Abstract
PURPOSE
To describe a technique to obtain combined images of vitreo-retino-choroidal structures using spectral-domain (SD) optical coherence tomography (OCT) and to evaluate applicability in normal eyes and limitations in eyes with cataract.
DESIGN
Prospective, observational case series.
METHODS
Three different foveal scans including conventional SD-OCT, enhanced depth imaging (EDI)-OCT and the novel method called “combined depth imaging” (CDI)-OCT were obtained in 42 eyes of healthy volunteers and in 26 eyes with cataract using Heidelberg Spectralis HRA. The CDI-OCT was manually obtained using an image modification process that enhances the vitreo-retinal interface first and then the choroid while averaging 100 separate OCT scans. The visualization of the inner border of the preretinal pocket and the outer border of the choroid was graded by independent masked observers for each OCT scan modality.
RESULTS
The CDI technique was able to create a good quality combined image of the posterior structures in all the eyes including eyes with cataract. The agreement between the grading performed by the independent observers was high for both the inner border of the vitreal pocket (kappa=0.86; P<0.001) and the outer choroidal border (kappa=0.90; P<0.001). CDI-OCT was equivalent to conventional SD-OCT in visualizing the vitreal pocket (P=0.445 for normal eyes, P=0.162 for eyes with cataract) and was equivalent to EDI-OCT in visualizing the outer choroidal border (P=0.660 for normal eyes, P=0.329 for eyes with cataract). CDI-OCT was superior to conventional SD-OCT and EDI-OCT to visualize both the structures (P<0.001).
CONCLUSIONS
The manual technique of CDI-OCT is highly sensitive to visualize posterior vitreo-retino-choroidal structures into a single full-depth image, and is not affected by mild to moderate cataract.
Introduction
Spectral-domain optical coherence tomography (SD-OCT) has emerged as the gold-standard non-invasive technique to visualize fine retinal details and to evaluate retinal structural changes for many ocular treatments. Commercially available SD-OCT devices achieve axial scanning speeds of 20,000 to 52,000 A-scans per second with an axial resolution of 5 µm to 7 µm in the eye1, allowing histological-like cross-sectional images of the vitreo-retinal interface and the retinal structures. High image quality may be achieved by the image averaging system; commercially available software averages 8 to 100 images, and increases signal-to-noise ratio in proportion to the square root of the number of images averaged.1 Some SD-OCT devices have automated eye-tracking as a feature to ensure that all the images to be averaged are taken from the same retinal location.
For clinical ophthalmic purposes, typical SD-OCT instruments use near-infrared wavelengths around 850 nm that penetrate well into the retina and the subretinal space, while deeper penetration is limited because of scattering induced by the retinal pigment epithelium and the vascular nature of the choroid.2 Other reasons for the choice of wavelengths around 850 nm versus 1,000 or 1,300 nm are the better contrast of retinal structures and better axial resolution. Moreover, typically the point of maximum sensitivity on SD-OCT (known as the “zero delay line”) is located in the vitreous.1, 2 As a result, with increasing depth into the tissue, the signal is reduced and details of the choroid are not visible.
More recently, the ability to visualize the choroidal anatomy has been improved with the development of the “Enhanced Depth Imaging” (EDI) technique on SD-OCT (EDI-OCT)2, and with the new generation of experimental prototypes of swept-source OCT (SS-OCT).3–5 The EDI acquisition software automatically captures cross-sectional images with the choroid close to the zero delay line to maximize sensitivity on the outer limit of the choroid, using the current 830–880 nm probing light of the conventional SD-OCT. Alternatively, SS-OCT prototypes (not yet commercially available) use a longer center wavelength (1,040 to 1,060 nm) and higher scan rate (100,000 Hz) of the light source, allowing deeper penetration into the choroid and the sclera. Clearly, these new techniques allow a great detection of the choroidal borders and fine detail; however, detection of the inner retinal surface is reduced using the EDI-OCT and the posterior vitreous is not visible. Moreover, so far no SS-OCT device has been approved by the Food and Drug Administration (FDA). In addition, the axial resolution and retinal contrast depends on the wavelength; at higher wavelengths the resolution deceases and retinal contrast reduces.
