Abstract
Purpose
Whole-body irradiated individuals are at increased risk of infection in the acute phase, whereas pulmonary complications are associated with late events. This study addressed whether irradiation-induced changes in the lung environment alter responses to a viral challenge delivered late after exposure, but prior to the appearance of late lung radiation injury.
Methods and Materials
C57BL/6 mice received either lung alone or combined lung + whole-body irradiation (0–15 Gy). At 10 weeks post-irradiation, animals were infected with 120 HAU influenza virus strain A/HKx31. Innate and adaptive immune cell recruitment was determined using flow cytometry. Cytokine and chemokine production and protein leakage into the lung following infection were assessed.
Results
Prior irradiation led to a dose-dependent failure to regain body weight post-infection, exacerbated mortality, but it did not affect virus-specific immune responses or virus clearance. Surviving irradiated animals displayed a persistent increase in total protein in bronchoalveolar lavage fluid and edema.
Conclusions
Lung irradiation increased susceptibility to death following infection with influenza virus and impaired the ability to complete recovery. This altered response does not appear due to a radiation effect on the immune response, but it may possibly be an effect on epithelial repair.
INTRODUCTION
It is well known that individuals receiving whole-body irradiation (WBI) are at increased risk of infections within the first weeks post-exposure, when critical immune cells are depleted (1). However, hematopoeitic-driven events appear less involved with WBI-associated late effects, with complications arising in classic late-responding organs, such as the lung. Pneumonitis and fibrosis are the most commonly observed complications in normal lung tissue following irradiation (2). While these clinical endpoints do not appear until months to years after exposure, persistent and fluctuating expression of cytokines and chemokines have been observed during the so-called latent period, suggesting a chronically altered environment exists prior to the appearance of overt injury (3, 4).
The pulmonary epithelium is in constant contact with the external environment, making the lung susceptible to pathogen exposure and infection. Following an infection, the pulmonary microenvironment initiates an immune response in which cytokines and chemokines play a regulatory role, controlling subsequent inflammatory and tissue healing responses (5). Although we are unaware of a literature indicating that patients who have undergone irradiation for thoracic neoplasms are at risk of late complications from pulmonary infections, there are significant indications of such a risk in recipients of bone marrow transplants, whose preparatory regimen can include WBI (6).
We hypothesize that chronic alterations in the lung microenvironment following irradiation may affect inflammatory and immune responses to later infection with a respiratory pathogen. To test this hypothesis, 10 weeks after exposure to either lung irradiation alone or combined lung + WBI, mice were infected with influenza A virus. Radiation effects on morbidity and mortality, innate and adaptive immune responses, and recovery from infection were assessed.
Methods and Materials
Radiation exposure and infection
The Institution’s Committee on Animal Resources approved all animal exposures. C57BL/6J mice (female, 6–8 weeks of age; Jackson Laboratory, Bar Harbor, ME) were housed in microisolator units and supplied with standard laboratory diet and water ad libitum. Mice were acclimated for one week before experimentation.
A Cesium-137 γ-ray source (~2.0 Gy/min) was used for radiation exposure. For whole-lung irradiation (5, 10, 15 Gy), mice were individually confined in plastic jigs and oriented so that only the thoracic region was within the exposure field. For combined irradiation, mice first received a whole-body dose (5 Gy), followed immediately by a 10 Gy “top-up” dose to the lung. Control mice were sham-irradiated by identical handling without exposure to the radiation source. At 10 weeks post-irradiation, sham and irradiated animals were intranasally infected with 120 HAU influenza virus A/HK×31 (H3N2) in 25 μL sterile PBS. Mock-infected controls received 25 μL of sterile PBS alone. Treatment groups included: sham irradiation plus mock (S+M) or influenza A virus (S+F) infection, whole-lung irradiation plus mock (WL+M) or influenza A virus (WL+F) infection, and combined irradiation plus mock (C+M) or influenza A virus (C+F) infection. Following infection, survival and body weight was monitored for three weeks, adequate time for complete resolution of virus infection (5).
