Abstract
Background
The differential susceptibility hypothesis states that some genetic variants that confer risk in adverse environments are beneficial in normal or nurturing environments. The cholinergic system is promising as a source of susceptibility genes because of its involvement in learning and neural plasticity. The cholinergic receptor gene CHRNA4 has been linked to characteristics related to the personality traits Neuroticism and Openness/Intellect.
Methods
The effects of interaction between CHRNA4 genotype and maltreatment status on child personality were examined in a well-matched sample of 339 maltreated and 275 nonmaltreated children (aged 8–13yrs.).
Results
Variation in CHRNA4 interacted with childhood maltreatment to predict personality in a manner indicating differential susceptibility. The interaction of CHRNA4 and maltreatment status predicted Neuroticism and Openness/Intellect. Maltreated children with the rs1044396 T/T genotype scored highest on Neuroticism and showed no effect of genotype on Openness/Intellect. Nonmaltreated children with this genotype scored lowest on Neuroticism and highest on Openness/Intellect.
Conclusion
Variation in CHRNA4 appears to contribute to personality by affecting degree of developmental sensitivity to both normal and adverse environments.
Keywords: Personality, genetics, CHRNA4, Differential Susceptibility, Neuroticism, Openness/Intellect
Many studies have been conducted to find genetic variants that confer risk for disorders or maladaptive traits. Evidence has been found for many such effects, but failures to replicate have also been common (Chanock et al., 2007; Duncan & Keller, 2011). One explanation offered for the difficulty in finding these effects reliably is the importance of gene by environment interactions, in which a particular genotype confers risk only under harsh environmental conditions (e.g. Caspi et al., 2002). A more nuanced perspective suggests that genes conferring risk in harsh environments may confer benefits in normal or nurturing environments (Belsky & Pluess, 2009). This differential susceptibility hypothesis is appealing in part because it offers one explanation for why genetic variants associated with negative outcomes would remain in the gene pool. Differential susceptibility implies that some genotypes render individuals more likely to be influenced, for better or for worse, by their environments. Ample evidence for differential susceptibility has been found using several genes in the dopaminergic and serotonergic systems (Belsky et al., 2009; Belsky & Pluess, 2009). The present study took a theoretically guided approach to identifying a novel differential susceptibility gene, examining a biological system crucially involved in sensitivity to environmental conditions.
The cholinergic system is an excellent candidate for genes producing differential susceptibility because it is strongly involved in neural plasticity and learning. The neurotransmitter acetylcholine is released in novel environments and during task-related attention shifting and associative learning. Acetylcholine plays a broad role in modulating cortical responsiveness to the contents of attention (Sarter, Hasselmo, Bruno, & Givens, 2005). Its release lowers the threshold for firing in neurons involved in forming new associations with the contents of the external world (especially thalamocortical pathways) while at the same time selectively suppressing intracortical communication pathways that might bias interpretation of novel events (Dani & Bertrand, 2007; Sarter, Hasselmo, Bruno, & Givens, 2005). Artificial stimulation of acetylcholine release increases experience-dependent neural plasticity (Bakin & Weinberger, 1996), and depletion of acetylcholine suppresses this effect (Baskerville, Schweitzer, & Herron, 1997).
Inputs that activate the cholinergic system can be broadly categorized as “expected uncertainty,” which characterizes contexts where uncertainty exists but is anticipated (Yu & Dayan, 2005). This is in contrast to unexpected uncertainty, or strong violation of expectations, which appears to be related primarily to the neurotransmitter norepinephrine (Yu & Dayan, 2005). In other words, the cholinergic system is most active in situations when the individual can predict that learning is possible.
Because acetylcholine induces neural plasticity and is associated with learning from the environment, genetic variation in the cholinergic system is likely to be associated with differential susceptibility—that is, with individual differences in susceptibility to environmental influences. In people with more active cholinergic systems, increased influence from harsh environments may lead to poor outcomes, but increased influence from normal or nurturing environments may lead to better outcomes.
Existing research allows us to form a hypothesis about what aspects of personality are likely to be affected by cholinergic genes, namely those related to automatic responses to uncertainty, such as anxiety, curiosity, and attention. Unpredictable and novel contexts, which generate uncertainty, function simultaneously as threats and incentive rewards (Gray & McNaughton, 2000; Peterson & Flanders, 2002). When the significance of an environment or stimulus is uncertain, both caution and exploration are adaptive. Thus, traits related to both positive and negative responses to uncertainty or novelty are most likely to be affected by cholinergic genes, and the harshness of the environment during development is likely to determine whether individuals tend to find expected uncertainty more threatening than promising, or vice versa.
We utilized these observations to predict the effects of childhood maltreatment and variation in cholinergic genes on the Big Five personality traits: Extraversion, Neuroticism, Conscientiousness, Agreeableness, and Openness/Intellect. The Big Five constitute the most widely used and well validated taxonomy of personality in adulthood (John, Naumann, & Soto, 2008) and appear to provide an effective model of childhood personality as well (Caspi & Shiner, 2006). Studies of twins indicate that the Big Five are strongly genetically influenced, with heritability estimates ranging from .40 to .80, depending on trait and method (Riemann, Angleitner, & Strelau, 1997). In the present study we examined the effects of genetic variation on the Big Five, in children who were maltreated and a closely matched comparison group. Maltreatment has been associated with differences in every Big Five dimension except Extraversion (Rogosch & Cicchetti, 2004). We hypothesized that cholinergic genetic variation would moderate the effects of maltreatment on Neuroticism and Openness/Intellect because these are the Big Five traits most linked to anxiety, curiosity, and response to uncertainty and novelty.
