Abstract
Acute pulmonary vasodilator testing (AVT) is essential to determining the initial therapy for children with pulmonary arterial hypertension (PAH). This study aimed to report the initial experience with inhaled treprostinil used for AVT in children with PAH and to evaluate the hemodynamic change after inhaled treprostinil compared with inhaled nitric oxide. This prospective cohort study was designed for 13 children who underwent AVT with inhaled treprostinil or oxygen plus inhaled nitric oxide (iNO) during catheterization. Inhaled treprostinil was delivered during cardiac catheterization by adapting the Optineb ultrasonic nebulizer via either a flow-inflating bag or the manual mode of the anesthesia system. The median age of the patients was 10 years (range 4–17 years). The etiologies of PAH included idiopathic PAH and associated PAH. All the patients tolerated inhaled treprostinil without marked clinical worsening and received six or nine breaths (36 or 54 µg) of treprostinil. The median of the total treprostinil doses was 1.53 µg/kg (range 0.71–2.89 µg/kg). Inhaled treprostinil was administrated via an endotracheal tube (n = 8), anesthesia mask (n = 3), or laryngeal mask airway (n = 2). Inhaled nitric oxide (iNO) and inhaled treprostinil significantly decreased the mean pulmonary artery pressure and the pulmonary vascular resistance index compared with baseline. Three adverse events were reported after inhaled treprostinil, including cough and mild to moderate hypotension with higher doses. All adverse events resolved without any intervention. This study report is the first to describe the use of inhaled treprostinil for AVT in children with PAH. In this small pediatric cohort, inhaled treprostinil was effectively delivered and well tolerated and may be useful for AVT.
Keywords: Acute pulmonary vasodilator testing, Children, Iloprost, Inhaled treprostinil, Nitric oxide, Pulmonary arterial hypertension
Introduction
Advances in pulmonary vascular biology have led to novel therapies for pulmonary arterial hypertension (PAH), which have led to improved survival in children. Epoprostenol, a prostacyclin analog, has been used for almost two decades to treat of PAH with great success. Currently, another prostacyclin analog, treprostinil, which may be given as subcutaneous, intravenous, or inhaled therapy, has demonstrated clinical efficacy in children with PAH [14, 18, 20]. Treprostinil has stability at room temperature, smaller pump options, and a longer half-life compared with epoprostenol, which are seen as advantages.
Both prostacyclin analog therapies are effective for improving symptoms and hemodynamics and for slowing disease progression in children with idiopathic PAH or PAH associated with congenital heart disease [3, 5, 14, 17–20, 26, 28]. However, intravenous therapy carries the risks associated with chronic intravenous access such as infection and catheter thrombosis, as well as temporary interruption of the infusion due to pump malfunction and systemic hypotension [9, 16]. Inhaled agents have several advantages over intravenous infusion therapy including less risk of systemic hemodynamic concerns and minimized effect on ventilation-perfusion mismatch [12, 13].
Iloprost, a synthetic prostacyclin analog with vasodilatory properties, has been approved for treatment of adults with PAH. In previous pediatric studies, iloprost improved hemodynamics and World Health Organization functional class in idiopathic PAH and PAH associated with congenital heart disease [15, 21, 25]. However, iloprost therapy requires six to nine inhalation treatments daily and thus may have a potential risk for worse compliance in the pediatric population. In contrast, inhaled treprostinil requires only four inhalation treatments daily, which offers an advantage over iloprost. Inhaled treprostinil therapy has demonstrated clinical efficacy in adult patients with PAH [8, 27]. A single report describes its chronic use in pediatric patients with PAH [18].
Acute pulmonary vasodilator testing (AVT) is essential to determining initial therapy for children with PAH. The currently recommended AVT agents include short-acting vasodilators such as inhaled nitric oxide (iNO), inhaled iloprost, intravenous epoprostenol, and intravenous adenosine [1, 2, 4, 10, 11, 15, 25]. This study report aims to describe the initial experience with inhaled treprostinil used for AVT in children with PAH.
Materials and Methods
This prospective cohort study included children followed at the University of Colorado School of Medicine, Children’s Hospital Colorado. All the children were prospectively enrolled in an institutional review board (IRB)-approved protocol. All the children had a previous diagnosis of PAH and were receiving chronic therapy before catheterization.
