Abstract
Objectives
Enhanced recovery after surgery (ERAS) or fast-track protocols have been implemented in different fields of surgery to attenuate the surgical stress response and accelerate recovery. The objective of this study was to systematically review the literature on outcomes of ERAS protocols applied in liver surgery.
Methods
The MEDLINE, EMBASE, PubMed and Cochrane Library databases were searched for randomized controlled trials (RCTs), case–control studies and case series published between January 1966 and October 2011 comparing adult patients undergoing elective liver surgery in an ERAS programme with those treated in a conventional manner. The primary outcome measure was hospital length of stay (LoS). Secondary outcome measures were time to functional recovery, and complication, readmission and mortality rates.
Results
A total of 307 articles were found, six of which were included in the review. These comprised two RCTs, three case–control studies and one retrospective case series. Median LoS ranged from 4 days in an ERAS group to 11 days in a control group. Morbidity, mortality and readmission rates did not differ significantly between the groups. Only two studies assessed time to functional recovery. Functional recovery in these studies was reached 2 days before discharge.
Conclusions
This systematic review suggests that ERAS protocols can be successfully implemented in liver surgery. Length of stay is reduced without compromising morbidity, mortality or readmission rates.
Introduction
Liver resection is the preferred treatment for a variety of primary and secondary liver tumours. Major abdominal surgical procedures such as hepatic resections cause a considerable surgical stress reaction and possible derangements in metabolic and pulmonary functions. Specific complications after hepatic resection include postoperative haemorrhage in the first hours to days after surgery, biliary leakage, intra-abdominal abscess and liver failure in a later postoperative stage.1 Improved operative techniques and insight into perioperative management have lowered mortality after liver resection to its current level of well below 5%, but morbidity rates remain high and range between 30% and 50%.2–4
In the past decade, multimodal enhanced recovery after surgery (ERAS) protocols or fast-track pathways have been applied in different forms of surgery. These pathways were developed to attenuate the surgical stress response and improve recovery, thereby decreasing postoperative complications and postoperative length of stay (LoS) in hospital.5 ERAS protocols have gained territory quickly because of the associated cost efficiency derived from the reduction in LoS, an important issue in today's context of rapidly increasing health care costs and the consequent need for optimization. To date, studies that show ERAS protocols that reduce LoS and morbidity rates and improve patient satisfaction have been published in the contexts of vascular surgery,6,7 musculoskeletal surgery,8 breast surgery9 and different forms of abdominal surgery.10–12
Enhanced recovery after surgery protocols have also been implemented in liver surgery, but their effectiveness has not been studied extensively. The present systematic review was performed to evaluate the effects of ERAS protocols in liver surgery on time to recovery following surgery and postoperative hospital LoS, and to examine the effects of the implementation of such protocols on complication and readmission rates following liver surgery.
Materials and methods
Search strategy
A systematic search was performed in PubMed, the Cochrane Library, EMBASE and MEDLINE for studies published between January 1966 and October 2011. Languages were restricted to English, Dutch and German. The following search terms were applied using the Boolean operators ‘AND’ and ‘OR’: ‘clinical pathway’, ‘critical pathway’, ‘enhanced recovery’, ‘accelerated’, ‘perioperative’ and ‘fast track’, combined with ‘liver’, ‘hepatic’ and ‘resection’. Synonyms of terms were also used in the search. The reference lists of selected papers were hand-searched for articles that were not retrieved in the database search. If necessary, authors of relevant articles were contacted to obtain additional information.
Inclusion and exclusion criteria
Studies were considered eligible for inclusion if they met all of the following inclusion criteria: (i) they reported on adult patients undergoing elective open or laparoscopic liver surgery; (ii) they described an enhanced recovery programme with at least four different perioperative elements, and (iii) they reported outcomes including LoS, postoperative morbidity and mortality, and readmission rates. Studies were excluded if they: (i) described a single intervention in perioperative care rather than a group of interventions combined in an enhanced recovery programme; (ii) reported on emergency, non-elective or transplantation surgery, and (iii) reported a non-systematic review. Table 1 lists a summary of ERAS items applicable to liver surgery. The items are supported by varying levels of evidence.13 Perioperative care is considered to fall within an ERAS protocol when at least four different items are included, covering the pre-, intra- and postoperative periods.14,15
Table 1.
