Abstract
Protein aggregation within the central nervous system has been recognized as a defining feature of neurodegenerative diseases since the early 20th century. Since that time, there has been a growing list of neurodegenerative disorders, including Parkinson disease, which are characterized by inclusions of specific pathogenic proteins. This has led to the long-held dogma that these characteristic protein inclusions, which are composed of large insoluble fibrillar protein aggregates and visible by light microscopy, are responsible for cell death in these diseases. However, the correlation between protein inclusion formation and cytotoxicity is inconsistent suggesting another form of the pathogenic proteins may be contributing to neurodegeneration. There is emerging evidence implicating soluble oligomers, smaller protein aggregates not detectable by conventional microscopy, as potential culprits in the pathogenesis of neurodegenerative diseases. The protein α-synuclein is well recognized to contribute to the pathogenesis of Parkinson disease and is the major component of Lewy bodies and Lewy neurites. However, α-synuclein also forms oligomeric species with certain conformations being toxic to cells. The mechanisms by which these α-synuclein oligomers cause cell death are being actively investigated as they may provide new strategies for diagnosis and treatment of Parkinson disease and related disorders. Here we review the possible role of α-synuclein oligomers in cell death in Parkinson disease and discuss the potential clinical implications.
Introduction
It has long been appreciated that neurodegenerative diseases share a common theme of abnormal protein aggregation. Early work by neuropathologists demonstrated that insoluble aggregates of misfolded proteins, observable under the light microscope, are associated with many neurodegenerative conditions including Parkinson disease (PD).1 Subsequent work in the genetics underlying familial forms of these conditions and use of immunohistochemical methods led to identification of the major protein components of these inclusions. From these discoveries, the concept of proteinopathies has evolved with classification of neurodegenerative disorders based on the primary aggregated protein: synucleinopathies, amyloidopathies, tauopathies, prionopathies, trinucleotide repeat disorders, and TDP-43 proteinopathies (Table). The central feature of protein inclusions among neurodegenerative diseases supported a causal relationship with neuronal death. However, further neuropathological observations, as well as characterization of animal models of these diseases, have challenged the dogma that protein inclusions alone lead to neurodegeneration. Investigations into protein aggregation using biochemical and high resolution microscopic techniques have revealed that, in addition to large insoluble inclusion bodies, many of these disease-associated proteins have a propensity to form small soluble aggregates, or oligomers. Although limited primarily to in vitro experiments, these initial findings suggested that oligomers may contribute to the pathogenicity of these proteins. More recent discoveries from studies with in vivo models and human patients are providing further support for this hypothesis. In this review, we focus on the potential role of α-synuclein (α-syn) oligomers as mediators of neurodegeneration in PD. There is increasing evidence that certain α-syn oligomeric species confer toxicity to cells through a variety of possible mechanisms. This emerging paradigm implicates α-syn oligomers in the early stages of neurodegeneration, opening new areas of investigation into biomarkers and therapeutics for PD and possibly other synucleinopathies.
Table 1.
Table Overview of current evidence for the role of oligomers in neurodegenerative diseases
| Proteinopathies | Toxic Protein | Protein Inclusions | Evidence for Toxic Oligomers |
|---|---|---|---|
|
| |||
| Synucleinopathies | α-Synuclein | Neuronal | In vitro10 |
| PD, DLB, MSA | Lewy bodies | Cell culture16 | |
| Lewy neurites | Animal models21,88 | ||
| Glial | Postmortem brain22,91 | ||
| Glial cytoplasmic inclusions | |||
|
| |||
| Amyloidopathies | β-Amyloid | Extracellular | In vitro9,149 |
| AD, Down syndrome | Amyloid plaques | Cell culture149,150 | |
| Vascular | Animal models149,151 | ||
| Cerebral amyloid angiopathy | Postmortem brain149,152 | ||
|
| |||
| Tauopathies | Tau | Neuronal | In vitro153 |
| AD, FTLD, FTDP-17, PSP, CBD | Neurofibrillary tangles | Cell culture154 | |
| Pick bodies | Animal models155,156 | ||
| Corticobasal bodies | Postmortem brain157–159 | ||
| Glial | |||
| Tufted astrocytes | |||
| Astrocytic plaques | |||
| Oligodendroglial coiled bodies | |||
|
| |||
| Prionopathies | Prion protein | Extracellular | In vitro160,161 |
| CJD, GSS, FFI, Kuru | Kuru plaques | Cell culture161,162 | |
| Florid plaques | Animal models160–162 | ||
| Postmortem brain163 | |||
|
| |||
| Trinucleotide repeat disorders | PolyQ protein | Neuronal | HD/Huntingtin |
| HD, DRPLA, SBMA, SCAs | Intranuclear inclusions | In vitro13,164 | |
| Cell culture164,165 | |||
| Animal models166,167 | |||
| Postmortem brain167,168 | |||
| DRPLA/Atrophin-1 | |||
| Cell culture169 | |||
| SBMA/Androgen receptor | |||
| In vitro170 | |||
| Animal model171 | |||
|
| |||
| TDP-43 proteinopathies | TDP-43 | Neuronal | – |
| FTLD, ALS | Cytoplasmic inclusions | ||
AD, Alzheimer disease; ALS, amyotrophic lateral sclerosis; CBD, corticobasal degeneration; CJD, Creutzfeld-Jakob disease; DLB, dementia with Lewy bodies; DRPLA, dentatorubral-pallidoluysian atrophy; FFI, fatal familial insomnia; FTDP-17, frontotemporal dementia with parkinsonism-17; FTLD, frontotemporal lobar degeneration; GSS, Gerstmann-Straussler-Scheinker syndrome; HD, Huntington disease; MSA, multiple system atrophy; PD, Parkinson disease; polyQ, polyglutamine; PSP, progressive supranuclear palsy; SBMA, spinobulbar muscular atrophy; SCAs, spinocerebellar ataxias; TDP-43, TAR DNA binding protein
α-Synuclein aggregation: from monomers to oligomers to inclusions
α-Syn is a 14-kDa neuronal protein from a family of structurally related proteins that are highly expressed in the brain.2,3 Under physiological conditions, α-syn is enriched at presynaptic terminals where it promotes the assembly of the SNARE machinery6 and is proposed to play a role in neurotransmitter release,5 as well as protection of nerve terminals against injury.4 α-Syn has been widely accepted to have a natively unfolded tertiary structure although recent studies have suggested it to be an α-helically folded tetramer in its native conformation.5,6 However, these findings have not been widely replicated and the main physiological form of α-syn in the brain appears to be an unfolded monomer.7 How pathogenic proteins such as α-syn proceed from monomers to inclusions is proposed to be a multistep process (Fig 1A).8 Aggregation of two or more monomers leads to the formation of soluble oligomeric species, which have also been termed protofibrils because they are fibrillization intermediates.9 Initial characterization of α-syn oligomers came from in vitro experiments in which recombinant α-syn was found to spontaneously aggregate.10 This occurred in the absence of other proteins and the propensity to aggregate increased with higher concentrations of α-syn. High resolution microscopic techniques, such as atomic force microscopy, allowed for direct visualization of the oligomers which range in size from 4 to 24 nm. Various morphologies were observed including spherical, chain-like, annular, and tubular oligomeric structures.11 These oligomers disappear upon formation of amyloid fibrils in vitro. The time course of α-syn oligomers and their morphology resemble those observed for in vitro oligomers of β-amyloid12 and mutant huntingtin,13 supporting a common aggregation process.
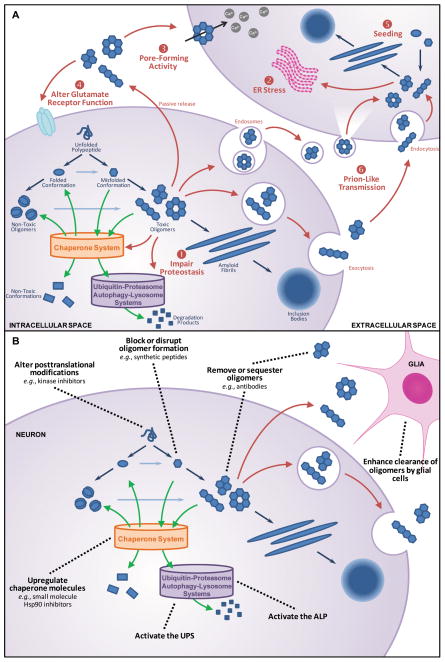
Figure 1. α-Syn oligomers and Parkinson disease.
(A) Formation and toxicity of α-syn oligomers. Protein aggregation in neurodegenerative diseases is initiated by aberrant protein folding which leads to the formation of oligomers and eventually amyloid fibrils and inclusions bodies (blue arrows). Certain oligomeric species are toxic to cells by mechanisms that include (1) impairment of proteostasis, (2) chronic endoplasmic reticulum (ER) stress, (3) pore formation, (4) glutamate receptor dysfunction, (5) seeding with (6) prion-like transmission (red arrows), all of which may combine in the pathogenic process of cell death and transmission. Endogenous cellular systems which can reduce oligomer levels are the chaperone network, the ubiquitin-proteasomal system (UPS), and the autophagy-lysosomal pathway (ALP) (green arrows).
(B) Potential treatment strategies which reduce toxic α-syn oligomers to slow or prevent neurodegeneration. These strategies target different steps along the protein aggregation pathway, as well as intracellular and/or extracellular pools of α-syn oligomers. The primary goal of treatment is to reduce toxic oligomer levels directly or indirectly by preventing oligomer formation, disrupting already formed oligomers, promoting degradation of toxic oligomers or conversion of toxic oligomers to non-toxic oligomers, and sequestering or clearing oligomers by antibody or cell-based mechanisms to prevent cell-to-cell transmission (black dotted lines).