Sample collection
On days 3, 6, 9, 14, or 21 post-infection, mice were sacrificed. Following anesthesia, whole blood was collected in EDTA-coated BD Microtainer® tubes. Complete blood counts were obtained using the Heska HemaTrue™ Hematology Analyzer (Loveland, CO). Plasma was collected following centrifugation. Left lung lobes were immediately frozen in liquid nitrogen. Right lung lobes were perfused with saline and used either for histology or flow cytometry. For histology, lobes were inflation-fixed with zinc-buffered formalin. Tissue was fixed overnight, processed, and embedded into paraffin blocks. 5 μm thick sections were cut and stained with CAT Hematoxylin and Reuben’s Eosin-Phloxine (H&E) (Biocare Medical, Concord, CA). Lungs used for flow cytometry, were first subjected to bronchoalveolar lavage (BAL) with saline wash. BAL fluid was collected following cell separation by centrifugation. Lungs were then instilled with collagenase, minced, and incubated at 37°C for digestion. A single cell suspension was prepared following filtration. Lavaged cells were combined with digested cells from the same lung.Red blood cells were lysed with ammonium chloride. Cells were counted using a hemacytometer and 1–2 million cells were subsequently stained for flow cytometric analysis.
Flow cytometry
Allophycocyanin-labeled MHC class I tetramer and peptide corresponding to an immunodominant viral epitope recognized by CD8+ T cells from C57BL/6J mice (Db/NP366–374) was used to identify influenza virus-specific CD8+ T cells. Fluorochrome-conjugated antibodies against cell surface antigens, Gr-1, NK1.1, CD3ε, CD4, and CD8α, were purchased from BD Biosciences (San Jose, CA). Nonspecific staining was blocked with 50 μg/mL rat IgG containing 0.5 μg/μL anti-mouse CD16/32 (BD Biosciences). Cells were incubated with antibodies for one hour at room temperature. Data were acquired from 50,000 to 200,000 events with a FACSCanto flow cytometer (BD Biosciences) and analyzed using FlowJo software (Tree Star, Inc., Ashland, OR).
Antibody analysis
Influenza virus-specific IgG antibody levels were measured by ELISA in serially diluted plasma samples using purified x31 antigen (Charles River, CT) and HRP-conjugated goat anti-mouse IgG secondary antibody. Dilution factor was interpolated from the titration curve at an OD value of 1.5.
Viral titer
Virus titer was determined using Madin-Darby Canine Kidney (MDCK) followed by a standard hemagglutination assay. Titer is expressed as the dilution of lung homogenate at which 50% of the MDCK cells revealed virus growth (7).
Protein and mRNA expression
Coomassie Plus Assay and Pierce© BCA Protein Assay (Thermo Scientific, Hanover Park, IL) were used to determine total protein concentration in the BAL fluid and lung homogenates, respectively. Duo-set ELISA kits (R&D Systems, Minneapolis, MN) were used to determine keratinocyte chemoattractant (KC) concentration in the BAL fluid and interferon (IFN)-γ concentration in lung homogenates. IFN-γ concentration was normalized to total protein concentration.
Messenger RNA was isolated from lung tissue with Trizol reagent and cDNA synthesis was performed using the Superscript III First-Strand Synthesis System (Invitrogen, San Diego, CA). Real-time PCR was performed on an iCycler MyiQ2 (Biorad, Hercules, CA).
Statistical analysis
Data are expressed as mean±SEM. Two-way analysis of variance with post-hoc test was performed in Statview (SAS Institute Inc., Cary NC). Differences were considered significant when p<0.05.
See Supplemental Material for more detailed information on Methods. (www.redjournal.org).
