Abstract
While traditional models of carcinogenesis have largely focused on neoplastic cells, converging data have revealed the importance of non-neoplastic stromal cells in influencing tumor growth and progression. Leveraging a genetically-engineered mouse model of NF1-associated optic glioma, we now demonstrate that stromal microglia express the CX3CR1 chemokine receptor, such that reduced CX3CR1 expression decreases optic nerve microglia. Moreover, genetic reduction of Cx3cr1 expression in Nf1 optic glioma mice delays optic glioma formation. Coupled with previous findings demonstrating that microglia maintain optic glioma growth, these new findings provide a strong preclinical rationale for the development of future stroma-directed glioma therapies in children.
Keywords: genetically-engineered mice, monocyte, astrocytoma, brain tumor, fractalkine
Introduction
Neurofibromatosis type 1 (NF1) is the most common inherited tumor predisposition syndrome in which affected children develop low-grade glial neoplasms (pilocytic astrocytomas). These gliomas primarily form in the optic pathway, such that tumor progression is often associated with reduced visual acuity.1 Histological analysis reveals a heterogeneous cellular composition, including neoplastic cells (astroglial cells) lacking NF1 gene expression and nonneoplastic cells (microglia and endothelial cells) with reduced NF1 gene expression. Since NF1-optic pathway gliomas (OPGs) are rarely resected, mechanistic insights have largely derived from studies using Nf1 genetically-engineered mouse (GEM) strains.
One of the important observations to emanate from the analysis of Nf1 GEM models is that Nf1 inactivation in astroglial progenitors is insufficient for gliomagenesis unless coupled with reduced Nf1 gene expression (Nf1+/- microenvironment).2 The requirement for Nf1+/- stromal cells in gliomagenesis, together with similar findings using Nf1 GEM models of plexiform neurofibroma,3 establishes a critical role for a supportive microenvironment in NF1-associated tumorigenesis.
Based on the abundance of microglia in human sporadic and NF1-associated pilocytic astrocytomas,4 we have previously demonstrated that optic glioma proliferation (maintenance) is attenuated by pharmacologic or genetic inhibition of microglia function.5,6 In the current study, we apply a genetic approach using Nf1 GEM strains to show that CX3CR1+ microglia are required for optic glioma formation in vivo.
Methods
Mice
All mice were maintained on a C57BL/6 background in accordance with established Washington University Animal Studies protocols. The mice employed in this study were generated by successive intercrossing (Supplementary Table 1).
Optic nerve measurements
Following paraformaldehyde fixation, optic nerves/chiasms were microdissected and photographed.7 Optic nerve diameters were measured at the chiasm and at 150, 300, and 450 μm anterior to the chiasm, and the volumes of the three 150 μm cones summed to calculate total optic nerve volume.
Immunohistochemistry and immunofluorescence
Following anesthetization, mice were transcardially perfused with Ringer's solution and then 4% paraformaldehyde in 0.1M sodium phosphate buffer (pH 7.4). Optic nerves with intact chiasms were post-fixed overnight and processed for either paraffin or OCT embedding. Immunohistochemistry was performed using appropriate antibodies (Supplementary Table 2) for 3,3’-diaminobenzidine development or immunofluorescence microscopy. Images were acquired on a Nikon Eclipse TE300 microscope (Tokyo, Japan) equipped with an optical camera (Optronics, Goleta, CA). Image J image analysis (http://rsbweb.nih.gov/ij/; National Institute of Mental Health, Bethesda, MD) and MetaMorph Microscopy Automation & Image Analysis (Molecular Devices, LLC, Sunnyvale, CA) software were used to obtain single collapsed fluorescence images.
Apoptosis was assessed by terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labeling (TUNEL) staining according to the manufacturer's instructions (Roche Diagnostics, Indianapolis, IN).
Flow cytometry (FACS)
Microglia from pools of 7-10 mice per experiment were collected and processed for antibody-mediated flow cytometry (Supplementary Table 2) using appropriate controls for gating. 6
Statistical analyses
Investigators were blinded to mouse genotypes and treatments, and percentages of positive cells were calculated using the total number of cells in each image (hematoxylin or DAPI nuclear staining). Data were analyzed with Graphpad Prism 5 software using a Student's t-test, and outliers were excluded using Grubbs’ test. Data are displayed as mean ± S.E.M.