Early investigations into oligomer formation of these proteins were restricted to in vitro studies. However, increasing evidence demonstrates that formation of oligomers is not simply an artifact of in vitro recombinant proteins but also occurs within cellular and in vivo models. A novel assay based on protein-fragment complementation14 has permitted the detection and measurement of intracellular α-syn oligomers in living cells including cultured cell lines15–18 and neurons,19 as well as extracellular α-syn oligomers in conditioned cell culture media.19 Furthermore, biochemical studies using methods that can separate protein complexes, such as size exclusion chromatography or non-denaturing protein gel electrophoresis, have provided evidence for endogenous α-syn oligomers in brain extracts from rodent models.20,21 Initial evidence for the presence of α-syn oligomers in the central nervous system of PD patients came from their detection in brain extracts from postmortem samples.22 More recently, α-syn oligomers have been identified in plasma23 and CSF24 of PD patients. Definitive evidence for their presence in the brains of PD patients in vivo – for example, with radioligand-based imaging technologies – remains to be obtained (see discussion on biomarkers below).
Oligomeric species can self-associate to form a nucleus or seed. Seed formation is a rate-limiting step after which aggregate growth proceeds rapidly with the further addition of monomers or other oligomers to the nucleus of proteins. This nucleation-dependent step results in the formation of amyloid fibrils which are insoluble large protein aggregates with a cross-β architecture formed by β-strands organized perpendicular to the long axis of the fibril.8 Amyloid fibrils display a characteristic fibrillar structure observed by electron microscopy. They also bind to Congo red dye resulting in green birefringence under cross-polarized light and stain with the fluorescent dye thioflavin S. α-Syn, as well as a number of proteins involved in neurodegeneration, are capable of forming these ordered aggregates. Amyloid fibrils are common to the proteinopathies being the primary component of most inclusions regardless of the associated protein (Table).25 In PD, α-syn amyloid fibrils are the major constituents of Lewy bodies (LBs) and Lewy neurites (LNs), the protein inclusions characteristic of the disease.26
Factors which are thought to promote the cascade of α-syn aggregation include missense mutations in the α-syn gene (SNCA), such as those associated with rare familial autosomal dominant forms of PD (A53T, A30P, E46K).10,27–30 Mutations associated with familial PD in which SNCA is duplicated or triplicated31,32 also increase the propensity of the protein to aggregate, likely as a result of increased α-syn protein concentrations and consequent macromolecular crowding.33 In idiopathic PD, polymorphisms in SNCA have been identified as risk factors for development of the disease.34,35 The effects of these genetic variations on the protein remain to be fully elucidated; however, some polymorphisms may be associated with increased α-syn protein expression36,37 and therefore may have consequences similar to SNCA multiplications. Aggregation of α-syn protein can also be influenced by its posttranslational modifications. For instance, α-syn phosphorylation on serine 129 promotes aggregation,38,39 whereas ubiquitination23 or nitration22 of α-syn is associated with reduced aggregation.18,40 α-Syn aggregation can occur not only from perturbations of the protein itself but also due to dysfunction of the cellular machinery which has evolved to handle detrimental proteins. This includes the chaperone networks which regulate protein folding and refolding of misfolded proteins,41 as well as the ubiquitin-proteasomal system (UPS) and autophagy-lysosomal pathway (ALP) which are responsible for elimination of harmful proteins (Fig 1A).42 Evaluation of human postmortem tissue has demonstrated that UPS components and activity, as well as constituents of the ALP,43–45 are reduced in the substantia nigra of PD patients compared to controls. These systems may fail due to saturation as in the case of increased protein expression. Failure may also occur as a result of faulty components; for example, mutation in the gene encoding the lysosomal enzyme glucocerebrosidase is the most common genetic risk factor for PD.46 If not affected by saturation or mutations, these systems may simply fail over time. Aging is a major risk factor for PD with multiple contributors, including genetic predisposition and environmental insults, altering the normal course of age-related neuronal dysfunction.47,48 The efficacy of the chaperone network, UPS, and ALP are known to decline with age49 and this may facilitate accumulation of α-syn in PD.
α-Synuclein oligomers and neurodegeneration
Lewy pathology is a feature of idiopathic and most autosomal dominantly inherited forms of PD.1,50,51 LBs are intracytoplasmic inclusions 5 to 25 μm in diameter, which are found within the soma of neurons, and LNs are dystrophic neuronal processes. Although Lewy pathology is a hallmark of PD, there is accumulating evidence that these insoluble α-syn-containing inclusions alone do not explain the pathogenesis of the disease. Perhaps the first piece of evidence that these protein inclusions do not invariably cause neurodegeneration was the observation of incidental LBs at autopsy of aged individuals without clinical features of PD or another neurodegenerative disease.52 Incidental LBs without degeneration in asymptomatic individuals are not an uncommon finding and constituted approximately 12% in a series of over 1200 consecutive autopsy cases.53 Although this is often proposed to be a presymptomatic stage of PD, the presence of LBs is not directly linked to neuronal loss. Further evidence that protein inclusions are related to, but separable from, neurodegeneration in PD comes from neuropathological analysis of genetic forms of the disease.51 The most common form of autosomal recessive early-onset PD is associated with mutations in the parkin gene. LBs or LB-like α-syn-positive inclusions have been found in association with neurodegeneration in a minority of cases of parkin-associated parkinsonism54–56 with most cases reported to date lacking LBs or LNs.57–63 Heterogeneity of neuropathological findings is seen in PD due to mutations in the gene encoding leucine-rich repeat kinase 2 (LRRK2). LRRK2 mutations are the most common known genetic cause of PD occurring in approximately 10% of patients with familial autosomal dominant PD and in close to 4% of patients with sporadic PD.64 Postmortem analysis of PD patients with LRRK2 mutations have demonstrated neurodegeneration both with and without Lewy pathology.65–80 Further studies comparing LRRK2 mutation cases with and without Lewy pathology are expected to help elucidate the contribution of these insoluble α-syn aggregates to neurodegeneration in these genetic forms of PD. Similarly, obtaining a larger number of well described neuropathological cases of LRRK2 and other genetic forms of PD may assist in explaining why certain gene mutations are associated with Lewy pathology, others without Lewy pathology, and still others with heterogeneous pathologic features.
The inconsistent association between Lewy pathology and neuronal death seen in human autopsy studies is recapitulated in PD animal models. For example, the classic toxin-induced model using 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is associated with motor symptoms and dopaminergic neurodegeneration in primates and mice without convincing Lewy pathology.81 Although α-syn inclusions are absent in this model, α-syn is required for MPTP-induced neurodegeneration as demonstrated by the resistance of α-syn null mice to MPTP intoxication.82 In contrast, chronic treatment with the toxin rotenone leads to nigrostriatal pathway neurodegeneration with nigral inclusions similar to Lewy bodies in a subset of treated rats.81,83 Targeted overexpression of wild-type or mutant α-syn within the nigrostriatal system using viral delivery in primates or rats induces progressive degeneration of dopaminergic neurons associated with non-fibrillar α-syn inclusions but not LBs or LNs.84 In general, transgenic PD models of autosomal dominant forms of PD (α-syn, LRRK2) and knockout models of autosomal recessive forms (parkin, PINK1, DJ-1) lack both dopaminergic neuronal loss and Lewy pathology.85 The transgenic animals with one of the most severe phenotypes to date are mice expressing human A53T mutant α-syn under the control of the prion-related protein promoter.86,87 These animals display neurodegeneration with fibrillary α-syn inclusions but also exhibit a range of α-syn species, including insoluble aggregates and soluble oligomeric forms.86 Taken together, the neuropathological findings in human postmortem studies and animal models demonstrate that Lewy pathology alone cannot account for α-syn-associated neuronal death. Thus, a different form of α-syn must also contribute to toxicity caused by the protein.
Evidence for α-synuclein oligomer toxicity
An emerging hypothesis proposes that there are toxic oligomeric species of α-syn. Studies using protein-fragment complementation assays have demonstrated that α-syn oligomers are associated with enhanced toxicity in cell culture models as measured by release of adenylate kinase from damaged cells, ATP levels, or caspase 3/7 activity.15–17,19 To examine the contribution of α-syn oligomerization to cell toxicity in animal models, various α-syn mutants have been designed to possess different aggregation properties. Introduction of one or more missense mutations involving specific alanine residues (i.e., A56P or triple mutant A30P/A56P/A76P) which participate in α-syn amyloid fibril formation generates mutant forms of α-syn with decreased capacity to form fibrils but increased propensity to form soluble oligomers.88 These design mutants were expressed in two different invertebrate model organisms: Drosophila melanogaster (fruit fly) and Caenorhabditis elegans (nematode worm). There was increased loss of dopaminergic neurons in flies expressing either of the oligomer-prone mutants. Those mutants causing the most neuronal loss also demonstrated the greatest motor deficits as measured by a climbing assay. Similarly, worms expressing either of the α-syn mutants also had pronounced dopaminergic neurodegeneration associated with significant impairment in dopamine-mediated behaviors. Interestingly, neurodegeneration associated with expression of these mutants was not restricted to dopaminergic neurons in this model; expression in other neuronal populations resulted in death of non-dopaminergic neurons.88 This finding is important in view of the increasing recognition that PD is a multisystem disorder affecting many non-dopaminergic systems.89 A similar approach was used to study α-syn oligomer toxicity in a mammalian model.21 Different α-syn mutants were designed which included oligomer-forming variants (E35K, E57K) and fibril-promoting variants (α-syn(30–110)). E35K and E57K were devised based on the prediction that these mutations would disrupt salt bridges between β-strands of α-syn and thereby interfere with formation of α-syn amyloid fibrils. Consistent with this prediction, neither of these two mutants showed amyloid fibrils visible by electron microscopy but they each formed pore-like, or annular, oligomeric structures. The α-syn(30–110) variant includes the residues required for amyloid fibril formation but lacks the N- and C-terminal residues which interfere with aggregation. This variant had an increased rate of formation of fibrils similar in structure to those found in LBs. A rat model was used in which viral-mediated overexpression of each of the α-syn mutants in the substantia nigra was achieved by stereotactic injection of lentivirus. E35K and E57K were found to induce the largest amount of dopaminergic neuronal loss in the substantia nigra whereas α-syn(30–110) did not demonstrate a significant toxic effect.21 The findings from these three different animal models support a role for α-syn oligomers in mediating cell death in vivo with consequences for dopaminergic and possibly non-dopaminergic systems.