RESULTS
Weight loss and mortality were used to assess influenza A virus infection severity. All virus-infected animals demonstrated significant weight loss at day 3 post-infection compared to mock-infected controls. The 15 Gy WL+F group lost more body weight during infection compared to S+F animals (30% versus 25%) (Fig 1A). After 14 days, weight gain reached a plateau in both groups; however, weights of 15 Gy WL+F animals remained significantly lower compared to both S+F and WL+M animals, whereas the difference in weight between S+F and S+M animals was not significantly different after day 9 (Fig. 1A). In lower dose studies, 5 Gy WL+F animals did not differ from sham, whereas 10 Gy WL+F animals showed exacerbated weight loss, but still regained body weight (Figure 1C). Exacerbated morbidity was associated with mortality in both 15 Gy (Fig. 1B) and 10 Gy (Fig. 1D) WL+F groups.
Figure 1. Weight loss and mortality following influenza a virus infection in sham and whole lung irradiated mice.
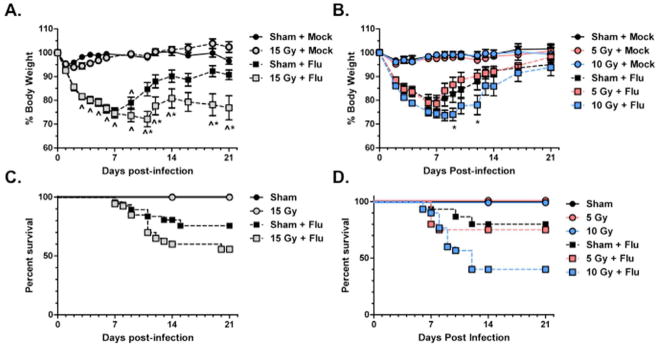
(A) and (B) Weight loss is expressed as a percentage of the starting weight. (*p<0.05 versus sham-irradiated infected, ^p<0.05 versus irradiated mock-infected, n=30 for virus-infected groups, n=10 for mock-infected groups) (C) and (D) Kaplan-Meier survival curve.
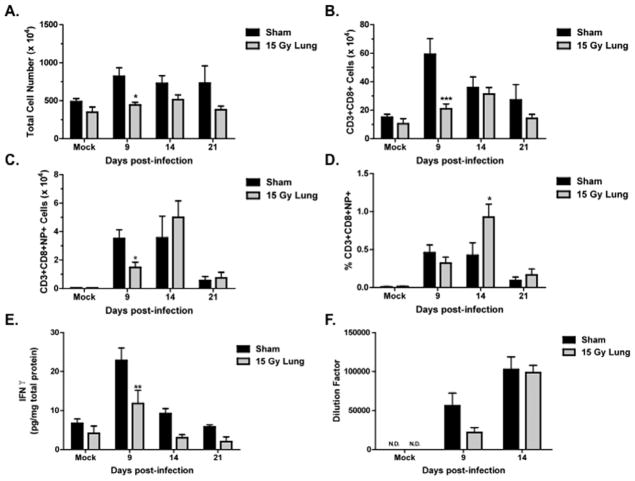
A time course following infection was used to evaluate both the innate and adaptive phases of the immune response. On days 3, 6, 9, and 14 post-infection, neutrophils and natural killer (NK) cells recruited into the lungs were measured by flow cytometry, using antibodies to the Gr-1 and NK1.1 antigens, respectively. No significant differences in Gr-1+ and NK1.1+ cell numbers were observed at any time post-infection between sham and irradiated animals (Fig. 2A and 2B). Levels of the neutrophil chemoattractant, KC, in the BAL fluid (Fig. 2C), and expression of the monocyte chemokine, MCP-1 (Fig. 2D), in the lung, also did not differ between sham and irradiated animals.
Figure 2. Innate immune responses to influenza a virus infection in sham and 15 Gy whole lung irradiated mice.