At cardiac catheterization, the patients underwent either general anesthesia with low concentrations of inhaled volatile anesthetics or moderate to deep sedation with combinations of low-dose propofol, fentanyl, and/or midazolam. Airway and ventilation management included either an endotracheal tube with controlled mechanical ventilation or a laryngeal mask airway with spontaneous ventilation for anesthetized patients and a natural airway with spontaneous ventilation for sedated patients.
Using a flow-directed Swan-Ganz catheter and a systemic arterial line for monitoring, we measured mean right atrial pressure, mean pulmonary artery pressure, pulmonary capillary wedge pressure, and mean systemic blood pressure. Accordingly, cardiac output was obtained using thermodilution, and the cardiac index was calculated. If a significant intracardiac defect remained, cardiac output was obtained by the Fick method, with oxygen consumption assumed by the LaFarge equation. The pulmonary vascular resistance index was calculated as the mean pulmonary artery pressure minus the mean pulmonary wedge pressure divided by the cardiac index. We also calculated the systemic vascular resistance index and the pulmonary/systemic vascular resistance index ratio. The acute response to inhaled treprostinil was compared with the effects of oxygen plus iNO.
A positive response to the vasodilator test in pediatric patients is defined as a reduction in mean pulmonary artery pressure of 20 % or more, an increase or no change in the cardiac index, and a decrease or no change in the ratio of the pulmonary-to-systemic vascular resistance index [2, 5, 10]. After evaluation of hemodynamics at baseline (21 % oxygen level), the acute response to iNO at 40 ppm plus oxygen was assessed.
After iNO administration and after the return of the hemodynamic status to baseline, inhaled treprostinil was administered. Initially, three to six breaths (18–36 µg) of inhaled treprostinil were delivered, followed by observation of hemodynamic measurements and adverse events. If no significant change in pulmonary artery pressure occurred after 5 min and the patient did not demonstrate tolerability issues, three to six additional breaths were administered to a maximum of nine breaths (54 µg).
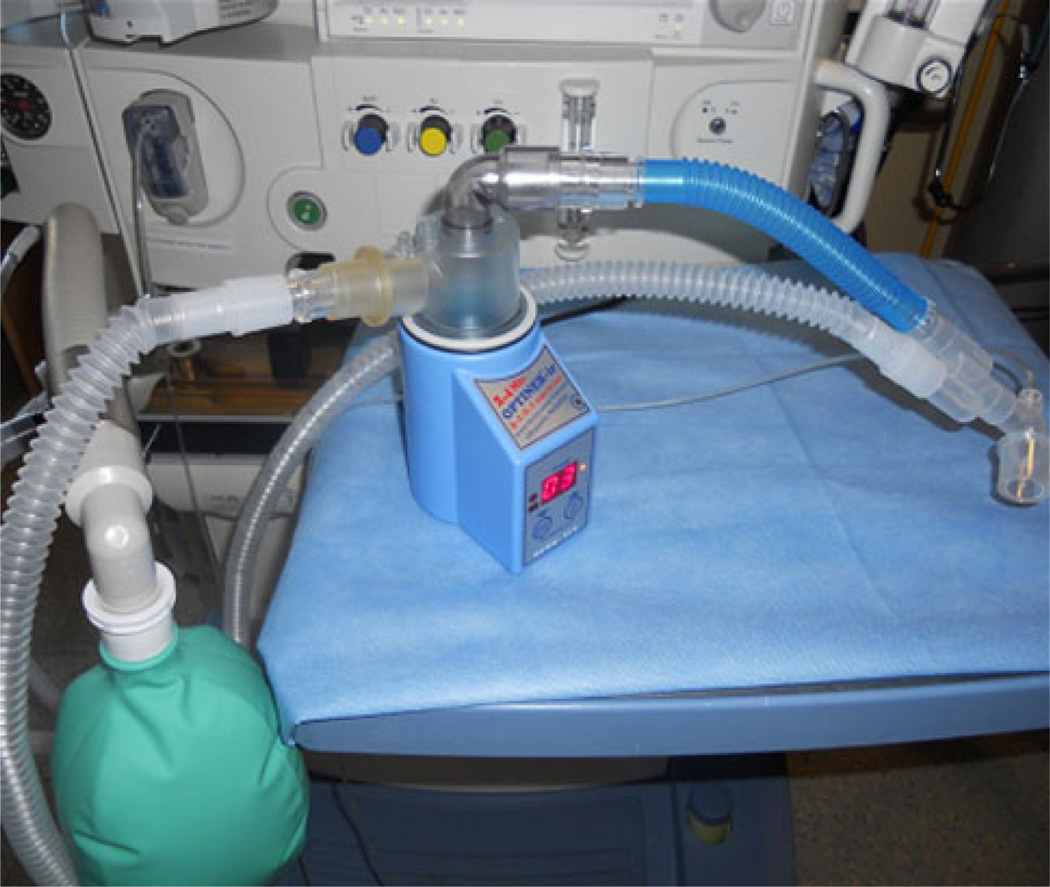
Inhaled treprostinil was delivered by the OPTINEB-ir Model ON-100/7 ultrasonic nebulizer (Metropolitan Medical, Inc., Winchester, VA, USA) via an anesthesia mask with a flow-inflating bag (for sedated patients breathing through a natural airway) or the manual mode of the anesthesia system in synchrony with the OPTINEB’s inhalation indicator (for anesthetized patients breathing through an endotracheal tube or laryngeal mask airway).