We examined the polymorphism rs1044396 in the nicotinic acetylcholine receptor alpha-4 subunit gene (CHRNA4), which produces a component of one major acetylcholine receptor. This single nucleotide polymorphism (SNP), in which one letter of the genetic code is changed, consists of a C→T transposition in the exonic region of CHRNA4. Although it does not alter the amino acid sequence coded by the gene, the repeated associations of this SNP with behavioral and neural characteristics (e.g., Espeseth, Sneve, Rootwelt, & Laeng, 2010; Winterer et al., 2007) suggest it is in linkage disequilibrium with some functional variation or has some direct effect on transcription. In keeping with our hypothesis, previous studies have linked rs1044396 to Neuroticism and to cognitive functions related to Openness/Intellect.
Neuroticism reflects the tendency to experience negative affect and related cognitive processes, including anxiety, depression, irritability, and self-consciousness. It has been linked to sensitivity to threat and punishment both psychologically and biologically (DeYoung & Gray, 2009), and it has been linked specfically to feeling threatened by uncertainty (Gray & McNaughton, 2000; Hirsh & Inzlicht, 2008). Animal models have implicated CHRNA4 in anxiety, and, in a healthy human population, the C/C genotype of rs1044396 was associated with higher Neuroticism than genotypes containing the T allele (Markett, Montag, & Reuter, 2011).
Openness/Intellect is probably best described in terms of cognitive exploration of both inner and outer experience, and curiosity is central to this trait (DeYoung, Grazioplene, & Peterson, 2012; DeYoung, Peterson, & Higgins, 2005). Individuals high in Openness/Intellect tend to be imaginative, artistic, intellectual, and perceptive, suggesting an underlying tendency to seek out, explore, and utilize novel information. The compound label for this trait reflects the fact that it involves both Openness to Experience, which reflects engagement with sensory information, and Intellect. which reflects engagement with abstract information (Johnson, 1994; DeYoung et al., 2009, 2012). Openness/Intellect is the only Big Five trait that is related to tests of attention and working memory capacity (DeYoung et al., 2005, 2009), and variation in CHRNA4 rs1044396 has been implicated in attentional function and working memory (Espeseth et al., 2010; Greenwood et al., 2008; Markett, Montag, Walter, & Reuter, 2010; Parasuraman, Greenwood, Kumar, & Fossella, 2005). Because these cognitive functions have been hypothesized to be integral components of the cognitive substrate of Openness/Intellect (DeYoung et al., 2005, 2009, 2012), CHRNA4 is likely to be an important genetic influence on this personality trait.
The hypothesis that variation in rs1044396 moderates the effects of maltreatment on both Neuroticism and Openness/Intellect was tested in a large sample of children enrolled in a week-long day camp research program. Roughly half of these children were selected because they had been maltreated, as determined from records of the Department of Human Services (DHS), whereas the other half were from the same socioeconomic background but were carefully screened to exclude any maltreated children. Although an ideal situation for examining differential susceptibility would involve a more detailed measure of variability in parental nurturing in the nonmaltreated group, the present sample nonetheless provides a good test of the differential susceptibility hypothesis because differences in environment were clearly defined, rigorously assessed, and dramatic (Ellis, Boyce, Belsky, Bakermans-Kranenburg, & van Ijzendoorn, 2011). Further, all children were assessed in the context of a novel, information-rich camp environment likely to be ideally suited to observation of individual differences associated with cholinergic function.
We did not expect CHRNA4 to moderate the effects of maltreatment on Conscientiousness or Agreeableness. Regarding the last of the Big Five, Extraversion, we formulated no strong hypothesis. No existing evidence directly links CHRNA4 to Extraversion or related characteristics; however, Extraversion is related to the tendencies to engage in approach behavior and to experience positive affect and is linked to the biological substrates of reward (DeYoung & Gray, 2009). Whereas Openness/Intellect is associated with cognitive exploration, Extraversion reflects the tendency to explore the world behaviorally. CHRNA4 might influence Extraversion because acetylcholine is heavily involved in modulating striatal dopamine function (Miwa, Freedman, & Lester, 2011), which governs sensitivity to cues of reward and appears to be related to Extraversion (DeYoung & Gray, 2009). One study found no association between rs1044396 and Extraversion (Markett et al., 2011); however, they examined only main effects, and thus could not have detected gene × environment interaction effects like those we examine here.
Methods
Participants
Participants were 614 children (age range = 8–13 years, M = 11.3, SD = 1.0), who were recruited from an urban setting in upstate New York to participate in a week-long day camp program. 339 of these children had been maltreated (169 girls, 170 boys), and 275 had not (142 girls, 133 boys). The sample was racially and ethnically diverse, and was categorized to allow separation of groups of mixed race/ethnicity. The sample was 60% Black, 11% Hispanic, 10% White, 2.5% Hispanic/Black, 3.5% Hispanic/White, 5.5% Black/White, and 4% other. (All results remained the same if we used the simpler racial and ethnic categorization system employed in previous research on this sample; DeYoung et al., 2011.) The camp program was designed for comparison of developmental processes and functioning in maltreated and nonmaltreated children. All participants came from low-income homes (Cicchetti & Manly, 1990). In the recruitment process, a liaison from the DHS contacted families with a child meeting research criteria, provided information about the camp and associated research, and asked families for written permission to have their names released to project staff. (Due to confidentiality, the DHS liaison was not able to provide information regarding families who were not interested in participation.) Subsequently, parents of all participating children provided informed consent for their child’s participation, as well as consent for examination of any DHS records associated with the family; children provided assent. Children attended the camp free of charge and received small prizes for completing research measures; mothers received compensation ($25) for completing a research interview. The procedures in this investigation were approved by the Research Subjects Review Board of the University of Rochester.
Children in the maltreated group were recruited based on DHS records indicating they had experienced maltreatment. Those in the nonmaltreated (comparison) group did not have records of maltreatment; these children were additionally screened through checks of the child abuse registry as well as through interviews with their mothers (utilizing the Maternal Maltreatment Classification Interview; Cicchetti, Toth, & Manly, 2003) to verify lack of DHS involvement and absence of maltreatment experiences. Children in the comparison group were well matched in age, socioeconomic status, and race. In order to avoid inclusion of unidentified maltreatment in the comparison group, additional screening excluded families who received preventive services through DHS due to concerns over risk for maltreatment.
