Abstract
Objective:
This study examined the efficacy of a commercially available, portion-controlled diet (PCD) on body weight and HbA1c over 6 months in obese patients with type 2 diabetes.
Research Design and Methods:
One-hundred participants with a mean±s.d. age of 55.6±10.6 year, body weight of 102.9±18.4 kg and HbA1c of 7.7±1.3% were randomly assigned to a 9-session group lifestyle intervention that included a PCD or to a 9-session group program of diabetes self-management education (DSME). Participants in the two groups were prescribed the same goals for energy intake (1250–1550 kcal per day) and physical activity (200 min per week).
Results:
While both groups produced significant improvements in weight and HbA1c after 6 months of treatment, PCD participants lost 7.3 kg [95% confidence interval (CI): −5.8 to −8.8 kg], compared with 2.2 kg (95% CI: −0.7 to −3.7 kg) in the DSME group (P<0.0001). Significantly more PCD than DSME participants lost ⩾5% of initial weight (54.0% vs 14.0%, P<0.0001) and ⩾10% (26.0% vs 6.0%, P<0.0001). HbA1c declined by 0.7% (95% CI: −0.4 to −1.0%) in the PCD group, compared with 0.4% (95% CI: −0.1 to −0.7%) in DSME (P<0.026). Across both groups, larger weight losses were associated with greater reductions in HbA1c (r=0.52, P<0.0001).
Conclusions:
These findings demonstrate that a commercially available portion-controlled meal plan can induce clinically meaningful improvements in weight and glycemic control in obese individuals with type 2 diabetes. These data have implications for the management of obesity in primary care, as now provided by the Centers for Medicare and Medicaid Services.
Keywords: portion control, diabetes, obesity, glycemic control, diabetes education
Introduction
A 5–10% reduction in initial weight dramatically decreases the risk of developing type 2 diabetes in overweight persons with impaired glucose tolerance1, 2 and improves glycemic control in individuals who already have diabetes.3, 4, 5 Comprehensive programs of lifestyle modification reliably produce these improvements in weight (and glycemic control), but such interventions are often very intensive and limited to academic medical centers.6, 7, 8 Less intensive yet structured weight-loss programs, which incorporate lifestyle modification and portion-controlled meal replacement products potentially offer an important treatment option for overweight individuals with diabetes, as suggested by a recent study.9 Individuals who were provided weekly group lifestyle modification classes for 3 months and a diet of portion-controlled foods lost 7.1% of initial weight and reduced their hemoglobin A1c (HbA1c) by 0.88%. Control participants, who received three standard diabetes education classes, achieved significantly smaller reductions of 0.4% in initial weight and 0.03% in HbA1c.9
The present 6-month randomized trial extends the prior study by standardizing the duration and intensity of the group treatment to isolate the effects of the portion-controlled diet (PCD) on the observed improvements in weight and glycemic control. To this end, overweight individuals with type 2 diabetes in each of the two treatment groups were prescribed the same calorie intake and physical activity goals and received the same number of group treatment sessions. Holding these variables constant across the two groups allowed for a clearer assessment of the effects of the PCD. We also elected in the present study to decrease the intensity (frequency) of the lifestyle intervention, a change that potentially could increase the dissemination of this approach.10
Materials and Methods
Participants
Participants were a total of 100 men and women who were recruited and treated at two medical centers in Philadelphia. The two sites followed identical protocols and met regularly to review study implementation. Participants were recruited from newspaper advertisements, flyers and physician referrals. Inclusion criteria included a body mass index of 25−50 kg m−2, age of 21−75 year, and a screening HbA1c ⩾6.5% and <12.0%. Use of all classes of diabetes medications was permitted (including insulin), provided they had been prescribed at stable doses for ⩾3 months (or ⩾6 months in the case of incretin mimetics and pramlintide), as was the case for medications for other conditions (for example, hypertension, dyslipidemia). Exclusion criteria included significant cardiovascular, pulmonary, hepatic, renal or gastrointestinal disease; uncontrolled hypertension (⩾160/100 mm Hg); history of alcohol or drug abuse; significant psychiatric conditions thought to impair the candidate's safe or successful study participation; use of psychiatric medications (except for selective serotonin reuptake inhibitors); pregnancy or lactation; bariatric surgery; use of weight-loss inducing medications or supplements in the prior 3 months; or weight loss ⩾5 kg in prior 6 months. All applicants completed a history and physical examination, performed by their own primary care provider (PCP), who forwarded findings to the study physicians. (If the applicant's PCP was unavailable, the examination was completed by one of the study's nurse practitioners or physicians.) The study physicians provided final approval for applicants to participate.