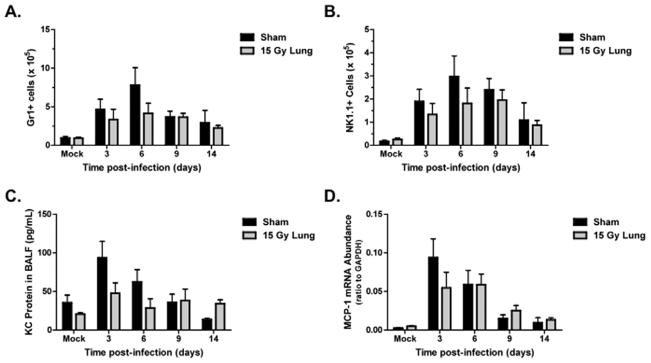
(A) Neutrophil cell counts, identified by positive Gr1 staining. (B) NK cell counts, identified by positive NK1.1 staining. (C) KC protein and (D) MCP-1 mRNA expression in the lung. No significant differences were observed between infected groups (n=4–6 per group per timepoint)
The adaptive immune response generates virus-specific cytotoxic CD8+ T cells and virus-specific antibodies. On days 9, 14, and 21 post-infection, the CD8+ T cell response was assessed by flow cytometry. On day 9 post-infection, total cells isolated (Fig. 3A) and number of CD8+ T cells (Fig. 3B) were significantly reduced in the irradiated animals. Virus-specific CD8+ T cells in WL+F animals were also significantly reduced at day 9 but reach numbers comparable to S+F animals by day 14 (Fig. 3C). The percentage of virus-specific CD8+ T cells in irradiated animals was significantly higher than in the sham group at this time (Fig. 3D). A decrease in lung IFN-γ concentration was also observed on day 9 post-infection in irradiated animals (Fig. 3E). IgG antibody levels in WL+F animals were reduced at day 9 (p=0.08), but reached levels comparable to S+F animals at day 14 post-infection (Fig. 3F). At this time, both S+F and WL+F groups had non-detectable levels of virus in the lung (Fig. 4).
Figure 3. Adaptive responses to influenza a virus in sham and 15 Gy whole lung irradiated mice.
(A) Total cell number isolated from the lung. (B) CD8+ T cell number in the lung, identified by CD3+ and CD8+ staining. (C) Virus-specific CD8+ T cell counts, identified by positive staining for CD3, CD8 and the T cell receptor recognizing the Db/NP366–374 epitope (NP+). (D) Percentage of virus-specific CD8+ T cells. (E) IFNγ protein concentration in the lung. (F) Virus-specific IgG antibody levels in plasma. (***p<0.001*p<0.05 versus sham-infected; n=4–6)
Figure 4. Lung viral titers in influenza a virus infected sham and whole lung irradiated mice.
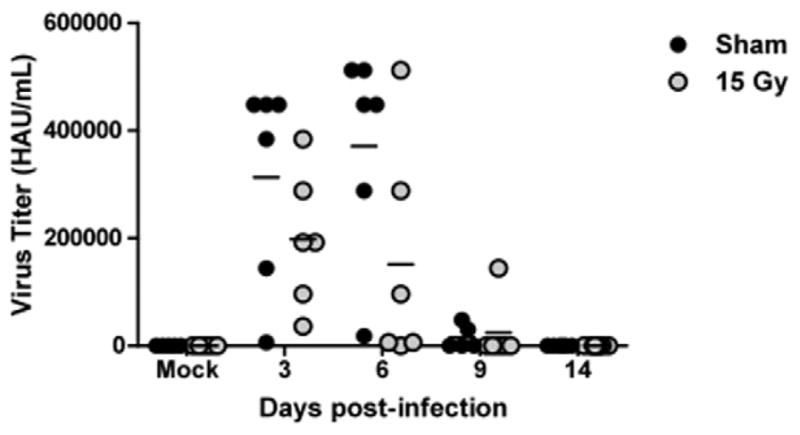
Detection limit of the hemaggluttination assay was 250 HAU/mL. The horizontal line represents the mean, and each symbol represents one animal within the group. (n=4–6)