Children attended the program for a week and participated in research assessments. While at camp, children were assigned to groups of eight (4 maltreated, 4 nonmaltreated) same-age and same-sex peers. Each group was led by three trained camp counselors, who were unaware of the maltreatment status of children and the hypotheses of the study. Camp lasted 7 hrs/day for five days, providing 35 hours of interaction between children and counselors.
Maltreatment
Descriptions of maltreatment in DHS records were used to identify, for each child, the presence of sexual abuse, physical abuse, neglect, and/or emotional maltreatment. Trained raters coded DHS records using the operational criteria of the Maltreatment Classification System (Barnett, Manly, & Cicchetti, 1993), a well-validated approach for classifying maltreatment experiences. Among the maltreated children, 8.6% had experienced sexual abuse, 28.6% physical abuse, 78.5% neglect, and 52.2% emotional maltreatment; most children (59.1%) had experienced more than one type of maltreatment.
Personality
The Big Five personality traits were assessed using two instruments: the Big Five scales derived from the California Child Q-sort (CCQ; John, Caspi, Robins, Moffitt, & Stouthamer-Loeber, 1994) and a set of 46 trait descriptive adjectives (TDA) designed for assessment of the Big Five in children (Hagekull & Bohlin, 1998). The CCQ comprises 100 personality descriptive items that are sorted according to a fixed distribution into 9 categories, representing the degree to which each is characteristic of the child. The TDA comprises 46 items rated on a 5-point Likert scale. Two adult camp counselors completed each of these two instruments after 35 hours of extensive observation and interaction with participants. Counselors were trained in use of the instruments, but were unaware of research hypotheses and maltreatment status. Interrater agreement was high, with the average intraclass correlation among pairs of raters ranging from .85 to .87 for the CCQ and from .74 to .89 for the TDA scales. Ratings for each item by each of the two raters were averaged before deriving scale scores for each instrument.
Big Five scores from the CCQ and TDA were standardized separately in order to combine scores across the two instruments. The standardized scores were then averaged, restandardized, and recentered by adding 1 (recentering was performed for clarity of graphical representation). Composite scores from these two inventories were very reliable, with Cronbach’s Alphas as follows: Extraversion: .95 (18 items), Agreeableness: .96 (25 items), Conscientiousness: .91 (18 items), Neuroticism: .90 (20 items), Openness/Intellect: .75 (10 items). (The lower Alpha for Openness/Intellect is attributable to its relatively fewer items.) Three items (one each from Agreeableness, Conscientiousness, and Openness/Intellect) were excluded from the calculation of trait scores because their correlations with the scale total (calculated without the item in question) were near zero and their inclusion reduced Cronbach’s Alpha. Scores calculated without these items correlated at .99 or higher with scores including them.
Neuroticism
Items in the Neuroticism scale from the CCQ were, “Is fearful and anxious”; “Tends to brood and ruminate and worry”; “Tends to become rigidly repetitive or immobilized under stress”; “Can recoup or recover after stressful experiences” (reversed); “Tends to go to pieces under stress”; “Seeks reassurance from others about his/her worth”; “Has bodily symptoms as a function of tension and conflict”; “Becomes anxious if the environment is unpredictable or poorly structured”; “Appears to feel unworthy, thinks of self as bad”; and “Is easily offended, sensitive to ridicule or criticism.” Items in the Neuroticism scale from the TDA were “nervous,” “tense,” “anxious,” “worries about things,” “fearful,” “relaxed” (reversed), “content” (reversed), “self-confident” (reversed), “oversensitive,” and “calm and stable” (reversed).
Openness/Intellect
Items from the Openness/Intellect scale of the CCQ were “Is curious and exploring; eager for new experiences”; “Appears to have high intellectual capacity”; “Is verbally fluent”; “Becomes strongly involved in what (s)he does”; “Is creative in perception, thought, work, or play”; “Has an active fantasy life”. TDA Openness/Intellect items were, “imaginative,” “curious,” “creative,” and “tries new activities.”
Genotyping
DNA was collected from all children using the Buccal Amp Kit (Epicentre, Cat. No. BQ0901SSC) and amplified using the Repli-g kit (Qiagen, Catalog No. 150043) per the kit instructions. DNA was whole-genome amplified to ensure the availability of data over the long term for this valuable sample. Amplified samples were then diluted to a working concentration and genotyped using an assay for SNP rs1044396 purchased from Applied Biosystems, Inc. (ABI). Individual allele determinations were made using TaqMan Genotyping Master Mix (Applied Biosystems, Catalog 4371357) with amplification in an ABI 9700 thermal cycler and analyzing the endpoint fluorescence using a Tecan M200. No other polymorphisms in the cholinergic system were genotyped. Genotypes in other neurotransmitter systems have been examined in this sample (DeYoung, Cicchetti, & Rogosch, 2011; DeYoung et al., 2011); these genotypes were not included in our primary analysis, but we did conduct a secondary analysis to test whether the effects of CHRNA4 were independent of the previously identified effects of other genes.
If a genotype was unable to be determined after the first run, then it was repeated up to 4 times. If the null result persisted, then the whole-genome amplification reaction was repeated along with subsequent genotyping until a genotype could be confidently assigned to a participant. The resultant genotyping data were subjected to quadratic discriminant analysis using JMP statistical software from SAS. Samples with a predicted probability of 0.95 or less were repeated. All DNA samples were genotyped in duplicate for quality control. In addition, human DNA from cell lines was purchased from Coriell Cell Repositories for all representative genotypes in duplicate and genotypes confirmed by sequencing using DTCS chemistry on an ABI 3130×1. The call rate for rs1044396 for CHRNA4 was 100%. There were no missing results. Rs1044396 did not differ significantly from Hardy-Weinberg equilibrium χ2(1) = 1.77, p = .77.