Summary of elements in an enhanced recovery after surgery (ERAS) programme applicable to liver surgery
| Evidence-based factors | Probably useful factors |
|---|---|
| No oral bowel preparation | Preoperative counselling |
| Preoperative feeding: carbohydrate loading up to 2 h before surgery | Provision of i.v. analgesia |
| No pre-anaesthetic medication | Stimulation of bowel movement with laxatives |
| Anti-thrombotic prophylaxis | Early and scheduled mobilization |
| Single-dose antibiotics | Audit |
| Epidural analgesia | |
| Prevention of postoperative nausea and vomiting | |
| Avoidance of hypothermia | |
| No routine drainage of peritoneal cavity | |
| No postoperative nasogastric intubation | |
| Good fluid balance | |
| Removal of urinary catheter on day 1 | |
| Normal food at will after surgery from day 1 | |
Evidence-based factors: separate items are graded as being supported by level 1 or level 2 evidence (according to the guidelines of the Oxford Centre for Evidence-Based Medicine13).
Probably useful factors: evidence is less strong, but these factors are felt to be useful because the items are most probably quality-enhancing, are associated generally with a low incidence of adverse effects and low costs.
Outcome measures
The primary outcome measure of this systematic review was hospital LoS. Secondary outcome measures were time to functional recovery, complication rates, readmissions and mortality rates. Criteria for functional recovery were: good pain control with oral analgesia only; tolerance for solid food; no requirement for i.v. fluids; passage of stool, and independent mobility at the preoperative level.16
Study selection and data selection
Abstracts and titles of studies identified by the search were read by two authors (MMEC and AAvdW), each of whom independently made a first selection of studies. These first selections were compared and, in the event that the inclusion of a study required discussion, a third reviewer (RMvD) was consulted. Second and final selections were made independently by each of the two authors after reading the full-text articles. Both randomized as well as non-randomized studies were eligible for inclusion as long as they met the inclusion criteria. The methodological quality of the included studies was assessed using the MINORS (methodological index for non-randomized studies) criteria,17 a checklist scoring eight methodological items for non-comparative studies (maximum of 16 points) and an additional four items for comparative studies (maximum of 24 points). Missing data were obtained by contacting the authors of the relevant studies.
Data on the following factors were extracted from the included articles: postoperative LoS; number of patients included; patient ages; types of surgery; discharge criteria; functional recovery; mortality; morbidity; readmissions, and protocol adherence.
Statistical analysis
As the search strategy did not identify any randomized controlled trials (RCTs) evaluating the outcomes of ERAS protocols against those of traditional care, the MOOSE (meta-analysis of observational studies in epidemiology) checklist for meta-analysis of observational studies was used to assess the possibility of conducting a meta-analysis.18 The included studies were considered to be too heterogeneous to support this and therefore no attempt at meta-analysis was made. Results are subsequently presented in tables and figures.
Results
Selected articles and characteristics of the studies
The literature search produced 307 articles, of which 300 were excluded after their abstracts had been read in the first round of selection because they did not concern the evaluation of a fast-track programme in liver surgery (Fig. 1). After evaluation of the remaining seven papers, one was excluded because it was a non-systematic review.19
Figure 1.