The standard OPTINEB®-ir configuration was adapted by removing the inhalation piece normally used by an awake patient and both the inhalation and exhalation filters from the dome assembly (Fig. 1). A 90° elbow connector (Portex, Inc., Keene, NH, USA) was placed in the top of the dome assembly followed by a 15-mm (inner diameter)/22-mm (outer diameter) multi-adapter and an Airlife 15 mm Inspiratory Line Extension (Allegiance Healthcare Corp., McGaw Park, IL, USA). A 22-mm silicone adapter (Vacumed, Ventura, CA, USA) was placed on the inlet of the OPTINEB-ir dome assembly and connected to either a flow-inflating bag or the inspiratory limb of the anesthesia circuit proximal to the patient Y. A custom connector for the inlet of the OPTINEB dome (Hans Rudolph Inc., Shawnee, KS, USA) was later acquired to provide a more secure fit. The inhalations were delivered using the manual breath system of the anesthesia machine in synchrony with the OPTINEB’s inhalation indicator.
Fig. 1.
Placement of refined inline system with custom part in circuit. Photograph shows incorporation of an inhaled treprostinil nebulizer (OPTINEB-ir Model ON-100/7 ultrasonic nebulizer) into a ventilatory circuit in the intensive care setting
Statistical Analysis
Descriptive statistics were calculated using median with range or mean. The statistical difference in hemodynamics with inhaled treprostinil or iNO compared with baseline was calculated using Student’s t tests for comparisons of data with a normal distribution. If the data did not show a normal distribution, a nonparametric test was used for analysis (Mann–Whitney’s U test). The level of statistical significance was defined as a p value of 0.05. Analyses were conducted using Statmate IV for Windows (Atoms Co., Tokyo, Japan).
Results
Patient Characteristics
The study enrolled 13 children (6 girls) who underwent AVT. The median age of the children was 10 years (range 4–17 years). The etiologies of PAH included idiopathic PAH (n = 7), PAH associated with congenital heart disease (n = 4), PAH associated with interstitial lung disease (n = 1), and PAH associated with hereditary hemorrhagic telangectasia (n = 1). The patient’s demographic data are listed in Table 1. Four children (31 %) were treated with inhaled treprostinil before the AVT evaluation, and two children received Iloprost therapy. These therapies were held longer than 6 h before the procedure.
Table 1.