Analysis
To test gene by environment interaction effects for each of the Big Five, we conducted a single MANCOVA with five criterion variables to control for multiple comparisons. CHRNA4 genotype, maltreatment status, sex, and race were entered as fixed factors, and age was included as a continuous covariate. Additionally, the interaction of genotype × maltreatment status was entered as the effect of interest for our hypothesis.
Results
Table 1 shows allele frequencies, comparing maltreated and nonmaltreated children, by sex and race. The maltreated and nonmaltreated groups did not differ by genotype, which indicates absence of gene-environment correlation, χ2(1, N = 614) = 1.10, p = .30. In other words, CHRNA4 genotype did not influence the likelihood that children would be maltreated. Sex was also unrelated to genotype, χ2(1, N = 614) = 1.13, p = .29; race was significantly associated with CHRNA4 genotype, χ2(3, N = 614) = 150.1, p <0.01, making it important to control for race in all analyses to account for potential population stratification.
Table 1.
CHRNA4 rs1044396 genotype frequencies (% of total N) by sex and race/ethnicity.
| Maltreated (N = 339) | Nonmaltreated (N = 275) | |||||
|---|---|---|---|---|---|---|
| C/C | C/T | T/T | C/C | C/T | T/T | |
| Sex | ||||||
| Female | 99 (16.1%) | 62 10.1%) | 8 (1.3%) | 84 (13.7%) | 46 (7.5%) | 12(2.0%) |
| Male | 112 (18.2%) | 52 (8.3%) | 8 (1.1%) | 79 (12.9%) | 44 (7.2%) | 10(1.6%) |
| Race/Ethnicity | ||||||
| Black | 166 (27%) | 43 (7.0%) | 1 (0.2%) | 126 (20.5%) | 44(7.2%) | 3(0.5%) |
| White | 10 (1.6%) | 23 (3.7) | 8 (1.3%) | 0 (0%) | 11(1.8%) | 12(2%) |
| Hispanic | 11 (1.8%) | 15 (2.4%) | 3 (0.5%) | 22 (3.6%) | 19(3.1%) | 1(0.2%) |
| Hispanic–Black | 7 (1.1%) | 5 (0.8%) | 0 (0%) | 5 (0.8%) | 4(0.7%) | 2(0.3%) |
| Hispanic–White | 1 (0.2%) | 7 (1.1%) | 1 (0.2%) | 1 (0.2%) | 3(0.5%) | 2(0.3%) |
| Black–White | 10 (1.6%) | 13 (2.1%) | 1 (0.2%) | 4 (0.7%) | 6(1.0%) | 1(0.2%) |
| Other | 6 (1.0%) | 7 (1.1%) | 2 (0.3%) | 4 (0.7%) | 2(0.3%) | 0(0%) |
Table 2 shows means and standard deviations for the Big Five in each group. As reported in previous work, maltreated children in this sample exhibited higher Neuroticism and lower Agreeableness, Conscientiousness, and Openness/Intellect than nonmaltreated children (DeYoung et al., 2011).
Table 2.
Means and standard deviations of the Big Five in maltreated and nonmaltreated children.
| Maltreated (N = 339) |
Nonmaltreated (N = 275) |
||||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t(612) | p | d | |
| Neuroticism | 1.10 | 1.03 | 0.88 | 0.95 | 2.70 | .01 | 0.22 |
| Extraversion | 1.02 | 1.01 | 0.97 | 0.98 | 0.66 | .51 | 0.05 |
| Agreeableness | 0.83 | 1.02 | 1.21 | 0.94 | −4.72 | < .001 | 0.39 |
| Conscientiousness | 0.82 | 1.02 | 1.22 | 0.94 | −4.95 | <.001 | 0.39 |
| Openness/Intellect | 0.92 | 0.97 | 1.10 | 1.02 | −2.13 | .03 | 0.17 |
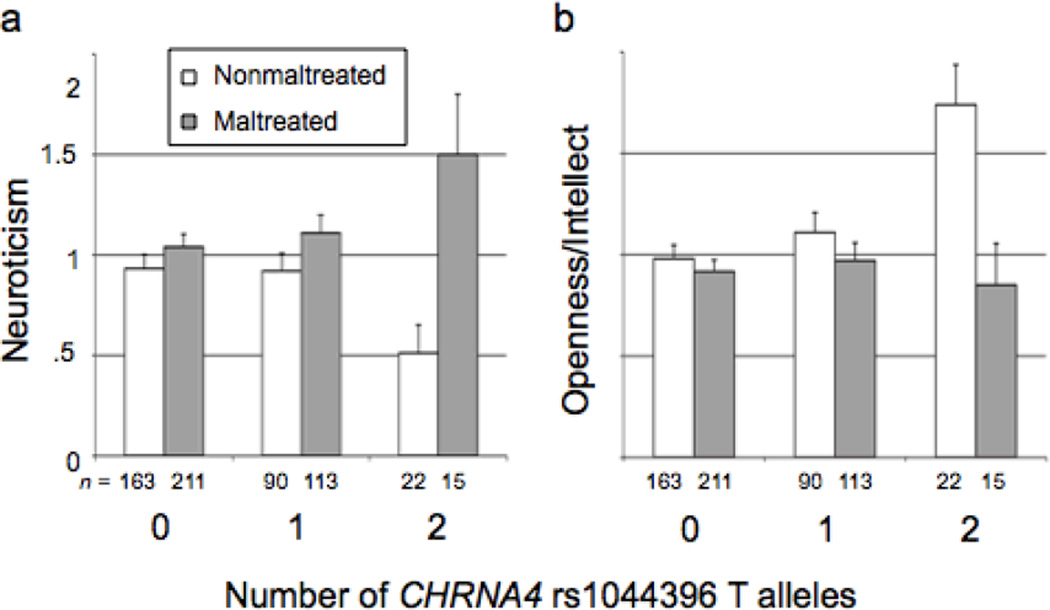
Results of MANCOVA are shown in Table 3 for Neuroticism, Openness/Intellect, and Extraversion. Significant gene × environment interaction effects were present for Neuroticism and Openness/Intellect (Figure 1). Individuals who were maltreated and possessed two copies of the T allele had the highest levels of Neuroticism, whereas nonmaltreated individuals with that genotype had the lowest levels of Neuroticism (Figure 1a). Nonmaltreated individuals with the T/T genotype also had the highest levels of Openness/Intellect (Figure 1b). Simple effects analysis of Neuroticism revealed a significant difference between maltreated and nonmaltreated children in the T/T genotype group, t(35) = 3.26, p = .003, but no significant difference in Neuroticism in the C/T group, t(201) = 1.48, p = .25, or in the C/C group, t(372) = 1.34, p = .18). The same pattern of simple effects was observed for Openness/Intellect when comparing maltreated to nonmaltreated groups in each genotype, T/T: t(35) = 3.04, p = .005; C/T: t(201) = 1.09, p = .28; C/C: t(372) = .72, p = .47)). The interaction effect was not significant for Extraversion (p = .23), nor was there any main effect of CHRNA4 genotype on Extraversion, after removing the interaction term from the model, p = .68. As expected there was no effect of CHRNA4 genotype on Conscientiousness or Agreeableness, either as a main effect or in interaction with maltreatment, all p > .22.