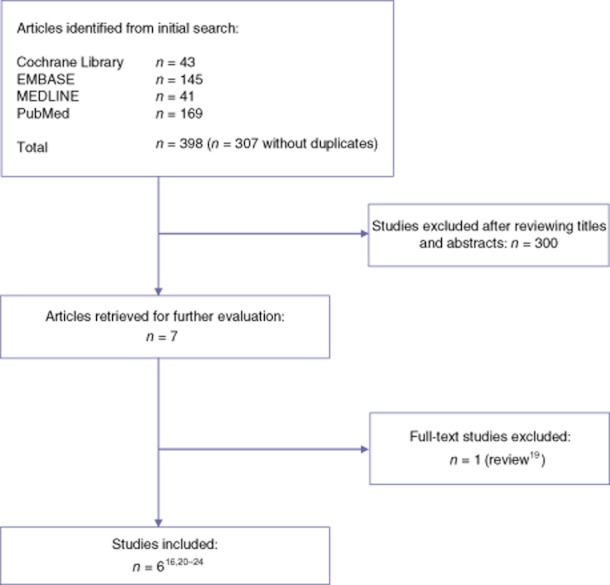
Selection of studies for systematic review
Finally, six papers were included in this systematic review. The details of the included studies are shown in Table 2. There were no reports of RCTs evaluating the outcomes of an ERAS programme against those of traditional care. In two RCTs, both study groups were treated in an ERAS programme. One of these RCTs evaluated the use of laxatives and oral nutritional supplements within an ERAS programme20 and one assessed different forms of postoperative analgesia in two groups managed in a fast-track programme.21 Three case–control studies and one retrospective case series were also included.16,22–24 All studies included patients undergoing various forms of liver resection, including (extended) hemi-hepatectomy, metastasectomy, sectionectomy, central resection and repeat hepatectomy. One study did not include major hepatectomies; all patients in this study underwent laparoscopic liver resection.20
Table 2.
Study characteristics and quality assessment
| Study | Type of surgery | Study design | Patients in study/control groups, n | Consecutive series of patients | Length of follow-up | Age, years, median (range) | MINORS score | |
|---|---|---|---|---|---|---|---|---|
| ERAS group | Control group | |||||||
| van Dam et al. 200816 | HE, EHE, ME, SE, CR, RHE | Case–control | 61/100 | Yes | 30 days | 62 (24–82) | 60 (20–81) | 18/24 |
| Lin et al. 201122 | SE, HE, EHE, CR | Case–control | 56/61 | Yes | 30 days | 57 (23–73) | 55 (22–81) | 17/24 |
| Stoot et al. 200923 | Laparoscopic: ME, SE, LLS | Case–control | 13/13 | Yes | 3–6 months | 55 (34–82) | 45 (26–70) | 19/24 |
| Hendry et al. 201020 | HE, ME, SE, CR | RCT | 68a | Yes | 30 days | 62 (53–69) | – | 13/16 |
| Koea et al. 200921 | HE, EHE, ME, SE | RCT | 100a | Yes | 30 days | 60 (23–83) | – | 11/16 |
| MacKay & O'Dwyer 200824 | HE, SE | Retrospective case series | 12 | Yes | Unknown | 60 (43–74) | – | 8/16 |
Patients in the control and experimental arms were all treated according to ERAS protocols.
ERAS, enhanced recovery after surgery; HE, hemi-hepatectomy; EHE, extended hemi-hepatectomy; ME, metastasectomy; SE, segmentectomy; CR, central resection; RHE, repeat hemi-hepatectomy; LLS, left lateral sectionectomy; RCT, randomized controlled trial.
All studies included a consecutive series of patients. Follow-up was 30 days in four studies and 3–6 months in one study. One study did not report the duration of follow-up.
Age and other patient characteristics did not differ significantly among the patient groups described in the selected studies. Methodological quality assessed using the MINORS criteria was scored in the range of 17–19 points (of a maximum of 24 points) in case–control studies. Non-comparative studies achieved MINORS scores in the range of 8–13 points (of a maximum of 16 points).
Most studies described the enhanced recovery programme in detail. A summary of the specific ERAS elements included in the different studies is shown in Table 3. Fourteen protocol elements were identified. Most studies included the majority of these elements; one study included only seven elements.
Table 3.
Summary of elements in an enhanced recovery after surgery (ERAS) programme included in each study
| Study | Preoperative | Perioperative | Postoperative | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Counselling | Feeding | No pre-med | Thrombo-embolic prophylaxis | No bowel prep | No drain | Epidural analgesia | Fluid restriction | Prevention of hypothermia | No NG tube | Early oral feeding (from PoD 1) | Mobilization from PoD 1 | Laxatives | Early removal of bladder catheter | |
| van Dam et al. 200816 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Lin et al. 201122 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |||
| Stoot et al. 200923 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Hendry et al. 201020 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Koea et al. 200921 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | ||||
| MacKay & O'Dwyer 200824 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |||||||
PoD, postoperative day.
Primary and secondary outcome measures
Table 4 outlines postoperative outcomes after implementation of an ERAS programme. Hospital LoS decreased significantly in the three comparative studies after ERAS implementation, in which median LoS was 5–7 days in the ERAS groups and 7–11 days in the traditional care groups.