Results
Previous studies from our laboratory have demonstrated that short-term pharmacologic (minocycline) inhibition or genetic ablation (ganciclovir treatment of CD11b-TK-expressing mice) of microglia at 3 months of age was sufficient to reduce tumor proliferation in Nf1+/-GFAPCKO mice.5,6 To determine whether microglia are also critical for tumor formation (gliomagenesis), we used Nf1+/-GFAPCKO-TK mice to reduce CD11b+ cells (microglia) following ganciclovir (GCV) treatment during glioma formation (6 weeks to 3 months of age; Supplementary Fig 1). While we found that long-term GCV treatment reduced optic glioma proliferation, interpretation was confounded due to the anti-proliferative properties of GCV in Nf1+/-GFAPCKO mice lacking the CD11b-TK transgene (data not shown). Thus, we sought an alternative method to target microglia.
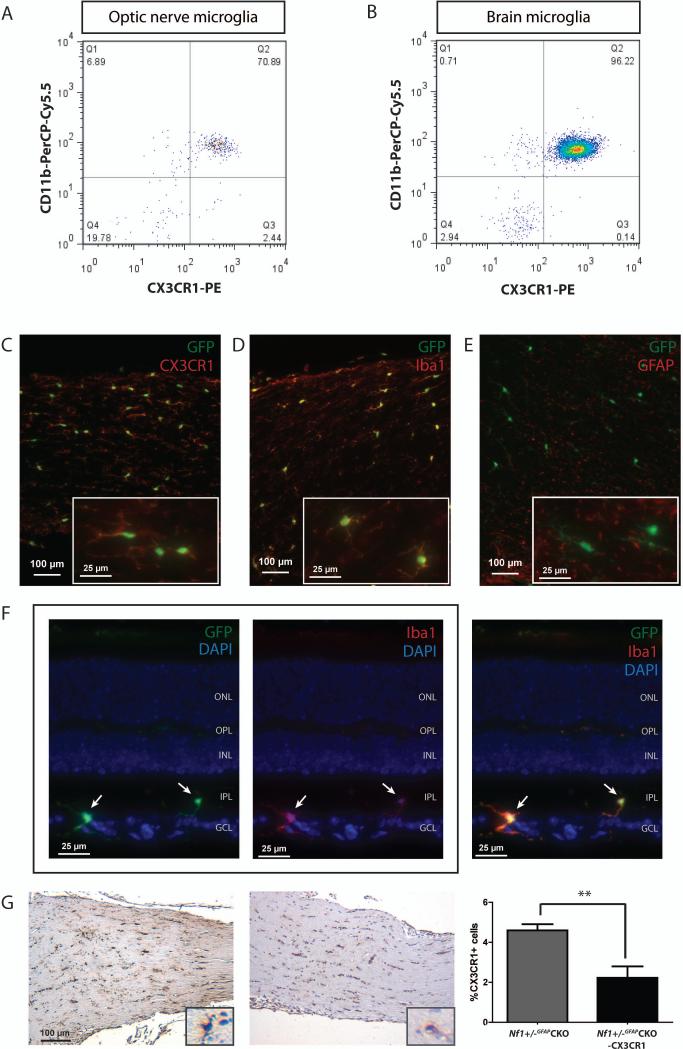
Microglia infiltration and function is partly driven by chemokines acting on their cognate receptors, including the CX3CL1 receptor, CX3CR1.8 Consistent with previous studies, CX3CL1 is found in abundant levels in the optic nerve and retina, both in wild-type and Nf1+/-GFAPCKO mice (Supplementary Fig 2A). Using FACS, CD45low microglia in the mouse optic nerve and brainstem co-express CX3CR1 and CD11b (Fig 1A and 1B). Employing a mouse strain in which one copy of the Cx3cr1 gene is inactivated following germline insertion of a green fluorescent protein (GFP) gene,8 double-labeling immunofluorescence reveals that all microglia identified with Iba1 or CX3CR1 antibodies in the optic nerves of Cx3cr1+/gfp mice also co-express GFP (Fig 1C-D, Supplementary Fig 2B-D), and that GFAP+ cells (astrocytes) and retinal ganglion cells lack GFP expression (Fig 1E-1F, Supplementary Fig 2E). Importantly, no reduction in optic nerve proliferation or microglia (%Iba1+ cells) was observed following targeted reduction of CX3CR1 expression in 3-month-old wild-type or Nf1+/- mice (Supplementary Fig 3A and 3B).
FIGURE 1.
