Abstract
Objectives
Sinusoidal injury (SI) after oxaliplatin-based therapies for colorectal liver metastasis (CRLM) can increase postoperative morbidity. Preoperative methods to estimate SI are lacking. The aim of this study was to identify SI by evaluating portal vein haemodynamics.
Methods
Magnetic resonance imaging flowmetry (MRIF) was used to estimate portal vein haemodynamics in 29 patients with CRLM before liver surgery. Sinusoidal injury was evaluated from resected non-tumorous liver parenchyma according to the combined vascular injury (CVI) score of ≥3.
Results
All patients with SI (six of 29) received oxaliplatin; however, a significant association could not be proven (P= 0.148). Oxaliplatin-treated patients showed portal vein dilatation in both the SI and non-SI groups compared with patients who had not received oxaliplatin (Bonferroni corrected P= 0.003 and P= 0.039, respectively). Mean portal velocity tended to be lower in patients with SI compared with oxaliplatin-treated patients without SI (Bonferroni corrected P= 0.087). A mean portal velocity of ≤14.35 cm/s together with a cross-section area of ≥1.55 cm2 was found to predict SI with sensitivity of 100% and specificity of 78%.
Conclusions
Oxaliplatin treatment was associated with portal vein dilatation. Patients with SI showed a tendency towards decreased mean portal flow velocity. This may indicate that SI is associated with an increased resistance to blood flow in the liver parenchyma. Portal vein haemodynamic variables estimated by MRIF can identify patients without SI non-invasively.
Introduction
During the last decade, the introduction of new chemotherapeutic agents has altered the management of patients with colorectal liver metastasis (CRLM). At present, approximately 70% of CRLM patients at Uppsala University Hospital receive neoadjuvant chemotherapy prior to liver surgery. Oxaliplatin- or irinotecan-based chemotherapies increase resectability rates by downsizing initially non-resectable tumours and provide overall survival benefits after liver surgery.1 In patients with resectable CRLM, neoadjuvant chemotherapy increases disease-free survival2 and probably allows for the identification and selection of patients with favourable tumour biology.3 Neoadjuvant chemotherapy can induce a complete radiological response, which is unfavourable when localizing tumours perioperatively,4 and also has various adverse effects on the liver parenchyma. Irinotecan is associated with chemotherapy-associated steatohepatitis and increased postoperative mortality caused by liver failure.5 Oxaliplatin has been reported to cause sinusoidal injury (SI) in 5–50% of patients.6–8 Sinusoidal injury is associated with a higher need for perioperative blood transfusions9 and increased postoperative morbidity,10,11 and may have a negative impact on longterm prognosis.12 However, other studies have not been able to show an increase in postoperative morbidity or mortality after oxaliplatin-based chemotherapy.13
Several studies have attempted to identify predictive factors for SI.14–18 A non-invasive and accurate assessment for SI is lacking. Preoperative biopsy of liver parenchyma is invasive and is not recommended because the amounts of tissue achieved are small and sampling error is possible.19 Studies of SI are usually based on non-tumorous liver tissue derived from resection.7,8 Intra- and interobserver variation can be minimized by using a semi-quantitative scoring system for combined vascular injury (CVI).8 Laboratory parameters such as a gamma-glutamyl transpeptidase (GGT) level that is > 1.5 times higher than the normal range14 or an aspartate aminotransferase to platelet ratio index (APRI) that is > 0.3617 can be used to predict SI preoperatively. Increased spleen size has been reported after oxaliplatin-based therapy20 and patients with an increase in spleen size of >50% have been reported to be at high risk for SI.16 The hypothesis that oxaliplatin-induced SI can lead to portal hypertension and subsequently splenomegaly and thrombocytopoenia has been proposed.21 Two case reports showed that oxaliplatin-based chemotherapy might induce significant portal hypertension as verified by direct measurement of portal venous pressure.21,22 Portal flow haemodynamics can also be evaluated non-invasively using Doppler ultrasonography or magnetic resonance imaging flowmetry (MRIF).23 Superparamagnetic iron oxide-enhanced MRI has been used to evaluate liver parenchyma for sinusoidal changes.15,18
The aim of the present study was to investigate whether portal vein haemodynamics as measured by MRIF were changed in patients with SI or in patients who had undergone oxaliplatin-based treatment for CRLM. A secondary aim was to evaluate preoperative MRIF as a predictive tool for SI.