Table 3.
Analysis of Variance: Effects of CHRNA4 genotype and maltreatment on Neuroticism, Openness/Intellect, and Extraversion
| Neuroticism | Openness/Intellect | Extraversion | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | df | p | η2 | F | df | p | η2 | F | df | p | η2 | |
| Age | 3.32 | 1 | .13 | .004 | 0.08 | 1 | .77 | <.001 | 2.5 | 1 | .11 | .004 |
| Sex | 0.04 | 1 | .85 | <.001 | 0.38 | 1 | .54 | .001 | 3.23 | 1 | .07 | .005 |
| Race | 1.97 | 6 | .07 | .019 | 2.74 | 6 | .01 | .011 | 0.36 | 6 | .91 | .004 |
| Maltreatment | 15.12 | 1 | <.001 | .025 | 9.61 | 1 | <.001 | .021 | 0.85 | 1 | .36 | .001 |
| CHRNA4 | 0.20 | 2 | .82 | .001 | 0.34 | 2 | .71 | .001 | 0.77 | 2 | .46 | .003 |
| Maltreatment × CHRNA4 | 4.45 | 2 | .01 | .015 | 4.23 | 2 | .02 | .014 | 1.59 | 2 | .21 | .005 |
Figure 1.
Levels of Neuroticism and Openness/Intellect associated with CHRNA4 genotype for maltreated and nonmaltreated children.
DeYoung, Cicchetti, and Rogosch (2011) demonstrated that variation in the corticotropin-releasing hormone receptor 1 gene (CRHR1), a component of the stress-response system, moderated the effect of maltreatment on Neuroticism. DeYoung et al. (2011) demonstrated that variations in the dopamine D4 receptor gene (DRD4) and the catechol-O-methyltransferase gene (COMT) had main effects on Openness/Intellect. In order to test for any influence of these previous findings on the findings of the present study, we ran an additional model in which CRHR1, DRD4, and COMT genotypes, and their interactions with maltreatment, were all entered into the same MANCOVA that we used to test the effect of CHRNA4. The effects reported in the present study remained significant when controlling for the previously demonstrated effects of other genes, and those other genes predicted variance in Neuroticism and Openness/Intellect independently of the effects of CHRNA4. (Further information regarding these latter analyses is available from the corresponding author upon request.)
Discussion
Results confirmed our hypothesis that variation in CHRNA4 is associated with differential susceptibility to environmental influences. The polymorphism rs1044396 moderated the association between childhood maltreatment and childhood personality. When compared to children with at least one copy of the C allele, individuals with the T/T genotype at rs1044396 appeared to be more susceptible to the conditions of their rearing environment. They were higher in Neuroticism when exposed to maltreatment, but lower in Neuroticism and higher in Openness/Intellect when not maltreated. This gene ×environment interaction effect was independent of age, race, and sex, and it explained approximately 1% of the variance in both Openness/Intellect and Neuroticism. This is the first study to examine differential susceptibility effects for cholinergic genes, which are plausible because of the importance of acetylcholine in learning and neural plasticity.
Effect sizes around 1% or less are to be expected for prediction of complex traits by single polymorphisms (Ioannidis,Trikalinos, & Khoury, 2006). Genetic research increasingly indicates that most complex traits are massively polygenic, influenced by large numbers of common genetic variants (e.g., Davies et al., 2011). Any given polymorphism, therefore, is likely to account for only a small fraction of trait variance. Our results are consistent with these observations, given that the effect of CHRNA4 was independent of previously reported effects (in this sample) on Openness/Intellect and Neuroticism by DRD4, COMT, and CRHR1 (DeYoung et al., 2011). It would appear, therefore, that genes in both the cholinergic and dopaminergic systems influence Openness/Intellect, and that genes in both the cholinergic and corticotropin systems influence Neuroticism.