CX3CR1 is expressed by microglia in the optic nerve. Flow cytometry demonstrates that nearly 100% of monocytes in wild-type (A) optic nerves (7-10 pooled optic nerves) or (B) brainstem are double-positive for both CD11b and CX3CR1 expression. Immunofluorescence analysis of paraformaldehyde-fixed tissue cryosections using CX3CR1 (C, red) or Iba1 antibodies (D, red) and endogenous GFP (green) shows that GFP is expressed by microglia in Cx3cr1+/gfp mouse optic nerves, but not by GFAP-expressing cells (E, red). Insets show representative labeled cells. (F) GFP (green) is also only expressed in microglia (Iba1+ cells, red) in the Nf1+/-GFAPCKO-CX3CR1 mouse retina (outer nuclear layer, ONL; outer plexiform layer, OPL; inner nuclear layer, INL; inner plexiform layer, IPL; ganglion cell layer, GCL). The two left panels show GFP or Iba1 immunofluorescence in paraformaldehyde-fixed tissue cryosections with DAPI (blue) nuclear staining, while the right panel shows the merged triple channel fluorescence. The white arrows denote GFP+ and Iba1+ double-labeled cells. (G) CX3CR1 immunohistochemistry reveals a 52% reduction in the percent of CX3CR1+ cells in the Nf1+/-GFAPCKO-CX3CR1 mouse optic nerve at 3 months of age (n=9, p = 0.0012). **, p<0.01.
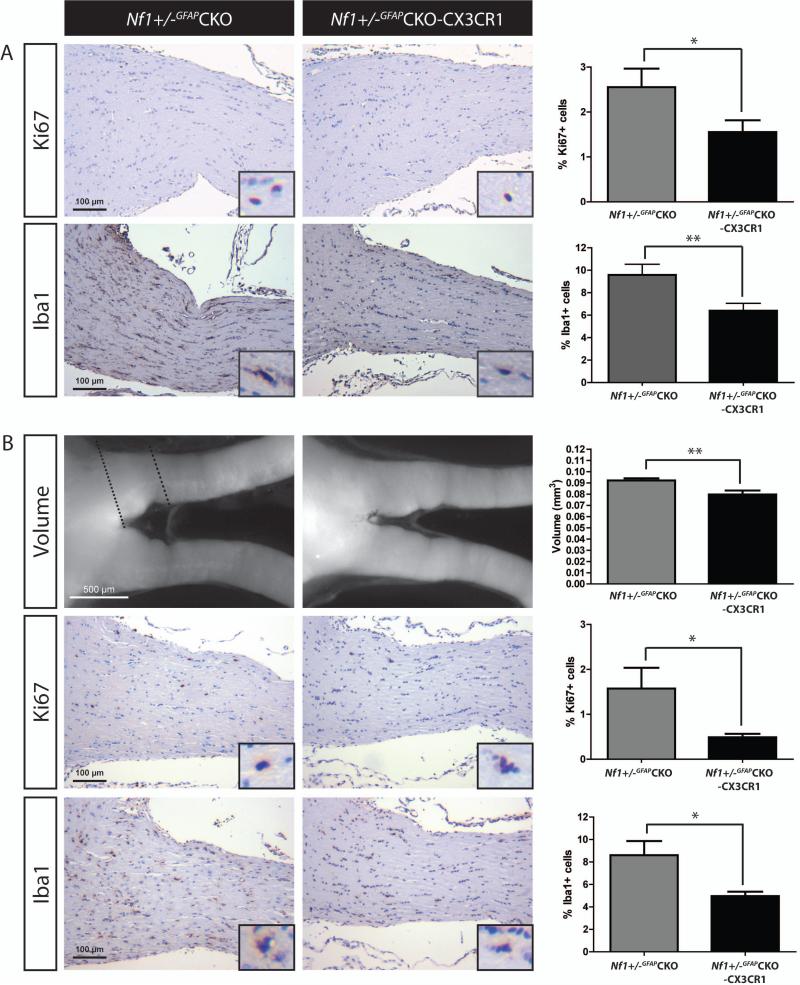
Next, we generated Nf1+/-GFAPCKO mice bearing one inactive Cx3cr1 allele (Nf1+/-GFAPCKO-CX3CR1 mice). Using a specific CX3CR1 antibody, Nf1+/-GFAPCKO-CX3CR1 optic nerves exhibited a 52% decrease in the percent of CX3CR1+ cells relative to Nf1+/-GFAPCKO mice (Fig 1G). To determine whether reduced CX3CR1 expression impaired microglia-mediated optic glioma formation, Nf1+/-GFAPCKO-CX3CR1 mice were examined at 6 weeks, 3 months, and 4 months of age. At 6 weeks of age (prior to obvious glioma development), the increased percent of microglia present in the Nf1+/-GFAPCKO mouse optic nerve 5 was reduced in Nf1+/-GFAPCKO-CX3CR1 mice concomitant with decreased optic nerve proliferation (%Ki67+ cells, n=9 mice; Fig 2A). Similarly, at 3 months of age, Nf1+/-GFAPCKO-CX3CR1 mice (n=10 mice) also had reduced optic nerve volumes, proliferation, and microglia (Fig 2B, Supplementary Fig 4) relative to Nf1+/-GFAPCKO mice. Importantly, unlike Nf1+/-GFAPCKO mice, there was no evidence of optic glioma formation in Nf1+/-GFAPCKO-CX3CR1 mice, demonstrating that CX3CR1+ microglia are critical for optic gliomagenesis.