Materials and methods
Patients
All patients scheduled for CRLM resection at the study institution were prospectively evaluated during 2007–2009. The principal inclusion criterion was a planned minimum two-segment resection in order to derive a sufficient amount of non-tumorous liver tissue for histopathology. Exclusion criteria included the presence of general contraindications to MRI. All patients who met the inclusion criteria and provided written informed consent were included in the study, provided the MRI facility was available. Patients were examined by MRIF 1 day prior to planned liver surgery. Preoperative blood tests and liver function tests were conducted routinely. The study was approved by the regional ethics committee (protocol no. 2007/020). The study cohort was a subgroup of patients reported in an earlier study by this group on liver steatosis and steatohepatitis prediction by proton magnetic resonance spectroscopy.24
Magnetic resonance imaging flowmetry
Magnetic resonance imaging flowmetry was measured using a 3T Achieva MRI scanner (Philips Medical Systems Nederland BV, Best, the Netherlands) using the SENSE-CARDIAC coil, with the patient in a supine position under basal fasting conditions. Axial and coronal steady-state free precession images of the portal venous system were used to establish the correct orientation of the oblique section required for flow measurements. The axial and coronal (in parenthesis) localizer images were taken at a repetition time of 2.5 ms (2.6 ms), an echo time of 1.2 ms (1.3 ms), a matrix size of 275 × 288 (217 × 320), a field of view of 40 cm (45 cm) and a flip angle of 40 °. These localizing images were acquired in 15 s (10 s). Cine phase-contrast (CPC) imaging was performed during a breath hold using triggering by vector electrocardiogram. The oblique CPC image used for flow calculations was oriented perpendicular to flow in the main portal vein before bifurcation to the left and right portal branches. Acquisition was taken with a repetition time 5.6 ms, an echo time of 3.6 ms, a flip angle of 10 °, a velocity encoding of 50 cm/s, a matrix size of 112 × 256 and a field of view of 30 cm. The number of signal averages was two, and section thickness was 6 mm. Flow encoding was selected parallel with the direction of blood flow. Acquisition time was 18 s. Using scanner-associated software (Philips Medical Systems Nederland BV), CPC flow rates were calculated by integrating the velocity product of 17 velocity images spanning the cardiac cycles. Regions of interest encompassing the portal vein cross-section areas were manually placed on the oblique CPC images. Mean flow rates were derived from the average flow rate during each phase of the cardiac cycle; portal flow, cross-section area, mean and peak velocities were calculated. These processes were performed by a radiologist blinded to clinical and histopathological data.
Histopathology
Non-tumorous liver specimens were obtained from the resected portion of the liver directly after surgery. Effort was made to retrieve parenchymal blocks measuring approximately 40 × 40 × 7 mm, avoiding big vessels and large bile duct structures, at ≥20 mm from the peritoneal liver surface, the resection margin and the metastases. The samples were directly fixed in 10% neutral buffered formalin (4% formaldehyde), embedded in paraffin blocks, cut into thicknesses of 3 µm and stained with haematoxylin and eosin, van Gieson and Gordon–Sweets reticulin stains. For immunohistochemical stainings, the sections were heated in Tris EDTA buffer, pH 9 (S2367; Dako Denmark A/S, Glostrup, Denmark) in a microwave oven for 10 min at 750 W followed by 15 min at 350 W for antigen retrieval. Tissue sections were stained by immunohistochemistry or immunofluorescence techniques with antibodies against ki-67 (clone MIB-1; Dako Denmark A/S) (ready to use: IR626). Antibody detection was performed using the REAL Envision Peroxidase DAB Detection System (Dako Denmark A/S) and haematoxylin was used for counterstaining. Figure 1 shows example findings in stained tissue. All liver samples were evaluated by an experienced liver pathologist blinded to clinical and MRIF data. Sinusoidal injury was graded according to CVI score (Table 1), for which the minimum and maximum number of points were 0 and 13, respectively. A CVI score of ≥3 was recognized as indicative of clinically relevant SI.8 Fibrosis stages were assessed using the scoring system described by Kleiner et al. (0–4 points).25
Figure 1.
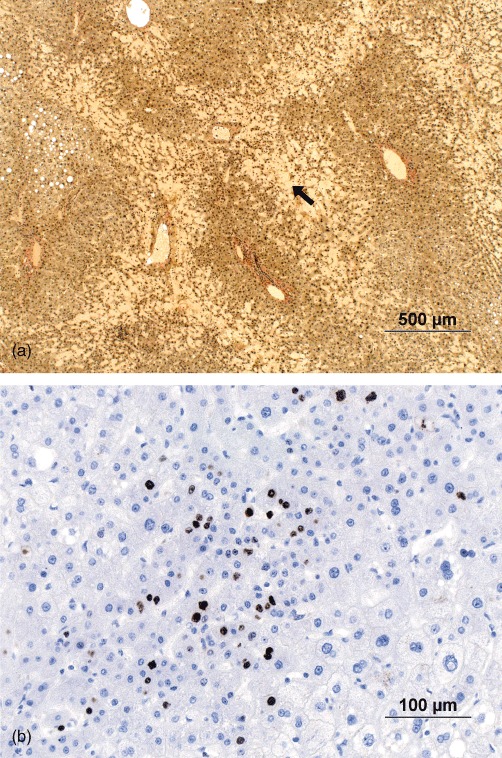
Histopathological findings in a patient with sinusoidal injury in addition to sinusoidal dilatation. (a) An extensive parenchymal extinction lesion (PEL) (black arrow) is seen in non-tumorous parenchyma. The border between the normal liver parenchyma and PEL is characterized by hepatocyte plate disruption. In addition, a small area with steatosis can be seen. (Van Gieson stain; original magnification ×4.) (b) Immunohistochemical staining for ki-67 was utilized to judge cases with suspicious nodular regenerative hyperplasia. Groups of smaller hepatocytes positive for ki-67 confirmed the presence of a focus of subtle nodular regenerative hyperplasia. (Original magnification ×20)
Table 1.