Further support for a role of oligomers in neurodegeneration is the observation that higher oligomer levels are associated with disease. For instance, the amount of α-syn oligomers is higher in transgenic mice expressing A53T mutant α-syn compared to transgenic mice expressing wild-type α-syn90 and in mouse models of Gaucher disease versus wild-type mice.20 Moreover, α-syn oligomer levels are increased in the cerebral cortex of patients with idiopathic PD or dementia with LBs, a related synucleinopathy, compared to age-matched normal controls.22,91 The presence of endogenous α-syn oligomers in common genetic forms of PD has not yet been established. However, patients with parkinsonism and Gaucher disease (to date, universally associated with Lewy pathology92–96) have been found to have increased levels of α-syn oligomers compared to those without parkinsonism or to healthy controls without mutations associated with Gaucher disease.20 Unlike LBs and LNs which are visualized in situ with conventional light microscopy at autopsy, α-syn oligomers have only been detected in brain tissue following protein extraction. Novel methods will need to be developed to definitively demonstrate elevated α-syn oligomer levels in intact brains of PD patients (see discussion below on radioligand-based brain imaging). Regardless, these findings suggest a relationship between endogenous α-syn oligomers and the neurodegeneration of PD.
Potential mechanisms of α-synuclein oligomer toxicity
How α-syn oligomers mediate cell death has not yet been fully elucidated but likely involves a number of different intracellular and extracellular mechanisms (Fig 1A). Within the cytoplasm, proteostasis(i.e., protein homeostasis) is maintained by the cell’s ability to refold misfolded proteins or target those proteins which are not amenable to refolding for degradation. Maintenance of proteostasis is accomplished through the cooperation of the chaperone system with the cell’s protein degradation systems mediated by proteasomes and lysosomes. α-Syn oligomers may promote the demise of the cell by inhibiting these critical systems and disrupting proteostasis. In a bacterial system, α-syn oligomers inhibited the protein refolding rate of the Hsp70 chaperone system whereas monomeric α-syn did not inhibit chaperone function nor did other types of protein aggregates.97 Similar findings were observed when the human Hsp70 chaperone system was reconstituted in vitro. Overexpression of α-syn is known to suppress the UPS98,99 and this may be attributed, at least in part, to oligomeric α-syn. Soluble α-syn oligomers can impair the chymotrypsin-like protease activity of the proteasome in vitro whereas β-synuclein, another member of the synuclein protein family, does not affect proteasomal function.100 Overexpression of α-syn also inhibits the lysosomal enzyme glucocerebrosidase20 but the effects of α-syn oligomers on the ALP require further investigation. Impaired proteostasis can lead to chronic endoplasmic reticulum (ER) stress which can induce cell death.101 Studies in yeast and other cells have demonstrated an association between α-syn-mediated toxicity and chronic ER stress.102,103 Recent investigations using transgenic α-syn mice suggest that α-syn oligomers can form within the lumen of the ER, accumulate within the ER, and sensitize neurons to ER stress.104,105 Furthermore, postmortem examination of brainstem specimens of PD patients revealed accumulation of α-syn oligomers within the ER compartment105 suggesting that toxic α-syn oligomers in the intracellular space may mediate neurodegeneration by causing chronic ER stress.
α-Syn oligomers in the extracellular space appear to have different mechanisms of cell toxicity depending on their morphology (Fig 1A). Oligomers with an annular structure have pore-forming activity.106 Dopaminergic cell lines or primary neuronal cultures treated with α-syn oligomers enriched in the annular conformation display an increase in intracellular calcium (whereas α-syn monomers have no effect).107 This is associated with caspase activation and a reduction in cell number, both surrogate measures of cell death. Thus annular oligomers may confer cell toxicity by forming a pore which disrupts the cell membrane, similar to the membrane attack complex of the complement system, resulting in influx of extracellular calcium and leading to neuronal death. On the other hand, the globular type of α-syn oligomers may act at synapses to impair neuronal function by altering the function of the glutamatergic receptors. Extracellular application of these oligomers onto cultured hippocampal neurons resulted in increased amplitudes of AMPA receptor-mediated excitatory postsynaptic currents.108 The amplitudes of NMDA receptor-mediated currents were unaffected. The consequences of AMPA receptor activation by α-syn oligomers remain to be further elucidated but may include dysfunctional synaptic signaling or activation of intracellular pathways leading to excitotoxicity and neuronal death. Evidence for altered synaptic signaling associated with extracellular α-syn oligomers comes from recent studies of long-term potentiation (LTP), a cellular model of synaptic plasticity, in which α-syn oligomers (but not monomers or amyloid fibrils) were found to abolish hippocampal LTP in organotypic brain slices.109
Globular, as well as protofibrillar, oligomers may also promote neuronal death by seeding, in which soluble oligomers act as nuclei from which insoluble aggregates form (Fig 1A). When applied extracellularly to cultured cells, these forms of α-syn oligomers directly entered cells and lead to intracellular seeding.107 Seeding is a key step in prion-like mechanisms and thus has been implicated in disease propagation within the nervous system for many neurodegenerative diseases.110 Support for α-syn seeding and a prion-like process in PD comes from the demonstration by multiple groups that healthy dopaminergic neurons transplanted into PD brains can eventually form LBs.111,112 This has been replicated in animal models.113,114 In addition to seeding, prion-like propagation requires intercellular transfer of the pathogenic protein. This has been demonstrated for α-syn oligomers in cell culture.19,115 The way in which α-syn from the intracellular compartment is released into the extracellular space may occur through passive release; for example, membrane disruption and leakage of cellular contents associated with cell death. Active processes, such as exocytosis116 and calcium-dependent exosomal mechanisms,117 have also been shown in cell culture systems to mediate release of intracellular α-syn. Endocytosis appears to be the mechanism by which extracellular α-syn is taken up by neighboring cells.115,118,119 Taken together, these findings implicate certain α-syn oligomeric species in prion-like propagation of neurodegeneration in PD. This topic has recently been reviewed in detail (see 120,121).
Clinical implications for Parkinson disease
Important caveats of α-syn oligomer toxicity have been discovered. First, the toxicity of α-syn oligomers is specific to certain oligomeric species.107;122 Next, toxic α-syn oligomers can cause cell death whether they are formed within the intracellular environment15–17 or exist within the extracellular space.19 Finally, oligomer-mediated cytotoxicity can be regulated by altering the levels of α-syn oligomers. For instance, molecular chaperones, such as Hsp7017,19 and C-terminus of Hsp70-Interacting Protein (CHIP),16,18 reduce α-syn oligomer levels and enhanced expression of these chaperone molecules decrease cell death due to α-syn oligomers. These caveats will have implications for clinical applications which are discussed below.
α-Synuclein oligomers as potential biomarkers
If indeed α-syn oligomer toxicity initiates neuronal death in PD by the mechanisms described in the previous section, or by additional undiscovered mechanisms, these toxic oligomers must be present prior to onset of clinical symptoms. This has important implications for diagnosis and treatment of people with PD. At present, the clinical diagnosis of PD relies on the presence of the disease’s cardinal motor features: rest tremor, rigidity, bradykinesia, and loss of postural reflexes.123 This method of diagnosis is particularly limited during early stages of the disease when diagnostic accuracy is 90% at best.124 The more significant limitation is that the neurodegenerative process associated with PD precedes onset of motor symptoms with approximately 70% of neurons in the ventral lateral substantia nigra being lost before motor features appear.125 It has been increasingly recognized that certain non-motor features can antedate the motor symptoms and therefore may have the potential to allow for earlier diagnosis of PD.89 However, there are limitations to this approach because most non-motor features are not specific or sensitive for PD or underlying Lewy pathology. Thus, α-syn oligomers could serve as a biomarker that would allow for identification of at-risk individuals before clinical diagnosis. Detection of α-syn oligomers may also provide a means to monitor disease progression and to follow response to treatments.
For α-syn oligomers to be a useful biomarker, methods for reliably measuring oligomer levels in brains of patients are necessary and ideally should be inexpensive and non-invasive. The development of surrogate measures of brain α-syn oligomer levels is in its early stages but recent studies demonstrate the feasibility of detecting oligomers in plasma or CSF.23,24 A sandwich enzyme-linked immunosorbent assay (ELISA) method was used to detect soluble α-syn oligomers. PD patients had higher levels of α-syn oligomers in plasma23 or CSF24 compared to age-matched controls. Depending on the cutoff values selected for a positive test result, sensitivity and specificity of the test measuring oligomers in plasma was 85% and 53%, respectively, for a diagnosis of PD using clinical diagnosis based on the United Kingdom PD Society Brain Bank criteria126 as the gold standard. The assay for α-syn oligomers in CSF had a diagnostic sensitivity of 87% and specificity of 75%. The accuracy of these tests may be improved by modifying the methods (for example, developing antibodies specific for toxic α-syn oligomers) or by using the tests in conjunction with other measures (such as neuroimaging techniques described below). Further validation in larger well-controlled prospective studies will be required to determine whether plasma and/or CSF α-syn oligomers will be useful as biomarkers to diagnose PD in its early stages or to predict which asymptomatic patients are going to develop the disease. Other fluid biomarkers which have not yet been explored include measuring α-syn oligomers in saliva or urine. However, reliability and utility of assays to measure α-syn oligomer levels in bodily fluids may be limited by the inherent propensity of α-syn to spontaneously form oligomers in vitro in a time- and concentration-dependent manner.
A more robust and useful technique would directly measure α-syn oligomers in vivo. Radioligand-based brain imaging technologies, such as PET and SPECT, could provide a non-invasive method for visualizing α-syn oligomers within the brains of patients. Significant advances have been made in the development of methods to image protein inclusions in AD. For example, ligands for β-amyloid such as Pittsburgh Compound-B (PIB)127 and florbetapir F18128 are now widely used to image amyloid plaques in vivo. Radioligand-based imaging for PD is less advanced with α-syn ligands for direct visualization of LBs and LNs still being developed and characterized.129,130 In the absence of specific α-syn ligands, a possible method to indirectly image Lewy pathology could be to measure total amyloid deposits in the brain with a generic amyloid ligand (for example, a thioflavin S derivative) and then subtract out non-α-syn-containing inclusions (for example, β-amyloid plaques measured with PIB). Development of α-syn ligands with specificity for α-syn oligomers may also pose a challenge since the usefulness of radioligand-based methods would require the ability to discriminate between α-syn oligomers and oligomers composed of other proteins (Table). Again, indirectly measuring α-syn oligomers by developing a pan-oligomer ligand and then subtracting out non-α-syn-containing oligomers could be a useful approach. An additional challenge is developing ligands that allow for distinguishing between toxic and non-toxic α-syn oligomeric species. Regardless, the presence of toxic α-syn oligomers in the extracellular space and their potential role in the early pathogenesis of PD may make development of ligands for oligomeric α-syn rather than intracellular fibrillar α-syn both more feasible and clinically useful.