FIGURE 2.
Targeted reduction of CX3CR1 expression reduces microglia content and optic glioma proliferation. (A) Optic nerves from Nf1+/-GFAPCKO-CX3CR1 mice (n=9) exhibit a 39% reduction in the percent of Ki67+ cells relative to Nf1+/-GFAPCKO (n=9) at 6 weeks of age (p = 0.0463) as well as a 33% reduction in the percent of Iba1+ cells (p = 0.0086). (B) 3-month-old Nf1+/-GFAPCKO-CX3CR1 mice (n=10) have smaller optic nerve volumes relative to Nf1+/-GFAPCKO mice (n=10, 13% reduction, p = 0.0039) and exhibit a 65% reduction in proliferation (%Ki67+ cells; p = 0.0358) as well as a 42% reduction in the percentage of Iba1+ microglia (p = 0.0114), relative to Nf1+/-GFAPCKO mice. Dashed lines delineate the representative area of the optic nerves used to calculate volume. Insets show representative positively-labeled cells. *, p<0.05; **, p<0.01.
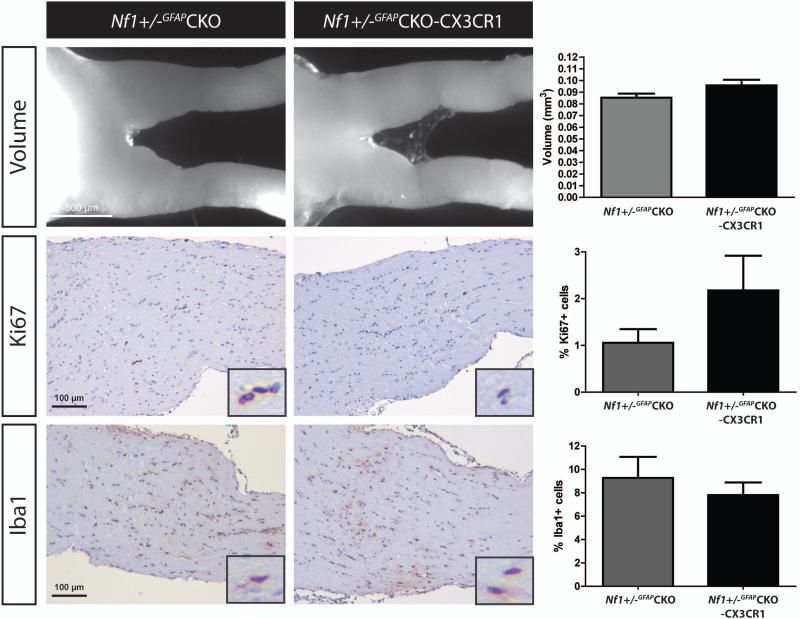
Since optic gliomas are found in the majority of Nf1+/-GFAPCKO mice by 3 months of age, 2 we sought to determine whether gliomagenesis was delayed, rather than prevented, by reduced CX3CR1 expression. Examination of Nf1+/-GFAPCKO-CX3CR1 mice (n=6 mice) at 4 months of age revealed optic nerve volumes, proliferation and microglia content indistinguishable from Nf1+/-GFAPCKO mice (Fig 3). In contrast, retinal ganglion cell death (TUNEL+ cells) in Nf1+/-GFAPCKO-CX3CR1 mice was not significantly different from Nf1+/-GFAPCKO mice at any age (Supplementary Fig 5A-C). Collectively, these observations establish microglia as essential drivers of gliomagenesis, such that reducing CX3CR1+ microglia function delays Nf1 murine optic glioma formation.
FIGURE 3.