Demographic data
| No. | Age (years) |
Gender | Diagnosis | Weight (kg) | Concomitant vasodilator therapies |
|---|---|---|---|---|---|
| 1 | 4 | M | APAH-HHT | 18.7 | iTre, PDE-5I |
| 2 | 5 | F | IPAH | 20.2 | ERA, PDE-5I |
| 3 | 6 | M | IPAH | 18.9 | iTre, ERA, PDE-5I |
| 4 | 6 | M | IPAH | 20.9 | iTre, ERA, PDE-5I |
| 5 | 9 | F | IPAH | 23.2 | CCB, PDE-5I |
| 6 | 9 | F | IPAH | 35.2 | CCB, IV-PGI2, PDE-5I, ERA |
| 7 | 10 | F | APAH-CHD | 24.5 | ERA, PDE-5I |
| 8 | 12 | F | IPAH | 55.2 | ERA, PDE-5I |
| 9 | 12 | M | APAH-CHD | 45.6 | ERA, PDE-5I |
| 10 | 13 | M | APAH-ILD | 47.6 | ERA, PDE-5I |
| 11 | 15 | M | IPAH | 72.9 | CCB, ilo, ERA, PDE-5I |
| 12 | 15 | F | APAH-CHD | 76.4 | iTre, PDE-5I |
| 13 | 17 | M | APAH-CHD | 37.6 | Ilo, PDE-5I |
F female, M male, PAH pulmonary arterial hypertension, APAH associated PAH, HHT hereditary hemorrhagic telangectasia, iTre inhaled treprostinil, PDE-5I phosphodiesterase inhibitors, IPAH idiopathic PAH, ERA endothelin receptor antagonist, CCB calcium-channel blocker, IV-PGI2 intravenous prostacyclin (epoprostenol), CHD congenital heart disease, ILD interstitial lung disease, ilo iloprost
Acute Pulmonary Vasodilator Testing
During AVT, younger children (n = 8) were administered iNO and inhaled treprostinil by endotracheal tube, whereas older children (n = 3) used a anesthesia mask or a laryngeal mask airway (n = 2) (Table 2). All the patients tolerated inhaled treprostinil during AVT without marked adverse events and eventually received six or nine breaths (36 or 54 µg) of treprostinil. The median treprostinil dose was 1.53 µg/kg (range 0.71–2.89 µg/kg).
Table 2.
Inhaled treprostinil doses during acute pulmonary vasodilator testing
| No. | Delivery method |
Total inhalations (6 µg/ inhalation) |
Total dose (µg/kg) |
Adverse events |
|---|---|---|---|---|
| 1 | ETT | 6 (36) | 2.89 | Cough |
| 2 | ETT | 6 (36) | 1.78 | – |
| 3 | ETT | 9 (54) | 2.86 | Moderate hypotension |
| 4 | ETT | 9 (54) | 2.58 | – |
| 5 | ETT | 6 (36) | 1.56 | – |
| 6 | ETT | 9 (54) | 1.53 | – |
| 7 | ETT | 6 (36) | 1.47 | Mild hypotension |
| 8 | Mask | 9 (54) | 0.98 | – |
| 9 | LMA | 9 (54) | 1.18 | – |
| 10 | ETT | 9 (54) | 1.13 | – |
| 11 | Mask | 9 (54) | 0.74 | – |
| 12 | Mask | 9 (54) | 0.71 | – |
| 13 | LMA | 9 (54) | 1.44 | – |
ETT endotracheal tube, LMA laryngeal mask, Mask face mask
Hemodynamic Change
During AVT, iNO and inhaled treprostinil significantly decreased mean pulmonary artery pressure compared with baseline (median baseline, 37 mmHg; range 21–56 mmHg; iNO, 31 mmHg; range 16–45 mmHg; p < 0.01; treprostinil, 33 mmHg; range 15–42 mmHg; p < 0.05) and the pulmonary vascular resistance index (median baseline, 6.7 U·m2; range 4.0–12.6 U·m2; iNO, 4.8 U·m2; range 2.6–11.0 U·m2; p < 0.01; treprostinil, 4.8 U·m2; range 3.4–11.1 U·m2; p < 0.05) (Fig. 2a, b). These hemodynamic reductions after inhaled treprostinil were similar to the level achieved with iNO.
Fig. 2.
