Abstract
Objective:
The aim of this study was to determine whether the quantity of fat is different across the central (that is, android, trunk) and peripheral (that is, arm, leg and gynoid) regions among young African-American (AA), Asian (AS), Hispanic (HI) and non-Hispanic White (NHW) men.
Subjects and Methods:
A cohort of 852 men (18–30 years; mean total body fat percent (TBF%)=18.8±7.9, range=3.7–45.4) were assessed for body composition in five body regions via dual-emission X-ray absorptiometry (DXA).
Results:
HI men (21.8±8.3) had higher TBF% than AA (17.0±10.0), NHW (17.9±7.2) and AS (18.9±8.0) groups (P-values <0.0001). AS had a lower BMI (23.9±3.4) than all other groups, and NHW (24.7±3.2) had a lower BMI than HI (25.7±3.9) and AA (26.5±4.7; P-values<0.0001). A linear mixed model (LMM) revealed a significant ethnicity by region fat% interaction (P<0.0001). HI men had a greater fat% than NHW for every region (adjusted means (%); android: 29.6 vs 23.3; arm: 13.3 vs 10.6; gynoid: 27.2 vs 23.8; leg: 21.2 vs 18.3; trunk: 25.5 vs 20.6) and a greater fat% than AA for every region except the arm. In addition, in the android and trunk regions, HI had a greater fat% than AS, and AS had a higher fat% than AA. Finally, the android fat% for AS was higher than that of NHW. When comparing the region fat% within ethnicities, the android region was greater than the gynoid region for AS and HI, but did not differ for AA and NHW, and the arm region had the least fat% in all ethnicities.
Conclusions:
Fat deposition and body fat patterning varies by ethnicity.
Keywords: ethnicity, DXA, android fat, body fat distribution, gynoid fat
Introduction
The specific pattern of fat distribution may be more important for an individual's health than their relative quantity of fat mass. Specifically, having excess fat in the central regions of the body (for example, trunk, android fat) is more strongly associated with deleterious health outcomes than indicators of total body fatness.1 For example, fat patterning in this area is a sex-independent risk factor for mortality, cardiovascular disease and insulin resistance.2, 3 More recently, it has been demonstrated that fat deposition in the gluteofemoral or gynoid area, particularly as it contrasts to android fat deposition, is associated with enhanced metabolic health.4, 5, 6 Given these associations, it is important to examine predictors of different distributions of fat mass.
One's ethnic and racial background is a key determinant of body fat patterning and is predictive of one's obesity status. Stults-Kolehmainen et al., for example, found that young women from African-American (AA), Hispanic (HI), non-Hispanic White (NHW) and Asian (AS) ethnic groups each distribute fat differently among five major body regions, and that total body fat percentage (TBF%) decreases in the following order: AA, HI, AS and NHW (the latter two being statistically equal).7 Among men, epidemiological studies report that obesity disproportionately affects the HI ethnicity, which is higher in TBF% than AA, NHW and AS.8, 9, 10, 11, 12 Several investigations have tried to ascertain whether these ethnicities also vary in measures of specific regions of body fat, with equivocal results. For instance, there are ethnic differences in measurements of central adiposity, with men of Chinese,13 Puerto Rican12 and Mexican14 descent having higher truncal fat% than NHW men. When examining total body visceral fat, AA men have lower measures of fat as compared with NHW8, 15 and HI16 even when this measure is corrected for TBF.17, 18 At the same time, AA have significantly higher measurements of subcutaneous adipose tissue than NHW, when adjusted for TBF% and TBF.17 Nevertheless, these same measurements for AA are significantly lower than those of NHW, when adjusted for BMI or sagittal diameter.17 There are likely differences between races/ethnicities in other trends and in male fat distribution. AA men, for example, have on average a greater subscapular–suprailiac skinfold ratio than NHW men, indicating greater upper body fat deposition.19 The methods employed in these studies along with other measurement techniques, such as CT scans, have varying degrees of both error and ability to assess fat in the abdomen.
Dual-emission X-ray absorptiometry (DXA) technology provides valid and reliable methodology to measure all fat in the viscera and subcutaneous areas.20 Indeed, as a reflection of the technology's overall accuracy, DXA fat measurements have been shown to outperform standard anthropometric measurements in their ability to predict risk factors for disease, such as elevated HDL and triglyceride levels, in obese populations.21 Standard software provided by the manufacturer calculates body fat patterning for not only the android and gynoid regions but also for the arm (arms and shoulders), trunk (neck, trunk and pelvis) and leg (legs and lateral hip area) regions.20, 22 Surprisingly, we are not aware of any study that has explored differences between ethnicities in fat deposition across all five body regions calculated by DXA in a sample of adult males. This technology was used to determine body composition in the current study.
The primary aim of the current investigation was to determine whether central obesity differs among men of various ethnicities (a region fat% by ethnicity interaction). We hypothesize that HI will have more android and trunk fat than other ethnicities and that NHW will have more fat in these central areas than AA. The second aim was to determine whether fat mass is distributed differently across the periphery: arm, leg and gynoid regions, among men of various ethnicities (a region fat% by ethnicity interaction). We hypothesize that there are ethnic differences in relative fat mass for each of these regions. The third aim was to determine how fat within ethnicities is distributed across the body (a region fat% by region interaction). Specifically, we hypothesize that the android region will have the greatest relative fat and the arm region will have the lowest relative fat compared to other regions for all ethnicities.