Direct effects of rs1044396 variation have previously been reported for individual differences in cognitive function and Neuroticism (Espeseth et al., 2010; Greenwood et al., 2008; Markett et al., 2011; Parasuraman et al., 2005); the present study suggests that detection of such associations may be facilitated by considering environmental conditions during development. Neuroticism was previously found to be lower for the T/T than the C/C genotype (Markett et al., 2011). We found a similar effect for children who had not been maltreated, whereas for children who had been maltreated the T/T genotype was associated with higher Neuroticism. The effect of genotype on Neuroticism observed in the study by Markett et al. suggests that the sample they analyzed, which consisted primarily of students, was more similar to our nonmaltreated than our maltreated group, which would not be surprising given that they are university students. Thus, in populations with normal rearing environments, the T allele may be beneficial, encouraging lower levels of Neuroticism and higher levels of Openness/Intellect. This pattern of personality traits suggests decreased anxiety and increased curiosity and cognitive engagement in response to situations containing expected uncertainty, where learning is likely to be potentiated by acetylcholine.
Acetylcholine is increasingly appreciated for its role in the etiology of mental illnesses such as schizophrenia and affective disorders (Miwa et al. 2011). Since childhood maltreatment is a known risk factor for psychiatric illness, the present findings may be of clinical importance for early intervention and treatment of mental illness in children who have been maltreated (Cicchetti & Valentino, 2006; Cicchetti & Rogosch, 2001). Genetic markers for variation in cholinergic function could potentially be used in tailoring pharmacological and therapeutic interventions (Kirchheiner et al., 2004). The present results are particularly relevant for clinical phenomena because Neuroticism is the major personality risk factor for most forms of psychopathology, including anxiety and schizophrenia-spectrum disorders (Griffith et al., 2010).
Although the association of Neuroticism and Openness/Intellect with CHRNA4 genotype and childhood maltreatment fits well with the emerging picture of acetylcholine function in development and disease, this research is not without limitations. The T/T genotype of rs1044396, which represents the “susceptible” group in the present analyses and hence drives the interaction effect, is relatively infrequent; the small size of this group in our study makes replication an important goal of future research. Additionally, the functional implications of variation at rs1044396 are not known; thus, it is not possible to determine whether the T allele increases or decreases acetylcholine binding.
Limitations exist for our non-genetic measures as well, including those of personality, race, and environment. The personality measures available in this sample assessed the Big Five only, without breaking those broad traits down into narrower facets. Additional research would be necessary to detect whether CHRNA4 has different effects on subtraits within Neuroticism and Openness/Intellect. Controlling for race using self-reported race and ethnicity is less desirable than the use of direct genetic markers, especially for the relatively heterogeneous group identifying as “Hispanic” (Cabellero, 2011). We attempted to capture some of this heterogeneity by creating separate categories for those of mixed race/ethnicity, but the lack of genetic markers for race remains a limitation. Finally, we acknowledge that inclusion of an assessment of parental nurturing in the nonmaltreated group would have enabled a more thorough test of differential susceptibility. Because “normal” parenting is likely to vary widely in quality, our data do not shed light on the effects of CHRNA4 on personality in average relative to more nurturing rearing environments. Given this limitation, it is compelling that we nonetheless found a differential susceptibility effect. Our ability to detect this effect was probably due in part to the rigor with which maltreatment was assessed and to the dramatic difference between the environments of maltreated and nonmaltreated children.
Another important question for future research is to address whether the associations observed in a child sample would carry through into adulthood. Evidence from twin studies indicates that many behavioral phenotypes become more heritable over the course of late adolescence and young adulthood (Bergen, Gardner, & Kendler, 2007). Because children who possess genetic variants associated with differential susceptibility are likely to be more malleable to environmental circumstances, they may undergo substantial personality change across the course of later development.
Conclusion
One important objective of research on childhood maltreatment is to understand the mechanisms by which abuse and neglect alter mental functioning in the course of development. The present findings suggest that genetic variation in the cholinergic system alters the degree to which children are influenced by their environments. The cholinergic system was identified as a likely candidate for differential susceptibility genes because of its role in learning and neural plasticity. Children with the T/T genotype of CHRNA4 rs1044396 who reside in harsh environments may be more likely to learn anxious and fearful responses (associated with Neuroticism) to situations with increased uncertainty. However, children with this same genotype may be more likely to exhibit curiosity and cognitive engagement (associated with Openness/Intellect) in response to uncertainty if they have been reared in normal or nurturing environments. We acknowledge that the relatively low frequency of the T/T genotype encourages a cautious interpretation of these results, but we emphasize that the finding that CHRNA4 genotype and maltreatment status interact to influence childhood personality adds to a growing body of evidence suggesting that some genetic variants confer differential susceptibility to environmental influence. Although the T/T genotype may be maladaptive in the presence of extreme environmental stressors, it may in fact contribute to the optimal developmental outcomes when combined with normal or nurturing rearing conditions.
Key Points.
The differential susceptibility hypothesis posits that some genetic polymorphisms that confer risk in harsh environments are beneficial (linked to positive outcomes) in normal or nurturing environments.
Understanding individual differences in susceptibility to environmental conditions is a crucial step in understanding how early maltreatment leads to risk for psychopathology.
The present results indicate that genetic variation in the cholinergic system interacts with childhood maltreatment to affect personality (Openness/Intellect and Neuroticism) in a pattern indicating differential susceptibility associated with the polymorphism rs1044396 in the CHRNA4 gene.
When examining genetic and environmental correlates of clinical and nonclinical traits, future research should consider the role of variation in genes likely to influence individual differences in learning and neural plasticity.
Acknowledgements
This article was supported by grants awarded to Dante Cicchetti and Fred A. Rogosch from the National Institute on Drug Abuse (DA12903, DA17741) and the Spunk Fund, Inc. We would like to thank the children, families, counselors, and research staff at the Mt. Hope Family Center, Rochester, New York, who participated in this work.
Abbreviations
- CHRNA4
nicotinic acetylcholine receptor alpha-4 subunit gene
- CCQ
California Child Q-sort
- TDA
trait descriptive adjectives
Footnotes
The authors have declared that they have no competing or potential conflicts of interest.