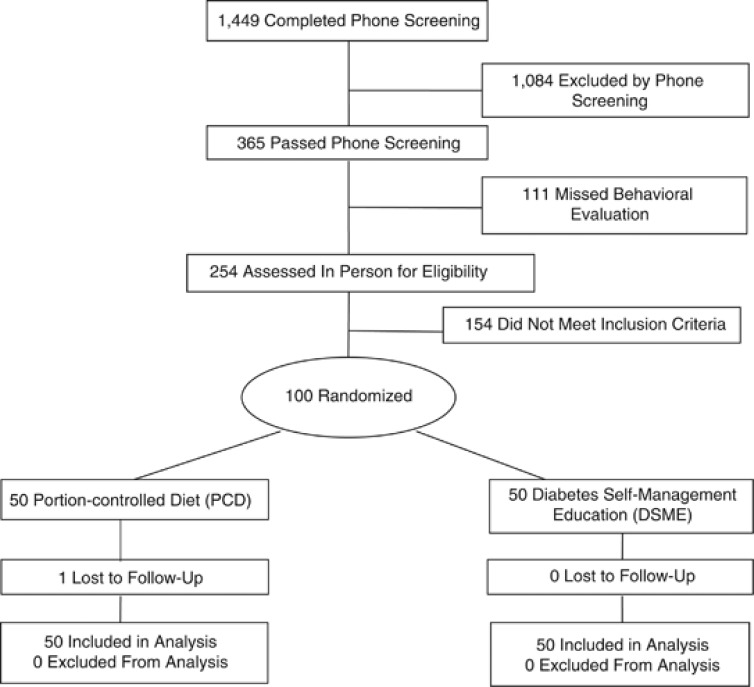
All participants gave written informed consent to take part in the study, which was approved by the Institutional Review Boards at Temple University and the University of Pennsylvania. The first participant was randomized on 7 July 2010, and final outcome measures were obtained on 26 July 2011. Figure 1 shows the flow of participants through the study.
Figure 1.
The flow of participants through the study.
Procedures
Participants were randomly assigned within site (stratified for insulin use), via a random-number generator, to: (1) a lifestyle intervention that included a prepackaged, PCD; or (2) a program of diabetes self-management education (DSME). The study statistician generated the random allocation sequence, and research coordinators enrolled participants and randomly assigned them to treatment conditions. The study's primary outcome was change in weight at 6 months. The principal secondary outcome was change in HbA1c at 6 months.
Interventions
Participants were randomized to one of two treatment conditions: Lifestyle intervention, PCD program or a DSME program.
Common elements
Participants in both treatment conditions attended group sessions (at weeks 0, 1, 2, 4, 8, 12, 16, 20 and 24) which lasted 90 min, included 8–12 persons, and were led by experienced practitioners (that is, lifestyle interventionists or certified diabetes educators, as appropriate). Participants in both treatment conditions were instructed to consume ∼1250 kcal per day (women) or 1550 kcal per day (men) and were provided a calorie-counting guide11 to facilitate this goal. Participants were instructed to gradually increase their walking (or other aerobic activity) to ⩾200 minutes per week.
All participants were instructed to monitor and record their blood glucose at least twice daily for 1 week before beginning their intervention. They were provided with a glucometer (OneTouch, LifeScan, Inc., Milpitas, CA, USA) and test strips for this purpose. Participants continued to monitor their blood glucose at least twice daily throughout the study, and records were reviewed by study staff at each session. If participants reported repetitive episodes of hyper- (three or more episodes ⩾300 mg dl−1) or hypoglycemia (three or more episodes ⩽60 mg dl−1), they received counseling regarding appropriate lifestyle strategies and medication adherence. Hypo- and hyperglycemia also prompted a referral back to PCP for medication adjustment. All changes in diabetic medications were managed by the participants' providers.
Participants in both treatment conditions met with a study physician at week 16 to review any changes in their health, including in blood pressure and serum chemistries (for example, triglycerides and total cholesterol) that were observed at the 3-month study assessment. Interventions differed from each other as described below.
Lifestyle intervention, PCD program
Half of the participants (N=50) were assigned to a lifestyle intervention that included the use of a prepackaged, PCD (Nutrisystem D, Fort Washington, PA, USA). Lifestyle intervention topics covered at group sessions included self-monitoring of food intake and physical activity, stimulus control, goal setting, problem solving, cognitive restructuring and relapse prevention.6, 7 This curriculum closely resembles that of the lifestyle modification guide included with the PCD program. Sessions began with a review of participants' progress from the prior meeting, followed by the introduction of a new topic in weight management.
Participants who received the PCD were instructed to supplement the prepackaged foods (which typically provided three entrées and one snack daily) with conventional foods (for example, fruits, vegetables, dairy items and lean protein), in accordance with the PCD program. Women's and men's meal plans provided ∼1250 and 1550 kcal per day, respectively, with ∼55% of total energy from the packaged foods and 45% from supplemental grocery items. The plans were structured to provide ∼50–55% of energy from carbohydrate, 20–25% from fat and 20–25% from protein, and to contain ⩽2300 mg per day of sodium. The glycemic index of the diet (including both the packaged and conventional foods) was ∼34 (on the glucose scale), which falls in the low range.12, 13
DSME program
The remaining participants (N=50) received a multi-faceted diabetes education program, which addressed topics that included goal setting, nutrition, weight loss, blood glucose monitoring, physical activity, prevention and management of hyperglycemia and hypoglycemia, sick day management, prevention of complications, medication management and barriers, and coping with diabetes. The program was based on the American Diabetes Association's National Standards for DSME.14 At the initial session each participant selected a specific goal. Sessions, led by a certified diabetes educator, were conducted in a format similar to the PCD group, beginning with a review of the prior session's readings and homework and followed by the introduction of a new topic. The DSME was intended to model structured diabetes self-management training programs provided by certified diabetes educators in medical settings.