Nf1 optic glioma formation is delayed by targeted reduction of CX3CR1 expression. At 4 months of age, Nf1+/-GFAPCKO-CX3CR1 mice (n=6) have optic nerves with similar volumes, proliferation (%Ki67+ cells), and microglia (%Iba1+ cells) as Nf1+/-GFAPCKO mice. Insets depict representative positively-labeled cells.
Discussion
Accumulating evidence from other solid tumors has revealed the importance of non-neoplastic cells in the tumor microenvironment for the maintenance of cancer growth.9 In brain cancer (glioma), microglia represent one of the main stromal cells.4 Consistent with previous immunohistochemistry and FACS studies demonstrating monocyte/microglia infiltration using Iba1, CD11b, CD163, CX3CR1, CD45, and CD68 antibodies, Nf1 GEM optic gliomas exhibit increased numbers of Iba1+, CD11b+ and CX3CR1+ cells with low CD45 expression. Based on these findings, we utilized a genetic targeting strategy to impair microglia function (reduced CX3CR1 expression) and delay the formation of Nf1 optic gliomas. These new observations have broad implications for glioma biology.
First, microglia are emerging as key stromal cells important for dictating both glioma formation and maintenance. We have previously shown that genetic or pharmacologic inhibition of microglia function reduces tumor proliferation, supporting a role for microglia in glioma maintenance. Similarly, others have reported that microglia ablation impairs glioma growth by limiting apoptosis and metalloproteinase-mediated tumor invasion.10,11 In addition, our new results using Nf1+/-GFAPCKO-CX3CR1 mice, coupled with earlier reports demonstrating that targeted disruption of IL-6 expression in a GFAP-v-src glioma model prevents glioma formation12 and monocyte chemoattractant protein-1-induced monocyte infiltration increases transplanted glioma growth, 13 argue that microglia also serve as essential mediators of glioma formation.
Second, CX3CL1 is one of the most abundantly expressed chemokines in the nervous system and regulates communication between neurons, glia, and microglia through activation of the CX3CR1 receptor. CX3CR1 function mediates pathology in many GEM models of neurological disease. In this regard, CX3CR1 loss in Alzheimer Disease (AD) mouse models attenuates neuronal loss 14 and beta-amyloid deposition through increased microglial phagocytosis.15 Additionally, reduced CX3CR1 expression results in smaller infarct volumes following brain ischemia.16 The observation that CX3CR1 reduction alone in Nf1 GEM delayed optic glioma formation establishes that CX3CL1/CX3CR1 axis function is also critical for microglia function relevant to brain tumor biology. While one report showed that high-grade glioma growth following implantation was not affected by host CX3CR1 loss,17 single nucleotide polymorphisms within the human CX3CR1 locus are associated with increased survival of individuals with high-grade glioblastoma and decreased microglial infiltration.18 CX3CR1 variants have been found to contribute to defective adhesive function, fractalkine binding, signaling 19, and impaired migration. 20 Taken together, these findings support a model in which chemokine-regulated microglia function represents a prime determinant of glioma biology.
As we enter an era of rational therapeutics, these data provide a scientific rationale for the design of future treatments that consider stromal cell (microglia) and stroma-derived factor (chemokines) targets. The combination of neoplastic and non-neoplastic cell therapies offers the potential of more durable outcomes with reduced toxicity to the normal cells within the developing brains of children.
Supplementary Material
Acknowledgments
The authors thank Dr. Daniel Littman (New York University) and Jean-Pierre Julien (Laval University) for providing the Cx3cr1+/gfp and CD11b-TK mice, respectively, and Dr Toshio Imai (Kan Research Institute, Kyoto) for the generous gift of the CX3CR1 antibody. We appreciate access to the Alvin J. Siteman Comprehensive Cancer Center High-Speed Cell Sorter Core facility.
Financial support: This work was supported by grants from the National Cancer Institute (U01-CA141549-01 and R01-CA136573 to DHG), the National Eye Institute (Vision Core Grant; EY02687), and the W.M. Keck Foundation (Postdoctoral Fellowship in Molecular Medicine to WWP).