Materials and methods
Subjects
A cohort of 852 men was assessed for body composition from June 2003 to May 2008. Participants were eliminated if they described themselves as being of ‘other' ethnicity. Exclusion criteria also included: (a) rods/metal in body, (b) limbs outside of scanned image area or (c) inability to distinguish anatomical landmarks to determine the regional fat analysis. All subjects were recruited from physical activity courses (for example, weight training and aerobics), which met twice weekly at The University of Texas at Austin. No subjects were intercollegiate athletes. Study personnel did not incentivize participation in the study with extra credit or other compensation. An e-mail was sent to all students enrolled in these courses inviting them to participate, which was followed by a short in-class presentation. Individuals participating were ethnically representative of the student population: AA (7.9% in the study vs 4.4% in the general student population), AS (23.7 vs 15.1%), HI (17.7 vs 15.9%) and NHW (50.7 vs 54.5%). These participants filled out a health questionnaire and were free from chronic conditions and recent illness. All individuals were under 30 years of age (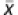 =20.9±2.0 years). Ethnicity was determined from self-selection by the individual from a list of five ethnic groups. The Institutional Review Board of the university approved the study and a written informed consent was obtained from each individual.
=20.9±2.0 years). Ethnicity was determined from self-selection by the individual from a list of five ethnic groups. The Institutional Review Board of the university approved the study and a written informed consent was obtained from each individual.
Protocol
Height and weight
Stature was measured to the nearest 0.1 cm on a stadiometer (Perspective Enterprises, Portage, MI, USA). Participants were instructed to stand with their back to the stadiometer and assume a neutral standing position with flat feet and good posture. Body mass was measured to the nearest 0.25 pound on an upright physician's scale (Health-O-Meter, Model 402KL, Bedford Heights, OH, USA). Subjects wore light exercise clothing and removed footwear for all testing.
Dual-emission X-ray absorptiometry
Body composition was determined from DXA technology using a Lunar Prodigy (G. E. Medical Systems, Madison, WI, USA). All selected data were analyzed with enCORE software (version 11.0; G. E. Medical Systems, Madison, WI, USA). Subjects removed all metal jewelry, plastic and rubber materials that could affect the X-ray beam. Quality control and bone mineral calibration were performed using a spine phantom made of calcium hydroxyapatite and embedded in a lucite block. Scans of the phantom spine occurred every other day according to the manufacturer's guidelines. The bone mineral density values obtained from calibration were stable over the entire study period (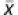 =0.993 g cm−2, coefficient of variation=0.09%).
=0.993 g cm−2, coefficient of variation=0.09%).
DXA regional fat analysis
Prodigy enCORE software automatically demarcates the regional boundaries. A protocol was established to manually refine these demarcations. Eleven well-trained technicians independently adjusted the demarcations according to a standardized checklist. Inter-tester reliability of regional fat quantification in a group of 216 women from this cohort and 41 women from a separate group was excellent (intra-class correlations >0.99).7, 20
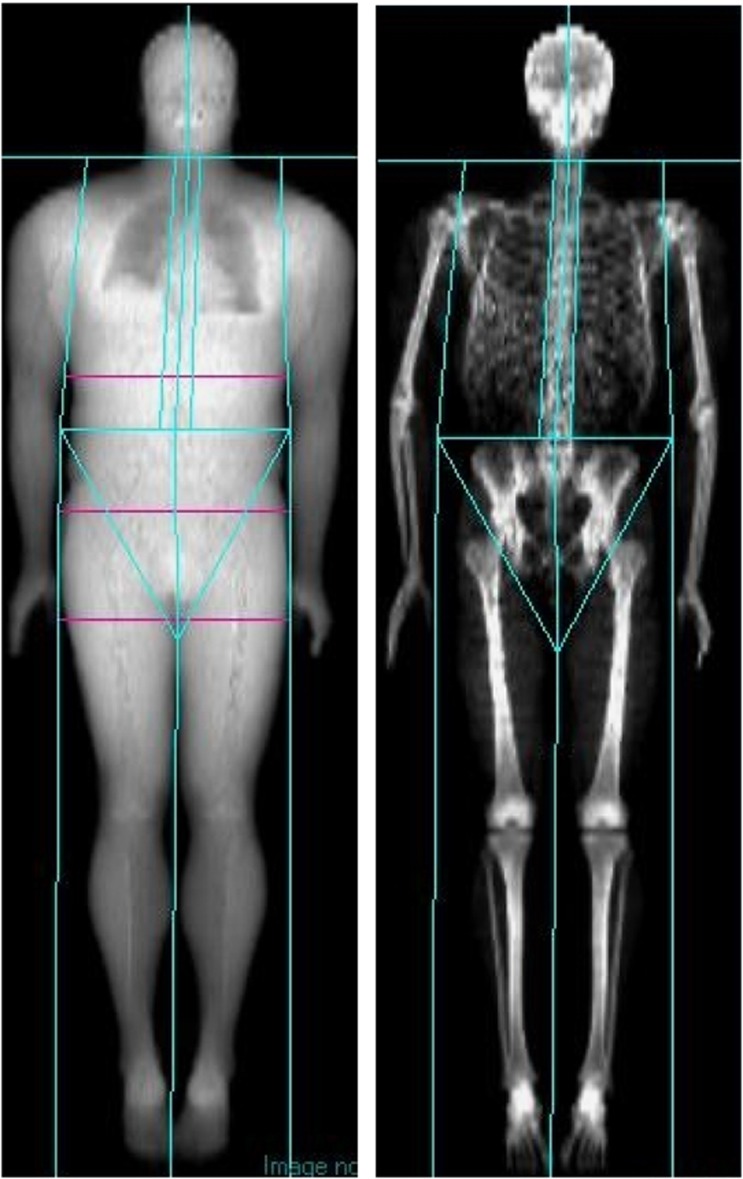
The arm region (Figure 1) is comprised of the arm and shoulder area formed by placing a line from the crease of the axilla and through the glenohumeral joint. The trunk region includes the neck, chest, abdominal and pelvic areas. Its upper perimeter is the inferior edge of the chin and the lower borders intersect the middle of the femoral necks without touching the brim of the pelvis. The leg region includes all of the area below the lines that form the lower borders of the trunk. The android region is the area between the ribs and the pelvis, and is totally enclosed by the trunk region. The upper demarcation is 20% of the distance between the iliac crest and the neck. The lower demarcation is at the top of the pelvis. The gynoid region includes the hips and upper thighs, and overlaps both the leg and trunk regions. The upper demarcation is below the top of the iliac crest at a distance of 1.5 times the android height. The total height of the gynoid region is two times the height of the android region. More detail concerning the analysis of regional body composition has been described in previous papers.22
Figure 1.