Table 4.
Postoperative outcome after implementation of a clinical pathway
| Study | Type of surgery | Hospital LoS, days, study group versus control group | Morbidity, %, study group versus control group | Mortality, %, study group versus control group | Readmissions, %, study group versus control group |
|---|---|---|---|---|---|
| van Dam et al. 200816 | HE, EHE, ME, SE, CR, RHE | 6 versus 8 (P < 0.001) | 41 versus 31 (NS) | 0 versus 2 (NS) | 13 versus 10 (NS) |
| Lin et al. 201122 | SE, HE, EHE, CR | 7 versus 11 (P < 0.001) | 46.4 versus 43.3 (NS) | 1.8 versus 1.6 (NS) | 7.1 versus 3.3 (NS) |
| Stoot et al. 200923 | Laparoscopic: ME, SE, LLS | 5 versus 7 (NS) | 15.3 versus 15.3 (NS) | 0 versus 0 (NS) | 0 versus 0 (NS) |
| Functional recovery: 3 versus 5 (P < 0.04) | |||||
| Hendry et al. 201020 | HE, ME, SE, CR | 6 | 17 | 2 | 5 |
| Functional recovery: 4 | |||||
| Koea et al. 200921 | HE, EHE, ME, SE | 4.7 ± 0.9 (intrathecal morphine) 6.8 ± 1.2 (epidural) | 19 | 0 | 4 |
| MacKay & O'Dwyer 200824 | HE, SE | 4 (2–7) | 16.6 | 0 | 0 |
LoS, length of stay; HE, hemi-hepatectomy; EHE; extended hemi-hepatectomy; ME, metastasectomy; SE, segmentectomy; CR, central resection; RHE; repeat hemi-hepatectomy; LLS, left lateral sectionectomy; NS, not significant.
In the non-comparative studies, postoperative LoS after liver resection ranged between 4 days and 7 days. In the study by Stoot et al.,21 all patients underwent laparoscopic liver resection. The median postoperative LoS was 5 days in the ERAS group and 7 days in the traditional care group; however, this difference was not statistically significant and the study did not include major liver resections. It is noteworthy that only two studies17,20 assessed time to functional recovery and the reasons for delayed discharge. In both studies time to functional recovery was achieved 2 days prior to actual discharge from hospital. The main reasons for later discharge were concern for complications or extensive surgery, low patient confidence, and transport-related or other social problems.
Table 5 shows the extent of protocol adherence. The level of adherence to protocol was moderate in the studies included. Generally, nasogastric tubes were either not used or were immediately removed after surgery. The proportion of patients requiring the reinsertion of a nasogastric tube was low. In the study groups, intra-abdominal drains were used in only 2–13% of patients. The majority of patients resumed oral fluid intake on the day of surgery and achieved a normal diet on days 1 or 2. The percentage of patients mobilized on the first postoperative day was low, with rates of 20–28% reported in only two studies.20,21 In one study full mobilization was achieved on day 3 by 85% of patients.16
Table 5.
Adherence to an enhanced recovery after surgery (ERAS) protocol
| Protocol element | van Dam et al. 200816 (ERAS versus control) | Lin et al. 201122 (ERAS versus control) | Stoot et al. 200923 (ERAS versus control) | Koea et al. 200921 (ERAS, n = 100) | Hendry et al. 201020 (ERAS, n = 68) | MacKay & O'Dwyer 200824 (ERAS, n = 12) |
|---|---|---|---|---|---|---|
| No NG tube or removed directly after surgery | 92% versus 0% | NA | 0% versus 38% | 100% | 100% | NA |
| NG tube reinserted | 4% versus 0% | 3.5% versus 1.6% | 0% versus 15% | NA | NA | NA |
| Intra-abdominal drain | 2% versus 66% | 0% versus 1.6% | 0% versus 46% | 2% | 13% | 0% |
| Oral fluid intake PoD 0 (ERAS), % or days, median (range) | 92% | NA | 1 (0–2) versus 1 (0–6) | NA | 94% | 100% |
| Resumption of normal food, % or days, median (range) | 1 (0–3) versus 3 (0–14) | NA | 1 (0–2) versus 1 (0–6) | 20% on day 1 | 37% on day 1 | NA |
| 91% on day 2 | ||||||
| Full mobilization (ERAS) | 85% on day 3 | NA | NA | 20% on day 1 | 28% on day 1 | NA |
| Functional recovery criteria met on day | NA | NA | 3 versus 5 | NA | 4 | NA |
NG, nasogastric; PoD, postoperative day; NA, data not available.