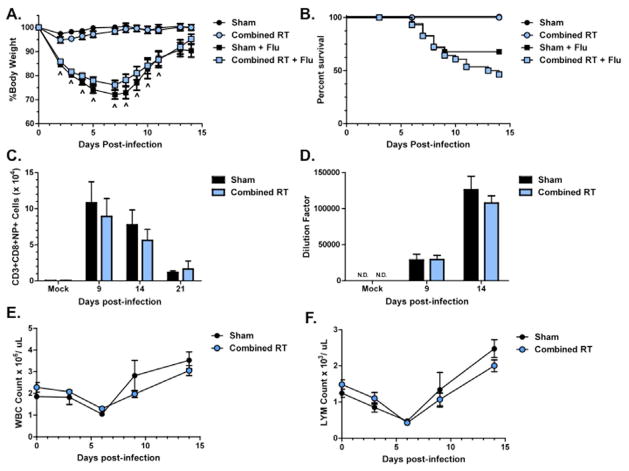
To assess if the immune response to influenza A virus would be different if hematopoietic stem cells were also exposed to radiation, we repeated this experiment using a combined lung + WBI exposure model. Interestingly, surviving C+F animals regained body weight (Fig. 5A). Increased mortality was also observed in this group compared to S+F animals, which exhibited greater mortality than previously observed (Fig. 5B). Consistent with our observations following whole-lung irradiation, C+F animals generated similar numbers of virus-specific CD8+ T cells in the lung (Fig. 5C) and produced comparable amounts of virus-specific IgG antibody as S+F animals (Fig. 5D). C+F animals effectively cleared the virus by day 14 (data not shown). Additionally, no radiation effect on the total number of white blood cells (Fig 5E), including lymphocytes (Fig. 5F), in the circulation was observed.
Figure 5. The response to influenza A virus in sham and combined irradiated C57BL/6J mice.
(A) Weight loss and (B) survival following infection. (C) Virus-specific CD8+ T cell counts. (D) Virus-specific IgG antibody titer. (E) Total white blood cell counts and (F) lymphocyte counts in the peripheral blood. No significant differences were observed (n=4–6).
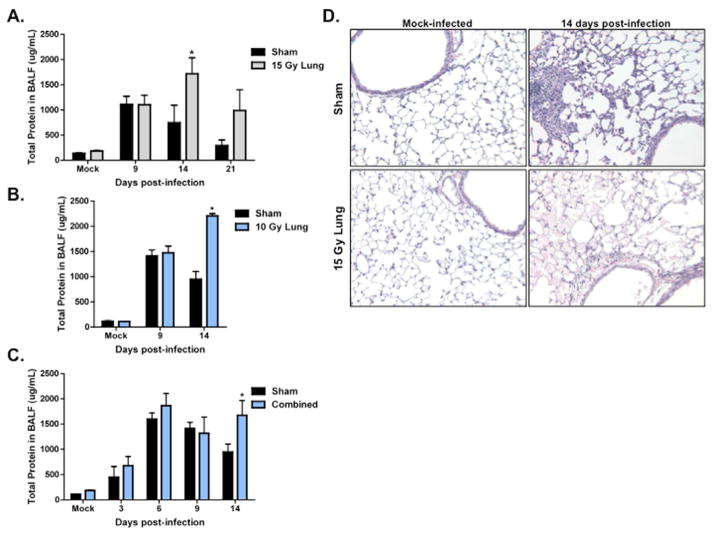
Epithelial integrity after infection was assessed by measuring total protein in the BAL fluid. Infection caused an increase in BAL protein. In 15 Gy WL+F animals, total protein in the lavage was significantly increased compared to S+F animals at day 14 post-infection and remained elevated at day 21 post-infection (Fig. 6A). Similarly, an increase in protein was observed at day 14 in 10 Gy WL+F animals (Fig. 6B) and in C+F animals (Fig. 6C). This is consistent with histological observations. At day 14 post-infection, H&E staining identified dramatic eosinophilic staining in the airspace of 15 Gy WL+F animals, indicating the presence of proteinaceous edema (Fig. 6D). This was not observed in the S+F animals.