Sample DXA scan showing demarcations between body regions generated by enCORE software.
Statistical analysis
Percent fat for each region was calculated in the following manner: fat%=(fat mass/(fat mass+lean soft mass+bone mineral content)) × 100. These masses, determined by DXA, were specific to each region. Subject characteristics, such as height, total body mass and TBF%, were analyzed using separate one-way analysis of variance and Tukey post hoc tests when necessary (SAS 9.2, Cary, NC, USA). A restricted maximum-likelihood linear mixed model (LMM) regression analysis with a compound symmetric heterogeneous variance–covariance matrix structure was performed to determine whether ethnicities differed in fat mass distribution and in the percentage of fat in arm, leg, trunk, android and gynoid regions. Analysis was conducted with the PROC MIXED procedure (SAS 9.2). This analysis was selected because participants had multiple measurements (one for each region) and because regional fat percentages were highly correlated (r-values >0.87). This statistical analysis is less challenged by muticollinearity between highly related body composition variables. The outcome variable of interest was region fat%. The independent variables were ethnicity and region. Ethnicity was dummy coded with NHW as the reference group. Region was also dummy coded with android as the reference group. After examining Q–Q plots and residual plots for each region fat%, it was determined that data were both normal and of equal variance, thus, meeting basic analysis assumptions.
LMM was used to determine the main effect of ethnicity and region on region fat%, as well as a region by ethnicity interaction. Nonsignificant interactions were then eliminated to produce a final LMM model. To determine significance of pairwise comparisons, region was fixed to determine ethnic differences in region fat%. Finally, ethnicity was fixed to determine region differences in region fat%. Regression coefficients were tested using a t-value generated for each comparison. The empirical cutoff value designating significance for all tests was set to P<0.01.
Results
Reliability measures for regional body composition measures were obtained from a separate sample of men. Measures collected from eight subjects scanned three times in a single day exhibited coefficient of variation values of 0.43, 0.35, 0.49, 0.54, 0.30 and 0.42 for android, gynoid, arm, leg, trunk and TBF mass, respectively. These are better than values observed for similar investigations, which report coefficient of variation values ranging from 1.0 to 6.0%.6, 7, 20, 23, 24 Intra-class correlations for these repeated measurements were >0.97 for all regions. The R2 between measured (scale) and DXA total body mass was >0.99.
Descriptive statistics for each ethnicity are contained in Table 1. The mean TBF% (18.8±7.9, range=3.7–45.4) indicated sufficient variability for the aims. All subject characteristics, except for age (P>0.196), significantly differed by ethnicity. AA and NHW were significantly taller than HI and AS (P-values<0.0001, except between AA and HI, P<0.01), but there was no difference between AA and NHW (P>0.05), and HI and AS (P>0.01). For total body mass, all ethnicities were greater than AS (P-values <0.0001) and AA were greater than HI and NHW (P-values <0.01). For TBF%, all other ethnicities were less than HI (P-values<0.0001, except in the case of AS, P<0.001); otherwise, there were no significant differences between ethnicities (P-values>0.05). For BMI, AA (P<0.0001) and HI (P<0.01) were both greater than NHW, who were in turn greater than AS (P<0.01). Additionally, AS were less than both AA and HI (P-values<0.0001), and there was no difference between AA and HI (P>0.1). AS and HI men had less lean soft mass and bone mineral content than AA (P-values<0.0001) and NHW (P-values<0.01) men.
Table 1. Subject characteristics by ethnicity.
| African-American (n=67) | Asian (n=202) | Hispanic (n=151) | NHW (n=432) | All ethnicities (n=852) | |
|---|---|---|---|---|---|
| Age (year) | 21.3±2.7 | 21.2±2.2 | 21.2±2.0 | 21.4±2.2 | 21.32±2.2 |
| Height (cm) | 177.5±5.9a,b | 173.0±8.1c | 174.4±5.8c | 179.1±6.5 | 176.7±7.3 |
| Total body mass-DXA (kg) | 83.4±14.8a,b,d | 71.4±10.4c,e | 78.4±12.6 | 79.2±11.4 | 77.5±12.2 |
| Total body fat% | 17.0±10.0e | 18.9±8.0f | 21.8±8.3c | 17.9±7.2 | 18.8±7.9 |
| Body mass index (kg·m−2) | 26.5±4.7a,c | 23.9±3.4d,e | 25.7±3.9d | 24.7±3.2 | 24.8±3.6 |
| Fat mass (kg) | 15.4±11.5 | 14.0±7.6e | 17.8±9.1c | 14.6±7.6 | 15.1±8.3 |
| Lean soft mass (kg) | 64.4±7.2a,e,g | 54.3±5.9c,e | 57.4±6.5c | 61.2±7.1 | 59.2±7.4 |
| Bone mineral content (kg) | 3.6±0.5a,c,e | 3.0±0.4c,f | 3.2±0.5d | 3.3±0.5 | 3.2±0.5 |
Abbreviations: DXA, dual-emission X-ray absorptiometry; NHW = non-Hispanic White. Values are mean±s.d.