Criteria used to derive a combined vascular injury score (0–13)8
| Histopathological feature | Score |
|---|---|
| Diffuse sinusoidal dilation | |
| None | 0 |
| <1/3 of a lobule | 1 |
| 1/3–2/3 of a lobule | 2 |
| Entire lobule | 3 |
| Small vessel loss/obliteration | |
| None | 0 |
| <1/3 of vessels | 1 |
| 1/3–2/3 of vessels | 2 |
| >2/3 of vessels | 3 |
| Focal hepatocyte plate disruption | |
| None | 0 |
| Present | 1 |
| Parenchymal extinction lesion | |
| None | 0 |
| Rare | 1 |
| Frequent | 2 |
| Nodular regenerative hyperplasia | |
| None | 0 |
| Subtle | 1 |
| Obvious | 2 |
| Peliosis | |
| None | 0 |
| Present | 1 |
| Veno-occlusive disease-like change | |
| None | 0 |
| Present | 1 |
Statistical analysis
Quantitative variables were expressed as the median and interquartile range (IQR). Summary statistics were compared in univariate analysis using Fisher's exact test, Mann–Whitney test or Kruskal–Wallis test as appropriate. P-values derived from multiple comparison tests were adjusted according to Bonferroni correction. Associations between quantitative variables were tested using Spearman correlations. All data were analysed using IBM® SPSS® Statistics Version 20 (IBM Corp., Armonk, NY, USA). The receiver operating characteristic (ROC) method was used to evaluate haemodynamic variables and their combinations in a predictive model associated with SI by calculating the area under the ROC curve, sensitivity, specificity and their 95% confidence intervals (CIs) using R software Version 2.14.1 [R Foundation for Statistical Computing (http://www.r-project.org/)]. A two-sided P-value of <0.05 was considered to indicate statistical significance.
Results
Patients and clinical data
During the study period, 105 patients were scheduled for CRLM resection. The number of patients included was mostly restricted by limits to access to the MRI facility for research purposes. Thirty patients were not eligible because their planned liver resections were small and three patients were excluded for contraindications to MRI. A total of 33 patients were examined by MRIF and 30 fulfilled the MRI protocol. Appropriate liver tissue samples were obtained in 29 patients (20 male, nine female) and were analysed in the study. The most important clinical characteristics of these patients are demonstrated in Table 2. Three patients had diabetes mellitus. A total of 21 patients had received oxaliplatin-based chemotherapy, seven had received irinotecan-based chemotherapy, mostly in combination with 5-fluorouracil/leucovorin (FLV) or Xeloda, and two patients had received FLV alone. Five patients had received no chemotherapy.
Table 2.
Clinical characteristics of patients in the three study groups defined according to whether or not patients received oxaliplatin-based chemotherapy and experienced sinusoidal injury
| Characteristic | All (n= 29) | No-OX (n= 8) | OX + no-SI (n= 15) | OX + SI (n= 6) | P-valuea |
|---|---|---|---|---|---|
| Age, years, median (IQR) | 62 (56–71) | 73 (61–77) | 59 (55–66) | 57 (55–63) | 0.044 |
| Body mass index, kg/m2, median (IQR) | 27.2 (25.1–29.8) | 27.8 (24.2–37.4) | 27.8 (26.0–30.0) | 25.9 (23.7–27.3) | 0.270 |
| Synchronous CRLM, n | 16 | 2 | 9 | 5 | 0.082 |
| Number of tumours, median (IQR) | 2 (1–3) | 1 (1–3) | 2 (1–4) | 3 (1–6) | 0.209 |
| Maximal tumour diameter, mm, median (IQR) | 25 (19–50) | 23 (20–54) | 25 (20–49) | 20 (10–60) | 0.687 |
| Platelet count, ×109/l, median (IQR) | 236 (203–270) | 257 (224–308) | 237 (200–281) | 206 (190–212) | 0.064 |
| Number of OX cycles, median (IQR) | 5 (4–7) | – | 5 (4–7) | 6 (4–7) | 0.843 |
| Interval between OX treatment and surgery, weeks, median (IQR) | 6 (4–34) | – | 6 (4–29) | 18 (4–109) | 0.613 |
Kruskal–Wallis test was used to compare the distributions of continuous variables and the chi-squared test was used to compare nominal variables among the three groups.
No-OX, patients who did not receive oxaliplatin-based chemotherapy; OX + no-SI, patients who received oxaliplatin-based chemotherapy without sinusoidal injury; OX + SI, patients who received oxaliplatin-based chemotherapy with sinusoidal injury; IQR, interquartile range; CRLM, colorectal liver metastasis.