Discussion
This systematic review examined the use of ERAS protocols in liver surgery in three case–control studies, two RCTs and one case series. The results suggest that an enhanced recovery protocol can be successfully implemented in liver surgery. Hospital LoS was reduced and functional recovery was accelerated without compromising morbidity or mortality rates, and readmission rates were not significantly increased. The present results are in line with a recent review describing the use of fast-track protocols in hepatopancreatic resections.25
At least four items in the pre-, peri- and postoperative periods must be included in an ERAS protocol for the protocol to be considered of value.14,15 The studies in this review incorporated an average of 12 of 14 items (range: 7–14 items). In large series of patients undergoing liver surgery, LoS varies between 8 days and 14 days.3,4 All of the studies included in this review reported a shorter LoS in the ERAS study group. Two studies assessed time to functional recovery, which was significantly lower than total LoS. In many studies, LoS is reported as a primary outcome parameter. However, the use of this outcome may not always be appropriate as discharge is often delayed by a variety of other factors that may be unrelated to the true outcomes of the procedure.26 The present authors therefore propose that time to functional recovery should be used as an outcome measure rather than LoS.
Morbidity rates reported in the literature vary from 38% to 45%4,27 and are comparable with the complication rates reported in the studies in the present review. However, it should be noted that complications in the studies included here were not always reported using a validated classification system (e.g. Clavien–Dindo or Accordion classification28,29). This makes it more difficult to make meaningful comparisons of morbidity among the different centres.
The reporting of adherence to the various elements of the protocol was rather low in the included studies, especially as far as the introduction of normal diet and fluids was concerned. As Maessen et al.30 have observed, the reporting of adherence to protocol seems to be problematic in a considerable number of international studies. This impedes comparisons among studies.30 The use of self-report patient diaries and continuous education of nurses and staff may represent strategies for overcoming this difficulty.
Overall, the methodological quality of the studies included in the present review, as assessed according to the MINORs criteria, was acceptable. However, this systematic review is limited by the fact that no RCTs comparing fast-track with standard care were available for inclusion (the RCTs included treated both the patient and control groups according to an ERAS protocol) and only case series and comparative studies using historical controls were included. The studies included were considered to be too heterogeneous to allow a meta-analysis. Another limitation of this review is that the individual studies used slightly different study protocols, with the result that the items incorporated in the various protocols are not identical and thus these studies are not fully comparable. However, a recent study by Ahmed et al.31 compared adherence to protocol in two groups of patients undergoing colorectal surgery and showed that outcome was unaltered in the study group in which adherence to some elements of the study protocol (e.g. preoperative carbohydrate loading and early fluid and diet introduction) was significantly lower. From this, it seems reasonable to conclude that not every item of an ERAS protocol makes an independent contribution to enhanced recovery, but, rather, it is the combination of different items in a structured care pathway that determines the outcome. This might also to some degree reflect a Hawthorne, or trial, effect, indicating a positive effect resulting from the implementation per se of a complex and comprehensive intervention.
Kehlet first introduced ERAS protocols in colon surgery in 1997.32 Now, 15 years later, several items drawn from ERAS protocols are increasingly implemented in modern care worldwide. However, in many surgical fields, ERAS protocols have not yet been accepted as standard care. In the context of liver surgery, ERAS was first described in 2008,24 since when only five studies examining an ERAS protocol in this field have been published16,20–23 and three of these were performed by the same study group.16,21,23 This seems to illustrate a limited international implementation of ERAS protocols in liver surgery.
Although the methodology used in the studies included is not optimal, the results are consistent and seem to indicate clear advantages in terms of recovery. Although most centres today perform a proportion of resections laparoscopically, the present results serve to illustrate what can be achieved in open surgery and hence serve as a backdrop against which advances in technique and subsequent results can be compared.