Figure 6. Evaluation of epithelial integrity in irradiated animals infected with influenza A virus at ten weeks post-irradiation.
Total protein concentration in BAL fluid in (A) 15 Gy and (B) 10 Gy whole lung, and (C) combined irradiated infected animals (*p<0.05 versus sham-infected; n=4–6). (D) 10X H&E images from sham and 15 Gy whole lung irradiated animals mock-infected, 14, and 21 days post-infection.
DISCUSSION
This study demonstrates prior lung irradiation increases morbidity and mortality following a delayed pulmonary viral challenge. This increase in morbidity and mortality is not due to an inability to clear the virus, however persistent epithelial damage may play a role.
In humans, the development of pneumonitis and fibrosis following lung irradiation is preceded by an asymptomatic period, in which no overt lung injury is present. Unfortunately, mouse strains vary in their radiation sensitivities and one has yet to be selected as the most appropriate to model the human response. Here, the C57Bl/6J mouse strain was selected because it also exhibits a high sensitivity to fibrosis following a considerable time delay (8). More recent work associates this strain with late pleural effusions, which may interfere with the evaluation of radiation endpoints (9). These outcomes do not become apparent until approximately 26 weeks post-irradiation (8, 9). Therefore, at the time chosen for infection in this study, C57Bl/6J mice show no discernible injury, allowing us to test the tolerance of the lung to infection at this time. Pneumonitis-susceptible strains would be expected to express radiation injury at this time (9). We infected at 10 weeks post-irradiation because this is adequate time for the acute depletion of immune cells to be restored (1, 10). Thus, our model allows us to identify sub-clinical radiation damage through altered responses to secondary challenges, without acute or late radiation injury complicating the assessment.
In the C57Bl/6J model, a 15 Gy dose is sufficient to cause late radiation injury (8). The altered response to infection observed suggests the presence of sub-clinical injury in the lung, which becomes apparent following a secondary challenge. Consistent with the presence of cryptic damage, we also observed altered responses in 10 Gy irradiated animals, a dose generally considered below the threshold for late lung injury development (8). Furthermore, radiation exposures resulting from a radiological attack or accidental release are likely to be heterogenous and involve systemic irradiation and lung irradiation (11), these observations also suggest that an individual whose lungs have been irradiated as part of WBI will continue to be at risk for complications from pulmonary infections even after survival of the acute radiation syndrome.
Following infection, viral clearance is mediated by coordinated innate and adaptive immune responses. During the innate response, cytokines and chemokines are produced to regulate inflammatory cell recruitment, including neutrophils and NK cells, into the lung to limit viral replication (12). The adaptive immune response produces virus-specific CD8+ T cells, responsible for clearance of virus and virus-infected cells, and virus-specific antibodies (13, 14). An effective immune response leads to complete viral clearance from the lung. Our results show viral clearance occurred in both whole-lung and combined irradiation models, indicating innate and adaptive immune responses to influenza virus remain effective in both. No observed differences in the number of virus-specific CD8+ T cells recruited into the lung and virus-specific IgG antibody production between sham and irradiated groups supports this conclusion. Furthermore, systemic irradiation, which exposes the hematopoietic stem cells to radiation, did not have any additional affect on function or number of immune cells.
Although we did not observe a radiation effect on virus-specific responses and viral clearance, we did see a trend toward attenuated peak innate immune responses, and the peak CD8+ T cell response was significantly reduced. This is consistent with radiation-exposed individuals having decreased percentages of naïve CD8+ T lymphocytes (15). The reduced number of CD8+ T cells provides a possible explanation for the decreased production of IFNγ in irradiated animals following infection because these are one of the primary sources of this cytokine (5). Despite an attenuation in peak immune responses with radiation, our data find this is still efficient for viral clearance, suggesting the normal response is very robust.