Participants were instructed to consume a balanced deficit diet consistent with recommendations of the Food Guide Pyramid15 and the American Diabetes Association.16 The suggested macronutrient content was 15–25% of calories from protein, <30% from fat (<7% from saturated fat) and the remainder from carbohydrate, with 30 g per day of fiber. Meal plans were provided to help participants meet their nutrition and calorie goals.
Outcomes
The following outcomes were assessed at baseline and 3 and 6 months.
Weight and height
Weight was measured on a calibrated electronic scale (Detecto 758C Digital weight indicator, Webb City, MO, USA), with participants dressed in light clothing, without shoes. Height was measured at baseline using a wall-mounted stadiometer (Holtain Limited Harpenden Stadiometer, Crymych Dyfed, UK). Body mass index was calculated as weight (kg) divided by height (m)2.
Waist circumference
Waist circumference was measured in centimeters using a standard tape measure (Gulick II, Country Technology, Gays Mills, WI, USA). Participants remained standing while the tape was placed around the abdomen horizontally at the midpoint between the highest point of the iliac crest and the lowest part of the costal margin. Waist circumference was measured three times, and the average of the three readings was used.
Blood pressure
Blood pressure was assessed using an automated instrument (Dinamap ProCare 200, GE Medical Systems, Milwaukee, WI, USA) with cuff sizes based on measured arm circumference. After sitting quietly for 5 min, two readings were taken, separated by a 1-minute rest period. The average of the two readings was used.
Serum chemistries
HbA1c, glucose, hs-CRP and lipid values were measured from samples obtained after participants fasted overnight (12 h). Samples, other than HbA1c, were centrifuged to separate serum and were shipped overnight to a commercial lab where the assays were performed (Quest Diagnostics, Horsham, PA, USA). (Details of each assay are described at http://www.questdiagnostics.com/hcp/qtim/testMenuSearch.do.) HbA1c was assayed at Temple University using high-performance liquid chromatography.
Diabetes medication regimen
At baseline, participants reported all medications that they took for diabetes. The name, dosage, frequency, and start date of each drug was recorded. Throughout the trial, changes in diabetes medication use were recorded in the study medical record. At 3 and 6 months, a reduction in the intensity of the diabetes medication regimen was operationalized as taking fewer medications or a lower dosage, compared with baseline. By contrast, an increase was defined as the addition of new medication or a higher dosage, compared with the baseline regimen. When a participant had changes in both directions (for example, discontinued one medication and started another), the net change in treatment intensity was adjudicated by a study physician who was masked to the patient's identity or treatment condition.
Statistical analyses
Differences between the groups on baseline characteristics were assessed using independent samples t-tests for continuous variables and chi square χ2-tests for categorical variables. The primary outcome was the change in body weight (kg) at month 6, as determined using a linear mixed-effects model with time, treatment and a time by treatment interaction included as explanatory variables. This model posited an unrestricted structure on the variance-covariance matrix of the residuals on all 100 participants. All continuous secondary outcomes were analyzed in the same manner as body weight. Categorical secondary outcomes (for example, changes in medication regimen intensity, achievement of clinically meaningful targets for weight loss and HbA1c) were analyzed using χ2-tests or Fisher's Exact Test. Alpha was set at P<0.05 for the comparison of the two treatment conditions on the primary outcome (weight change), as well as on all secondary outcomes. All statistical analyses were performed with SAS 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Participants' baseline characteristics
Study participants were 59 women and 41 men with a mean±s.d. age of 55.6±10.6 years, weight of 102.9±18.4 kg, body mass index of 35.8±5.3 kg m−2, and HbA1c of 7.7±1.3%. The sample included 59 African Americans, 36 Caucasians and 3 Asian Americans, as ascertained by self-report. The two treatment conditions did not differ significantly on any baseline characteristics (as shown in Table 1). As shown in Figure 1, 49 of 50 participants in PCD completed the 6-month outcome, as did 50 of 50 in DSME.