α-Synuclein oligomers as potential treatment targets
The clinical importance of biomarkers for early diagnosis of PD lies in the anticipated discovery of neuroprotective treatments. As with other neurodegenerative diseases such as Alzheimer disease and Huntington disease, diagnostic and treatment approaches for PD are shifting toward attempts at diagnosing very early in order to treat with disease-modifying therapies before more severe neurodegeneration has become well established. The discovery of a role for α-syn oligomers in neuronal loss in PD opens a new area of investigation into disease-modifying therapies for PD and related synucleinopathies using α-syn oligomeric species as the treatment target. The main goal of these therapies would be to reduce toxic α-syn oligomer levels by preventing their formation, disassembling already formed oligomers, or sequestering or removing them from the intracellular and/or extracellular space. Multiple approaches aimed at different steps along the protein aggregation pathway and at various aspects of oligomer toxicity are currently being examined in basic research and preclinical studies. These approaches include altering the posttranslational modification state of α-syn, directly blocking or disrupting oligomer formation, preventing cell-to-cell transmission of α-syn oligomers, upregulating the chaperone system, or promoting the clearance of toxic oligomeric species (Fig 1B).
Posttranslational modifications of α-syn can influence its propensity to form oligomers. For example, phosphorylation of α-syn on serine 129 promotes α-syn aggregation.39 Protein phosphorylation is regulated by two opposing classes of enzymes: protein kinases, which catalyze the transfer of phosphate to proteins, and protein phosphatases, which dephosphorylate proteins. Thus kinase inhibitors or phosphatase activators can reduce the phosphorylation state of a protein. Several kinase inhibitors have been developed for treatment of non-neurological disorders, especially cancer.131 Extension of this work to develop specific inhibitors of the kinase that mediates serine α-syn phosphorylation may lead to the development of oligomer-lowering drugs (Fig 1B).132 In contrast to phosphorylation, ubiquitination of α-syn can be associated with decreased oligomer levels.18 Ubiquitination is also regulated by two enzyme families, ubiquitin ligases and deubiquitinases, which add and remove the ubiquitin moiety to and from proteins, respectively. Similar to kinase inhibitors, drugs that inhibit the deubiquitinating enzyme which mediates α-syn ubiquitination may reduce oligomer levels. Although less advanced than kinase inhibitor development, drug discovery programs for the ubiquitin system are expected to expand.131 The major challenge for this strategy is that phosphorylation and ubiquitination regulate most aspects of cell life and therefore specificity of inhibition is critical to prevent dysregulation of other important cellular functions.
Directly blocking or disrupting α-syn oligomer formation using small molecules or synthetic peptides is another strategy to reduce oligomer-mediated neurodegeneration (Fig 1B). The small molecule curcumin, a polyphenolic compound found in the spice turmeric, was recently demonstrated to bind with high affinity to α-syn monomers.133 Upon binding, curcumin promotes a conformation of α-syn with reduced propensity to aggregate and thereby prevents formation of α-syn oligomers and fibrils. Polyphenols are a structural class of organic chemicals characterized by multiple aromatic rings and produced by many different plants. Other polyphenolic compounds are able to inhibit α-syn oligomer formation and even disaggregate preformed α-syn oligomeric structures in vitro, possibly by interacting with the aromatic amino acids of α-syn that facilitate protein aggregation.134 Synthetic peptides may also be useful to reduce oligomer levels by interacting with specific regions of α-syn. Peptides can interfere with protein-protein interactions if designed to match the specific amino acid sequence to which a protein binds. The sequence within α-syn responsible for its self-association has been identified as amino acids 64 to 86.135 Short synthetic peptides containing part of this sequence (amino acids 69 to 72) bind to full-length α-syn and preclude the assembly of oligomers and fibrils in vitro. Membrane-permeable versions of these peptides have been developed135 but their effects on α-syn oligomers in cell culture or animal models have not yet been reported. At present, a major limitation of oligomer-targeting small molecules and synthetic peptides as a treatment for PD is attaining adequate permeability through the blood-brain barrier. Additional limitations include bioavailability and metabolic turnover of small molecules, as well as generation of an immune response to peptides or degradation of peptides by endogenous proteases. Moreover, the approaches to targeting α-syn oligomers described above may not discriminate between different forms of α-syn oligomers.
Treatments which target the specific α-syn oligomeric species that are toxic to neurons are expected to reduce neurodegeneration as well as avoid side effects that may result from inhibiting non-toxic α-syn oligomers. The possibility that native cellular α-syn exists as an α-helically folded tetramer and not all α-syn oligomers are toxic5 suggests that indiscriminate targeting of α-syn oligomers may impact the physiologic function of endogenous α-syn. Unlike small molecules and synthetic peptides which typically recognize specific amino acids or short sequences within a protein, antibodies can be designed against protein conformations and therefore are conducive for development as oligomer-specific targeting therapies. Monoclonal mouse antibodies have been generated against α-syn oligomers and a clone which can be internalized by cells to reduce intracellular levels of α-syn oligomers in cell culture has been identified.136 Similarly, phage display technology has been used to isolate single chain variable fragments, which are fusion proteins of the heavy and light chains of immunoglobulins, against specific oligomeric conformations of α-syn.137,138 Using this technique, two different single chain variable fragments were found to recognize distinct populations of toxic α-syn oligomers. Antibodies also provide a potential means by which intercellular transmission of α-syn oligomers can be blocked, thereby inhibiting prion-like propagation.
Preclinical studies support the potential for immunotherapy as an approach to treat PD. These include studies investigating active immunization using a mouse model of PD.139 Specifically, transgenic mice overexpressing human α-syn were vaccinated with recombinant human α-syn. Vaccinated mice which produced high relative affinity anti-α-syn antibodies were found to have reduced levels of α-syn oligomers. Passive immunization of these transgenic mice by injection of a monoclonal anti-α-syn antibody also resulted in a decrease in α-syn oligomer levels.140 This was associated with improvement in motor behavior, as well as learning and memory tasks, suggesting specificity of targeting toxic oligomeric species. Translation of immunization strategies from animal models to clinical trials are just beginning with the first phase I study investigating active immunization using an α-syn-based peptide vaccine in PD patients (ClinicalTrials.gov, NCT01568099). Clinical trials are further advanced in the field of AD where β-amyloid immunotherapy is being developed.141 β-Amyloid is a suitable target for antibody-based therapies because of its extracellular site of action whereas targeting α-syn may be more challenging because of its intracellular and extracellular pools. There have been setbacks for β-amyloid immunotherapy including early termination of phase II clinical trials testing active immunization due to a small but significant occurrence of meningoencephalitis. There have also been conflicting results regarding the clinical benefit of β-amyloid immunization for AD patients. Regardless, immunotherapy remains a promising area of investigation for AD and an emerging strategy for treatment of PD. It is anticipated that knowledge gained from β-amyloid vaccination in humans will allow for optimization of similar vaccines to avoid detrimental CNS inflammatory reactions. This may not even be a concern for PD if the inflammatory response observed in AD was related to coexistence of cerebral amyloid angiopathy; unlike β-amyloid, α-syn does not deposit in leptomeningeal and cortical blood vessels. Furthermore, using antibodies which are designed to target toxic oligomers, instead of antibodies which do not recognize conformational epitopes, is expected to more specifically treat the underlying pathological process and translate to improved efficacy as well as safety.
An indirect strategy to reduce toxic α-syn oligomeric species is to upregulate the intracellular and extracellular systems responsible for regulating oligomer levels (Fig 1B). Specific chaperone molecules, such as Hsp7017,19 and CHIP,16,18 can reduce α-syn oligomers likely by multiple mechanisms including refolding misfolded proteins, disrupting protein aggregates, and promoting protein clearance. Several brain permeable small molecules that increase Hsp70 levels have been studied in PD models.142 In particular, small molecule inhibitors of the chaperone molecule Hsp90, which induce Hsp70 expression, can decrease α-syn oligomerization and rescue α-syn-induced toxicity.17 Additional strategies to enhance chaperone activity and reduce α-syn levels include viral-mediated gene therapy or transduction-based peptide delivery to increase chaperone levels or to modulate chaperone activity.142 The ALP is also important in regulating α-syn oligomer levels by clearing oligomers within the intracellular compartment.143 Investigations into drugs that can enhance this pathway and reduce toxic α-syn species are being initiated.144,145 Areas for future investigation include strategies to discriminately activate the UPS to expedite the elimination of intracellular α-syn oligomers146 and to upregulate glia-mediated mechanisms for specific clearance of extracellular α-syn oligomers.147,148
Conclusions
Protein aggregation remains a unifying theme among neurodegenerative diseases but our understanding of the relationship between aggregates of pathogenic proteins and neurodegeneration continues to evolve. As illustrated by ongoing research into the role of α-syn in PD pathogenesis, there has been a shift in focus from protein inclusions to oligomers. In PD and other neurodegenerative conditions, protein inclusions do not represent the sole neurotoxic protein entity. Oligomers have emerged as important contributors to toxicity and therefore their role in neurodegeneration warrants further investigation. If the oligomer hypothesis turns out to be correct, it will have the potential to lead to neuroprotective therapies which, to date, have remained elusive for PD and all other neurodegenerative diseases.
Acknowledgments
LVK is supported by a Canadian Institutes of Health Research (CIHR) Clinician-Scientist Award. PJM is supported by a NIH grant (NS 063963). AML is a Tier 1 Canada Research Chair in Neuroscience at the University of Toronto and holds the RR Tasker Chair in Functional Neurosurgery at the University Health Network. AEL holds the Jack Clark Chair in Parkinson’s Disease Research at the University of Toronto. This work was partially supported by the National Parkinson Foundation Center of Excellence Award.