Studies investigating radiation effects on responses to infection are limited. Consistent with our data, Hasegawa et al. (16) showed irradiation exacerbated weight loss and decreased survival after influenza A virus infection. Based on their findings, irradiation caused a persistent infection and an inability to produce antibody-forming cells. Irradiation also exacerbated lipopolysaccharide (LPS)-induced weight loss and reduced increases in spleen mass, effects that were attributed to a radiation-induced reduction of white blood cells in the blood and spleen (17). Similarly, depletion in the alveolar macrophage population by radiation caused an attenuated response to subsequent E. coli challenge (18). The main difference between these studies and ours is the time at which challenge occurred after irradiation. These studies challenged within ten days of radiation exposure, a time when immune cell depletion is still evident (1). Thus, these studies only support current knowledge that, early after irradiation, infections may be exacerbated due to a suppressed immune system. In contrast, the novel findings from our study show immune responses to an infection acquired later after radiation exposure are effective, but an inability to recover from infection persists. This is consistent with our previous data showing that a low dose WBI results in increased inflammatory cell recruitment and a persistent inflammatory response to LPS challenge at one year post-irradiation (19). Together, these findings suggest mechanisms leading to altered responses to infection depend on the time post-irradiation challenge occurs and the type of secondary challenge.
Influenza A virus preferentially targets epithelial cells for infection (20), requiring epithelial regeneration for complete recovery from infection. It is evident from the persistent low body weight that irradiated infected animals display an inability to recover. The prolonged increased protein levels in the lavage following infection also suggest a persistent increase in epithelial permeability, possibly due to inadequate repair of the pulmonary epithelium. This increase in epithelial permeability is likely contributing to the increased morbidity and mortality observed in irradiated animals. Furthermore, this effect may become apparent only following a secondary pulmonary epithelial challenge, such as influenza a virus, in which epithelial cells are stimulated to respond. Ongoing studies aim to determine the effect of radiation on epithelial repair processes following injury.
CONCLUSION
This study demonstrates that sub-clinical lung damage exists following irradiation and becomes apparent following a secondary injury, such as infection. Furthermore, our data has shown that the radiation-induced altered response to a late-occuring influenza infection is not due to defective immune responses, but suggests inadequate epithelial repair. Finally, this study also suggests that whole-body irradiated individuals who have recovered from acute bone marrow damage may continue to be at risk for infection-related complications in the lung.
Supplementary Material
Summary.
We hypothesized that radiation-induced damage may affect the lung’s ability to respond to insult. Using a mouse model, we demonstrated that a delayed challenge with influenza A virus led to a highly exacerbated response, which was likely due to a direct effect on repair of the epithelium. Our study suggests that, at a time when overt lung damage cannot be observed, the presence of sub-clinical lung damage can cause altered responses to a secondary challenge.
Acknowledgments
The authors thank Amy K. Huser for editorial assistance. This research was supported by NIAID U19 AI091036-01, HL066988, ES-01247, and ES-T32 07026.
Footnotes
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Waselenko JK, MacVittie TJ, Blakely WF, et al. Medical management of the acute radiation syndrome: recommendations of the Strategic National Stockpile Radiation Working Group. Ann Intern Med. 2004;140:1037–1051. doi: 10.7326/0003-4819-140-12-200406150-00015. [DOI] [PubMed] [Google Scholar]
- 2.Carver JR, Shapiro CL, Ng A, et al. American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol. 2007;25:3991–4008. doi: 10.1200/JCO.2007.10.9777. [DOI] [PubMed] [Google Scholar]