To overcome this imaging limitation and to obtain a single comprehensive image of both vitreo-retinal interface and choroid, we developed a novel imaging method using a commercially available SD-OCT device.6 The aims of the present study are: 1) to describe in detail this technique; 2) to test its ability to visualize vitreo-retino-choroidal structures in a series of normal eyes; and 3) to evaluate its limitations in eyes with mild to moderate cataract.
Methods
Patients
For this prospective observational study, 42 eyes of 21 healthy volunteers with no known eye disease were consecutively included, as well as 26 eyes of 17 patients with known cataract. Exclusion criteria included known posterior vitreous detachment and diffuse corneal opacity. After obtaining written informed consent to participate in this research, all subjects underwent axial length measurement using ocular biometry (IOLMaster; Carl Zeiss Meditec, Jena, Germany), and pupillary dilation with Tropicamide 1%. In the group of patients with cataract, lens opacities were graded after dilation according to the Lens Opacities Classification System III (LOCS III).7 This prospective study was approved by the Institutional Review Board of the University of California San Diego (UCSD) and was conducted at the UCSD Shiley Eye Center in adherence to the tenets of the Declaration of Helsinki.
Derivation of the Combined Depth Imaging technique
This simple technique, that we called “Combined Depth Imaging” (CDI), is an image process modification that combines conventional SD-OCT B-scan with EDI-OCT B-scan into a single image. The Spectralis HRA (Heidelberg Engineering, Carlsbad, CA) is a SD-OCT device that has real-time eye-tracking system (TruTrack™ active eye-tracking) and is capable of averaging up to 100 separate OCT scans at any arbitrary location. The device achieves axial scanning speed of 40,000 A-scans/second with an axial digital resolution of 3.9 µm and an axial optical resolution of 5 to 7 µm. By convention, structures nearer to the zero delay are imaged at the top of the screen and deeper structures are imaged further down in the screen. By default, the device operates with the inner retina closest to the zero delay line to maximize the sensitivity of the retina and vitreo-retinal interface. To enhance the choroid, the EDI acquisition software is available on the device, with a dedicated button on the keypad; when the EDI button is selected the choroid is placed closer to the zero delay line. The CDI-OCT image derives from an image modification process that enhances first the vitreo-retinal interface and then the choroid while averaging 100 separate OCT scans. Indeed, while averaging at least 50 conventional SD-OCT scans the vitreo-retinal interface is highly enhanced; by selecting the EDI button after averaging 50 scans, the choroid of the subsequent scans gets enhanced and the device is able to merge conventional scans with EDI-OCT scans into a single comprehensive image showing good sensitivity through the full imaging depth.
Optical Coherence Tomography scanning protocol
All patients were imaged using the Spectralis HRA SD-OCT device through dilated pupils, by a single experienced physician (GB). The Spectralis HRA was set to perform a 9-mm high-resolution horizontal B-scan, centered on the fovea. An internal fixation light was used to center the scanning line on the fovea. The averaging system was set to 100 OCT scans. A sequence of 3 different images was performed for each eye of the patients:
-
▪
Conventional OCT: After positioning the OCT B-scan at the upper half of the screen, the operator activated the averaging system of the device, and the image was captured after reaching at least 50% of the averaging.
-
▪
Enhanced Depth Imaging OCT: After positioning the OCT B-scan at the lower half of the screen, the operator activated the EDI acquisition software and subsequently the averaging system of the device. The image was captured after reaching at least 50% of the averaging.