Surgeries included 23 major resections (more than three segments) and six resections of two segments. No mortality was observed within 90 days of surgery. Morbidity defined as a Clavien score26 of ≥3b was observed in four of 29 patients and consisted of biliary leakages (n= 2), liver failure (n= 1) and haemorrhage (n= 1).
Histopathology
Histopathological analysis observed SI defined as a CVI score of ≥3 (Table 1) in six of 29 patients. The median CVI score in the group of patients with SI was 5 (IQR: 4–6). Twenty patients had no fibrosis at all. The remaining nine patients had zone 3 perisinusoidal fibrosis that was mild (n= 8) (stage 1a) or moderate (n= 1) (stage 1b). All patients with SI had received oxaliplatin-based chemotherapy; however, Fisher's exact test failed to prove a significant association (P= 0.148). In order to make further comparisons, three groups of patients were defined as, respectively: patients who had not received oxaliplatin-based chemotherapy (no-OX); patients who had received oxaliplatin-based therapy without SI (OX + no-SI), and patients who had received oxaliplatin-based therapy with SI (OX + SI) (Tables 2 and 3). Patients who had not received oxaliplatin-based chemotherapy were older than those treated with oxaliplatin (P= 0.044) as a result of selection prior to treatment.
Table 3.
Results of magnetic resonance imaging flowmetry in the three study groups defined according to whether or not patients received oxaliplatin-based chemotherapy and experienced sinusoidal injury
| Characteristic | All (n= 29) | No-OX (n= 8) | OX + no-SI (n= 15) | OX + SI (n= 6) | P-valuea |
|---|---|---|---|---|---|
| Portal flow, ml/s, median (IQR) | 17.9 (13.5–20.8) | 15.5 (11.0–19.1) | 19.6 (16.2–21.3) | 19.8 (14.8–23.2) | 0.241 |
| Cross-section area, cm2, median (IQR) | 1.5 (1.2–1.8) | 1.2 (0.9–1.2) | 1.7 (1.2–2.0) | 1.7 (1.6–2.1) | 0.005 |
| Mean velocity, cm/s, median (IQR) | 14.0 (12.2–16.3) | 16.3 (13.4–18.5) | 14.3 (12.7–17.4) | 12.0 (10.2–13.5) | 0.060 |
| Peak velocity, cm/s, median (IQR) | 23.2 (19.9–26.6) | 25.5 (20.3–29.3) | 24.3 (21.4–26.5) | 19.6 (18.2–26.4) | 0.311 |
Kruskal–Wallis test was used to compare distributions among the three groups.
No-OX, patients who did not receive oxaliplatin-based chemotherapy; OX + no-SI, patients who received oxaliplatin-based chemotherapy without sinusoidal injury; OX + SI, patients who received oxaliplatin-based chemotherapy with sinusoidal injury; IQR, interquartile range.
Blood tests
All patients underwent routine blood tests [aspartate aminotransferase (AST), alanine aminotransferase, alkaline phosphatase, bilirubin, prothrombin time and platelet count] in which results were found to be within the normal reference intervals the day before surgery. Prothrombin time showed a statistically significant (P= 0.040) but clinically unimportant difference in distribution among the groups but remained within the normal reference interval. A trend towards a decreased platelet count (P= 0.064) was observed across the groups; however, this parameter also remained within the normal reference interval (Table 2).
Portal vein haemodynamics as measured by MRIF
The portal vein cross-section area differed significantly among the groups (P= 0.005) (Table 3). Portal vein dilatation was observed in the OX + SI group (Bonferroni corrected P= 0.003) and the OX + no-SI group (Bonferroni corrected P= 0.039) compared with the no-OX group. However, no difference was observed between the OX + SI and OX + no-SI groups (Fig. 2).
Figure 2.
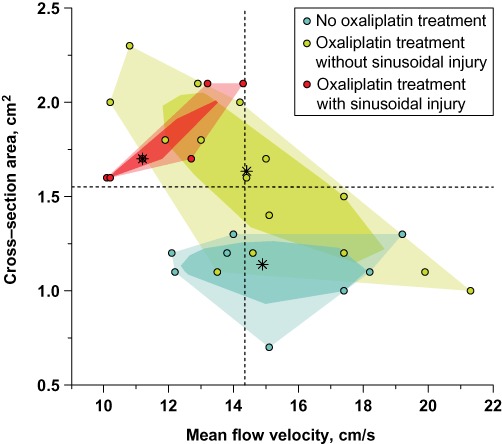
Bagplot for mean portal flow velocity and cross-section area. A bagplot is a bivariate generalization of the boxplot. For each of the patient groups, the two-dimensional median (*) is approximated; the darker convex polygon, called the ‘bag’, contains 50% of all points and the lighter convex hull contains all the points. Actual values are shown as points on an ordinary scatterplot. Dotted lines shows best performing threshold values for mean portal flow velocity (14.35 cm/s) and cross-section area (1.55 cm2) estimated by receiver operating characteristic analysis
Mean portal vein blood flow velocity showed a tendency to differ among the groups (P= 0.060). The OX + SI group showed a tendency towards a decreased mean flow velocity compared with the OX + no-SI group (Bonferroni corrected P= 0.087) (Fig. 2). No differences between the OX + no-SI and OX + SI groups compared with the no-OX group were observed (Bonferroni corrected P= 1.000 and P= 0.129, respectively).