References
- Bakin JS, Weinberger NM. Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(20):11219–11224. doi: 10.1073/pnas.93.20.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett D, Manly JT, Cicchetti D. Defining child maltreatment: The interface between policy and research. In: Cicchetti D, Toth SL, editors. Child abuse, child development, and social policy. Norwood, NJ: Ablex; 1993. pp. 7–73. [Google Scholar]
- Baskerville KA, Schweitzer JB, Herron P. Effects of cholinergic depletion on experience-dependent plasticity in the cortex of the rat. Neuroscience. 1997;80:1159–1169. doi: 10.1016/s0306-4522(97)00064-x. [DOI] [PubMed] [Google Scholar]
- Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R. Vulnerability genes or plasticity genes? Molecular Psychiatry. 2009;14(8):746–754. doi: 10.1038/mp.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Pluess M. The nature (and nurture?) of plasticity in early human development. Psychological Science. 2009;4(4):345–351. doi: 10.1111/j.1745-6924.2009.01136.x. [DOI] [PubMed] [Google Scholar]
- Bergen SE, Gardner CO, Kendler KS. Age-related changes in heritability of behavioral phenotypes over adolescence and young adulthood: a meta-analysis. Twin Research and Human Genetics. 2007;10(3):423–433. doi: 10.1375/twin.10.3.423. [DOI] [PubMed] [Google Scholar]
- Caballero AE. Understanding the Hispanic/Latino patient. The American Journal of Medicine. 2011;124:S10–S15. doi: 10.1016/j.amjmed.2011.07.018. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 5582. Vol. 297. New York, N.Y.: 2002. pp. 851–854. [DOI] [PubMed] [Google Scholar]
- Caspi A, Shiner RL. Personality development. In: Damon W, Lerne R, Eisenberg N, editors. Handbook of child psychology: Vol. 3. Social, emotional, and personality development. 6th edn. New York: Wiley; 2006. pp. 300–365. (Series Eds.),(Vol. Ed.) [Google Scholar]
- Chanock SJ, Manolio T, Boehnke M, Boerwinkle E, Hunter DJ, Thomas G, Hirschhorn JN, et al. Replicating genotype-phenotype associations. Nature. 2007;447(7145):655–660. doi: 10.1038/447655a. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Manly JT. A personal perspective on conducting research with maltreating families: Problems and solutions. In: Brody G, Sigel I, editors. Methods of family research: Families at risk. vol. 2. Hillsdale, NJ: Erlbaum; 1990. pp. 87–133. [Google Scholar]
- Cicchetti D, Toth SL, Manly JT. Maternal Maltreatment Interview. Rochester, NY: 2003. Unpublished manuscript. [Google Scholar]
- Cicchetti D, Valentino K. An ecological transactional perspective on child maltreatment: Failure of the average expectable environment and its influence upon child development. In: Cicchetti D, Cohen DJ, editors. Developmental Psychopathology. 2nd ed. Vol. 3. New York: Wiley; 2006. pp. 129–201. [Google Scholar]
- Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annual Review of Pharmacology and Toxicology. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- Davies G, Tenesa A, Payton A, Yang J, Harris SE, Liewald D, Ke X, Le Hellard S, Christoforou A, Luciano M, McGhee K, Lopez L, Gow AJ, Corley J, Redmond P, Fox HC, Haggarty P, Whalley LJ, McNeill G, Goddard ME, Espeseth T, Lundervold AJ, Reinvang I, Pickles A, Steen VM, Ollier W, Porteous DJ, Horan M, Starr JM, Pendleton N, Visscher PM, Deary IJ. Genome-wide association studies establish that human intelligence is highly heritable and polygenic. Molecular Psychiatry. 2011;16:996–1005. doi: 10.1038/mp.2011.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoung CG, Cicchetti D, Rogosch FA. Moderation of the association between childhood maltreatment and neuroticism by the corticotropin-releasing hormone receptor 1 gene. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2011;52(8):898–906. doi: 10.1111/j.1469-7610.2011.02404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoung CG, Cicchetti D, Rogosch FA, Gray JR, Eastman M, Grigorenko EL. Sources of cognitive exploration: Genetic variation in the prefrontal dopamine system predicts Openness/Intellect. Journal of Research in Personality. 2011;45:364–371. doi: 10.1016/j.jrp.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoung CG, Gray JR. Personality neuroscience: Explaining individual differences in affect, behaviour and cognition, 323–346. In: Corr PJ, Matthews G, editors. The Cambridge handbook of personality psychology. New York: Cambridge University Press; 2009. pp. 323–346. (2009) [Google Scholar]
- DeYoung CG, Grazioplene RG, Peterson JB. From madness to genius: The Openness/Intellect trait domain as a paradoxical simplex. Journal of Research in Personality. 2012;46:63–78. [Google Scholar]
- DeYoung CG, Peterson JB, Higgins DM. Sources of Openness/Intellect: cognitive and neuropsychological correlates of the fifth factor of personality. Journal of Personality. 2005;73(4):825–858. doi: 10.1111/j.1467-6494.2005.00330.x. [DOI] [PubMed] [Google Scholar]