-
▪
Combined Depth Imaging OCT: After positioning the OCT B-scan in the middle of the screen, the operator firstly activated the averaging system of the device using the conventional SD-OCT (as described in point 1). After reaching 50% of the averaging, he manually activated the EDI acquisition software using the dedicated button on the keypad. The combined image that resulted was captured as soon as a good-quality image was visualized in the middle of the screen.
Optical Coherence Tomography imaging analysis
The three images for each eye were saved as maximum quality jpeg image files in native resolution. All images were then masked removing features identifying OCT scan modality and patient information, and were then randomly mixed. Two independent masked physicians (SE, LG) reviewed each image on the same monitor with same resolution at different time points, and graded visualization of the inner border of the preretinal pocket and of the outer border of the choroid, separately. Grade 0 was defined when the border was not seen; grade 1 when the border was barely seen; grade 2 when the border was clearly seen.
Statistical Analysis
The inter-observer agreement for the grading of the inner border of the vitreal pocket and the outer border of the choroid was assessed using Cohen’s Kappa. Bland-Altman plot was used to assess the clinically relevant magnitude of the difference between grading and observers.8 A paired samples test was used to compare grading of the inner border of the vitreal pocket and the outer border of the choroid among the 3 OCT images for each eye. Statistical analysis was performed using SPSS statistical software version 20.0 (SPSS Inc., Chicago, IL). A p-value <0.05 was considered to be statistically significant.
Results
Normal volunteers had mean age of 38 years (range, 23 to 64 years) and mean axial length of 23.9 mm (range, 21.9 to 26.4 mm). Subjects with cataract had mean age of 66 years (range, 52 to 82 years) and mean axial length of 23.4 mm (range, 20.9 to 24.9 mm). Cataract grading ranged from LOCS III grade NO2-NC2 to grade NO4-C3.
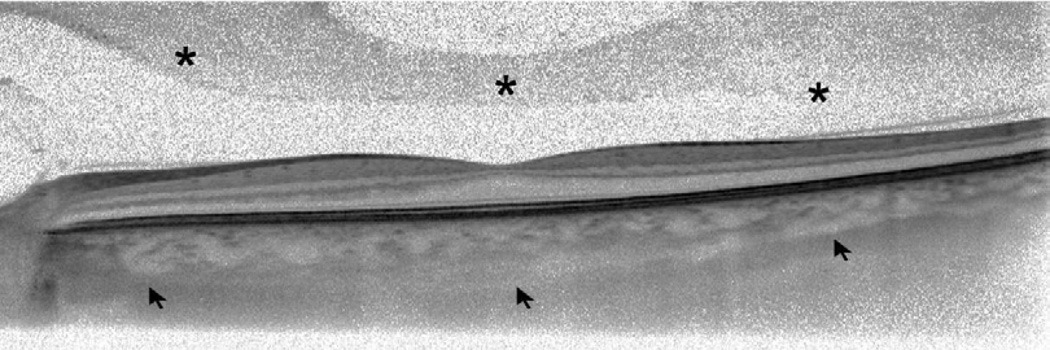
In all the eyes, normal or with cataract, the Heidelberg Spectralis HRA was able to create a good quality combined image of the posterior structures in a few seconds during image averaging using the CDI technique. Examples of comparison between different OCT modalities are shown in Figure 1.
Figure 1.
Comparative optical coherence tomography (OCT) sections through the fovea obtained with various OCT modalities in two different eyes. (Top row) Conventional spectral domain OCT scans show good visualization on posterior vitreous structures (asterisks) but poor visualization of the outer border of the choroid. (Middle row) Enhanced depth imaging OCT scans improve visualization of the outer choroidal border (arrows) but can’t visualize details of the posterior vitreous pocket. (Bottom row) Combined depth imaging OCT scans are able to enhance visualization of both the vitreal pocket (asterisks) and the outer border of the choroid (arrows).