Portal blood flow and peak portal blood flow velocity did not differ among the groups (P= 0.241 and P= 0.311, respectively).
No significant correlation was observed between fasting time and measured haemodynamic variables although fasting time varied among patients with median 6.5 h (IQR: 4.9–8.0 h).
Diagnostic accuracy of MRIF
The diagnostic accuracy of MRIF was evaluated in all patients with CVI scores of ≥3 and < 3, respectively, because the present data were unable to show a significant association between oxaliplatin-based treatment and SI. The ROC method identified best discriminating threshold values for haemodynamic variables (Table 4). The combination of mean flow velocity and cross-section area used in the SI prediction model (Fig. 3) showed the highest discriminative ability according to the area under the ROC curve, but the present data were unable to demonstrate significant differences in performance among variables and their combinations. According to this model, in patients with a mean flow velocity of ≤14.35 cm/s and a cross-section area of ≥1.55 cm2, sensitivity was 100%, specificity reached 78%, the positive predictive value reached 50% and the negative predictive value reached 100% for a CVI score of ≥3 (Table 4).
Table 4.
Receiver operating characteristic analysis results for predicting a combined vascular injury score of ≥3
| Variable and threshold | AUC (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | PPV | NPV |
|---|---|---|---|---|---|
| Cross-section area ≥1.55 cm2 | 0.775 (0.603–0.948) | 100 (100–100) | 65 (48–83) | 43 | 100 |
| Mean velocity ≤14.35 cm/s | 0.815 (0.637–0.994) | 100 (100–100) | 52 (30–74) | 35 | 100 |
| Mean velocity ≤14.35 cm/s and cross-section area ≥1.55 cm2 | 0.855 (0.718–0.993) | 100 (100–100) | 78 (61–96) | 50 | 100 |
AUC, area under the receiver operating characteristic curve; 95% CI, 95% confidence interval; PPV, positive predictive value; NPV, negative predictive value.
Figure 3.
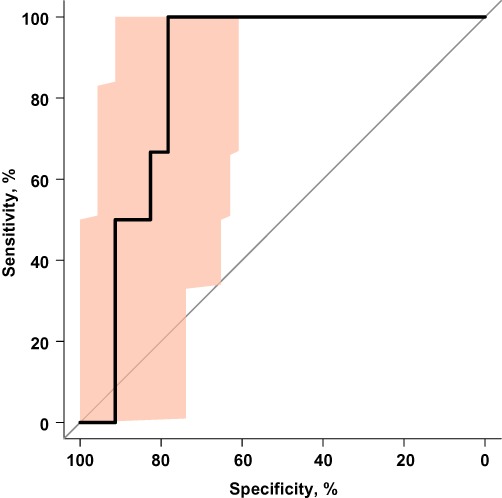
Receiver operating characteristic (ROC) curve for sinusoidal injury (defined as a combined vascular injury score of ≥3) prediction utilizing the cross-section area and mean portal flow velocity measured by magnetic resonance imaging flowmetry. Shading indicates the 95% confidence interval of the ROC curve
Discussion
The present authors believe this to be the first study to utilize a non-invasive method, MRIF, to evaluate changes in portal vein haemodynamic variables associated with oxaliplatin-based chemotherapy for CRLM with or without SI. The present data demonstrate that oxaliplatin-based chemotherapy is associated with portal vein dilatation measured by MRIF. In patients who developed SI (CVI scores: ≥3), portal vein dilatation and a trend towards a non-significant decrease in mean portal vein flow velocity was observed, but no changes in portal vein flow were seen.
The present MRIF findings are consistent with the proposed model for SI effects after oxaliplatin-based treatment (Fig. 4).21 Portal vein dilatation alone and in combination with a trend towards a change in mean flow velocity can be interpreted as an indirect sign of higher liver parenchyma resistance to blood flow in the sinusoids. Increased liver parenchyma resistance is usually documented in cases of high-grade fibrosis and cirrhosis,23 which were not observed in the present data, which supports the role of SI in the changes observed in portal vein haemodynamics. The present authors are unaware of any other reports of directly measured portal dilatation in association with oxaliplatin-based treatment or SI. An increase in spleen size after oxaliplatin-based chemotherapy20 as a consequence of SI16 may be regarded as an indirect sign of congestion in the portal system (Fig. 4). This reversible, oxaliplatin dose-dependent increase in splenic size is also documented as one of the reasons for associated thrombocytopoenia,16,20 which directly influences the APRI value, another predictor of SI.17,27 Cases of clinically manifested symptoms21,28 and liver vein wedge catheter-verified portal hypertension22 have been reported after oxaliplatin-based chemotherapy induced SI. The present findings support the hypothesis that oxaliplatin-based treatment and subsequent SI lead to blood congestion in the portal system. Portal haemodynamics are balanced in a ‘new’ steady state, maintaining equivalent liver perfusion with an unchanged portal blood flow.