In summary, this systematic review shows that it is feasible and safe to implement an ERAS protocol in hepatic surgery. The available evidence suggests that LoS is shortened without comprising morbidity, mortality or readmission rates. In view of the limited number of studies and the discrepancies in reporting among them, the present authors recommend the application of a standardized system of classifying complications, the accurate reporting of adherence to protocol, and the use of time to functional recovery as a primary outcome measure in future studies in order to enhance quality and comparability.
Conflicts of interest
None declared.
References
- 1.van den Broek MA, van Dam RM, van Breukelen GJ, Bemelmans MH, Oussoultzoglou E, Pessaux P, et al. Development of a composite endpoint for randomized controlled trials in liver surgery. Br J Surg. 2011;98:1138–1145. doi: 10.1002/bjs.7503. [DOI] [PubMed] [Google Scholar]
- 2.Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38–46. doi: 10.1016/s1072-7515(00)00261-1. [DOI] [PubMed] [Google Scholar]
- 3.Dimick JB, Wainess RM, Cowan JA, Upchurch GR, Jr, Knol JA, Colletti LM. National trends in the use and outcomes of hepatic resection. J Am Coll Surg. 2004;199:31–38. doi: 10.1016/j.jamcollsurg.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1803 consecutive cases over the past decade. Ann Surg. 2002;236:397–407. doi: 10.1097/01.SLA.0000029003.66466.B3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg. 2002;183:630–641. doi: 10.1016/s0002-9610(02)00866-8. [DOI] [PubMed] [Google Scholar]
- 6.Brustia P, Renghi A, Gramaglia L, Porta C, Cassatella R, De Angelis R, et al. Mininvasive abdominal aortic surgery. Early recovery and reduced hospitalization after multidisciplinary approach. J Cardiovasc Surg (Torino) 2003;44:629–635. [PubMed] [Google Scholar]
- 7.Podore PC, Throop EB. Infrarenal aortic surgery with a 3-day hospital stay: a report on success with a clinical pathway. J Vasc Surg. 1999;29:787–792. doi: 10.1016/s0741-5214(99)70204-1. [DOI] [PubMed] [Google Scholar]
- 8.Barbieri A, Vanhaecht K, Van Herck P, Sermeus W, Faggiano F, Marchisio S, et al. Effects of clinical pathways in the joint replacement: a meta-analysis. BMC Med. 2009;7:32. doi: 10.1186/1741-7015-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arsalani-Zadeh R, ElFadl D, Yassin N, MacFie J. Evidence-based review of enhancing postoperative recovery after breast surgery. Br J Surg. 2011;98:181–196. doi: 10.1002/bjs.7331. [DOI] [PubMed] [Google Scholar]
- 10.Rawlinson A, Kang P, Evans J, Khanna A. A systematic review of enhanced recovery protocols in colorectal surgery. Ann R Coll Surg Engl. 2011;93:583–588. doi: 10.1308/147870811X605219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCarty TM, Arnold DT, Lamont JP, Fisher TL, Kuhn JA. Optimizing outcomes in bariatric surgery: outpatient laparoscopic gastric bypass. Ann Surg. 2005;242:494–498. doi: 10.1097/01.sla.0000183354.66073.4c. discussion 498–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirsh EJ, Worwag EM, Sinner M, Chodak GW. Using outcome data and patient satisfaction surveys to develop policies regarding minimum length of hospitalization after radical prostatectomy. Urology. 2000;56:101–106. doi: 10.1016/s0090-4295(00)00594-x. discussion 106–107. [DOI] [PubMed] [Google Scholar]
- 13.Phillips B, Ball C, Sackett D, Badenoch D, Straus S, Haynes B, et al. Levels of evidence and grades of recommendations. 2011. Oxford Centre for Evidence-Based Medicine. Available at http://www.cebm.net/index.aspx?o=1025 (last accessed 19 September 2012)
- 14.Varadhan KK, Neal KR, Dejong CH, Fearon KC, Ljungqvist O, Lobo DN. The enhanced recovery after surgery (ERAS) pathway for patients undergoing major elective open colorectal surgery: a meta-analysis of randomized controlled trials. Clin Nutr. 2010;29:434–440. doi: 10.1016/j.clnu.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Wind J, Polle SW, Fung Kon Jin PH, Dejong CH, von Meyenfeldt MF, Ubbink DT, et al. Systematic review of enhanced recovery programmes in colonic surgery. Br J Surg. 2006;93:800–809. doi: 10.1002/bjs.5384. [DOI] [PubMed] [Google Scholar]