Table 1. Baseline characteristics of participants.
| Measures | PCD | DSME | All |
|---|---|---|---|
| N | 50 | 50 | 100 |
| Gender, no. of participants (%) | |||
| Male | 21 (42.0) | 20 (40.0) | 41 (41.0) |
| Female | 29 (58.0) | 30 (60.0) | 59 (59.0) |
| Race, no. of participants (%) | |||
| White | 16 (32.0) | 20 (40.0) | 36 (36.0) |
| African-American | 32 (64.0) | 27 (54.0) | 59 (59.0) |
| Asian | 2 (4.0) | 1 (2.0) | 3 (3.0) |
| Hispanic/Latino | 1 (2.0) | 1 (2.0) | 2 (2.0) |
| Other/more than on race | 0 (0.0) | 2 (4.0) | 2 (2.0) |
| Age (year) | 55.5±10.3 | 55.7±11.0 | 55.6±10.6 |
| Body mass index (kg m−2) | 35.3±4.6 | 36.2±5.8 | 35.8±5.3 |
| Weight (kg) | 101.8±16.7 | 104.0±20.1 | 102.9±18.4 |
| Waist circumference (cm) | 115.9±12.1 | 118.4±14.8 | 117.1±13.5 |
| Systolic blood pressure (mm Hg) | 128.2±16.6 | 125.7±17.3 | 126.9±16.9 |
| Diastolic blood pressure (mm Hg) | 74.4±9.5 | 75.4±10.4 | 74.9±9.9 |
| Hemoglobin A1C (%) | 7.6±1.3 | 7.9±1.3 | 7.7 ±1.3 |
| Glucose (mg dl−1) | 144.2±44.9 | 161.5±59.9 | 152.9±53.4 |
| Total cholesterol (mg dl−1) | 172.3±39.8 | 173.1±38.0 | 172.7±38.8 |
| Triglycerides (mg dl−1) | 136.5±60.7 | 141.0±78.5 | 138.8±69.9 |
| High-density lipoprotein (mg dl−1) | 49.9±16.8 | 51.8±15.3 | 50.8±16.0 |
| Low-density lipoprotein (mg dl−1) | 96.0±36.4 | 94.3a±30.5 | 95.1±33.5 |
| hs-CRP (mg l−1) | 5.6a±7.4 | 5.8±6.0 | 5.7±6.7 |
Abbreviations: All, both groups combined; DSME, diabetes self-management education program; hs-CRP, high-sensitivity CRP; PCD, portion-controlled diet.
Data are means±or frequency (%). There were no significant differences between the two groups.
N=49.
Weight loss
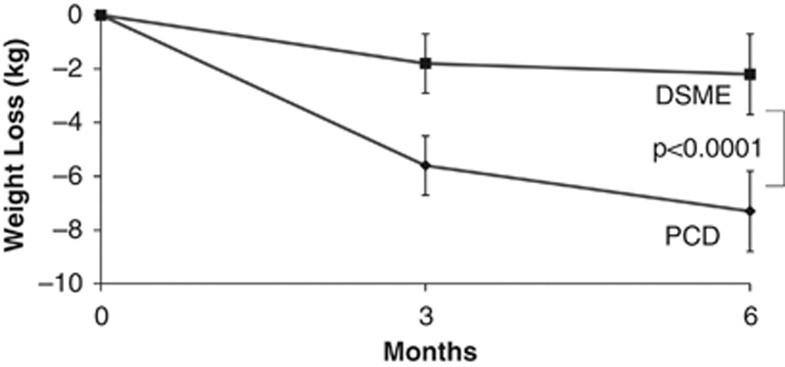
At month 6, PCD participants lost 7.3 kg (95% confidence interval (CI): −5.8 to −8.8 kg), compared with a significantly (P<0.0001) smaller 2.2 kg loss (95% CI: −0.7 to −3.7 kg) for DSME (see Table 2 and Figure 2). These losses corresponded to reductions in initial weight of 7.8% and 2.1% for PCD and DSME, respectively. Figure 2 shows that directionally similar differences were observed between the groups at month 3 (−5.6 vs −1.8 kg). A significantly greater percentage of participants in the PCD than DSME groups lost ⩾5% of initial weight at month 6 (54.0% vs 14.0%, P<0.0001), as well as ⩾10% (26.0% vs 6.0%, P<0.0001) (note: the percentage of participants who lost ⩾5% includes those who lost ⩾10%).
Table 2. Change in primary and secondary outcomes at month 6.