References
- 1.Lewy F. Paralysis agitans. In: Lewandowski M, editor. Handbuch der Neurologie. Berlin: Springer; 1912. pp. 920–933. [Google Scholar]
- 2.Ueda K, Fukushima H, Masliah E, et al. Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:11282–11286. doi: 10.1073/pnas.90.23.11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jakes R, Spillantini MG, Goedert M. Identification of two distinct synucleins from human brain. FEBS Lett. 1994;345:27–32. doi: 10.1016/0014-5793(94)00395-5. [DOI] [PubMed] [Google Scholar]
- 4.Chandra S, Gallardo G, Fernandez-Chacon R, et al. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;123:383–396. doi: 10.1016/j.cell.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 5.Bartels T, Choi JG, Selkoe DJ. alpha-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature. 2011;477:107–110. doi: 10.1038/nature10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang W, Perovic I, Chittuluru J, et al. A soluble alpha-synuclein construct forms a dynamic tetramer. Proc Natl Acad Sci U S A. 2011;108:17797–17802. doi: 10.1073/pnas.1113260108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fauvet B, Mbefo MK, Fares MB, et al. alpha-Synuclein in central nervous system and from erythrocytes, mammalian cells, and Escherichia coli exists predominantly as disordered monomer. J Biol Chem. 2012;287:15345–15364. doi: 10.1074/jbc.M111.318949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eichner T, Radford SE. A diversity of assembly mechanisms of a generic amyloid fold. Mol Cell. 2011;43:8–18. doi: 10.1016/j.molcel.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Walsh DM, Lomakin A, Benedek GB, et al. Amyloid beta-protein fibrillogenesis. Detection of a protofibrillar intermediate. J Biol Chem. 1997;272:22364–22372. doi: 10.1074/jbc.272.35.22364. [DOI] [PubMed] [Google Scholar]
- 10.Conway KA, Harper JD, Lansbury PT. Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat Med. 1998;4:1318–1320. doi: 10.1038/3311. [DOI] [PubMed] [Google Scholar]
- 11.Lashuel HA, Petre BM, Wall J, et al. Alpha-synuclein, especially the Parkinson’s disease-associated mutants, forms pore-like annular and tubular protofibrils. J Mol Biol. 2002;322:1089–1102. doi: 10.1016/s0022-2836(02)00735-0. [DOI] [PubMed] [Google Scholar]
- 12.Harper JD, Lieber CM, Lansbury PT., Jr Atomic force microscopic imaging of seeded fibril formation and fibril branching by the Alzheimer’s disease amyloid-beta protein. Chem Biol. 1997;4:951–959. doi: 10.1016/s1074-5521(97)90303-3. [DOI] [PubMed] [Google Scholar]
- 13.Wacker JL, Zareie MH, Fong H, et al. Hsp70 and Hsp40 attenuate formation of spherical and annular polyglutamine oligomers by partitioning monomer. Nat Struct Mol Biol. 2004;11:1215–1222. doi: 10.1038/nsmb860. [DOI] [PubMed] [Google Scholar]
- 14.Hu CD, Chinenov Y, Kerppola TK. Visualization of interactions among bZIP and Rel family proteins in living cells using bimolecular fluorescence complementation. Mol Cell. 2002;9:789–798. doi: 10.1016/s1097-2765(02)00496-3. [DOI] [PubMed] [Google Scholar]
- 15.Outeiro TF, Putcha P, Tetzlaff JE, et al. Formation of toxic oligomeric alpha-synuclein species in living cells. PLoS One. 2008;3:e1867. doi: 10.1371/journal.pone.0001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tetzlaff JE, Putcha P, Outeiro TF, et al. CHIP targets toxic alpha-Synuclein oligomers for degradation. J Biol Chem. 2008;283:17962–17968. doi: 10.1074/jbc.M802283200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Putcha P, Danzer KM, Kranich LR, et al. Brain-permeable small-molecule inhibitors of Hsp90 prevent alpha-synuclein oligomer formation and rescue alpha-synuclein-induced toxicity. J Pharmacol Exp Ther. 2010;332:849–857. doi: 10.1124/jpet.109.158436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalia LV, Kalia SK, Chau H, et al. Ubiquitinylation of alpha-synuclein by carboxyl terminus Hsp70-interacting protein (CHIP) is regulated by Bcl-2-associated athanogene 5 (BAG5) PLoS One. 2011;6:e14695. doi: 10.1371/journal.pone.0014695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danzer KM, Ruf WP, Putcha P, et al. Heat-shock protein 70 modulates toxic extracellular alpha-synuclein oligomers and rescues trans-synaptic toxicity. FASEB J. 2011;25:326–336. doi: 10.1096/fj.10-164624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mazzulli JR, Xu YH, Sun Y, et al. Gaucher disease glucocerebrosidase and alpha-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell. 2011;146:37–52. doi: 10.1016/j.cell.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winner B, Jappelli R, Maji SK, et al. In vivo demonstration that alpha-synuclein oligomers are toxic. Proc Natl Acad Sci U S A. 2011;108:4194–4199. doi: 10.1073/pnas.1100976108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharon R, Bar-Joseph I, Frosch MP, et al. The formation of highly soluble oligomers of alpha-synuclein is regulated sby fatty acids and enhanced in Parkinson’s disease. Neuron. 2003;37:583–595. doi: 10.1016/s0896-6273(03)00024-2. [DOI] [PubMed] [Google Scholar]
- 23.El-Agnaf OM, Salem SA, Paleologou KE, et al. Detection of oligomeric forms of alpha-synuclein protein in human plasma as a potential biomarker for Parkinson’s disease. FASEB J. 2006;20:419–425. doi: 10.1096/fj.03-1449com. [DOI] [PubMed] [Google Scholar]
- 24.Tokuda T, Qureshi MM, Ardah MT, et al. Detection of elevated levels of alpha-synuclein oligomers in CSF from patients with Parkinson disease. Neurology. 2010;75:1766–1772. doi: 10.1212/WNL.0b013e3181fd613b. [DOI] [PubMed] [Google Scholar]
- 25.Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004;10 (Suppl):S10–S17. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- 26.Spillantini MG, Schmidt ML, Lee VM, et al. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 27.Polymeropoulos MH, Lavedan C, Leroy E, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 28.Kruger R, Kuhn W, Muller T, et al. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 29.Zarranz JJ, Alegre J, Gomez-Esteban JC, et al. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 30.Greenbaum EA, Graves CL, Mishizen-Eberz AJ, et al. The E46K mutation in alpha-synuclein increases amyloid fibril formation. J Biol Chem. 2005;280:7800–7807. doi: 10.1074/jbc.M411638200. [DOI] [PubMed] [Google Scholar]
- 31.Singleton AB, Farrer M, Johnson J, et al. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 32.Chartier-Harlin MC, Kachergus J, Roumier C, et al. Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet. 2004;364:1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- 33.Minton AP. Influence of macromolecular crowding upon the stability and state of association of proteins: predictions and observations. J Pharm Sci. 2005;94:1668–1675. doi: 10.1002/jps.20417. [DOI] [PubMed] [Google Scholar]
- 34.Satake W, Nakabayashi Y, Mizuta I, et al. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson’s disease. Nat Genet. 2009;41:1303–1307. doi: 10.1038/ng.485. [DOI] [PubMed] [Google Scholar]
- 35.Simon-Sanchez J, Schulte C, Bras JM, et al. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat Genet. 2009;41:1308–1312. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fuchs J, Tichopad A, Golub Y, et al. Genetic variability in the SNCA gene influences alpha-synuclein levels in the blood and brain. FASEB J. 2008;22:1327–1334. doi: 10.1096/fj.07-9348com. [DOI] [PubMed] [Google Scholar]
- 37.Mata IF, Shi M, Agarwal P, et al. SNCA variant associated with Parkinson disease and plasma alpha-synuclein level. Arch Neurol. 2010;67:1350–1356. doi: 10.1001/archneurol.2010.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neumann M, Kahle PJ, Giasson BI, et al. Misfolded proteinase K-resistant hyperphosphorylated alpha-synuclein in aged transgenic mice with locomotor deterioration and in human alpha-synucleinopathies. J Clin Invest. 2002;110:1429–1439. doi: 10.1172/JCI15777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujiwara H, Hasegawa M, Dohmae N, et al. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4:160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 40.Yamin G, Uversky VN, Fink AL. Nitration inhibits fibrillation of human alpha-synuclein in vitro by formation of soluble oligomers. FEBS Lett. 2003;542:147–152. doi: 10.1016/s0014-5793(03)00367-3. [DOI] [PubMed] [Google Scholar]
- 41.Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475:324–332. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- 42.Tyedmers J, Mogk A, Bukau B. Cellular strategies for controlling protein aggregation. Nat Rev Mol Cell Biol. 2010;11:777–788. doi: 10.1038/nrm2993. [DOI] [PubMed] [Google Scholar]
- 43.Cook C, Petrucelli L. A critical evaluation of the ubiquitin-proteasome system in Parkinson’s disease. Biochim Biophys Acta. 2009;1792:664–675. doi: 10.1016/j.bbadis.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chu Y, Dodiya H, Aebischer P, et al. Alterations in lysosomal and proteasomal markers in Parkinson’s disease: relationship to alpha-synuclein inclusions. Neurobiol Dis. 2009;35:385–398. doi: 10.1016/j.nbd.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 45.Alvarez-Erviti L, Rodriguez-Oroz MC, Cooper JM, et al. Chaperone-mediated autophagy markers in Parkinson disease brains. Arch Neurol. 2010;67:1464–1472. doi: 10.1001/archneurol.2010.198. [DOI] [PubMed] [Google Scholar]