Hemodynamic change with inhaled nitric oxide and inhaled treprostinil. The figure displays the hemodynamic change in the mean pulmonary artery pressure, the pulmonary vascular resistance index, the pulmonary/systemic vascular resistance index ratio, and the cardiac index during acute pulmonary vasodilator testing with inhaled nitric oxide and inhaled treprostinil. Inhaled nitric oxide and inhaled treprostinil significantly decreased the mean pulmonary artery pressure and the pulmonary vascular resistance index, whereas no significant changes from baseline values for the cardiac index occurred with either inhaled nitric oxide or inhaled treprostinil. iNO, inhaled nitric oxide; NS not significant
The cardiac index was not significantly changed after inhalation of NO and treprostinil (Fig. 2c). However, inhaled treprostinil tended to decrease the pulmonary/systemic vascular resistance index ratio (median baseline, 0.5; range 0.26–1.10; treprostinil, 0.4; range 0.16–1.06; p = 0.11), whereas iNO significantly decreased the ratio (median iNO, 0.38; range 0.13–0.79; p < 0.01) (Fig. 2d). There were no significant changes from baseline values for right atrial pressure, pulmonary capillary wedge pressure, systemic blood pressure, or arterial pressure of carbon dioxide (PaCO2) during the AVT with either iNO or inhaled treprostinil. Of the 13 children, 8 (62 %) were acute responders to the AVT.
Adverse Events of Inhaled Treprostinil
Three adverse events were reported after inhalation of treprostinil, including cough and mild to moderate systemic hypotension. A 4-year-old boy with associated PAH and a history of asthma (patient 1) had cough after the initial 3 breaths but tolerated an additional three breaths without extended cough or signs of bronchospasm. Mild transient hypotension (baseline systemic blood pressure, 76/44 mmHg [57 mmHg] decreased to 70/40 mmHg [50 mmHg]) was observed in a 10-year old girl who had PAH associated with CHD (patient 7) after six inhalations (36 µg).
A 6 year-old boy with idiopathic PAH (patient 3) initially tolerated six breaths well, but moderate systemic hypotension was observed (baseline 62/39 mmHg [49 mmHg] decreased to 50/30 mmHg [38 mmHg]) after an additional three breaths (total dose, 2.86 µg/kg). The systemic blood pressure recovered to baseline without any intervention. Interestingly, the boy was treated with nine breaths of inhaled treprostinil before catheterization and reported dizziness after inhalation. After the catheterization, the dose of inhaled treprostinil was decreased to six breaths four times daily, and the dizziness resolved.
Discussion
This is the first reported study that examined the acute hemodynamic effects, safety, and tolerability of inhaled treprostinil in children with PAH. In this small cohort of 13 children with idiopathic and associated PAH, we found that inhaled treprostinil had acute hemodynamic effects similar to those of iNO and was well tolerated with an acceptable safety profile.
Acute pulmonary vasodilator testing is essential to determining initial therapy for patients with PAH. Current adult guidelines and good clinical practice with children recommend consideration of high-dose calcium-channel blocker agents for patients demonstrating a positive AVT response [13, 23]. Medications such as adenosine, epoprostenol, iNO, and inhaled iloprost have been used to assess AVT responses. However, systemic agents may potentially worsen ventilation-perfusion mismatch compared with inhaled medications [13]. In addition, recent pediatric registry data have demonstrated that maintenance of an acute vasodilator response is associated with improved survival [5, 10].
In our study, both iNO and inhaled treprostinil were equally effective in selectively lowering mean pulmonary artery pressure and the pulmonary vascular resistance index in children with PAH during AVT. Therefore, this result suggests that inhaled treprostinil could possibly be used to assess AVT in children with PAH. Although the exact treprostinil dose administered to the patient may have decreased slightly by the delivery system dead space, the hemodynamic response suggests adequate drug delivery. Use of inhaled treprostinil in other acute clinical settings, such as postoperative cardiac surgery, requires further investigation but is appealing due to the longer half-life.
We have previously reported that inhaled iloprost had a hemodynamic response similar to that of iNO during AVT. In this study, we did not compare the acute hemodynamic response of inhaled iloprost with that of inhaled treprostinil. However, an adult randomized, placebo-control study directly compared hemodynamic effects between inhaled treprostinil and iloprost in patients with pulmonary hypertension [27]. The results showed that inhaled treprostinil had a more sustained effect on pulmonary vascular resistance and cardiac output than iloprost with fewer systemic side effects.
Inhaled treprostinil, a long-acting prostacyclin analog, has demonstrated sustained pulmonary vasodilation with excellent tolerability at relatively low doses in adult patients with PAH [6–8, 24]. Currently, there is a single study of long-term outcome for children treated with inhaled treprostinil [18]. Inhaled iloprost has demonstrated improved hemodynamics in pediatric patients with PAH [11, 15, 21, 25]. However, due to its short half-life, patients need to inhale iloprost six to nine times per day, which poses practical limitations, especially in children. Thus, inhaled treprostinil has some advantages over iloprost therapy, may provide beneficial effects in the pediatric population, and requires further study.