| Measure | PCD | DSME | P-value |
|---|---|---|---|
| N, baseline (6 months) | 50 (49) | 50 (50) | |
| Weight (kg) | |||
| Baseline | 101.8±16.7 | 104.0±20.1 | |
| 6 Months | 93.9±14.7 | 101.8±19.4 | |
| Adjusted change | −7.3 (−8.8 to −5.8) | −2.2 (−3.7 to −0.7) | <0.0001 |
| Body mass index (kg m−2) | |||
| Baseline | 35.3±4.6 | 36.2±5.8 | |
| 6 Months | 32.6±4.2 | 35.5±5.8 | |
| Adjusted change | −2.5 (−3.0 to −2.0) | −0.7 (−1.2 to −0.2) | <0.0001 |
| Waist circumference (cm) | |||
| Baseline | 115.9±12.1 | 118.4±14.8 | |
| 6 Months | 108.9±11.1 | 115.5±14.9 | |
| Adjusted change | −6.5 (−7.8 to −5.3) | −2.9 (−4.1 to −1.7) | <0.0001 |
| Hemoglobin A1C(%) | |||
| Baseline | 7.6±1.3 | 7.9±1.3 | |
| 6 Months | 6.9±1.2 | 7.5±1.3 | |
| Adjusted change | −0.7 (−1.0 to −0.4) | −0.4 (−0.7 to −0.1) | 0.021 |
| Fasting glucose (mg dl−1) | |||
| Baseline | 144.2±44.9 | 161.5±59.9 | |
| 6 Months | 128.0±47.9 | 148.7±52.3 | |
| Adjusted change | −16.5 (−33.0 to +0.1) | −12.8 (−29.3 to +3.7) | 0.217a |
| Systolic blood pressure (mm Hg) | |||
| Baseline | 128.2±16.6 | 125.7±17.3 | |
| 6 Months | 121.4±16.2 | 123.8±13.8 | |
| Adjusted change | −6.6 (−11.0 to −2.2) | −1.9 (−6.2 to +2.5) | 0.044a |
| Diastolic blood pressure (mm Hg) | |||
| Baseline | 74.4±9.5 | 75.4±10.4 | |
| 6 Months | 72.4±11.2 | 74.1±9.8 | |
| Adjusted change | −2.2 (−4.6 to +3.0) | −1.2 (−3.6 to +1.2) | 0.359 |
| Triglycerides (mg dl−1) | |||
| Baseline | 136.5±60.7 | 141.0±78.5 | |
| 6 Months | 109.2±64.6 | 120.7±54.6 | |
| Adjusted change | −26.3 (−42.4 to −10.3) | −20.3 (−36.3 to −4.3) | 0.343a |
| Total cholesterol (mg dl−1) | |||
| Baseline | 172.3±39.8 | 173.1±38.0 | |
| 6 Months | 162.5±42.2 | 165.3±29.6 | |
| Adjusted change | −8.6 (−15.9 to −1.2) | −7.8 (−15.1 to −0.5) | 0.808 |
| High-density lipoprotein (mg dl−1) | |||
| Baseline | 49.9±16.8 | 51.8±15.3 | |
| 6 Months | 50.1±14.8 | 50.2±13.8 | |
| Adjusted change | −0.1 (−1.9 to +1.7) | −1.6 (−3.4 to +0.2) | 0.113 |
| Low-density lipoprotein (mg dl−1) | |||
| Baseline | 96.0±36.4 | 94.3±30.5b | |
| 6 Months | 90.3±36.1 | 93.0±26.6 | |
| Adjusted change | −4.3 (−11.0 to +2.4) | −0.8 (−7.6 to +5.9) | 0.320 |
| hs-CRP (mg l−1) | |||
| Baseline | 5.6±7.4b | 5.8±6.0 | |
| 6 Months | 4.4±5.8 | 4.7±4.8 | |
| Adjusted change | −1.0 (−2.5 to +0.5) | −1.2 (−2.6 to 0.3) | 0.883 |
Abbreviations: DSME, diabetes self-management education program; hs-CRP, high-sensitivity CRP; PCD, portion-controlled diet
Indicates that P-values were obtained from a linear mixed-effects model on the log-transformed outcome, which were similar in direction and significance to analyses performed on the raw outcome.
Data were obtained for only 49 of 50 participants.
Unadjusted baseline and 6-month data are reported as means±s.d.
Adjusted change is reported as means (95% confidence intervals) and was obtained from linear mixed-effects models with time, treatment, and a time by treatment interaction included as explanatory variables.
Figure 2.
The figure shows weight loss (in kg) in participants assigned to the PCD and DSME conditions.
Change in HbA1c
At month 6, HbA1c declined by 0.7% (95% CI: −0.4 to −1.0%) in PCD participants, compared with a significantly (P<0.026) smaller 0.4% decline (95% CI: −0.1 to −0.7%) in DSME participants (see Table 2). Similar reductions were observed in the two groups at month 3 (−0.8% vs −0.4%). At month 6, a significantly (P<0.0046) greater percentage of participants in the PCD than DSME groups (72.0 vs 44.0%) met the American Diabetes Association goal of tight blood sugar control (that is, HbA1c<7.0%).17 Corresponding values at baseline had been 38.0% and 36.0%, respectively.
Change in use of diabetes medications
At baseline, 94.0% of participants in both the PCD and DSME groups were taking an oral agent for their diabetes, the most common of which was metformin, used by 82% and 86% of participants in the two groups, respectively. Insulin was used by 18% and 22% of participants, respectively, and non-insulin injectables by 8% and 0% of individuals, respectively. At month 6, 28.0% of PCD participants, compared with 4.0% of DSME, had a reduction in the intensity of their diabetes medication regimen (P=0.0034). By contrast, 6.0% of PCD vs 12.0% of DSME participants had an intensification in their regimen.