Different from Asian, P < 0.0001.
Different from Hispanic, P < 0.01.
Different from NHW, P < 0.0001.
Different from NHW, P < 0.01.
Different from Hispanic, P < 0.0001.
Different from Hispanic, P < 0.001.
Different from NHW, P <0.001.
All terms in the LMM model were significant. There were significant main effects of region (df=4/3356, f=1807.2, P<0.0001) and ethnicity on region fat% (df=3/840, f=10.97, P<0.0001). There was also a significant ethnicity by region interaction (df=12/3356, f=14.43, P<0.0001). Because these findings may be confounded by indicators of total body adiposity, BMI and total fat mass were additionally added to the model as covariates to determine whether the associations were attenuated. In this new model, the significant main effects of region (df=4/3356, f=1837.07, P<0.0001), ethnicity (df=3/840, f=33.91, P<0.0001) and the ethnicity by region interaction were still observed (df=12/3356, f=14.65, P<0.0001).
Table 2 shows the region by ethnicity comparisons with both observed data and data adjusted for ethnicity. HI had a greater fat% than NHW for every region (adjusted means (%); android: 29.6 vs 23.3; arm: 13.3 vs 10.6; gynoid: 27.2 vs 23.8; leg: 21.2 vs 18.3; trunk: 25.5 vs 20.6) and a greater fat% than AA for every region except the arm (AA; android: 21.3; gynoid: 21.7; leg: 17.9; trunk: 19.0). In addition, in the android and trunk regions, HI had a greater fat% than AS (android: 26.2, trunk: 22.2), and AS had a higher fat% than AA. Finally, the android fat% for AS was higher than for NHW. AA and NHW were not significantly different in fat% for any region.
Table 2. Observed (non-adjusted) and adjusted estimates for fat% of the arm, leg, trunk, android and gynoid regions.
| AA | Asian | HI | NHW | All ethnicities | |
|---|---|---|---|---|---|
| Arm% | 10.2±7.7 | 11.8±6.5 | 13.3±6.8 | 10.6±6.1 | 11.3±6.5 |
| Arm adjusted% | 10.1±1.1 | 11.8±0.6 | 13.3±0.7a | 10.6±0.4 | 11.4±0.37 |
| Leg% | 18.1±10.0 | 18.8±7.6 | 21.2±7.7 | 18.3±7.0 | 18.9±7.6 |
| Leg adjusted% | 17.9±1.1b | 18.9±0.6 | 21.2±0.7c | 18.3±0.4 | 19.1±0.37 |
| Trunk% | 19.1±11.4 | 22.2±9.7 | 25.5±9.9 | 20.6±8.4 | 21.7±9.4 |
| Trunk adjusted% | 19.0±1.1b,d | 22.2±0.6b | 25.5±0.7e | 20.6±0.4 | 21.8±0.37 |
| Android% | 21.5±13.7 | 26.2±11.4 | 29.6±11.6 | 23.3±10.2 | 25.0±11.3 |
| Android adjusted% | 21.3±1.1f,h | 26.2±0.6e,g | 29.6±0.7e | 23.3±0.4 | 25.1±0.37 |
| Gynoid% | 21.8±10.7 | 24.8±8.3 | 27.2±8.4 | 23.8±7.8 | 24.4±8.4 |
| Gynoid adjusted% | 21.7±1.1h | 24.8±0.6 | 27.2±0. 7e | 23.8±0.4 | 24.4±0.37 |
Abbreviations: AA, African-American; HI, Hispanic; NHW, non-Hispanic White.
Observed (non-adjusted) data are mean±s.d. Adjusted means are fitted for ethnicity and region. Adjusted fat% values are mean±s.e.
Values are mean±s.d.
Different from NHW, P<0.01.
Different from HI, P<0.01.
Different from NHW, P<0.001.
Different from AS, P<0.01.
Different from NHW, P<0.0001.
Different from AS, P<0.0001.
Different from HI, P<0.001.
Different from HI, P<0.0001.
