Stereotactic body radiotherapy (SBRT), also known as stereotactic ablative radiotherapy (SABR), is rapidly becoming an accepted practice for the radiotherapy of certain tumors (1) including some lung cancers, liver metastases, brain metastases, recurrent brain tumors, spinal metastases, kidney and pancreatic tumors. Typically SBRT involves the delivery of one or a few large dose fractions of 8 to 30 Gy per fraction (1). This is a major paradigm shift from the practice of radiotherapy that has developed over the past 90 years when, with relatively large amounts of normal tissues receiving doses close to the prescribed tumor dose, the goal was to maximize tumor response for an acceptable level of normal tissue injury. It is uncontested that fractionation of the radiation dose is superior to single doses in achieving such differential sparing of normal tissue compared to tumor. That fractionation is effective in this situation has been demonstrated in numerous animal models and is concordant with clinical practice over many decades. The reason that SBRT can essentially ignore this classic fractionation paradigm is the result of technological advances in image guidance and treatment delivery techniques that enable the delivery of large doses to tumors with reduced margins with high gradients outside of the target, thereby minimizing doses to relatively large volumes of surrounding normal tissue. For many tumor sites, such as with tumors in the periphery of the lung this has reduced the concern about unacceptable normal tissue injury and allowed considerable dose escalation.
Concurrently with these clinical developments, laboratory studies have suggested that at high dose fractions (> 8–10 Gy) there may be additional biological processes resulting in enhanced tumor cell killing. For example, this has been suggested by studies from the laboratories of Fuks and Kolesnick who have reported that high radiation doses produce sphingomyelinase dependent rapid vascular collapse that markedly enhances the antitumor effect of radiation (2, 3). Second, it has been shown that radiation enhances the antigenicity of tumors (4) though it is not yet clear whether this is greater for large single doses compared to standard fractionation (5). Third, it has been suggested that high doses of radiation such as are delivered by SBRT induce vascular damage over several days that leads secondarily to tumor cell death (6). Any of these factors, colloquially termed a “new biology” (7) could make SBRT more effective than would be predicted from clinical experience with fractionated irradiation. The question addressed here is whether any or all of these processes, need to be invoked to explain the remarkable success of SBRT. In particular, for most successful SBRT fractionation schemes the calculated biological effective doses (BEDs) delivered to the tumor are extremely high. So the question becomes: Is the impressive efficacy of SBRT the result of a “new biology” or the result of the high BEDs delivered to the tumor?
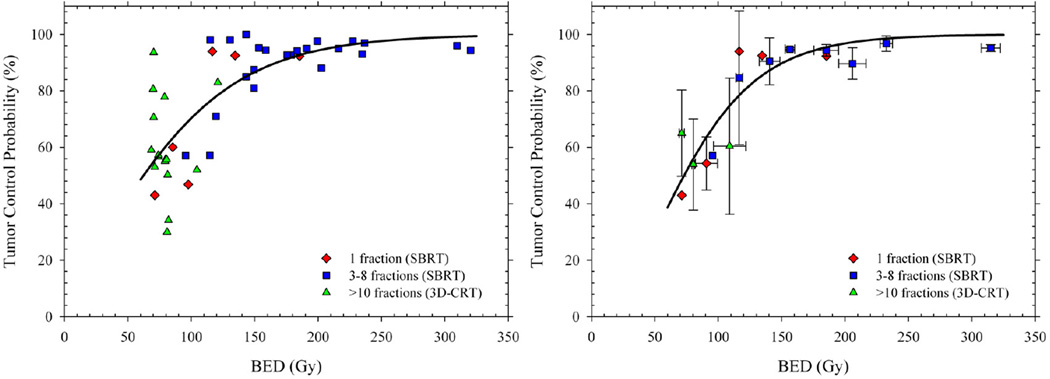
A recent publication by Mehta and colleagues (8) who reviewed the available clinical tumor control probability (TCP) data for SBRT and 3D conformal radiotherapy (3D-CRT) for stage 1 non-small cell lung cancer (NSCLC) sheds light on the “new biology” vs. high BED question. The authors did not address this question, however; rather they used the clinical data to determine the relationship between TCP and BED and to evaluate the impact of different models on calculated BED values assuming a tumor radiosensitivity of α/β = 8.6 Gy and no cellular proliferation. Fig. 1a is a replot of the Mehta et al. (8) data of TCP against BED for NSCLC with different symbols for regimes using 3D-CRT and SBRT with either 1 fraction or 3–8 fractions. The reader is reminded that BED is a linear-quadratic (LQ) model-based estimate of equivalent biological dose that corrects for dose fractionation (9). What can immediately be seen from Fig. 1a is that there is a monotonic relationship between TCP and BED for SBRT – a conclusion also recently reached by Ohri and colleagues (10). In other words, the higher the BED, the higher the TCP. More importantly the data for 3D-CRT also fall on this curve relating TCP to BED for SBRT regimes. We obtain the same conclusion if we bin the studies into BED intervals of 25 Gy (e.g. 75–100 Gy, 100–125 Gy, etc.) and produce weighted mean TCP probabilities (with standard deviations) to compensate for the different numbers of patients in each study (Fig. 1b). In other words, there is no indication from these data that SBRT and 3DCRT produce different TCP probabilities when adjusted for BED. It is also clear from the Figure that, once fractionation has been taken into account through the use of BED, there is no difference in tumor control for single-fraction SBRT compared with multi-fractioned SBRT. Thus the higher TCPs for SBRT can be fully explained by the much higher tumor BEDs delivered. For NSCLC, then, it follows that there is no need to invoke a “new biology” to explain the high tumor control rates.