- 46.Sidransky E, Nalls MA, Aasly JO, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N Engl J Med. 2009;361:1651–1661. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Calne DB, Langston JW. Aetiology of Parkinson’s disease. Lancet. 1983;2:1457–1459. doi: 10.1016/s0140-6736(83)90802-4. [DOI] [PubMed] [Google Scholar]
- 48.Collier TJ, Kanaan NM, Kordower JH. Ageing as a primary risk factor for Parkinson’s disease: evidence from studies of non-human primates. Nat Rev Neurosci. 2011;12:359–366. doi: 10.1038/nrn3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kourtis N, Tavernarakis N. Cellular stress response pathways and ageing: intricate molecular relationships. EMBO J. 2011;30:2520–2531. doi: 10.1038/emboj.2011.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Braak H, Del TK, Rub U, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 51.Cookson MR, Hardy J, Lewis PA. Genetic neuropathology of Parkinson’s disease. Int J Clin Exp Pathol. 2008;1:217–231. [PMC free article] [PubMed] [Google Scholar]
- 52.Forno LS. Concentric hyalin intraneuronal inclusions of Lewy type in the brains of elderly persons (50 incidental cases): relationship to parkinsonism. J Am Geriatr Soc. 1969;17:557–575. doi: 10.1111/j.1532-5415.1969.tb01316.x. [DOI] [PubMed] [Google Scholar]
- 53.Saito Y, Ruberu NN, Sawabe M, et al. Lewy body-related alpha-synucleinopathy in aging. J Neuropathol Exp Neurol. 2004;63:742–749. doi: 10.1093/jnen/63.7.742. [DOI] [PubMed] [Google Scholar]
- 54.Farrer M, Chan P, Chen R, et al. Lewy bodies and parkinsonism in families with parkin mutations. Ann Neurol. 2001;50:293–300. doi: 10.1002/ana.1132. [DOI] [PubMed] [Google Scholar]
- 55.Pramstaller PP, Schlossmacher MG, Jacques TS, et al. Lewy body Parkinson’s disease in a large pedigree with 77 Parkin mutation carriers. Ann Neurol. 2005;58:411–422. doi: 10.1002/ana.20587. [DOI] [PubMed] [Google Scholar]
- 56.Sasaki S, Shirata A, Yamane K, et al. Parkin-positive autosomal recessive juvenile Parkinsonism with alpha-synuclein-positive inclusions. Neurology. 2004;63:678–682. doi: 10.1212/01.wnl.0000134657.25904.0b. [DOI] [PubMed] [Google Scholar]
- 57.Matsumine H, Saito M, Shimoda-Matsubayashi S, et al. Localization of a gene for an autosomal recessive form of juvenile Parkinsonism to chromosome 6q25.2–27. Am J Hum Genet. 1997;60:588–596. [PMC free article] [PubMed] [Google Scholar]
- 58.Mori H, Kondo T, Yokochi M, et al. Pathologic and biochemical studies of juvenile parkinsonism linked to chromosome 6q. Neurology. 1998;51:890–892. doi: 10.1212/wnl.51.3.890. [DOI] [PubMed] [Google Scholar]
- 59.Yamamura Y, Kuzuhara S, Kondo K, et al. Clinical, pathologic and genetic studies on autosomal recessive early-onset parkinsonism with diurnal fluctuation. Parkinsonism Relat Disord. 1998;4:65–72. doi: 10.1016/s1353-8020(98)00015-7. [DOI] [PubMed] [Google Scholar]
- 60.Yamamura Y, Hattori N, Matsumine H, et al. Autosomal recessive early-onset parkinsonism with diurnal fluctuation: clinicopathologic characteristics and molecular genetic identification. Brain Dev. 2000;22 (Suppl 1):S87–S91. doi: 10.1016/s0387-7604(00)00130-3. [DOI] [PubMed] [Google Scholar]
- 61.Hayashi S, Wakabayashi K, Ishikawa A, et al. An autopsy case of autosomal-recessive juvenile parkinsonism with a homozygous exon 4 deletion in the parkin gene. Mov Disord. 2000;15:884–888. doi: 10.1002/1531-8257(200009)15:5<884::aid-mds1019>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 62.van de Warrenburg BP, Lammens M, Lucking CB, et al. Clinical and pathologic abnormalities in a family with parkinsonism and parkin gene mutations. Neurology. 2001;56:555–557. doi: 10.1212/wnl.56.4.555. [DOI] [PubMed] [Google Scholar]
- 63.Gouider-Khouja N, Larnaout A, Amouri R, et al. Autosomal recessive parkinsonism linked to parkin gene in a Tunisian family. Clinical, genetic and pathological study. Parkinsonism Relat Disord. 2003;9:247–251. doi: 10.1016/s1353-8020(03)00016-6. [DOI] [PubMed] [Google Scholar]
- 64.Corti O, Lesage S, Brice A. What genetics tells us about the causes and mechanisms of Parkinson’s disease. Physiol Rev. 2011;91:1161–1218. doi: 10.1152/physrev.00022.2010. [DOI] [PubMed] [Google Scholar]
- 65.Gilks WP, bou-Sleiman PM, Gandhi S, et al. A common LRRK2 mutation in idiopathic Parkinson’s disease. Lancet. 2005;365:415–416. doi: 10.1016/S0140-6736(05)17830-1. [DOI] [PubMed] [Google Scholar]
- 66.Khan NL, Jain S, Lynch JM, et al. Mutations in the gene LRRK2 encoding dardarin (PARK8) cause familial Parkinson’s disease: clinical, pathological, olfactory and functional imaging and genetic data. Brain. 2005;128:2786–2796. doi: 10.1093/brain/awh667. [DOI] [PubMed] [Google Scholar]
- 67.Giasson BI, Covy JP, Bonini NM, et al. Biochemical and pathological characterization of Lrrk2. Ann Neurol. 2006;59:315–322. doi: 10.1002/ana.20791. [DOI] [PubMed] [Google Scholar]
- 68.Rajput A, Dickson DW, Robinson CA, et al. Parkinsonism, Lrrk2 G2019S, and tau neuropathology. Neurology. 2006;67:1506–1508. doi: 10.1212/01.wnl.0000240220.33950.0c. [DOI] [PubMed] [Google Scholar]
- 69.Ross OA, Toft M, Whittle AJ, et al. Lrrk2 and Lewy body disease. Ann Neurol. 2006;59:388–393. doi: 10.1002/ana.20731. [DOI] [PubMed] [Google Scholar]
- 70.Gaig C, Marti MJ, Ezquerra M, et al. G2019S LRRK2 mutation causing Parkinson’s disease without Lewy bodies. J Neurol Neurosurg Psychiatry. 2007;78:626–628. doi: 10.1136/jnnp.2006.107904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Giordana MT, D’Agostino C, Albani G, et al. Neuropathology of Parkinson’s disease associated with the LRRK2 Ile1371Val mutation. Mov Disord. 2007;22:275–278. doi: 10.1002/mds.21281. [DOI] [PubMed] [Google Scholar]
- 72.Gaig C, Ezquerra M, Marti MJ, et al. Screening for the LRRK2 G2019S and codon-1441 mutations in a pathological series of parkinsonian syndromes and frontotemporal lobar degeneration. J Neurol Sci. 2008;270:94–98. doi: 10.1016/j.jns.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 73.Covy JP, Yuan W, Waxman EA, et al. Clinical and pathological characteristics of patients with leucine-rich repeat kinase-2 mutations. Mov Disord. 2009;24:32–39. doi: 10.1002/mds.22096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gaig C, Marti MJ, Ezquerra M, et al. G2019S LRRK2 mutation causing Parkinson’s disease without Lewy bodies. BMJ Case Rep. 2009 doi: 10.1136/bcr.08.2008.0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hasegawa K, Stoessl AJ, Yokoyama T, et al. Familial parkinsonism: study of original Sagamihara PARK8 (I2020T) kindred with variable clinicopathologic outcomes. Parkinsonism Relat Disord. 2009;15:300–306. doi: 10.1016/j.parkreldis.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marti-Masso JF, Ruiz-Martinez J, Bolano MJ, et al. Neuropathology of Parkinson’s disease with the R1441G mutation in LRRK2. Mov Disord. 2009;24:1998–2001. doi: 10.1002/mds.22677. [DOI] [PubMed] [Google Scholar]
- 77.Gomez A, Ferrer I. Involvement of the cerebral cortex in Parkinson disease linked with G2019S LRRK2 mutation without cognitive impairment. Acta Neuropathol. 2010;120:155–167. doi: 10.1007/s00401-010-0669-y. [DOI] [PubMed] [Google Scholar]
- 78.Kingsbury AE, Bandopadhyay R, Silveira-Moriyama L, et al. Brain stem pathology in Parkinson’s disease: an evaluation of the Braak staging model. Mov Disord. 2010;25:2508–2515. doi: 10.1002/mds.23305. [DOI] [PubMed] [Google Scholar]
- 79.Vitte J, Traver S, Maues De PA, et al. Leucine-rich repeat kinase 2 is associated with the endoplasmic reticulum in dopaminergic neurons and accumulates in the core of Lewy bodies in Parkinson disease. J Neuropathol Exp Neurol. 2010;69:959–972. doi: 10.1097/NEN.0b013e3181efc01c. [DOI] [PubMed] [Google Scholar]
- 80.Poulopoulos M, Cortes E, Vonsattel JP, et al. Clinical and Pathological Characteristics of LRRK2 G2019S Patients with PD. J Mol Neurosci. 2011;47:139–143. doi: 10.1007/s12031-011-9696-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bezard E, Przedborski S. A tale on animal models of Parkinson’s disease. Mov Disord. 2011;26:993–1002. doi: 10.1002/mds.23696. [DOI] [PubMed] [Google Scholar]
- 82.Dauer W, Kholodilov N, Vila M, et al. Resistance of alpha-synuclein null mice to the parkinsonian neurotoxin MPTP. Proc Natl Acad Sci U S A. 2002;99:14524–14529. doi: 10.1073/pnas.172514599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Betarbet R, Sherer TB, MacKenzie G, et al. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 84.Maries E, Dass B, Collier TJ, et al. The role of alpha-synuclein in Parkinson’s disease: insights from animal models. Nat Rev Neurosci. 2003;4:727–738. doi: 10.1038/nrn1199. [DOI] [PubMed] [Google Scholar]
- 85.Dawson TM, Ko HS, Dawson VL. Genetic animal models of Parkinson’s disease. Neuron. 2010;66:646–661. doi: 10.1016/j.neuron.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee MK, Stirling W, Xu Y, et al. Human alpha-synuclein-harboring familial Parkinson’s disease-linked Ala-53 --> Thr mutation causes neurodegenerative disease with alpha-synuclein aggregation in transgenic mice. Proc Natl Acad Sci U S A. 2002;99:8968–8973. doi: 10.1073/pnas.132197599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Giasson BI, Duda JE, Quinn SM, et al. Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron. 2002;34:521–533. doi: 10.1016/s0896-6273(02)00682-7. [DOI] [PubMed] [Google Scholar]