As previous adult studies have shown, common side effects due to inhaled treprostinil therapy include cough, headache, nausea, flushing, and sore throat [6, 22]. In our study, we had only three adverse events after inhalation of treprostinil during AVT. One patient with a history of asthma had transient cough after inhalation.
Inhaled treprostinil has a potential risk of worsening respiratory symptoms in patients with reactive airway disease. Lower airway reactivity may be a problem of inhaled agents. Thus, special caution should be required for children with concomitant lung disease during AVT with inhaled treprostinil. In the current study, two patients experienced systemic hypotension after higher doses of inhaled treprostinil were administered. Both cases resolved without interventions. Although the appropriate dose of inhaled treprostinil for pediatric patients is not known, clinicians should use higher doses of inhaled treprostinil with caution.
The limitations of this study included the small sample size, the relative heterogeneity of the patients, and the lack of a pharmacokinetic evaluation. A larger controlled study will be needed to determine whether these results observed in pediatric patients with PAH are seen in a larger population. Although the sample size was small, inhaled treprostinil improved hemodynamics during AVT in our pediatric patients. Moderate hypotension was observed in one patient after a higher dose of treprostinil was inhaled.
The appropriate dosing of inhaled treprostinil for pediatric patients remains unknown. Therefore, further studies are required to determine the optimal dose of inhaled treprostinil for AVT. Additionally, we did not evaluate the difference in clinical efficacy between inhaled treprostinil and iloprost during AVT in the pediatric population, which should be performed in future studies. Despite several limitations, our study suggests that inhaled treprostinil may be useful for AVT with tolerability and safety profiles and may become a new strategy for treatment of pediatric PAH in the critical care setting.
Acknowledgments
This study was supported by the Jayden DeLuca Foundation, the Leah Bult Foundation, the UL1 RR025780 Colorado Clinical Translational Science Institute, the National Center for Research Resources, and the National Institutes of Health.
Footnotes
Disclosures D. Dunbar Ivy serves as a member of the Gilead Sciences Research Scholars Program. The University of Colorado School of Medicine receives consultant fees from Actelion, Gilead, Pfizer, and United Therapeutics for Dr. Ivy to be a consultant. Aimee K. Doran is an employee of United Therapeutics.
Contributor Information
Shinichi Takatsuki, Pediatric Cardiology, Children’s Hospital Colorado, University of Colorado School of Medicine, 13123 East 16th Avenue, B100, Aurora, CO 80045, USA.
Donna K. Parker, Respiratory Care, Children’s Hospital Colorado, Aurora, CO, USA
Aimee K. Doran, United Therapeutics Corporation, Research Triangle Park, NC, USA
Robert H. Friesen, Pediatric Anesthesia, Children’s Hospital Colorado, University of Colorado School of Medicine, Aurora, CO, USA
D. Dunbar Ivy, Email: dunbar.ivy@childrenscolorado.org, Pediatric Cardiology, Children’s Hospital Colorado, University of Colorado School of Medicine, 13123 East 16th Avenue, B100, Aurora, CO 80045, USA.