Agreement between grading of the borders performed by the independent masked observers was excellent for both inner border of the vitreal pocket (kappa = 0.86; P<0.001) and outer choroidal border (kappa = 0.90; P<0.001). Bland-Altman plots of the differences between grading of visualization of the inner border of the vitreal pocket and the outer choroidal border by the two observers are shown in Figure 2.
Figure 2.
Bland-Altman plots showing the level of interobserver concordance between grading of vitreal pocket visualization (Left) and grading of outer choroidal border visualization (Right). Dashed lines show mean difference and 95% limits of agreement (1.96 ± SD).
Visualization of the inner border of the vitreal pocket was not different between conventional SD-OCT and CDI-OCT (P = 0.445 for normal eyes, P = 0.162 for eyes with cataract), but was significantly higher in both in comparison to EDI-OCT scan (P<0.001 for normal eyes and eyes with cataract).On the contrary, visualization of the outer choroidal border was not different between EDI-OCT and CDI-OCT (P = 0.660 for normal eyes, P = 0.329 for eyes with cataract), but was significantly better in both of them compared to conventional SD-OCT (P<0.001 for normal eyes and eyes with cataract). When merging results of grading for the vitreal pocket and the posterior choroid, the CDI-OCT was significantly better than conventional SD-OCT and EDI-OCT to visualize both the structures (P<0.001 for normal eyes and eyes with cataract).
Clinical example of use of the Combined Depth Imaging technique
A 65-year-old man treated with three bevacizumab intravitreal injections for wet age-related macular degeneration (AMD) was scanned with the Spectralis HRA to verify the treatment response after the loading phase. EDI-OCT scan was performed to study the subfoveal choroidal thickness, which may reduce after intravitreal treatment for wet AMD.9 The EDI-OCT scan through the fovea showed the presence of subfoveal cystic spaces in the inner retina, focal disruption of the outer retinal layers, juxtafoveal choroidal neovascularization mushrooming into the subretinal and intraretinal spaces, and normal choroidal thickness (Figure 3). According to the re-treatment guidelines used in recent randomized clinical trials, persistent intraretinal fluid is a criterion for ongoing intravitreal treatment for wet AMD.10–13 However, the conventional SD-OCT through the fovea showed that cystic intraretinal spaces were the mechanical result of a vitreo-macular traction syndrome that was not visible using the EDI-OCT; thus, the patient was not re-treated for wet AMD according to a pro-re-nata (PRN) regimen. To overcome misunderstanding of the patient’s eye condition, a single enhanced image of vitreo-retino-choroidal structures may be the best choice. Indeed, the CDI-OCT of the same eye showed good visualization of the posterior vitreous pocket, vitreo-macular adhesion, inner and outer retinal layers, and choroid.
Figure 3.
Results of loading phase with bevacizumab for choroidal neovascularization secondary to wet age-related macular degeneration. (Left) Near-infrared image with overlaying optical coherence tomography (OCT) line passing through the fovea: the image shows a subfoveal hyper-reflective area surrounded by multiple hyper and hypo-reflective dots. (Top right) Enhanced depth imaging (EDI)-OCT shows the presence of subfoveal cystic spaces in the inner retina, focal disruption of the outer retinal layers, juxtafoveal choroidal neovascularization mushrooming into the subretinal and intraretinal spaces, and normal choroidal thickness. (Middle right) Conventional spectral domain (SD)-OCT shows that the cystic intraretinal spaces were the mechanical result of a vitreo-macular traction syndrome that was not visible on EDI-OCT; the posterior border of the choroid is not clearly visible. (Bottom right) Combined depth imaging (CDI)-OCT is able to visualize all the posterior structures in a single comprehensive image, allowing a complete clinical evaluation of the case.
Discussion
The present study describes the “Combined Depth Imaging” technique on SD-OCT, and demonstrates that the CDI-OCT technique is able to visualize all posterior structures in a single image. Except in the cases of high myopia or albinism14, conventional SD-OCT alone is usually not able to obtain a comprehensive image because of intense scattering induced by the retinal pigment epithelium.2 On the other hand, EDI-OCT is not able to visualize the posterior vitreous because the deeper portions of the choroid are placed closer to the zero delay.2 Our new image process modification is simple and fast to perform, and not influenced by mild to moderate cataract.