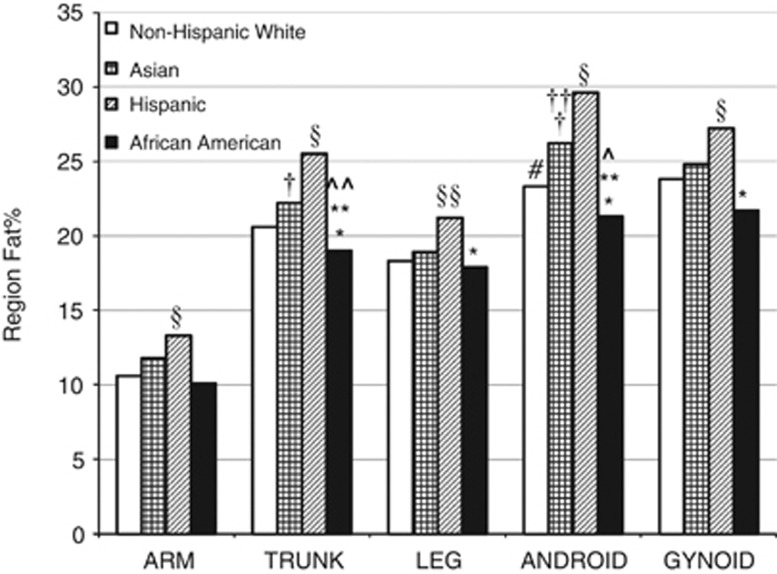
When comparing the region fat% within ethnicities, the android region was greater than the gynoid region for AS and HI (P-values<0.0001), but did not differ for AA (P>0.5) and NHW (P=0.035). Among AA, the leg region did not differ from the trunk region (P=0.039). In AS and HI ethnicities, all regions were significantly different from each other and differed in the following order from highest to lowest: android>gynoid>trunk>leg>arm (all P-values<0.0001). The arm region had the least fat% for all ethnicities (range for adjusted values,10.6–13.3% all P-values<0.0001). See Figure 2.
Figure 2.
Observed means for four ethnicities/races on region fat%. Each region is different from other regions within ethnicities (P<0.0001) except as indicated by symbols. Regions differ between ethnicities (P<0.01) as marked by symbols. *Difference between AA and HI for leg, trunk, android and gynoid regions. **Difference between AA and AS for trunk and android regions. †Difference between AS and HI for trunk and android regions. ††Difference between AS and NHW for android region. §Difference between HI and NHW for arm, trunk, android, gynoid and trunk regions. §§Difference between HI and NHW for leg region (P<0.001). ^No difference between android and gynoid for AA. ^^No difference between trunk and leg for AA. #No difference between android and gynoid for NHW.
Discussion
The central purpose of this study was to determine whether differences exist among ethnic and racial groups of young men in central (that is, android and trunk) and peripheral (that is, arm, leg and gynoid) regional fat mass. As with a previous study in women, these data reveal that fat in each of these regions varies by ethnicity. A primary finding of this study was that HI have higher central fat% measures than AA, AS and NHW. AS had a higher fat% than AA for the android and trunk regions. AA and NHW were not significantly different in fat% for any region, but android fat% for AS was higher than for NHW. Among peripheral regions, HI had greater fat% measures than NHW in the arm, leg and gynoid, and greater fat% measures than AA in the gynoid. This study, paired with a similar analysis in women, is the first to examine ethnic differences for fat% in all five body regions assessed by DXA technology.
Our hypotheses were largely verified. Although it was hypothesized that HI men would have higher central adiposity than other ethnicities, it was discovered that this group, in fact, was greater than AS, AA and NHW men in nearly every region fat% and in total adiposity. The present data support findings that assert that HI have a higher level of whole body adiposity, lower fat-free mass and bone mineral content compared with NHW, even when controlling for numerous factors.8, 10, 12 HI men were 2.9% higher in total fat% and 6.3% higher in android fat% and had 3.8 kg less lean soft mass than NHW men. AS men were higher than NHW men for android but not trunk fat%, which highlights the equivocal nature of comparisons between these groups for central adiposity.7, 25 Our observation of differences in fat distribution among ethnicities may be explained by a variety of different anthropometric, metabolic and behavioral mechanisms. For instance, differences between ethnicities exist for bone mineral content, limb length, muscle density and many other factors.7, 8, 26, 27, 28
Contrary to the hypothesis that NHW men would be higher than AA men in measures of central adiposity, there were surprisingly no group differences in fat% between these ethnicities for any region. Comparisons between these groups are most prevalent in the literature, with numerous studies finding ethnic differences.7, 8, 15, 17, 18, 19 In a study similar to our own, Wu et al.11 found that AA had lower measures of absolute trunk fat than NHW, and also had less fat in this area than AS and HI. The same study reported that AA also had the lowest TBF% of the four ethnicities examined. Additional studies have concluded that AA men have lower measures of abdominal visceral fat than NHW and HI men, even when controlling for total adipose tissue.8, 15, 16, 17, 18 Furthermore, AA individuals have more bone mineral content than other ethnicities, a finding consistent with the present data.26 Less clear is the influence of ethnicity on fat-free mass. The current study found that AA and NHW men were higher in fat-free mass than AS and HI ethnicities, but did not differ from each other. However, a different pattern of results is evident for women. When specifically examining appendicular muscle and skeletal mass (from DXA), AA women are higher than NHW women. Furthermore, Stults-Kolehmainen et al.7 found that AA have a higher TBF% than NHW (37.1 vs 29.5%), as well as a higher fat% in the android region (42.7 vs 32.3%). This contradictory set of results may be explained by sociocultural factors, patterns of physical activity, diet or may simply be due to genetic factors, such as regulation of adipose tissue deposition,29, 30 differences in resting energy expenditure,31 leptin concentration and/or androgenic activity.15, 28, 32
Making direct comparisons between the extant literature and our results, however, is hampered by two sets of issues. First, we did not adjust data for covariates of central fat mass, as is commonly reported. And second, other ethnic comparisons for body composition have typically employed skinfolds or measures of central adiposity determined from MRI or computed tomography. An important question of interest regarding our data, then, is whether our measurements of android fat as determined by DXA are a proxy for more-established measures of visceral fat.33 This is important because DXA measures the total fat of the entire region of interest, both visceral and subcutaneous. Nonetheless, it has been observed that the correlation between visceral fat and android fat is relatively strong (R=0.78).20