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
References
- 1.Listernick R, Ferner RE, Liu GT, Gutmann DH. Optic pathway gliomas in neurofibromatosis-1: controversies and recommendations. Ann Neurol. 2007;61(3):189–98. doi: 10.1002/ana.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bajenaru ML, Hernandez MR, Perry A, et al. Optic nerve glioma in mice requires astrocyte Nf1 gene inactivation and Nf1 brain heterozygosity. Cancer Res. 2003;63(24):8573–7. [PubMed] [Google Scholar]
- 3.Zhu Y, Ghosh P, Charnay P, et al. Neurofibromas in NF1: Schwann cell origin and role of tumor environment. Science. 2002;296(5569):920–2. doi: 10.1126/science.1068452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watters JJ, Schartner JM, Badie B. Microglia function in brain tumors. J Neurosci Res. 2005;81(3):447–55. doi: 10.1002/jnr.20485. [DOI] [PubMed] [Google Scholar]
- 5.Daginakatte GC, Gutmann DH. Neurofibromatosis-1 (Nf1) heterozygous brain microglia elaborate paracrine factors that promote Nf1-deficient astrocyte and glioma growth. Hum Mol Genet. 2007;16(9):1098–112. doi: 10.1093/hmg/ddm059. [DOI] [PubMed] [Google Scholar]
- 6.Simmons GW, Pong WW, Emnett RJ, et al. Neurofibromatosis-1 heterozygosity increases microglia in a spatially and temporally restricted pattern relevant to mouse optic glioma formation and growth. J Neuropathol Exp Neurol. 2011;70(1):51–62. doi: 10.1097/NEN.0b013e3182032d37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hegedus B, Banerjee D, Yeh T-H, et al. Preclinical cancer therapy in a mouse model of neurofibromatosis-1 optic glioma. Cancer Res. 2008;68(5):1520–8. doi: 10.1158/0008-5472.CAN-07-5916. [DOI] [PubMed] [Google Scholar]
- 8.Jung S, Aliberti J, Graemmel P, et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20(11):4106–14. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21(3):309–22. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 10.Markovic DS, Vinnakota K, Chirasani S, et al. Gliomas induce and exploit microglial MT1-MMP expression for tumor expansion. Proc Natl Acad Sci U S A. 2009;106(30):12530–5. doi: 10.1073/pnas.0804273106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhai H, Heppner FL, Tsirka SE. Microglia/macrophages promote glioma progression. Glia. 2011;59(3):472–85. doi: 10.1002/glia.21117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weissenberger J, Loeffler S, Kappeler A, et al. IL-6 is required for glioma development in a mouse model. Oncogene. 2004;23(19):3308–16. doi: 10.1038/sj.onc.1207455. [DOI] [PubMed] [Google Scholar]
- 13.Platten M, Kretz A, Naumann U, et al. Monocyte chemoattractant protein-1 increases microglial infiltration and aggressiveness of gliomas. Ann Neurol. 2003;54(3):388–92. doi: 10.1002/ana.10679. [DOI] [PubMed] [Google Scholar]
- 14.Fuhrmann M, Bittner T, Jung CKE, et al. Microglial Cx3cr1 knockout prevents neuron loss in a mouse model of Alzheimer's disease. Nat Neurosci. 2010;13(4):411–3. doi: 10.1038/nn.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Z, Condello C, Schain A, et al. CX3CR1 in microglia regulates brain amyloid deposition through selective protofibrillar amyloid-β phagocytosis. J Neurosci. 2010;30(50):17091–101. doi: 10.1523/JNEUROSCI.4403-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dénes A, Ferenczi S, Halász J, et al. Role of CX3CR1 (fractalkine receptor) in brain damage and inflammation induced by focal cerebral ischemia in mouse. J Cereb Blood Flow Metab. 2008;28(10):1707–21. doi: 10.1038/jcbfm.2008.64. [DOI] [PubMed] [Google Scholar]
- 17.Liu C, Luo D, Streit WJ, Harrison JK. CX3CL1 and CX3CR1 in the GL261 murine model of glioma: CX3CR1 deficiency does not impact tumor growth or infiltration of microglia and lymphocytes. J Neuroimmunol. 2008;198(1-2):98–105. doi: 10.1016/j.jneuroim.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodero M, Marie Y, Coudert M, et al. Polymorphism in the microglial cell-mobilizing CX3CR1 gene is associated with survival in patients with glioblastoma. J Clin Oncol. 2008;26(36):5957–64. doi: 10.1200/JCO.2008.17.2833. [DOI] [PubMed] [Google Scholar]
- 19.McDermott DH, Fong AM, Yang Q, et al. Chemokine receptor mutant CX3CR1-M280 has impaired adhesive function and correlates with protection from cardiovascular disease in humans. J Clin Invest. 2003;111(8):1241–50. doi: 10.1172/JCI16790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Combadière C, Feumi C, Raoul W, et al. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J Clin Invest. 2007;117(10):2920–8. doi: 10.1172/JCI31692. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.