References
- 1.Atz AM, Adatia I, Lock JE, Wessel DL. Combined effects of nitric oxide and oxygen during acute pulmonary vasodilator testing. J Am Coll Cardiol. 1999;33:813–819. doi: 10.1016/s0735-1097(98)00668-8. [DOI] [PubMed] [Google Scholar]
- 2.Barst RJ. Pharmacologically induced pulmonary vasodilatation in children and young adults with primary pulmonary hypertension. Chest. 1986;89:497–503. doi: 10.1378/chest.89.4.497. [DOI] [PubMed] [Google Scholar]
- 3.Barst RJ, Maislin G, Fishman AP. Vasodilator therapy for primary pulmonary hypertension in children. Circulation. 1999;99:1197–1208. doi: 10.1161/01.cir.99.9.1197. [DOI] [PubMed] [Google Scholar]
- 4.Barst RJ, Agnoletti G, Fraisse A, Baldassarre J, Wessel DL. Vasodilator testing with nitric oxide and/or oxygen in pediatric pulmonary hypertension. Pediatr Cardiol. 2010;31:598–606. doi: 10.1007/s00246-010-9645-5. [DOI] [PubMed] [Google Scholar]
- 5.Barst RJ, McGoon MD, Elliott CG, Foreman AJ, Miller DP, Ivy DD. Survival in childhood pulmonary arterial hypertension: insights from the registry to evaluate early and long-term pulmonary arterial hypertension disease management. Circulation. 2012;125:113–122. doi: 10.1161/CIRCULATIONAHA.111.026591. [DOI] [PubMed] [Google Scholar]
- 6.Benza RL, Seeger W, McLaughlin VV, Channick RN, Voswinckel R, Tapson VF, Robbins IM, Olschewski H, Rubin LJ. Long-term effects of inhaled treprostinil in patients with pulmonary arterial hypertension: the Treprostinil Sodium Inhalation Used in the Management of Pulmonary Arterial Hypertension (TRIUMPH) study open-label extension. J Heart Lung Transplant. 2011;30:1327–1333. doi: 10.1016/j.healun.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 7.Bourge RC, Tapson VF, Safdar Z, Benza RL, Channick RN, Rosenzweig EB, Shapiro S, James White R, Shane McSwain C, Karl Gotzkowsky S, Nelsen AC, Rubin LJ. Rapid transition from inhaled iloprost to inhaled treprostinil in patients with pulmonary arterial hypertension. Cardiovasc Therapeutics. 2012 doi: 10.1111/1755-5922.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Channick RN, Olschewski H, Seeger W, Staub T, Voswinckel R, Rubin LJ. Safety and efficacy of inhaled treprostinil as add-on therapy to bosentan in pulmonary arterial hypertension. J Am Coll Cardiol. 2006;48:1433–1437. doi: 10.1016/j.jacc.2006.05.070. [DOI] [PubMed] [Google Scholar]
- 9.Doran AK, Ivy DD, Barst RJ, Hill N, Murali S, Benza RL. Guidelines for the prevention of central venous catheter-related bloodstream infections with prostanoid therapy for pulmonary arterial hypertension. Int J Clin Pract Suppl. 2008;160:5–9. doi: 10.1111/j.1742-1241.2008.01811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Douwes JM, van Loon RL, Hoendermis ES, Vonk-Noordegraaf A, Roofthooft MT, Talsma MD, Hillege HL, Berger RM. Acute pulmonary vasodilator response in paediatric and adult pulmonary arterial hypertension: occurrence and prognostic value when comparing three response criteria. Eur Heart J. 2011 doi: 10.1093/eurheartj/ehr282. [DOI] [PubMed] [Google Scholar]
- 11.Hallioglu O, Dilber E, Celiker A. Comparison of acute hemodynamic effects of aerosolized and intravenous iloprost in secondary pulmonary hypertension in children with congenital heart disease. Am J Cardiol. 2003;92:1007–1009. doi: 10.1016/s0002-9149(03)00991-3. [DOI] [PubMed] [Google Scholar]
- 12.Ivy DD. Prostacyclin in the intensive care setting. Pediatr Crit Care Med. 2010;11:S41–S45. doi: 10.1097/PCC.0b013e3181d10845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ivy D. Advances in pediatric pulmonary arterial hypertension. Curr Opin Cardiol. 2012;27:70–81. doi: 10.1097/HCO.0b013e32835018cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ivy DD, Claussen L, Doran A. Transition of stable pediatric patients with pulmonary arterial hypertension from intravenous epoprostenol to intravenous treprostinil. Am J Cardiol. 2007;99:696–698. doi: 10.1016/j.amjcard.2006.09.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ivy DD, Doran AK, Smith KJ, Mallory GB, Jr, Beghetti M, Barst RJ, Brady D, Law Y, Parker D, Claussen L, Abman SH. Short- and long-term effects of inhaled iloprost therapy in children with pulmonary arterial hypertension. J Am Coll Cardiol. 