Beyond comparison between ethnic groups, a second purpose of this study was to determine whether regions differed in fat% within ethnicities/races. As expected, the arm region had the lowest fat% among all ethnicities observed, a finding true among all women's groups as well.7 Our data demonstrated that the android region was greater than the gynoid region in fat% among AS and HI, even though the two regions did not differ among AA and NHW. Android fat% among AA and NHW ethnicities differed from TBF% to a lesser degree than AS and HI (4.5% and 5.4% difference vs 7.3% and 7.8%, respectively). Therefore, our findings show that AS and HI men distribute fat differently than AA and NHW; the key difference being that AS and HI tend to store more fat in the lower torso relative to the hips and upper thighs. Body distribution of fat is important because, as mentioned above, fat deposited more centrally—and particularly visceral or intra-abdominal fat—is related to a number of chronic health conditions, such as insulin resistance and cardiovascular disease.2, 3 If this association is correct, our data suggest that AA, AS and HI might be at a higher risk for abdominal-fat-related chronic conditions. However, some data suggest that abdominal fat has an ethnic-dependent association with these chronic conditions.34 Given these associations, estimates of central adiposity derived from indirect measures, such as waist circumference, skinfolds or TBF, should be ethnic specific.35 An additional need is ethnic-specific clinical cutoffs developed for measures of central adiposity beyond waist–hip circumference.36
Limitations to the current study exist. First, despite the fact that our sample was ethnically representative from the larger university population, it is possible that it was not representative for obesity status or adiposity distribution. Participants were self selected and only a study design including a random sample would resolve this issue. It should also be noted that our sample was composed of young men, whereas many studies have utilized a much larger age range.11 Age may interact with ethnicity to predict regional body fat distribution; however, there was not a sufficient age range in this cohort to definitively address this issue. Another problem centers on the self report of ethnicity and race, and the lack of precise criteria to classify individuals into ethnic groupings.28 The HI ethnicity, for instance, is based mainly on a common surname and culture, and those who associate with this group are often a mixture of indigenous and European descent.8, 32 Those of AS Indian descent vary in body composition from individuals of other AS ancestry, but unfortunately, this was not queried in the current study.35 Future work must observe this important distinction.
We also did not assess behavioral factors, such as chronic physical activity status, which is a factor some studies have controlled.11 Ethnic differences in physical activity patterns are known to exist;37 however, all of our subjects were active at least two times a week over the course of a semester and were not athletes. SES and cultural factors are also likely relevant32 as are the experience of psychological stress and poor coping behaviors, which are related to central fat distribution.29 From a statistical modeling perspective, it must be noted that although regional differences among these groups are significant, previous studies demonstrate that variation in regional fat varies largely within ethnic groups as opposed to between groups.38 No consensus exists on the most valid and reliable way to determine android fat from DXA data. Consequently, the anatomical specification of the android region varies throughout the literature, and direct comparisons with other studies are not always possible.20, 38, 39 Finally, we did not analyze fat content of the head, which in at least one study was more predictive of insulin sensitivity than other regional measures of body composition.40
Despite the aforementioned limitations, this investigation, alongside a paired study in women,7 represents a strong methodological advance in the literature on ethnic differences in body composition. To our knowledge, this is the first study in men to utilize DXA technology to complete analyses of fat mass for five regions. Furthermore, the final sample included in this study was >800 men who represented a wide range of leanness, from very lean to morbidly obese. Finally, this is also one of few studies to compare four major ethnic groups. Specifically, investigations incorporating both AA and AS groups have been uncommon. Indeed, most studies have limited ethnicity comparisons,27, 28 a subject selection biased by the use of convenience groups, a focus on one obesity status (for example, overweight individuals),26 or examine only non-exercisers.11 A continuing concern in these studies is that significant differences may be reflective of outlying participants in the extremes for quantity of fat mass.38
Overall, the results suggest a pattern of ethnic/race-related influence on regional fat deposition and relative distribution in a large sample of young men. HI men are highest in fat% for the whole body and the central regions of the body: android and trunk. This stands in contrast to a recent study which found that among women, the AA ethnicity has the greatest total and central adiposity. There were no differences, however, between HI and AS in fat% among all peripheral regions (that is, arm, leg and gynoid). Interestingly, there were no differences observed between AA and NHW men, which contrast many previous findings. Finally, the arm had the lowest fat% of any region among all ethnicities studied, and the android region had greater fat% relative to the gynoid region among AS and HI men, but not AA or NHW. Future research needs to determine whether ethnic differences in central body fat modulate risk for suboptimal health outcomes. If such is the case, ethnic-specific cutoffs should be developed to improve risk assessment and intervention.
The authors declare no conflict of interest.