- 88.Karpinar DP, Balija MB, Kugler S, et al. Pre-fibrillar alpha-synuclein variants with impaired beta-structure increase neurotoxicity in Parkinson’s disease models. EMBO J. 2009;28:3256–3268. doi: 10.1038/emboj.2009.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lim SY, Fox SH, Lang AE. Overview of the extranigral aspects of Parkinson disease. Arch Neurol. 2009;66:167–172. doi: 10.1001/archneurol.2008.561. [DOI] [PubMed] [Google Scholar]
- 90.Tsika E, Moysidou M, Guo J, et al. Distinct region-specific alpha-synuclein oligomers in A53T transgenic mice: implications for neurodegeneration. J Neurosci. 2010;30:3409–3418. doi: 10.1523/JNEUROSCI.4977-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Paleologou KE, Kragh CL, Mann DM, et al. Detection of elevated levels of soluble alpha-synuclein oligomers in post-mortem brain extracts from patients with dementia with Lewy bodies. Brain. 2009;132:1093–1101. doi: 10.1093/brain/awn349. [DOI] [PubMed] [Google Scholar]
- 92.Tayebi N, Walker J, Stubblefield B, et al. Gaucher disease with parkinsonian manifestations: does glucocerebrosidase deficiency contribute to a vulnerability to parkinsonism? Mol Genet Metab. 2003;79:104–109. doi: 10.1016/s1096-7192(03)00071-4. [DOI] [PubMed] [Google Scholar]
- 93.Wong K, Sidransky E, Verma A, et al. Neuropathology provides clues to the pathophysiology of Gaucher disease. Mol Genet Metab. 2004;82:192–207. doi: 10.1016/j.ymgme.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 94.Neumann J, Bras J, Deas E, et al. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson’s disease. Brain. 2009;132:1783–1794. doi: 10.1093/brain/awp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Goker-Alpan O, Stubblefield BK, Giasson BI, et al. Glucocerebrosidase is present in alpha-synuclein inclusions in Lewy body disorders. Acta Neuropathol. 2010;120:641–649. doi: 10.1007/s00401-010-0741-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nishioka K, Ross OA, Vilarino-Guell C, et al. Glucocerebrosidase mutations in diffuse Lewy body disease. Parkinsonism Relat Disord. 2011;17:55–57. doi: 10.1016/j.parkreldis.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hinault MP, Cuendet AF, Mattoo RU, et al. Stable alpha-synuclein oligomers strongly inhibit chaperone activity of the Hsp70 system by weak interactions with J-domain co-chaperones. J Biol Chem. 2010;285:38173–38182. doi: 10.1074/jbc.M110.127753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Petrucelli L, O’Farrell C, Lockhart PJ, et al. Parkin protects against the toxicity associated with mutant alpha-synuclein: proteasome dysfunction selectively affects catecholaminergic neurons. Neuron. 2002;36:1007–1019. doi: 10.1016/s0896-6273(02)01125-x. [DOI] [PubMed] [Google Scholar]
- 99.Kalia SK, Lee S, Smith PD, et al. BAG5 inhibits parkin and enhances dopaminergic neuron degeneration. Neuron. 2004;44:931–945. doi: 10.1016/j.neuron.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 100.Lindersson E, Beedholm R, Hojrup P, et al. Proteasomal inhibition by alpha-synuclein filaments and oligomers. J Biol Chem. 2004;279:12924–12934. doi: 10.1074/jbc.M306390200. [DOI] [PubMed] [Google Scholar]
- 101.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 102.Smith WW, Jiang H, Pei Z, et al. Endoplasmic reticulum stress and mitochondrial cell death pathways mediate A53T mutant alpha-synuclein-induced toxicity. Hum Mol Genet. 2005;14:3801–3811. doi: 10.1093/hmg/ddi396. [DOI] [PubMed] [Google Scholar]
- 103.Cooper AA, Gitler AD, Cashikar A, et al. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson’s models. Science. 2006;313:324–328. doi: 10.1126/science.1129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Colla E, Jensen PH, Pletnikova O, et al. Accumulation of Toxic alpha-Synuclein Oligomer within Endoplasmic Reticulum Occurs in alpha-Synucleinopathy In Vivo. J Neurosci. 2012;32:3301–3305. doi: 10.1523/JNEUROSCI.5368-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Colla E, Coune P, Liu Y, et al. Endoplasmic Reticulum Stress Is Important for the Manifestations of alpha-Synucleinopathy In Vivo. J Neurosci. 2012;32:3306–3320. doi: 10.1523/JNEUROSCI.5367-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Volles MJ, Lee SJ, Rochet JC, et al. Vesicle permeabilization by protofibrillar alpha-synuclein: implications for the pathogenesis and treatment of Parkinson’s disease. Biochemistry. 2001;40:7812–7819. doi: 10.1021/bi0102398. [DOI] [PubMed] [Google Scholar]
- 107.Danzer KM, Haasen D, Karow AR, et al. Different species of alpha-synuclein oligomers induce calcium influx and seeding. J Neurosci. 2007;27:9220–9232. doi: 10.1523/JNEUROSCI.2617-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huls S, Hogen T, Vassallo N, et al. AMPA-receptor-mediated excitatory synaptic transmission is enhanced by iron-induced alpha-synuclein oligomers. J Neurochem. 2011;117:868–878. doi: 10.1111/j.1471-4159.2011.07254.x. [DOI] [PubMed] [Google Scholar]
- 109.Martin ZS, Neugebauer V, Dineley KT, et al. alpha-Synuclein oligomers oppose long-term potentiation and impair memory through a calcineurin-dependent mechanism: relevance to human synucleopathic diseases. J Neurochem. 2012;120:440–452. doi: 10.1111/j.1471-4159.2011.07576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jucker M, Walker LC. Pathogenic protein seeding in Alzheimer disease and other neurodegenerative disorders. Ann Neurol. 2011;70:532–540. doi: 10.1002/ana.22615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kordower JH, Chu Y, Hauser RA, et al. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med. 2008;14:504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 112.Li JY, Englund E, Holton JL, et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008;14:501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 113.Kordower JH, Dodiya HB, Kordower AM, et al. Transfer of host-derived alpha synuclein to grafted dopaminergic neurons in rat. Neurobiol Dis. 2011;43:552–557. doi: 10.1016/j.nbd.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hansen C, Angot E, Bergstrom AL, et al. alpha-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest. 2011;121:715–725. doi: 10.1172/JCI43366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Volpicelli-Daley LA, Luk KC, Patel TP, et al. Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72:57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu J, Zhang JP, Shi M, et al. Rab11a and HSP90 regulate recycling of extracellular alpha-synuclein. J Neurosci. 2009;29:1480–1485. doi: 10.1523/JNEUROSCI.6202-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Emmanouilidou E, Melachroinou K, Roumeliotis T, et al. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci. 2010;30:6838–6851. doi: 10.1523/JNEUROSCI.5699-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lee HJ, Suk JE, Bae EJ, et al. Assembly-dependent endocytosis and clearance of extracellular alpha-synuclein. Int J Biochem Cell Biol. 2008;40:1835–1849. doi: 10.1016/j.biocel.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 119.Desplats P, Lee HJ, Bae EJ, et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci U S A. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Angot E, Steiner JA, Hansen C, et al. Are synucleinopathies prion-like disorders? Lancet Neurol. 2010;9:1128–1138. doi: 10.1016/S1474-4422(10)70213-1. [DOI] [PubMed] [Google Scholar]
- 121.Goedert M, Clavaguera F, Tolnay M. The propagation of prion-like protein inclusions in neurodegenerative diseases. Trends Neurosci. 2010;33:317–325. doi: 10.1016/j.tins.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 122.Cremades N, Cohen SI, Deas E, et al. Direct observation of the interconversion of normal and toxic forms of alpha-synuclein. Cell. 2012;149:1048–1059. doi: 10.1016/j.cell.2012.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lang AE, Lozano AM. Parkinson’s disease. First of two parts. N Engl J Med. 1998;339:1044–1053. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- 124.Hughes AJ, Daniel SE, Lees AJ. Improved accuracy of clinical diagnosis of Lewy body Parkinson’s disease. Neurology. 2001;57:1497–1499. doi: 10.1212/wnl.57.8.1497. [DOI] [PubMed] [Google Scholar]
- 125.Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain. 1991;114:2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- 126.Gibb WR, Lees AJ. The significance of the Lewy body in the diagnosis of idiopathic Parkinson’s disease. Neuropathol Appl Neurobiol. 1989;15:27–44. doi: 10.1111/j.1365-2990.1989.tb01147.x. [DOI] [PubMed] [Google Scholar]
- 127.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 128.Clark CM, Schneider JA, Bedell BJ, et al. Use of florbetapir-PET for imaging beta-amyloid pathology. JAMA. 2011;305:275–283. doi: 10.1001/jama.2010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hefti F. PET Radiopharmaceuticals for Detection and Differential Diagnosis of Parkinson’s Disease. Harvard NeuroDiscovery Center Annual Symposium. Progress in Molecular Neurology: A Parkinson’s Disease Perspective; 2009. [Google Scholar]
- 130.Kikuchi A, Takeda A, Okamura N, et al. In vivo visualization of alpha-synuclein deposition by carbon-11-labelled 2-[2-(2-dimethylaminothiazol-5-yl)ethenyl]-6-[2-(fluoro)ethoxy]benzoxazole positron emission tomography in multiple system atrophy. Brain. 2010;133:1772–1778. doi: 10.1093/brain/awq091. [DOI] [PubMed] [Google Scholar]