Additional secondary outcomes
As shown in Table 2, participants in both conditions achieved significant reductions from baseline in body mass index, waist circumference, triglycerides and total cholesterol, with PCD participants achieving significantly greater reductions than DSME participants in waist circumference (P<0.0001) and in systolic blood pressure (P=0.044) at month 6. The former group generally achieved more favorable changes than the latter on additional measures of cardiovascular disease risk. However, differences between the groups were not statistically significant.
Relationship between weight change and HbA1cchange
The two treatment conditions were combined (N=100) to examine the relationship between changes in weight and HbA1c at month 6. Partial correlation analysis, which controlled for the effect of treatment condition, revealed that the more weight participants lost (kg), the greater their reduction in HbA1c (r=0.52, P<0.0001).
Adverse events
Two participants, both in the PCD group, experienced serious adverse events. The first had urinary retention and hematuria (at week 18). The second reported (at week 20) that he had experienced a myocardial infarction between his screening and baseline visits. (This information was withheld from study staff until week 20.) This individual also was hospitalized for atrial fibrillation at week 25 (1 week after the study ended; reported to study staff at week 28). None of the serious adverse events was determined to be related to study treatment.
Discussion
This study's principal finding was that a lifestyle intervention that incorporated a PCD of prepackaged foods produced a 7.3 kg weight loss in 6 months that was associated with a clinically significant reduction in HbA1c of 0.7% and with reductions in the use of diabetes medications. Improvements on all three measures were superior to those produced by a robust control intervention of DSME that also was associated with significant reductions in weight and HbA1c. A meta-analysis of diabetes self-management programs18 found a 0.73% decrease in HbA1c. It is important to note, however, that only 4 of the 29 studies had baseline HbA1c comparable to our study (that is,<8). In addition, DSME is typically applied in either newly diagnosed patients or to those experiencing complications. Thus, the higher initial HbA1c values in that meta-analysis likely led to greater reductions than were observed in our DSME group.
The low glycemic index (GI) of the PCD may have added to the effect of weight loss on HbA1c. A review of relatively small and short studies found significantly greater reductions in glycated proteins with lower-GI diets than higher-GI alternatives.19 More recently, Fabricatore et al.13 found a significantly greater reduction in HbA1c with a low-glycemic load diet (also low in GI) compared with an isoenergetic low-fat diet, despite equivalent weight loss, in a sample of obese adults with type 2 diabetes. Although we do not know whether or to what extent dietary GI differed between the two groups in the present study, the very low GI—34—of the PCD makes it reasonable to assume that the diet consumed in the DSME group was considerably higher in GI.
This study demonstrates the benefits of prescribing a low-calorie diet that incorporates prepared portion-controlled foods. At month 6, participants who were provided the PCD plan lost 5.1 kg more than individuals who were instructed to consume an equivalent-calorie diet of self-selected foods; both dietary interventions were consistent with recommendations of the American Diabetes Association.16 The present findings confirm that low-calorie portion-controlled foods, whether provided as liquid shakes and meal bars,20, 21 or as prepared servings of conventional foods,22 induce significantly greater weight losses than recommendations to consume an equivalent-calorie diet comprised entirely of self-selected foods. This finding has been observed in overweight and obese individuals with22, 23, 24 and without20, 25, 26 type 2 diabetes. PCDs help those trying to manage their weights meet their calorie goals by providing pre-measured amounts of food with a known energy intake.21, 27 By contrast, overweight and obese individuals typically underestimate their energy intake by 40% or more when consuming a self-selected diet of conventional foods.28
Participants in the PCD group achieved a 7 kg weight loss and clinically significant metabolic improvements with only nine group treatment sessions over 24 weeks. In most lifestyle modification trials, investigators typically have prescribed weekly counseling sessions for the first 16 to 26 weeks6, 7 or, as in the case of the Diabetes Prevention Program, 16 sessions over 26 weeks.1 The inclusion of a PCD would appear to allow participants to achieve clinically meaningful weight loss in fewer sessions than required if a traditional low-calorie diet is used. Fewer treatment sessions could be more convenient to patients, as well as reduce the cost of achieving clinically significant weight loss. The Centers for Medicare and Medicaid Services, for example, have agreed to reimburse the cost of lifestyle counseling for weight control.29 Patients will be provided 14 to 15 visits with a PCP over 6 months, with the goal of losing at least 3 kg. Inclusion of a PCD with lifestyle counseling would appear likely to increase the magnitude of the weight loss and, with it, improvements in health. A more rapid rate of weight loss also could possibly reduce the number of PCP visits required, thus reducing costs. The weight reduction achieved in the DSME group (2.2 kg with 9 sessions over 6 months) suggests the potential that diabetes educators who prescribed energy restriction through prepackaged meals would also induce a mean weight loss that exceeds the Centers for Medicare and Medicaid Service goal, if provided 14 sessions in which to deliver the intervention. Such an approach may be a suitable, lower-cost alternative to physician-delivered intensive behavioral counseling for obese individuals with type 2 diabetes.