References
- Shen W, Punyanitya M, Chen J, Gallagher D, Albu J, Pi-Sunyer X, et al. Waist circumference correlates with metabolic syndrome indicators better than percentage fat. Obesity. 2006;14:727–736. doi: 10.1038/oby.2006.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366:1640–1649. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- Lee CC, Glickman SG, Dengel DR, Brown MD, Supiano MA. Abdominal adiposity assessed by dual energy X-ray absorptiometry provides a sex-independent predictor of insulin sensitivity in older adults. J Gerontol Ser A-Biol Sci Med Sci. 2005;60:872–877. doi: 10.1093/gerona/60.7.872. [DOI] [PubMed] [Google Scholar]
- Folsom AR, Kushi LH, Anderson KE, Mink PJ, Olson JE, Hong CP, et al. Associations of general and abdominal obesity with multiple health outcomes in older women—the Iowa women's health study. Arch Intern Med. 2000;160:2117–2128. doi: 10.1001/archinte.160.14.2117. [DOI] [PubMed] [Google Scholar]
- Manolopoulos KN, Karpe F, Frayn KN. Gluteofemoral body fat as a determinant of metabolic health. Int J Obes. 2010;34:949–959. doi: 10.1038/ijo.2009.286. [DOI] [PubMed] [Google Scholar]
- Okura T, Nakata Y, Yamabuki K, Tanaka K. Regional body composition changes exhibit opposing effects on coronary heart disease risk factors. Arterioscler Thromb Vasc Biol. 2004;24:923–929. doi: 10.1161/01.ATV.0000125702.26272.f6. [DOI] [PubMed] [Google Scholar]
- Stults-Kolehmainen MA, Stanforth PR, Bartholomew JB. Fat in android, trunk, and peripheral regions varies by ethnicity and race in college aged women. Obesity. 2012;20:660–665. doi: 10.1038/oby.2011.300. [DOI] [PubMed] [Google Scholar]
- Malina RM.Variation in body composition associated with sex and ethnicityIn: Heymsfield SB, Lohman TG, Wang Z, Going SB (eds).Human Body Composition Human Kinetics: Champaign, IL; 2005. p271–298. [Google Scholar]
- Mott JW, Wang J, Thornton JC, Allison DB, Heymsfield SB, Pierson RN. Relation between body fat and age in 4 ethnic groups. Am J Clin Nutr. 1999;69:1007–1013. doi: 10.1093/ajcn/69.5.1007. [DOI] [PubMed] [Google Scholar]
- Wang J, Thornton JC, Burastero S, Shen J, Tanenbaum S, Heymsfield SB, et al. Comparisons for body mass index and body fat percent among Puerto Ricans, Blacks, Whites and Asians living in the New York City area. Obes Res. 1996;4:377–384. doi: 10.1002/j.1550-8528.1996.tb00245.x. [DOI] [PubMed] [Google Scholar]
- Wu CH, Heshka S, Wang J, Pierson RN, Heymsfield S, Laferrere B, et al. Truncal fat in relation to total body fat: influences of age, sex, ethnicity and fatness. Int J Obes. 2007;31:1384–1391. doi: 10.1038/sj.ijo.0803624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus MA, Wang J, Pi-Sunyer FX, Thornton JC, Kofopoulou I, Pierson RN. Effects of ethnicity, gender, obesity, and age on central fat distribution: comparison of dual x-ray absorptiometry measurements in White, Black, and Puerto Rican adults. Am J Hum Biol. 1998;10:361–369. doi: 10.1002/(SICI)1520-6300(1998)10:3<361::AID-AJHB11>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Wang D, Li YP, Lee SG, Wang L, Fan JH, Zhang G, et al. Ethnic differences in body composition and obesity related risk factors: study in Chinese and White males living in China. PLoS One. 2011;6:e19835. doi: 10.1371/journal.pone.0019835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleman-Mateo H, Lee SY, Javed F, Thornton J, Heymsfield SB, Pierson RN, et al. Elderly mexicans have less muscle and greater total and truncal fat compared to African-Americans and caucasians with the same BMI. J Nutr Health Aging. 2009;13:919–923. doi: 10.1007/s12603-009-0252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DJ, Wang ZM, Gallagher D, Heymsfield SB. Comparison of visceral adipose tissue mass in adult African Americans and whites. Obes Res. 2005;13:66–74. doi: 10.1038/oby.2005.9. [DOI] [PubMed] [Google Scholar]
- Carroll JF, Chiapa AL, Rodriquez M, Phelps DR, Cardarelli KM, Vishwanatha JK, et al. Visceral fat, waist circumference, and BMI: impact of race/ethnicity. Obesity. 2008;16:600–607. doi: 10.1038/oby.2007.92. [DOI] [PubMed] [Google Scholar]
- Hill JO, Sidney S, Lewis CE, Tolan K, Scherzinger AL, Stamm ER. Racial differences in amounts of visceral adipose tissue in young adults: the CARDIA (coronary artery risk development in young adults) study. Am J Clin Nutr. 1999;69:381–387. doi: 10.1093/ajcn/69.3.381. [DOI] [PubMed] [Google Scholar]
- Stanforth PR, Jackson AS, Green JS, Gagnon J, Rankinen T, Despres JP, et al. Generalized abdominal visceral fat prediction models for black and white adults aged 17-65 y: the HERITAGE Family Study. Int J Obes. 2004;28:925–932. doi: 10.1038/sj.ijo.0802563. [DOI] [PubMed] [Google Scholar]
- Zillikens MC, Conway JM. Anthropometry in blacks—applicability of generalized skinfold equations and differences in fat patterning between blacks and whites. Am J Clin Nutr. 1990;52:45–51. doi: 10.1093/ajcn/52.1.45. [DOI] [PubMed] [Google Scholar]
