cho KS, Bonnett EL, van Dommelen S, Shellam GR, Hill AB. Memory inflation during chronic viral infection is maintained by continuous production of short–lived, functional T cells. Immunity. 2008;29:650–659. doi: 10.1016/j.immuni.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurz SK, Rapp M, Steffens HP, Grzimek NK, Schmalz S, Reddehase MJ. Focal transcriptional activity of murine cytomegalovirus during latency in the lungs. J Virol. 1999;73:482–494. doi: 10.1128/jvi.73.1.482-494.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grzimek NK, Dreis D, Schmalz S, Reddehase MJ. Random, asynchronous, and asymmetric transcriptional activity of enhancer-flanking major immediate-early genes ie1/3 and ie2 during murine cytomegalovirus latency in the lungs. J Virol. 2001;75:2692–2705. doi: 10.1128/JVI.75.6.2692-2705.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simon CO, Holtappels R, Tervo HM, Bohm V, Daubner T, Oehrlein-Karpi SA, Kuhnapfel B, Renzaho A, Strand D, Podlech J, Reddehase MJ, Grzimek NK. CD8 T cells control cytomegalovirus latency by epitope-specific sensing of transcriptional reactivation. J Virol. 2006;80:10436–10456. doi: 10.1128/JVI.01248-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karrer U, Wagner M, Sierro S, Oxenius A, Hengel H, Dumrese T, Freigang S, Koszinowski UH, Phillips RE, Klenerman P. Expansion of protective CD8+ T-cell responses driven by recombinant cytomegaloviruses. J Virol. 2004;78:2255–2264. doi: 10.1128/JVI.78.5.2255-2264.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, Sleath PR, Grabstein KH, Hosken NA, Kern F, Nelson JA, Picker LJ. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med. 2005;202:673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boppana SB, Britt WJ. Recognition of human cytomegalovirus gene products by HCMV-specific cytotoxic T cells. Virology. 1996;222:293–296. doi: 10.1006/viro.1996.0424. [DOI] [PubMed] [Google Scholar]
- 13.McLaughlin-Taylor E, Pande H, Forman SJ, Tanamachi B, Li CR, Zaia JA, Greenberg PD, Riddell SR. Identification of the major late human cytomegalovirus matrix protein pp65 as a target antigen for CD8+ virus-specific cytotoxic T lymphocytes. J Med Virol. 1994;43:103–110. doi: 10.1002/jmv.1890430119. [DOI] [PubMed] [Google Scholar]
- 14.Wills MR, Carmichael AJ, Mynard K, Jin X, Weekes MP, Plachter B, Sissons JG. The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity, and T-cell receptor usage of pp65-specific CTL. J Virol. 1996;70:7569–7579. doi: 10.1128/jvi.70.11.7569-7579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Streblow DN, van Cleef KW, Kreklywich CN, Meyer C, Smith P, Defilippis V, Grey F, Fruh K, Searles R, Bruggeman C, Vink C, Nelson JA, Orloff SL. Rat cytomegalovirus gene expression in cardiac allograft recipients is tissue specific and does not parallel the profiles detected in vitro. J Virol. 2007;81:3816–3826. doi: 10.1128/JVI.02425-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodrum FD, Jordan CT, High K, Shenk T. Human cytomegalovirus gene expression during infection of primary hematopoietic progenitor cells: a model for latency. Proc Natl Acad Sci U S A. 2002;99:16255–16260. doi: 10.1073/pnas.252630899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holtappels R, Simon CO, Munks MW, Thomas D, Deegen P, Kuhnapfel B, Daubner T, Emde SF, Podlech J, Grzimek NK, Oehrlein-Karpi SA, Hill AB, Reddehase MJ. Subdominant CD8 T-cell epitopes account for protection against cytomegalovirus independent of immunodomination. J Virol. 2008;82:5781–5796. doi: 10.1128/JVI.00155-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wall EM, Milne K, Martin ML, Watson PH, Theiss P, Nelson BH. Spontaneous mammary tumors differ widely in their inherent sensitivity to adoptively transferred T cells. Cancer Res. 2007;67:6442–6450. doi: 10.1158/0008-5472.CAN-07-0622. [DOI] [PubMed] [Google Scholar]
- 19.Borst EM, Hahn G, Koszinowski UH, Messerle M. Cloning of the human cytomegalovirus (HCMV) genome as an infectious bacterial artificial chromosome in Escherichia coli: a new approach for construction of HCMV mutants. J Virol. 1999;73:8320–8329. doi: 10.1128/jvi.73.10.8320-8329.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cascio P, Hilton C, Kisselev AF, Rock KL, Goldberg AL. 26S proteasomes and immunoproteasomes produce mainly N-extended versions of an antigenic peptide. EMBO J. 2001;20:2357–2366. doi: 10.1093/emboj/20.10.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borst EM, Benkartek C, Messerle M. Use of bacterial artificial chromosomes in generating targeted mutations in human and mouse cytomegaloviruses. Curr Protoc Immunol. 2007;Chapter 10(Unit 10.32) doi: 10.1002/0471142735.im1032s77. [DOI] [PubMed] [Google Scholar]
- 22.Messerle M, Crnkovic I, Hammerschmidt W, Ziegler H, Koszinowski UH. Cloning and mutagenesis of a herpesvirus genome as an infectious bacterial artificial chromosome. Proc Natl Acad Sci U S A. 1997;94:14759–14763. doi: 10.1073/pnas.94.26.14759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obar JJ, Khanna KM, Lefrancois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28:859–869. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Redmond WL, Sherman LA. Peripheral tolerance of CD8 T lymphocytes. Immunity. 2005;22:275–284. doi: 10.1016/j.immuni.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 25.Srinivasan M, Frauwirth KA. Peripheral tolerance in CD8+ T cells. Cytokine. 2009;46:147–159. doi: 10.1016/j.cyto.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 26.Walker LS, Abbas AK. The enemy within: keeping self-reactive T cells at bay in the periphery. Nat Rev Immunol. 2002;2:11–19. doi: 10.1038/nri701. [DOI] [PubMed] [Google Scholar]
- 27.Kedl RM, Rees WA, Hildeman DA, Schaefer B, Mitchell T, Kappler J, Marrack P. T cells compete for access to antigen-bearing antigen-presenting cells. J Exp Med. 2000;192:1105–1113. doi: 10.1084/jem.192.8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holtappels R, Podlech J, Pahl-Seibert MF, Julch M, Thomas D, Simon CO, Wagner M, Reddehase MJ. Cytomegalovirus misleads its host by priming of CD8 T cells specific for an epitope not presented in infected tissues. J Exp Med. 2004;199:131–136. doi: 10.1084/jem.20031582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yewdell JW, Bennink JR. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu Rev Immunol. 1999;17:51–88. doi: 10.1146/annurev.immunol.17.1.51. [DOI] [PubMed] [Google Scholar]
- 30.Yewdell JW. Confronting complexity: real-world immunodominance in antiviral CD8+ T cell responses. Immunity. 2006;25:533–543. doi: 10.1016/j.immuni.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 31.Dekhtiarenko I, Jarvis MA, Ruzsics Z, Cicin-Sain L. The context of gene expression defines the immunodominance hierarchy of CMV Antigens. The Journal of Immunology. doi: 10.4049/jimmunol.1203173. In press. [DOI] [PubMed] [Google Scholar]