2008;51:161–169. doi: 10.1016/j.jacc.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ivy DD, Calderbank M, Wagner BD, Dolan S, Nyquist AC, Wade M, Nickels WM, Doran AK. Closed-hub systems with protected connections and the reduction of risk of catheter-related bloodstream infection in pediatric patients receiving intravenous prostanoid therapy for pulmonary hypertension. Infect Control Hosp Epidemiol. 2009;30:823–829. doi: 10.1086/605320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ivy DD, Rosenzweig EB, Lemarie JC, Brand M, Rosenberg D, Barst RJ. Long-term outcomes in children with pulmonary arterial hypertension treated with bosentan in real-world clinical settings. Am J Cardiol. 2010;106:1332–1338. doi: 10.1016/j.amjcard.2010.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krishnan U, Ivy DD, Takatsuki S, Kerstein J, Calderbank M, Rosenzweig EB. Effectiveness and safety of inhaled treprostinil for the treatment of pulmonary arterial hypertension in children. Am J Cardiol. 2012;110(11):1704–1709. doi: 10.1016/j.amjcard.2012.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lammers AE, Hislop AA, Flynn Y, Haworth SG. Epoprostenol treatment in children with severe pulmonary hypertension. Heart. 2007;93:739–743. doi: 10.1136/hrt.2006.096412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levy M, Celermajer DS, Bourges-Petit E, Del Cerro MJ, Bajolle F, Bonnet D. Add-on therapy with subcutaneous treprostinil for refractory pediatric pulmonary hypertension. J Pediatr. 2011;158:584–588. doi: 10.1016/j.jpeds.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 21.Limsuwan A, Khosithseth A, Wanichkul S, Khowsathit P. Aerosolized iloprost for pulmonary vasoreactivity testing in children with long-standing pulmonary hypertension related to congenital heart disease. Catheter Cardiovasc Interv. 2009;73:98–104. doi: 10.1002/ccd.21793. [DOI] [PubMed] [Google Scholar]
- 22.McLaughlin VV, Oudiz RJ, Frost A, Tapson VF, Murali S, Channick RN, Badesch DB, Barst RJ, Hsu HH, Rubin LJ. Randomized study of adding inhaled iloprost to existing bosentan in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2006;174:1257–1263. doi: 10.1164/rccm.200603-358OC. [DOI] [PubMed] [Google Scholar]
- 23.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, Rubin LJ, Tapson VF, Varga J. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53:1573–1619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 24.McLaughlin VV, Benza RL, Rubin LJ, Channick RN, Voswinckel R, Tapson VF, Robbins IM, Olschewski H, Rubenfire M, Seeger W. Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: a randomized controlled clinical trial. J Am Coll Cardiol. 2010;55:1915–1922. doi: 10.1016/j.jacc.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 25.Rimensberger PC, Spahr-Schopfer I, Berner M, Jaeggi E, Kalangos A, Friedli B, Beghetti M. Inhaled nitric oxide versus aerosolized iloprost in secondary pulmonary hypertension in children with congenital heart disease: vasodilator capacity and cellular mechanisms. Circulation. 2001;103:544–548. doi: 10.1161/01.cir.103.4.544. [DOI] [PubMed] [Google Scholar]
- 26.van Loon RL, Roofthooft MT, Delhaas T, van Osch-Gevers M, ten Harkel AD, Strengers JL, Backx A, Hillege HL, Berger RM. Outcome of pediatric patients with pulmonary arterial hypertension in the era of new medical therapies. Am J Cardiol. 2010;106:117–124. doi: 10.1016/j.amjcard.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 27.Voswinckel R, Enke B, Reichenberger F, Kohstall M, Kreckel A, Krick S, Gall H, Gessler T, Schmehl T, Ghofrani HA, Schermuly RT, Grimminger F, Rubin LJ, Seeger W, Olschewski H. Favorable effects of inhaled treprostinil in severe pulmonary hypertension: results from randomized controlled pilot studies. J Am Coll Cardiol. 2006;48:1672–1681. doi: 10.1016/j.jacc.2006.06.062. [DOI] [PubMed] [Google Scholar]
- 28.Yung D, Widlitz AC, Rosenzweig EB, Kerstein D, Maislin G, Barst RJ. Outcomes in children with idiopathic pulmonary arterial hypertension. Circulation. 2004;110:660–665. doi: 10.1161/01.CIR.0000138104.83366.E9. [DOI] [PubMed] [Google Scholar]