- 16.van Dam RM, Hendry PO, Coolsen MM, Bemelmans MH, Lassen K, Revhaug A, et al. Initial experience with a multimodal enhanced recovery programme in patients undergoing liver resection. Br J Surg. 2008;95:969–975. doi: 10.1002/bjs.6227. [DOI] [PubMed] [Google Scholar]
- 17.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ J Surg. 2003;73:712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 18.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 19.van Gulik T. Open versus laparoscopic resection for liver tumours. HPB. 2009;11:465–468. doi: 10.1111/j.1477-2574.2009.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hendry PO, van Dam RM, Bukkems SF, McKeown DW, Parks RW, Preston T, et al. Randomized clinical trial of laxatives and oral nutritional supplements within an enhanced recovery after surgery protocol following liver resection. Br J Surg. 2010;97:1198–1206. doi: 10.1002/bjs.7120. [DOI] [PubMed] [Google Scholar]
- 21.Koea JB, Young Y, Gunn K. Fast-track liver resection: the effect of a comprehensive care package and analgesia with single-dose intrathecal morphine with gabapentin or continuous epidural analgesia. HPB Surg. 2009;2009:271986. doi: 10.1155/2009/271986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin DX, Li X, Ye QW, Lin F, Li LL, Zhang QY. Implementation of a fast-track clinical pathway decreases postoperative length of stay and hospital charges for liver resection. Cell Biochem Biophys. 2011;61:413–419. doi: 10.1007/s12013-011-9203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoot JH, van Dam RM, Busch OR, van Hillegersberg R, De Boer M, Olde Damink SW, et al. The effect of a multimodal fast-track programme on outcomes in laparoscopic liver surgery: a multicentre pilot study. HPB. 2009;11:140–144. doi: 10.1111/j.1477-2574.2009.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacKay G, O'Dwyer PJ. Early discharge following liver resection for colorectal metastases. Scott Med J. 2008;53:22–24. doi: 10.1258/rsmsmj.53.2.22. [DOI] [PubMed] [Google Scholar]
- 25.Spelt L, Ansari D, Sturesson C, Tingstedt B, Andersson R. Fast-track programmes for hepatopancreatic resections: where do we stand? HPB. 2011;13:833–838. doi: 10.1111/j.1477-2574.2011.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maessen JM, Dejong CH, Kessels AG, von Meyenfeldt MF. Length of stay: an inappropriate readout of the success of enhanced recovery programmes. World J Surg. 2008;32:971–975. doi: 10.1007/s00268-007-9404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McColl RJ, You X, Ghali WA, Kaplan G, Myers R, Dixon E. Recent trends of hepatic resection in Canada: 1995–2004. J Gastrointest Surg. 2008;12:1839–1846. doi: 10.1007/s11605-008-0679-4. discussion 1846. [DOI] [PubMed] [Google Scholar]
- 28.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien–Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 29.Strasberg SM, Linehan DC, Hawkins WG. The accordion severity grading system of surgical complications. Ann Surg. 2009;250:177–186. doi: 10.1097/SLA.0b013e3181afde41. [DOI] [PubMed] [Google Scholar]
- 30.Maessen J, Dejong CH, Hausel J, Nygren J, Lassen K, Andersen J, et al. A protocol is not enough to implement an enhanced recovery programme for colorectal resection. Br J Surg. 2007;94:224–231. doi: 10.1002/bjs.5468. [DOI] [PubMed] [Google Scholar]
- 31.Ahmed J, Khan S, Gatt M, Kallam R, MacFie J. Compliance with enhanced recovery programmes in elective colorectal surgery. Br J Surg. 2010;97:754–758. doi: 10.1002/bjs.6961. [DOI] [PubMed] [Google Scholar]
- 32.Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. 1997;78:606–617. doi: 10.1093/bja/78.5.606. [DOI] [PubMed] [Google Scholar]