The single scans of a particular area, taken with conventional SD-OCT and EDI-OCT separately, may not precisely image the exactly same area, since after collecting the first image the patient may slightly change the fixation. Because of this, with a very small movement of the fixating point, the recorded image may not correspond to the same area. The CDI technique is useful in having a complete evaluation of the structures of the same area of interest, without being affected by fixation changes. This technique may be useful while imaging non-vitrectomized eyes or eyes with no posterior vitreous detachment, to obtain complete information about posterior vitreous, retina and choroid.
Mild to moderate cataract does not affect CDI-OCT image quality. However, from our experience and from the literature15, 16, dense cataract reduces signal strength on SD-OCT. Irregular cortical opacity can significantly scatter light, and advanced nuclear cataract or diffuse sub-posterior capsular opacity can block light. In these cases it’s difficult to obtain sharp visualization of the vitreo-retinal structures using conventional OCT, or clear detection of the chorio-scleral boundary using EDI-OCT. Therefore, we can assume that a full-depth image that combines conventional OCT and EDI-OCT has equally limited ability to visualize details of the posterior structures of the eye in cases of dense cataract.
There are important limitations of this method of imaging. First, this technique is currently only possible with the Spectralis HRA; no other commercial SD-OCT device is able to simultaneously enhance the choroid, track eye movements, and perform image averaging. Second, when performing the CDI-OCT procedure, we noticed that the quality of the conventional OCT image reduces with increase of the averaging of the superimposed EDI-OCT image; in other words, visualization of the posterior vitreous structures may reduce quickly after shifting to the EDI mode. Thus, we suggest capturing the CDI-OCT image as soon as a combined image is well visible on the screen. A third important limitation of the current technique is that the subject must be able to fixate reasonably well with at least one eye. In our experience, patients with unstable fixation required longer time to obtain a good averaging and this longer time may become burdensome. A forth limitation is that it is possible to obtain the combined image only when performing a single linear B-scan, while the automatic image capture when performing a raster scan does not currently allow manual activation of the EDI mode.
A new generation of OCT devices (SS-OCT), that use wavelength swept laser as light source, longer wavelengths and higher scanning speed, will likely be available soon for physicians to evaluate the posterior structures of the eye. This new technology allows much less roll-off in sensitivity with increasing depth as compared with conventional SD-OCT devices.17 However, a FDA-approved SS-OCT device is not yet commercially available, and the cost of these devices will likely be very high and not always affordable in the clinical practice. Furthermore, the longer wavelength of SS-OCT devices has an inherent reduced retinal contrast and worse axial resolution.
In conclusion, the present study demonstrates that the manual technique of CDI-OCT is easy, fast, and sensitive enough to visualize posterior vitreo-retino-choroidal structures into a single, comprehensive image, using a commercially available and widely used SD-OCT device. Further studies are necessary to evaluate usefulness of the CDI-OCT technique in eyes with retinal pathologies. Dedicated built-in software may be useful to obtain this full-depth imaging result automatically.
Acknowledgments
Funding/Support: This study was supported by NIH grants R01EY007366 and R01EY018589 (WRF), R01EY016323 (DUB), and "RPB incorporated and unrestricted funds from Jacobs Retina Center". The funding organizations had no role in the design or conduct of this research.
Biography

Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosure: All authors have completed and submitted the ICMJE form for disclosure of potential conflicts of interest. None of the authors have any financial interests to disclose.
Contributions of Authors: design and conduct of the study (GB, DUB, WRF); collection and management of the data (GB, SEE, LG); analysis and interpretation of the data (GB, SL); and preparation of the draft (GB, SEE, JC); review and approval of the manuscript (LC, DUB, WRF).