Overweight and obese individuals typically are advised to lose 5–10% of their initial weight to improve health complications.3, 30 The present findings support this recommendation and demonstrate the benefits of achieving larger weight loss, ∼10% of initial weight rather than 5%. Collapsing across the treatment groups we observed a strong, linear relationship between reductions in weight and HbA1c. The use of the PCD in the present study significantly increased the number of participants who lost 10% or more of weight, with the accompanying benefit of the reduction in HbA1c.
In addition to its strengths, this study also had some limitations, the principal of which was the absence of a follow-up assessment. We anticipate that participants would require continued intervention, as provided in the Look AHEAD study,5, 31 to maintain their full improvements in weight and HbA1c. The study also was underpowered to assess clinically significant differences in secondary outcomes. Moreover, 81% and 63% of participants in the total sample took medications for hypertension and dyslipidemia, respectively (data not shown), which helps to explain the relatively low baseline values we observed for these cardiovascular disease risk factors. A third limitation was the lack of a usual care control group. Given that fewer than 60% of adults with diabetes reported ever having attended a diabetes self-management class,32 both study conditions offered a stronger behavioral intervention than what most patients with type 2 diabetes are likely to receive in practice. Finally, the lack of self-reported data on physical activity and diet precluded an assessment of adherence to the prescribed regimens.
Conclusion
In summary, this study found that a lifestyle intervention that included a PCD induced significantly greater weight loss and improvements in HbA1c in 6 months than did a comprehensive diabetes self-management and education program that provided instruction in weight management and control of diabetes complications. These findings suggest that traditional diabetes education programs, as offered by diabetes educators (as in the present study), could be strengthened by the addition of a PCD.
Acknowledgments
This trial (ClinicalTrials.gov identifier: NCT01260337) was supported by research grants from Nutrisystem, Inc. to Temple University and the University of Pennsylvania. This study was also supported by LifeScan, Inc.
Dr Foster is a consultant with Nutrisystem. Dr Wadden is a consultant with BMIQ. All other authors declare no conflict of interest.
References
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- National Heart, Lung, and Blood Institute Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. Obes Res. 1998;6 (Suppl 2:51S–209S. [PubMed] [Google Scholar]
- Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diab Care. 2011;34:1481–1486. doi: 10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing RR. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med. 2010;170:1566–1575. doi: 10.1001/archinternmed.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadden TA, Webb V, Moran C, Bailer B. Lifestyle modification for obesity: new developments in diet, physical activity, and behavior therapy. Circulation. 2012;125:1157–1170. doi: 10.1161/CIRCULATIONAHA.111.039453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster GD, Makris AP, Bailer BA. Behavioral treatment of obesity. Am J Clin Nutr. 2005;82 (1 Suppl:230S–235SS. doi: 10.1093/ajcn/82.1.230S. [DOI] [PubMed] [Google Scholar]
- Butryn ML, Webb V, Wadden TA. Behavioral treatment of obesity. Psychiatr Clin North Am. 2011;34:841–859. doi: 10.1016/j.psc.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster GD, Borradaile KE, Vander Veur SS, Leh Shantz K, Dilks RJ, Goldbacher EM, et al. The effects of a commercially available weight loss program among obese patients with type 2 diabetes: a randomized study. Postgrad Med. 2009;121:113–118. doi: 10.3810/pgm.2009.09.2046. [DOI] [PubMed] [Google Scholar]
- Ackermann RT, Finch EA, Brizendine E, Zhou H, Marrero DG. Translating the Diabetes Prevention Program into the community. The DEPLOY Pilot Study. Am J Prev Med. 2008;35:357–363. doi: 10.1016/j.amepre.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borushek A. The CalorieKing Calorie, Fat & Carbohydrate Counter. Family Health Publications: Costa Mesa, CA, USA; 2011. [Google Scholar]
- Jenkins DJ, Kendall CW, McKeown-Eyssen G, Josse RG, Silverberg J, Booth GL, et al. Effect of a low-glycemic index or a high-cereal fiber diet on type 2 diabetes: a randomized trial. JAMA. 2008;300:2742–2753. doi: 10.1001/jama.2008.808. [DOI] [PubMed] [Google Scholar]
- Fabricatore AN, Wadden TA, Ebbeling CB, Thomas JG, Stallings VA, Schwartz S, et al. Targeting dietary fat or glycemic load in the treatment of obesity and type 2 diabetes: a randomized controlled trial. Diabetes Res Clin Pract. 2011;92:37–45. doi: 10.1016/j.diabres.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funnell MM, Brown TL, Childs BP, Haas LB, Hosey GM, Jensen B, et al. National standards for diabetes self-management education. Diab Care. 2010;33 (Suppl 1:S89–S96. doi: 10.2337/dc10-S089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietary Guidelines for Americans, 2005 In U.S. Department of Health and Human Services (ed)U.S. Government Printing Office: Washington, DC, USA; 2005 [Google Scholar]
- Bantle JP, Wylie-Rosett J, Albright AL, Apovian CM, Clark NG, Franz MJ, et al. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diab Care. 2008;31 (Suppl 1:S61–S78. doi: 10.2337/dc08-S061. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. Tight Diabetes Control [cited 2013 March 4] Available from http://www.diabetes.org/living-with-diabetes/treatment-and-care/blood-glucose-control/tight-diabetes-control.html .
- Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM. Self-management education for adults with type 2 diabetes: a meta-analysis of the effect on glycemic control. Diab Care. 2002;25:1159–1171. doi: 10.2337/diacare.25.7.1159. [DOI] [PubMed] [Google Scholar]
- Thomas D, Elliott EJ.Low glycaemic index, or low glycaemic load, diets for diabetes mellitus Cochrane Database Syst Rev 2009: CD006296. [DOI] [PMC free article] [PubMed]
- Ditschuneit HH, Flechtner-Mors M, Johnson TD, Adler G. Metabolic and weight-loss effects of a long-term dietary intervention in obese patients. Am J Clin Nutr. 1999;69:198–204. doi: 10.1093/ajcn/69.2.198. [DOI] [PubMed] [Google Scholar]
- Heymsfield SB, Van Mierlo CAJ, Van der Knaap HCM, Heo M, Frier HI. Weight management using a meal replacement strategy: meta and pooling analysis from six studies. Int J Obes. 2003;27:537–549. doi: 10.1038/sj.ijo.0802258. [DOI] [PubMed] [Google Scholar]
- Metz JA, Stern JS, Kris-Etherton P, Reusser ME, Morris CD, Hatton DC, et al. A randomized trial of improved weight loss with a prepared meal plan in overweight and obese patients: impact on cardiovascular risk reduction. Arch Intern Med. 2000;160:2150–2158. doi: 10.1001/archinte.160.14.2150. [DOI] [PubMed] [Google Scholar]
- Cheskin LJ, Mitchell AM, Jhaveri AD, Mitola AH, Davis LM, Lewis RA, et al. Efficacy of meal replacements versus a standard food-based diet for weight loss in type 2 diabetes: a controlled clinical trial. Diabetes Educ. 2008;34:118–127. doi: 10.1177/0145721707312463. [DOI] [PubMed] [Google Scholar]
- Li Z, Hong K, Saltsman P, DeShields S, Bellman M, Thames G, et al. Long-term efficacy of soy-based meal replacements vs an individualized diet plan in obese type II DM patients: relative effects on weight loss, metabolic parameters, and C-reactive protein. Eur J Clin Nutr. 2005;59:411–418. doi: 10.1038/sj.ejcn.1602089. [DOI] [PubMed] [Google Scholar]
- Hannum SM, Carson LA, Evans EM, Petr EL, Wharton CM, Bui L, et al. Use of packaged entrees as part of a weight-loss diet in overweight men: an 8-week randomized clinicial trial. Diabetes Obes Metab. 2006;8:146–155. doi: 10.1111/j.1463-1326.2005.00493.x. [DOI] [PubMed] [Google Scholar]
- Davis LM, Coleman C, Kiel J, Rampolla J, Hutchisen T, Ford L, et al. Efficacy of a meal replacement diet plan compared to a food-based diet plan after a period of weight loss and weight maintenance: a randomized controlled trial. Nutr J. 2010;9:11. doi: 10.1186/1475-2891-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai AG, Wadden TA. The evolution of very-low-calorie diets: an update and meta-analysis. Obesity (Silver Spring) 2006;14:1283–1293. doi: 10.1038/oby.2006.146. [DOI] [PubMed] [Google Scholar]
- Lichtman SW, Pisarska K, Berman ER, Pestone M, Dowling H, Offenbacher E, et al. Discrepancy between self-reported and actual caloric intake and exercise in obese subjects. N Engl J Med. 1992;327:1893–1898. doi: 10.1056/NEJM199212313272701. [DOI] [PubMed] [Google Scholar]
- Jacques L, Jensen TS, Schafer J, MClain S, Chin J.Final Coverage Decision Memorandum for Intensive Behavioral Therapy for Obesity (CAG-00423N)In: Centers for Medicare and Medicaid Services (eds)2011
- World Health Organization Obesity: preventing and managing the global epidemic Report of a WHO consultation; World Health Organ Tech Rep Ser 2000894i-xii1–253. [PubMed] [Google Scholar]
- Wadden TA, West DS, Delahanty L, Jakicic J, Rejeski J, Williamson D, et al. The look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring) 2006;14:737–752. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Age-Adjusted Percentage of Adults Aged 18 Years or Older with Diagnosed Diabetes Ever Attending a Diabetes Self-Management Class, United States, 2000–20102012 [cited 2012 February 1] Available from http://www.cdc.gov/diabetes/statistics/preventive/fy_class.htm .