Figure 4.
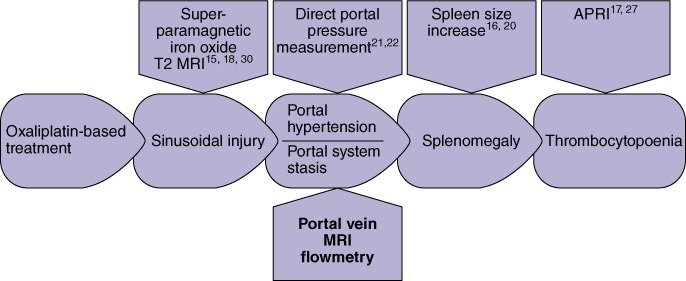
Theoretical model of sinusoidal injury effects caused by oxaliplatin-based treatment with methods for sinusoidal injury prediction. APRI, aspartate aminotransferase to platelet ratio index; MRI, magnetic resonance imaging
Recent clinical studies indicate that SI after oxaliplatin-based chemotherapy in patients undergoing liver resection for CRLM is associated with increased postoperative morbidity.9,11 The accurate and sensitive non-invasive prediction of SI may change strategies for the management of CRLM patients. Addition of MRIF portal flow measurement to routine tumour response evaluation may represent an affordable clinical tool with which to optimize the number of cycles of chemotherapy administered before liver surgery and the timing of liver surgery after chemotherapy.
Several clinical studies have tried to identify predictive factors associated with the development of SI after oxaliplatin-based therapy. The duration of chemotherapy,9,11 age,29 female sex,11 preoperatively elevated AST,11 GGT,12,14 initial non-resectability and synchronous metastases,14 tumour size of > 5 cm and positive nodes with primary tumour12 are associated with a high risk for the development of SI. The low sensitivities and specificities of these variables make them less useful for SI prediction. However, the APRI is a more sensitive and specific independent predictor of SI caused by oxaliplatin-based chemotherapy.17 The APRI is also reported to be a predictor of risk for oxaliplatin-induced splenomegaly before chemotherapy is started.27 Various reports on radiological methods utilizing superparamagnetic iron oxide-enhanced T2 MRI in SI prediction show wide discrepancy in their sensitivity and specificity results, which range from 87% and 89%,18 to 14% and 100%,15 to 75% and 100%30 for sensitivity and specificity, respectively. Doppler ultrasonography can evaluate portal flow haemodynamics, but interobserver variability and differences in patient anatomy and investigational conditions may cause non-reproducibility. Such drawbacks are eliminated by the more objective MRIF23 used in the present study. Portal vein dilatation and changes in mean flow velocity as measured by MRIF are sensitive indicators of SI, although they are less specific than the methods mentioned earlier.
There are several limitations to the present study. By contrast with other studies, the current study failed to show a direct association between oxaliplatin-based treatment and SI,7,8 probably as a result of its small sample size. However, this was not the aim of the study. In the present study, all of the patients with SI had received oxaliplatin-based chemotherapy, as they had in a large histopathological study conducted by Rubbia-Brandt et al.7 However, the period between the end of oxaliplatin treatment and liver surgery was relatively long in the present study (Table 2). It remains unclear how long the SI effect lasts after chemotherapy, but it is probably much longer than the commonly reported period of 5–6 weeks after neoadjuvant treatment. An increase in spleen size of ≥50% is strongly correlated with the occurrence of SI and remains for 6 months after cessation of oxaliplatin.16 Ryan et al. observed CVI scores of ≥3 in 11% of patients who did not receive any chemotherapy in the year prior to liver surgery, but did not describe their patients' past history of chemotherapy treatment.8 The analysis of diagnostic efficacy using ROC curves can be perceived as another drawback of the present study. Sensitivity and specificity values estimated by ROC analysis should be interpreted according to the terms of their confidence intervals, rather than as absolute values, given the relatively small sample size (Table 4).
Surveillance by MRIF of changes in portal vein haemodynamics is needed to be performed before, during and after oxaliplatin-based chemotherapy. The dynamic of any such changes can verify whether the onset of SI in the liver parenchyma is associated with the lowering of mean portal flow velocity in the dilated portal vein. The potential benefits of the method proposed should be studied in other prospective clinical studies.
In conclusion, oxaliplatin-based chemotherapy for CRLM, with or without SI, is associated with portal vein dilatation. In patients in whom SI develops, portal vein dilatation, together with a tendency towards a decreased mean portal flow velocity, was observed as a marker of changes in portal vein haemodynamics, whereas portal flow remained unchanged. This may indicate that oxaliplatin-based chemotherapy and subsequent SI are associated with an increase in liver parenchyma resistance to blood flow. The evaluation of portal vein haemodynamics by MRIF can identify patients without risk for SI with high accuracy in a non-invasive manner.