Figure 1.
Tumor control probability (TCP) as a function of biological effective dose (BED) for stage I NSCLC. Left panel shows crude local control rates (≥ 2 years) redrawn from a pooled analysis reported by Mehta et al. (8) with different symbols distinguishing 3D-CRT and SBRT regimens. Right panel shows weighted mean TCP probabilities calculated from the same data to compensate for the different numbers of patients in each study. Solid lines are TCP predictions for an LQ-based fit to the available data.
This conclusion does not necessarily rule out the existence of a “new biology” at high doses. It does however suggest that such new biological processes are unlikely to be contributing significantly to the remarkable success of SBRT, over and above the same primary biological mechanisms that dominate at conventional doses per fraction. The old paradigm, that successful radiotherapy involves putting as much dose into the tumor while depositing as little dose as possible to surrounding normal tissue, appears to remain unchanged. SBRT of NSCLC has taken this to its logical extreme, with dose distributions that are so good that normal tissue sequelae play a much smaller role in determining the maximum tumor dose that can be delivered.
It is important to note that this analysis was specifically of SBRT for early stage NSCLC, and whether these conclusions apply to other sites has yet to be determined. It is possible that the vascular, stromal, or immunological effects discussed here could affect SBRT TCP for other tumors; indeed, provocative data on the stimulation of antitumor immunity for melanoma treated with SBRT thereby leading to a systemic abscopal effect has recently been reported(11, 12). Nonetheless we suggest that the sort of dosimetric analysis described here for NSCLC could similarly resolve the question of “new biology” vs dose for other sites. Hopefully the present analysis will stimulate such comparisons
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest Notification
The authors have no conflict of interest
References
- 1.Lo SS, Fakiris AJ, Chang EL, Mayr NA, Wang JZ, Papiez L, et al. Stereotactic body radiation therapy: a novel treatment modality. Nature reviews Clinical oncology. 2010;7:44–54. doi: 10.1038/nrclinonc.2009.188. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz- Friedman A, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–1159. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 3.Fuks Z, Kolesnick R. Engaging the vascular component of the tumor response. Cancer Cell. 2005;8:89–91. doi: 10.1016/j.ccr.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 4.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigenspecific effector cells that traffic to the tumor. J Immunol. 2005;174:7516–7523. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 5.Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15:5379–5388. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park HJ, Griffin RJ, Hui S, Levitt SH, Song CW. Radiation-induced vascular damage in tumors: implications of vascular damage in ablative hypofractionated radiotherapy (SBRT and SRS) Radiat Res. 2012;177:311–327. doi: 10.1667/rr2773.1. [DOI] [PubMed] [Google Scholar]
- 7.Brown JM, Koong AC. High-dose single-fraction radiotherapy: exploiting a new biology? Int J Radiat Oncol Biol Phys. 2008;71:324–325. doi: 10.1016/j.ijrobp.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Mehta N, King CR, Agazaryan N, Steinberg M, Hua A, Lee P. Stereotactic body radiation therapy and 3-dimensional conformal radiotherapy for stage I non-small cell lung cancer: A pooled analysis of biological equivalent dose and local control. Practical Radiation Oncology. 2012;2:288–295. doi: 10.1016/j.prro.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. The British journal of radiology. 1989;62:679–694. doi: 10.1259/0007-1285-62-740-679. [DOI] [PubMed] [Google Scholar]
- 10.Ohri N, Werner-Wasik M, Grills IS, Belderbos J, Hope A, Yan D, et al. Modeling local control after hypofractionated stereotactic body radiation therapy for stage I nonsmall cell lung cancer: a report from the elekta collaborative lung research group. Int J Radiat Oncol Biol Phys. 2012;84:e379–e384. doi: 10.1016/j.ijrobp.2012.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiniker SM, Chen DS, Knox SJ. Abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:2035. doi: 10.1056/NEJMc1203984. author reply -6. [DOI] [PubMed] [Google Scholar]
- 12.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]