- 3.Schaue D, McBride WH. Links between innate immunity and normal tissue radiobiology. Radiat Res. 2010;173:406–417. doi: 10.1667/RR1931.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubin P, Johnston CJ, Williams JP, et al. A perpetual cascade of cytokines postirradiation leads to pulmonary fibrosis. Int J Radiat Oncol Biol Phys. 1995;33:99–109. doi: 10.1016/0360-3016(95)00095-G. [DOI] [PubMed] [Google Scholar]
- 5.Kohlmeier JE, Woodland DL. Immunity to respiratory viruses. Annu Rev Immunol. 2009;27:61–82. doi: 10.1146/annurev.immunol.021908.132625. [DOI] [PubMed] [Google Scholar]
- 6.Protheroe RE, Kirkland KE, Pearce RM, et al. The clinical features and outcome of 2009 H1N1 influenza infection in allo-SCT patients: a British Society of Blood and Marrow Transplantation study. Bone Marrow Transplant. 2012;47:88–94. doi: 10.1038/bmt.2011.12. [DOI] [PubMed] [Google Scholar]
- 7.Barrett T, Inglis SC. Growth, Purification and Titration of Influenza Viruses. In: Mahy BWJ, editor. Virology: A Practical Approach. Oxford, U.K: IRL Press; 1985. pp. 119–150. [Google Scholar]
- 8.Sharplin J, Franko AJ. A quantitative histological study of strain-dependent differences in the effects of irradiation on mouse lung during the intermediate and late phases. Radiat Res. 1989;119:15–31. [PubMed] [Google Scholar]
- 9.Jackson IL, Vujaskovic Z, Down JD. Revisiting Strain-Related Differences in Radiation Sensitivity of the Mouse Lung: Recognizing and Avoiding the Confounding Effects of Pleural Effusions. Radiation Research. 2010;173:10–20. doi: 10.1667/RR1911.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hahn I, Klaus A, Maus R, et al. Dendritic cell depletion and repopulation in the lung after irradiation and bone marrow transplantation in mice. Am J Respir Cell Mol Biol. 2011;45:534–541. doi: 10.1165/rcmb.2010-0279OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams JP, Brown SL, Georges GE, et al. Animal models for medical countermeasures to radiation exposure. Radiat Res. 2010;173:557–578. doi: 10.1667/RR1880.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tate MD, Brooks AG, Reading PC. The role of neutrophils in the upper and lower respiratory tract during influenza virus infection of mice. Respir Res. 2008;9:57. doi: 10.1186/1465-9921-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stambas J, Guillonneau C, Kedzierska K, et al. Killer T cells in influenza. Pharmacol Ther. 2008;120:186–196. doi: 10.1016/j.pharmthera.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Gerhard W. The role of the antibody response in influenza virus infection. Curr Top Microbiol Immunol. 2001;260:171–190. doi: 10.1007/978-3-662-05783-4_9. [DOI] [PubMed] [Google Scholar]
- 15.Yamaoka M, Kusunoki Y, Kasagi F, et al. Decreases in percentages of naive CD4 and CD8 T cells and increases in percentages of memory CD8 T-cell subsets in the peripheral blood lymphocyte populations of A-bomb survivors. Radiat Res. 2004;161:290–298. doi: 10.1667/rr3143. [DOI] [PubMed] [Google Scholar]
- 16.Hasegawa H, Kadowaki S, Watanabe I, et al. Persistent infection of influenza virus in irradiated mice and its prevention by intranasal vaccination. Vaccine. 2002;20:1050–1057. doi: 10.1016/s0264-410x(01)00447-9. [DOI] [PubMed] [Google Scholar]
- 17.Gridley DS, Miller GM, Pecaut MJ. Radiation and primary response to lipopolysaccharide: bone marrow-derived cells and susceptible organs. In Vivo. 2007;21:453–461. [PubMed] [Google Scholar]
- 18.Ishizaka A, Sayama K, Hasegawa N, et al. Attenuation of live E. coli-induced acute lung injury by X-ray irradiation in guinea pigs. Lung. 2000;178:331–340. doi: 10.1007/s004080000036. [DOI] [PubMed] [Google Scholar]
- 19.Johnston CJ, Manning C, Hernady E, et al. Effect of total body irradiation on late lung effects: Hidden dangers. Int J Radiat Biol. 2011 doi: 10.3109/09553002.2011.573439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson CI, Barclay WS, Zambon MC, et al. Infection of human airway epithelium by human and avian strains of influenza a virus. J Virol. 2006;80:8060–8068. doi: 10.1128/JVI.00384-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.