- DeYoung CG, Shamosh Na, Green AE, Braver TS, Gray JR. Intellect as distinct from Openness: differences revealed by fMRI of working memory. Journal of Personality and Social Psychology. 2009;97(5):883–892. doi: 10.1037/a0016615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan LE, Keller MC. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. American Journal of Psychiatry. 2011;168:1041–1049. doi: 10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, van Ijzendoorn MH. Differential susceptibility to the environment: an evolutionary-neurodevelopmental theory. Development and psychopathology. 2011;23:7–28. doi: 10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- Espeseth T, Sneve MH, Rootwelt H, Laeng B. Nicotinic receptor gene CHRNA4 interacts with processing load in attention. PLoS ONE. 2010;5(12):e14407. doi: 10.1371/journal.pone.0014407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood PM, Sundararajan R, Lin M-K, Kumar R, Fryxell KJ, Parasuraman R. Both a nicotinic single nucleotide polymorphism (SNP) and a noradrenergic SNP modulate working memory performance when attention is manipulated. Journal of Cognitive Neuroscience. 2009;21(11):2139–2153. doi: 10.1162/jocn.2008.21164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith JW, Zinbarg RE, Craske MG, Mineka S, Rose RD, Waters AM, Sutton JM. Neuroticism as a common dimension in the internalizing disorders. Psychological Medicine. 2010;40:1125–1136. doi: 10.1017/S0033291709991449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagekull B, Bohlin G. Preschool temperament and environmental factors related to the Five-Factor Model of personality in middle childhood. Merrill-Palmer Quarterly. 1998;44:194–215. [Google Scholar]
- Heath CJ, King SL, Gotti C, Marks MJ, Picciotto MR. Cortico-thalamic connectivity is vulnerable to nicotine exposure during early postnatal development through α4/β2/α5 nicotinic acetylcholine receptors. Neuropsychopharmacology. 2010;35(12):2324–2338. doi: 10.1038/npp.2010.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh JB, Inzlicht M. The devil you know Neuroticism predicts neural response to uncertainty. Psychological Science. 2008;19:962–967. doi: 10.1111/j.1467-9280.2008.02183.x. [DOI] [PubMed] [Google Scholar]
- Ioannidis JPa, Trikalinos Ta, Khoury MJ. Implications of small effect sizes of individual genetic variants on the design and interpretation of genetic association studies of complex diseases. American Journal of Epidemiology. 2006;164(7):609–614. doi: 10.1093/aje/kwj259. [DOI] [PubMed] [Google Scholar]
- John OP, Caspi A, Robins RW, Moffitt TE, Stouthamer-Loeber M. The ‘little five’: Exploring the nomological network of the five-factor model of personality in adolescent boys. Child Development. 1994;65:160–178. [PubMed] [Google Scholar]
- John OP, Naumann LP, Soto CJ. Paradigm shift to the integrative Big Five trait taxonomy: History: measurement, and conceptual issue. In: John OP, Robins RW, Pervin LA, editors. Handbook of Personality: Theory and research. New York: Guilford; 2008. pp. 114–158. [Google Scholar]
- Johnson JA. Clarification of factor five with the help of the AB5C model. European Journal of Personality. 1994;8:311–334. [Google Scholar]
- Kirchheiner J, Nickchen K, Bauer M, Wong M-L, Licinio J, Roots I, Brockmöller J. Pharmacogenetics of antidepressants and antipsychotics: the contribution of allelic variations to the phenotype of drug response. Molecular Psychiatry. 2004;9(5):442–473. doi: 10.1038/sj.mp.4001494. [DOI] [PubMed] [Google Scholar]
- Markett S, Montag C, Reuter M. The nicotinic acetylcholine receptor gene CHRNA4 is associated with negative emotionality. Emotion. 2011;11(2):450–455. doi: 10.1037/a0021784. [DOI] [PubMed] [Google Scholar]
- Markett S, Montag C, Walter NT, Reuter M. Evidence for the modality independence of the genetic epistasis between the dopaminergic and cholinergic system on working memory capacity. European Neuropsychopharmacology. 2010;21(2):216–220. doi: 10.1016/j.euroneuro.2010.10.011. [DOI] [PubMed] [Google Scholar]
- McNaughton N, Gray Ja. Anxiolytic action on the behavioural inhibition system implies multiple types of arousal contribute to anxiety. Journal of Affective Disorders. 2000;61(3):161–176. doi: 10.1016/s0165-0327(00)00344-x. [DOI] [PubMed] [Google Scholar]
- Miwa JM, Freedman R, Lester Ha. Neural systems governed by nicotinic acetylcholine receptors: Emerging hypotheses. Neuron. 2011;70(1):20–33. doi: 10.1016/j.neuron.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasuraman R, Greenwood PM, Kumar R, Fossella J. Beyond heritability – neurotransmitter genes differentially modulate visuospatial attention and working memory. Psychological Science. 2005;16:200–207. doi: 10.1111/j.0956-7976.2005.00804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JB, Flanders JL. Complexity management theory: Motivation for ideological rigidity and social conflict. Cortex. 2002;38:429–458. doi: 10.1016/s0010-9452(08)70680-4. [DOI] [PubMed] [Google Scholar]
- Riemann R, Angleitner A, Strelau J. Genetic and Environmental Influences on Personality: A Study of Twins Reared Together Using the Self- and Peer Report NEO-FFI Scales. Journal of Personality. 1997;65(3):449–475. [Google Scholar]
- Rogosch FA, Cicchetti D. Child maltreatment and emergent personality organization: perspectives from the five-factor model. Journal of Abnormal Child Psychology. 2004;32(2):123–145. doi: 10.1023/b:jacp.0000019766.47625.40. [DOI] [PubMed] [Google Scholar]
- Sarter M, Hasselmo ME, Bruno JP, Givens B. Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain Research Reviews. 2005;48(1):98–111. doi: 10.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Toth SL, Pickreign Stronach E, Rogosch FA, Caplan R, Cicchetti D. Illogical thinking and thought disorder in maltreated children. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50:659–668. doi: 10.1016/j.jaac.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Winterer G, Musso F, Konrad A, Vucurevic G, Stoeter P, Sander T. Association of attentional network function with exon 5 variations of the CHRNA4 gene. Human Molecular Genetics. 2007;16(18):2165–2174. doi: 10.1093/hmg/ddm168. [DOI] [PubMed] [Google Scholar]
- Yu AJ, Dayan P. Uncertainty, neuromodulation, and attention. Neuron. 2005;46(4):681–692. doi: 10.1016/j.neuron.2005.04.026. [DOI] [PubMed] [Google Scholar]