Acknowledgments
This project was funded by Avtal om Läkarutbildning och Forskning grants from the Departments of Surgery and Diagnostic Radiology, Uppsala University Hospital.
Conflicts of interest
None declared.
References
- 1.Lehmann K, Rickenbacher A, Weber A, Pestalozzi BC, Clavien PA. Chemotherapy before liver resection of colorectal metastases: friend or foe? Ann Surg. 2012;255:237–247. doi: 10.1097/SLA.0b013e3182356236. [DOI] [PubMed] [Google Scholar]
- 2.Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomized controlled trial. Lancet. 2008;371:1007–1016. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blazer DG, 3rd, Kishi Y, Maru DM, Kopetz S, Chun YS, Overman MJ, et al. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol. 2008;26:5344–5351. doi: 10.1200/JCO.2008.17.5299. [DOI] [PubMed] [Google Scholar]
- 4.Benoist S, Brouquet A, Penna C, Julie C, El Hajjam M, Chagnon S, et al. Complete response of colorectal liver metastases after chemotherapy: does it mean cure? J Clin Oncol. 2006;24:3939–3945. doi: 10.1200/JCO.2006.05.8727. [DOI] [PubMed] [Google Scholar]
- 5.Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065–2072. doi: 10.1200/JCO.2005.05.3074. [DOI] [PubMed] [Google Scholar]
- 6.Rubbia-Brandt L, Audard V, Sartoretti P, Roth AD, Brezault C, Le Charpentier M, et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460–466. doi: 10.1093/annonc/mdh095. [DOI] [PubMed] [Google Scholar]
- 7.Rubbia-Brandt L, Lauwers GY, Wang H, Majno PE, Tanabe K, Zhu AX, et al. Sinusoidal obstruction syndrome and nodular regenerative hyperplasia are frequent oxaliplatin-associated liver lesions and partially prevented by bevacizumab in patients with hepatic colorectal metastasis. Histopathology. 2010;56:430–439. doi: 10.1111/j.1365-2559.2010.03511.x. [DOI] [PubMed] [Google Scholar]
- 8.Ryan P, Nanji S, Pollett A, Moore M, Moulton CA, Gallinger S, et al. Chemotherapy-induced liver injury in metastatic colorectal cancer: semiquantitative histologic analysis of 334 resected liver specimens shows that vascular injury but not steatohepatitis is associated with preoperative chemotherapy. Am J Surg Pathol. 2010;34:784–791. doi: 10.1097/PAS.0b013e3181dc242c. [DOI] [PubMed] [Google Scholar]
- 9.Aloia T, Sebagh M, Plasse M, Karam V, Levi F, Giacchetti S, et al. Liver histology and surgical outcomes after preoperative chemotherapy with fluorouracil plus oxaliplatin in colorectal cancer liver metastases. J Clin Oncol. 2006;24:4983–4990. doi: 10.1200/JCO.2006.05.8156. [DOI] [PubMed] [Google Scholar]
- 10.Karoui M, Penna C, Amin-Hashem M, Mitry E, Benoist S, Franc B, et al. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases. Ann Surg. 2006;243:1–7. doi: 10.1097/01.sla.0000193603.26265.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakano H, Oussoultzoglou E, Rosso E, Casnedi S, Chenard-Neu MP, Dufour P, et al. Sinusoidal injury increases morbidity after major hepatectomy in patients with colorectal liver metastases receiving preoperative chemotherapy. Ann Surg. 2008;247:118–124. doi: 10.1097/SLA.0b013e31815774de. [DOI] [PubMed] [Google Scholar]
- 12.Tamandl D, Klinger M, Eipeldauer S, Herberger B, Kaczirek K, Gruenberger B, et al. Sinusoidal obstruction syndrome impairs longterm outcome of colorectal liver metastases treated with resection after neoadjuvant chemotherapy. Ann Surg Oncol. 2011;18:421–430. doi: 10.1245/s10434-010-1317-4. [DOI] [PubMed] [Google Scholar]
- 13.Pawlik TM, Olino K, Gleisner AL, Torbenson M, Schulick R, Choti MA. Preoperative chemotherapy for colorectal liver metastases: impact on hepatic histology and postoperative outcome. J Gastrointest Surg. 2007;11:860–868. doi: 10.1007/s11605-007-0149-4. [DOI] [PubMed] [Google Scholar]
- 14.Brouquet A, Benoist S, Julie C, Penna C, Beauchet A, Rougier P, et al. Risk factors for chemotherapy-associated liver injuries: a multivariate analysis of a group of 146 patients with colorectal metastases. Surgery. 2009;145:362–371. doi: 10.1016/j.surg.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 15.O'Rourke TR, Welsh FK, Tekkis PP, Lyle N, Mustajab A, John TG, et al. Accuracy of liver-specific magnetic resonance imaging as a predictor of chemotherapy-associated hepatic cellular injury prior to liver resection. Eur J Surg Oncol. 2009;35:1085–1091. doi: 10.1016/j.ejso.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Overman MJ, Maru DM, Charnsangavej C, Loyer EM, Wang H, Pathak P, et al. Oxaliplatin-mediated increase in spleen size as a biomarker for the development of hepatic sinusoidal injury. J Clin Oncol. 2010;28:2549–2555. doi: 10.1200/JCO.2009.27.5701. [DOI] [PubMed] [Google Scholar]