Other Acknowledgments: none.
References
- 1.Regatieri CV, Branchini L, Fujimoto JG, Duker JS. Choroidal imaging using spectral-domain optical coherence tomography. Retina. 2012;32(5):865–876. doi: 10.1097/IAE.0b013e318251a3a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146(4):496–500. doi: 10.1016/j.ajo.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 3.Povazay B, Bizheva K, Hermann B, et al. Enhanced visualization of choroidal vessels using ultrahigh resolution ophthalmic OCT at 1050 nm. Opt Express. 2003;11(17):1980–1986. doi: 10.1364/oe.11.001980. [DOI] [PubMed] [Google Scholar]
- 4.Huber R, Adler DC, Srinivasan VJ, Fujimoto JG. Fourier domain mode locking at 1050 nm for ultra-high-speed optical coherence tomography of the human retina at 236,000 axial scans per second. Opt Lett. 2007;32(14):2049–2051. doi: 10.1364/ol.32.002049. [DOI] [PubMed] [Google Scholar]
- 5.Hirata M, Tsujikawa A, Matsumoto A, et al. Macular choroidal thickness and volume in normal subjects measured by swept-source optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52(8):4971–4978. doi: 10.1167/iovs.11-7729. [DOI] [PubMed] [Google Scholar]
- 6.Barteselli G, Bartsch DU, Freeman WR. Combined Depth Imaging using optical coherence tomography as a novel imaging technique to visualize vitreo-retino-choroidal structures. Retina. 2012 doi: 10.1097/IAE.0b013e31826f5252. (forthcoming). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chylack LT, Jr, Wolfe JK, Singer DM, et al. The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. Arch Ophthalmol. 1993;111(6):831–836. doi: 10.1001/archopht.1993.01090060119035. [DOI] [PubMed] [Google Scholar]
- 8.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310. [PubMed] [Google Scholar]
- 9.Yamazaki T, Koizumi H, Yamagishi T, Kinoshita S. Subfoveal Choroidal Thickness after Ranibizumab Therapy for Neovascular Age-related Macular Degeneration: 12-Month Results. Ophthalmology. 2012;119(8):1621–1627. doi: 10.1016/j.ophtha.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 10.Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 11.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 12.Group CR, Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown DM, Heier JS, Ciulla T, et al. Primary endpoint results of a phase II study of vascular endothelial growth factor trap-eye in wet age-related macular degeneration. Ophthalmology. 2011;118(6):1089–1097. doi: 10.1016/j.ophtha.2011.02.039. [DOI] [PubMed] [Google Scholar]
- 14.Seo JH, Yu YS, Kim JH, Choung HK, Heo JW, Kim SJ. Correlation of visual acuity with foveal hypoplasia grading by optical coherence tomography in albinism. Ophthalmology. 2007;114(8):1547–1551. doi: 10.1016/j.ophtha.2006.10.054. [DOI] [PubMed] [Google Scholar]
- 15.Kim NR, Lee H, Lee ES, et al. Influence of cataract on time domain and spectral domain optical coherence tomography retinal nerve fiber layer measurements. J Glaucoma. 2012;21(2):116–122. doi: 10.1097/IJG.0b013e31820277da. [DOI] [PubMed] [Google Scholar]
- 16.Na JH, Sung KR, Lee Y. Factors associated with the signal strengths obtained by spectral domain optical coherence tomography. Korean J Ophthalmol. 2012;26(3):169–173. doi: 10.3341/kjo.2012.26.3.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wojtkowski M, Srinivasan V, Ko T, Fujimoto J, Kowalczyk A, Duker J. Ultrahigh-resolution, high-speed, Fourier domain optical coherence tomography and methods for dispersion compensation. Opt Express. 2004;12(11):2404–2222. doi: 10.1364/opex.12.002404. [DOI] [PubMed] [Google Scholar]