- 131.Cohen P, Tcherpakov M. Will the ubiquitin system furnish as many drug targets as protein kinases? Cell. 2010;143:686–693. doi: 10.1016/j.cell.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 132.Sato H, Arawaka S, Hara S, et al. Authentically phosphorylated alpha-synuclein at Ser129 accelerates neurodegeneration in a rat model of familial Parkinson’s disease. J Neurosci. 2011;31:16884–16894. doi: 10.1523/JNEUROSCI.3967-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ahmad B, Lapidus LJ. Curcumin Prevents Aggregation in alpha-Synuclein by Increasing Reconfiguration Rate. J Biol Chem. 2012;287:9193–9199. doi: 10.1074/jbc.M111.325548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Caruana M, Hogen T, Levin J, et al. Inhibition and disaggregation of alpha-synuclein oligomers by natural polyphenolic compounds. FEBS Lett. 2011;585:1113–1120. doi: 10.1016/j.febslet.2011.03.046. [DOI] [PubMed] [Google Scholar]
- 135.El-Agnaf OM, Paleologou KE, Greer B, et al. A strategy for designing inhibitors of alpha-synuclein aggregation and toxicity as a novel treatment for Parkinson’s disease and related disorders. FASEB J. 2004;18:1315–1317. doi: 10.1096/fj.03-1346fje. [DOI] [PubMed] [Google Scholar]
- 136.Nasstrom T, Goncalves S, Sahlin C, et al. Antibodies against alpha-synuclein reduce oligomerization in living cells. PLoS One. 2011;6:e27230. doi: 10.1371/journal.pone.0027230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Emadi S, Barkhordarian H, Wang MS, et al. Isolation of a human single chain antibody fragment against oligomeric alpha-synuclein that inhibits aggregation and prevents alpha-synuclein-induced toxicity. J Mol Biol. 2007;368:1132–1144. doi: 10.1016/j.jmb.2007.02.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Emadi S, Kasturirangan S, Wang MS, et al. Detecting morphologically distinct oligomeric forms of alpha-synuclein. J Biol Chem. 2009;284:11048–11058. doi: 10.1074/jbc.M806559200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Masliah E, Rockenstein E, Adame A, et al. Effects of alpha-synuclein immunization in a mouse model of Parkinson’s disease. Neuron. 2005;46:857–868. doi: 10.1016/j.neuron.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 140.Masliah E, Rockenstein E, Mante M, et al. Passive immunization reduces behavioral and neuropathological deficits in an alpha-synuclein transgenic model of Lewy body disease. PLoS One. 2011;6:e19338. doi: 10.1371/journal.pone.0019338. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 141.Wisniewski T, Konietzko U. Amyloid-beta immunisation for Alzheimer’s disease. Lancet Neurol. 2008;7:805–811. doi: 10.1016/S1474-4422(08)70170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kalia SK, Kalia LV, McLean PJ. Molecular chaperones as rational drug targets for Parkinson’s disease therapeutics. CNS Neurol Disord Drug Targets. 2010;9:741–753. doi: 10.2174/187152710793237386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lee HJ, Khoshaghideh F, Patel S, et al. Clearance of alpha-synuclein oligomeric intermediates via the lysosomal degradation pathway. J Neurosci. 2004;24:1888–1896. doi: 10.1523/JNEUROSCI.3809-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu K, Liu C, Shen L, et al. Therapeutic effects of rapamycin on MPTP-induced Parkinsonism in mice. Neurochem Int. 2011 doi: 10.1016/j.neuint.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 145.Lu JH, Tan JQ, Durairajan SS, et al. Isorhynchophylline, a natural alkaloid, promotes the degradation of alpha-synuclein in neuronal cells via inducing autophagy. Autophagy. 2012;8:98–108. doi: 10.4161/auto.8.1.18313. [DOI] [PubMed] [Google Scholar]
- 146.Olanow CW, McNaught KS. Ubiquitin-proteasome system and Parkinson’s disease. Mov Disord. 2006;21:1806–1823. doi: 10.1002/mds.21013. [DOI] [PubMed] [Google Scholar]
- 147.Halliday GM, Stevens CH. Glia: initiators and progressors of pathology in Parkinson’s disease. Mov Disord. 2011;26:6–17. doi: 10.1002/mds.23455. [DOI] [PubMed] [Google Scholar]
- 148.Zhu Y, Hou H, Rezai-Zadeh K, et al. CD45 deficiency drives amyloid-beta peptide oligomers and neuronal loss in Alzheimer’s disease mice. J Neurosci. 2011;31:1355–1365. doi: 10.1523/JNEUROSCI.3268-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Benilova I, Karran E, De SB. The toxic Abeta oligomer and Alzheimer’s disease: an emperor in need of clothes. Nat Neurosci. 2012;15:349–357. doi: 10.1038/nn.3028. [DOI] [PubMed] [Google Scholar]
- 150.Pike CJ, Walencewicz AJ, Glabe CG, et al. In vitro aging of beta-amyloid protein causes peptide aggregation and neurotoxicity. Brain Res. 1991;563:311–314. doi: 10.1016/0006-8993(91)91553-d. [DOI] [PubMed] [Google Scholar]
- 151.Walsh DM, Klyubin I, Fadeeva JV, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 152.McLean CA, Cherny RA, Fraser FW, et al. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann Neurol. 1999;46:860–866. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 153.Lasagna-Reeves CA, Castillo-Carranza DL, Guerrero-Muoz MJ, et al. Preparation and characterization of neurotoxic tau oligomers. Biochemistry. 2010;49:10039–10041. doi: 10.1021/bi1016233. [DOI] [PubMed] [Google Scholar]
- 154.Chun W, Waldo GS, Johnson GV. Split GFP complementation assay for quantitative measurement of tau aggregation in situ. Methods Mol Biol. 2011;670:109–123. doi: 10.1007/978-1-60761-744-0_9. [DOI] [PubMed] [Google Scholar]
- 155.Berger Z, Roder H, Hanna A, et al. Accumulation of pathological tau species and memory loss in a conditional model of tauopathy. J Neurosci. 2007;27:3650–3662. doi: 10.1523/JNEUROSCI.0587-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lasagna-Reeves CA, Castillo-Carranza DL, Sengupta U, et al. Tau oligomers impair memory and induce synaptic and mitochondrial dysfunction in wild-type mice. Mol Neurodegener. 2011;6:39. doi: 10.1186/1750-1326-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Maeda S, Sahara N, Saito Y, et al. Increased levels of granular tau oligomers: an early sign of brain aging and Alzheimer’s disease. Neurosci Res. 2006;54:197–201. doi: 10.1016/j.neures.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 158.Maeda S, Sahara N, Saito Y, et al. Granular tau oligomers as intermediates of tau filaments. Biochemistry. 2007;46:3856–3861. doi: 10.1021/bi061359o. [DOI] [PubMed] [Google Scholar]
- 159.Lasagna-Reeves CA, Castillo-Carranza DL, Sengupta U, et al. Identification of oligomers at early stages of tau aggregation in Alzheimer’s disease. FASEB J. 2012;26:1946–1959. doi: 10.1096/fj.11-199851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Silveira JR, Raymond GJ, Hughson AG, et al. The most infectious prion protein particles. Nature. 2005;437:257–261. doi: 10.1038/nature03989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Biasini E, Turnbaugh JA, Unterberger U, et al. Prion protein at the crossroads of physiology and disease. Trends Neurosci. 2012;35:92–103. doi: 10.1016/j.tins.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Simoneau S, Rezaei H, Sales N, et al. In vitro and in vivo neurotoxicity of prion protein oligomers. PLoS Pathog. 2007;3:e125. doi: 10.1371/journal.ppat.0030125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Minaki H, Sasaki K, Honda H, et al. Prion protein oligomers in Creutzfeldt-Jakob disease detected by gel-filtration centrifuge columns. Neuropathology. 2009;29:536–542. doi: 10.1111/j.1440-1789.2009.01007.x. [DOI] [PubMed] [Google Scholar]
- 164.Schaffar G, Breuer P, Boteva R, et al. Cellular toxicity of polyglutamine expansion proteins: mechanism of transcription factor deactivation. Mol Cell. 2004;15:95–105. doi: 10.1016/j.molcel.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 165.Lajoie P, Snapp EL. Formation and toxicity of soluble polyglutamine oligomers in living cells. PLoS One. 2010;5:e15245. doi: 10.1371/journal.pone.0015245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sathasivam K, Lane A, Legleiter J, et al. Identical oligomeric and fibrillar structures captured from the brains of R6/2 and knock-in mouse models of Huntington’s disease. Hum Mol Genet. 2010;19:65–78. doi: 10.1093/hmg/ddp467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Legleiter J, Mitchell E, Lotz GP, et al. Mutant huntingtin fragments form oligomers in a polyglutamine length-dependent manner in vitro and in vivo. J Biol Chem. 2010;285:14777–14790. doi: 10.1074/jbc.M109.093708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Shirendeb U, Reddy AP, Manczak M, et al. Abnormal mitochondrial dynamics, mitochondrial loss and mutant huntingtin oligomers in Huntington’s disease: implications for selective neuronal damage. Hum Mol Genet. 2011;20:1438–1455. doi: 10.1093/hmg/ddr024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Takahashi T, Kikuchi S, Katada S, et al. Soluble polyglutamine oligomers formed prior to inclusion body formation are cytotoxic. Hum Mol Genet. 2008;17:345–356. doi: 10.1093/hmg/ddm311. [DOI] [PubMed] [Google Scholar]
- 170.Jochum T, Ritz ME, Schuster C, et al. Toxic and non-toxic aggregates from the SBMA and normal forms of androgen receptor have distinct oligomeric structures. Biochim Biophys Acta. 2012;1822:1070–8. doi: 10.1016/j.bbadis.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 171.Li M, Chevalier-Larsen ES, Merry DE, et al. Soluble androgen receptor oligomers underlie pathology in a mouse model of spinobulbar muscular atrophy. J Biol Chem. 2007;282:3157–3164. doi: 10.1074/jbc.M609972200. [DOI] [PubMed] [Google Scholar]