- 17.Soubrane O, Brouquet A, Zalinski S, Terris B, Brezault C, Mallet V, et al. Predicting high grade lesions of sinusoidal obstruction syndrome related to oxaliplatin-based chemotherapy for colorectal liver metastases: correlation with post-hepatectomy outcome. Ann Surg. 2010;251:454–460. doi: 10.1097/SLA.0b013e3181c79403. [DOI] [PubMed] [Google Scholar]
- 18.Ward J, Guthrie JA, Sheridan MB, Boyes S, Smith JT, Wilson D, et al. Sinusoidal obstructive syndrome diagnosed with superparamagnetic iron oxide-enhanced magnetic resonance imaging in patients with chemotherapy-treated colorectal liver metastases. J Clin Oncol. 2008;26:4304–4310. doi: 10.1200/JCO.2008.16.1893. [DOI] [PubMed] [Google Scholar]
- 19.Zorzi D, Laurent A, Pawlik TM, Lauwers GY, Vauthey JN, Abdalla EK. Chemotherapy-associated hepatotoxicity and surgery for colorectal liver metastases. Br J Surg. 2007;94:274–286. doi: 10.1002/bjs.5719. [DOI] [PubMed] [Google Scholar]
- 20.Angitapalli R, Litwin AM, Kumar PR, Nasser E, Lombardo J, Mashtare T, et al. Adjuvant FOLFOX chemotherapy and splenomegaly in patients with stages II–III colorectal cancer. Oncology. 2009;76:363–368. doi: 10.1159/000210025. [DOI] [PubMed] [Google Scholar]
- 21.Slade JH, Alattar ML, Fogelman DR, Overman MJ, Agarwal A, Maru DM, et al. Portal hypertension associated with oxaliplatin administration: clinical manifestations of hepatic sinusoidal injury. Clin Colorectal Cancer. 2009;8:225–230. doi: 10.3816/CCC.2009.n.038. [DOI] [PubMed] [Google Scholar]
- 22.Tisman G, MacDonald D, Shindell N, Reece E, Patel P, Honda N, et al. Oxaliplatin toxicity masquerading as recurrent colon cancer. J Clin Oncol. 2004;22:3202–3204. doi: 10.1200/JCO.2004.99.106. [DOI] [PubMed] [Google Scholar]
- 23.Nanashima A, Shibasaki S, Sakamoto I, Sueyoshi E, Sumida Y, Abo T, et al. Clinical evaluation of magnetic resonance imaging flowmetry of portal and hepatic veins in patients following hepatectomy. Liver Int. 2006;26:587–594. doi: 10.1111/j.1478-3231.2006.01273.x. [DOI] [PubMed] [Google Scholar]
- 24.Urdzik J, Bjerner T, Wanders A, Weis J, Duraj F, Haglund U, et al. The value of preoperative magnetic resonance spectroscopy in the assessment of steatohepatitis in patients with colorectal liver metastasis. J Hepatol. 2012;56:640–646. doi: 10.1016/j.jhep.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for non-alcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 26.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miura K, Nakano H, Sakurai J, Kobayashi S, Koizumi S, Arai T, et al. Splenomegaly in FOLFOX-naive stage IV or recurrent colorectal cancer patients due to chemotherapy-associated hepatotoxicity can be predicted by the aspartate aminotransferase to platelet ratio before chemotherapy. Int J Clin Oncol. 2011;16:257–263. doi: 10.1007/s10147-010-0176-0. [DOI] [PubMed] [Google Scholar]
- 28.Agarwal V, Sgouros J, Smithson J, Lodge JP, Razack A, Campbell A, et al. Sinusoidal obstruction syndrome (veno-occlusive disease) in a patient receiving bevacizumab for metastatic colorectal cancer: a case report. J Med Case Reports. 2008;2:227. doi: 10.1186/1752-1947-2-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kandutsch S, Klinger M, Hacker S, Wrba F, Gruenberger B, Gruenberger T. Patterns of hepatotoxicity after chemotherapy for colorectal cancer liver metastases. Eur J Surg Oncol. 2008;34:1231–1236. doi: 10.1016/j.ejso.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 30.Shin NY, Kim MJ, Lim JS, Park MS, Chung YE, Choi JY, et al. Accuracy of gadoxetic acid-enhanced magnetic resonance imaging for the diagnosis of sinusoidal obstruction syndrome in patients with chemotherapy-treated colorectal liver metastases. Eur Radiol. 2012;22:864–871. doi: 10.1007/s00330-011-2333-x. [DOI] [PubMed] [Google Scholar]