- Hill AM, LaForgia J, Coates AM, Buckley JD, Howe PRC Estimating abdominal adipose tissue with DXA and anthropometry. Obesity. 2007;15:504–510. doi: 10.1038/oby.2007.629. [DOI] [PubMed] [Google Scholar]
- Rissanen P, Hamalainen P, Vanninen E, TenhunenEskelinen M, Uusitupa M. Relationship of metabolic variables to abdominal adiposity measured by different anthropometric measurements and dual-energy X-ray absorptiometry in obese middle-aged women. Int J Obes. 1997;21:367–371. doi: 10.1038/sj.ijo.0800414. [DOI] [PubMed] [Google Scholar]
- Mazess RB, Barden HS, Bisek JP, Hanson J. Dual-energy X-ray absorptiometry for total-body and regional bone-minearl and soft-tissue composition. Am J Clin Nutr. 1990;51:1106–1112. doi: 10.1093/ajcn/51.6.1106. [DOI] [PubMed] [Google Scholar]
- Kiebzak GM, Leamy LJ, Pierson LM, Nord RH, Zhang ZY. Measurement precision of body composition variables using the Lunar DPX-L densitometer. J Clin Densitom. 2000;3:35–41. doi: 10.1385/jcd:3:1:035. [DOI] [PubMed] [Google Scholar]
- Novotny R, Daida YHG, Grove JS, Le Marchand L, Vijayadeva V. Asian adolescents have a higher trunk: peripheral fat ratio than whites. J Nutr. 2006;136:642–647. doi: 10.1093/jn/136.3.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YW, Allison DB, Heymsfield SB, Gallagher D. Larger amounts of visceral adipose tissue in Asian Americans. Obes Res. 2001;9:381–387. doi: 10.1038/oby.2001.49. [DOI] [PubMed] [Google Scholar]
- Gasperino JA, Wang J, Pierson RN, Heymsfield SB. Age-related-changes in musculoskeletal mass between Black-and-White women. Metab Clin Exp. 1995;44:30–34. doi: 10.1016/0026-0495(95)90285-6. [DOI] [PubMed] [Google Scholar]
- Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationhip. Obes Rev. 2002;3:141–146. doi: 10.1046/j.1467-789x.2002.00065.x. [DOI] [PubMed] [Google Scholar]
- Wagner DR, Heyward VH. Measures of body composition in blacks and whites: a comparative review. Am J Clin Nutr. 2000;71:1392–1402. doi: 10.1093/ajcn/71.6.1392. [DOI] [PubMed] [Google Scholar]
- Bjorntorp P. The regulation of adipose tissue distribution in humans. Int J Obes. 1996;20:291–302. [PubMed] [Google Scholar]
- Malis C, Rasmussen EL, Poulsen P, Petersen I, Christensen K, Beck-Nielsen H, et al. Total and regional fat distribution is strongly influenced by genetic factors in young and elderly twins. Obes Res. 2005;13:2139–2145. doi: 10.1038/oby.2005.265. [DOI] [PubMed] [Google Scholar]
- Weyer C, Snitker S, Bogardus C, Ravussin E. Energy metabolism in African Americans: potential risk factors for obesity. Am J Clin Nutr. 1999;70:13–20. doi: 10.1093/ajcn/70.1.13. [DOI] [PubMed] [Google Scholar]
- Casas YG, Schiller BC, DeSouza CA, Seals DR. Total and regional body composition across age in healthy Hispanic and white women of similar socioeconomic status. Am J Clin Nutr. 2001;73:13–18. doi: 10.1093/ajcn/73.1.13. [DOI] [PubMed] [Google Scholar]
- Park YW, Heymsfield SB, Gallagher D. Are dual-energy X-ray absorptiometry regional estimates associated with visceral adipose tissue mass. Int J Obes. 2002;26:978–983. doi: 10.1038/sj.ijo.0801982. [DOI] [PubMed] [Google Scholar]
- Bacha F, Saad R, Gungor N, Janosky J, Arslanian SA. Obesity, regional fat distribution, and syndrome X in obese black versus white adolescents: race differential in diabetogenic and atherogenic risk factors. J Clin Endocrinol Metab. 2003;88:2534–2540. doi: 10.1210/jc.2002-021267. [DOI] [PubMed] [Google Scholar]
- O'Connor DP, Bray MS, McFarlin BK, Ellis KJ, Sailors MH, Jackson AS. Ethnic bias in anthropometric estimates of DXA abdominal fat: the TIGER Study. Med Sci Sports Exerc. 2011;43:1785–1790. doi: 10.1249/MSS.0b013e318216d90f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lear SA, James PT, Ko GT, Kumanyika S. Appropriateness of waist circumference and waist-to-hip ratio cutoffs for different ethnic groups. Eur J Clin Nutr. 2010;64:42–61. doi: 10.1038/ejcn.2009.70. [DOI] [PubMed] [Google Scholar]
- Gordon-Larsen P, Adair LS, Popkin BM. Ethnic differences in physical activity and inactivity patterns and overweight status. Obes Res. 2002;10:141–149. doi: 10.1038/oby.2002.23. [DOI] [PubMed] [Google Scholar]
- Thomas KT, Keller CS, Holbert KE. Ethnic and age trends for body composition in women residing in the US Southwest: I. regional fat. Med Sci Sports Exerc. 1997;29:82–89. doi: 10.1097/00005768-199701000-00012. [DOI] [PubMed] [Google Scholar]
- He Q, Horlick M, Thornton J, Wang J, Pierson RN, Heshka S, et al. Sex-specific fat distribution is not linear across pubertal groups in a multiethnic study. Obes Res. 2004;12:725–733. doi: 10.1038/oby.2004.85. [DOI] [PubMed] [Google Scholar]
- Sierra-Johnson J, Johnson BD, Bailey KR, Turner ST. Relationships between insulin sensitivity and measures of body fat in asymptomatic men and women. Obes Res. 2004;12:2070–2077. doi: 10.1038/oby.2004.258. [DOI] [PubMed] [Google Scholar]