Abstract
Objectives
The optimal incision for liver resection in living donors or patients with small tumours should be revisited. This study introduces the upper midline incision (UMI) above the umbilicus for various liver resections using a conventional open-surgery technique.
Methods
A retrospective study based on a prospectively collected database of 308 liver resections performed by a single surgeon was conducted to evaluate the feasibility, safety and applicability of the UMI.
Results
From September 2006 to September 2010, this incision was used successfully in 308 consecutive liver resections in all patients with tumours measuring ≤ 5 cm and all living donors without any extension of the incision. The median length of the incision was 16.4 cm (range: 12–20 cm).The median operating time was 189 min (range: 54–305 min). The median postoperative hospital stay was 8 days (range: 6–17 days). One patient died in the postoperative period from heart failure. All other patients fully recovered and returned to their previous level of activity. Over a median follow-up of 31 months (range: 20–68 months), 25 complications (8.1%) developed. Seven wound infections (2.3%) occurred with no incisional hernia.
Conclusions
The UMI can be used safely and effectively in conventional open surgery in various liver resections and should therefore be given priority as the first-line technique in living liver donors and patients with tumours measuring ≤ 5 cm.
Introduction
Liver resection to remove tumours or to source grafts from living donors is nowadays performed worldwide. The incisions most commonly used have included a bilateral rooftop incision with or without a vertical extension, a J-shaped incision and a reverse L-shaped incision with or without a left extension.1,2 The type of incision selected depends on the circumstances. In the presence of a large tumour or when bleeding near the inferior vena cava requires to be controlled, thoracic extension may be necessary. The importance of extending the incision without hesitation in order to increase safety and obtain a radical resection cannot be overemphasized. However, as long as safety and the completeness of resection are secured, a shorter length of incision will facilitate better patient recovery.
A laparoscopic approach to hepatectomy in living donors and patients with hepatic tumours has been shown to decrease morbidity and the invasiveness of liver resection.3–6 However, this approach remains feasible only in selected patients and donors, and is complex and expensive because it requires the surgeon to be conversant with both hepatectomy and laparoscopic surgery.
Initial experiences in the use of an upper midline incision (UMI) in living donor right hepatectomy and liver resection combined with laparoscopy-assisted colorectal resection have shown the UMI to be feasible, safe and effective.7,8 Subsequently, at this institution, the UMI has been successfully applied in various types of liver resection in all patients with tumours measuring ≤ 5 cm and in all living donors consecutively, without any laparoscopic assistance.
This paper reports on a single surgeon's experience of using the UMI above the umbilicus in various liver resections in conventional open surgery.
Materials and methods
A retrospective study based on a prospectively collected database of 328 liver resections performed by a single attending surgeon (SHK) at the National Cancer Centre, South Korea, from September 2006 to September 2010, was performed to evaluate the feasibility and safety of the UMI above the umbilicus. During a median follow-up of 31 months (range: 20–68 months), all complications were recorded prospectively and stratified according to Clavien's system of classification. Postoperative mortality was defined as death within 90 days of surgery.9
Subjects
The criteria indicating the use of a UMI in liver resection were a tumour size of ≤ 5 cm and living-donor donation (Fig. 1a). Cirrhosis, previous abdominal surgery, the presence of multiple tumours and a high body mass index (BMI) of > 30 kg/m2 were not considered as exclusion criteria. However, 20 patients were excluded from this study because they demonstrated tumours of > 5 cm in size or tumour invasion of the diaphragm; in these patients, a reverse L incision with or without a left extension was used in order to avoid iatrogenic tumour rupture during liver mobilization and to circumvent the difficulty of extracting resected liver through a smaller-than-usual incision (Fig. 1b).
Figure 1.

Operative wounds after right hepatectomy using (a) an upper midline incision and (b) a reverse L incision
Postoperative i.v. analgesia with fentanyl and morphine was administered to all donors and patients using a patient-controlled pump.
Surgical techniques
A detailed technical description of liver resection under a short UMI in living donors and patients with liver metastasis of colorectal cancer has been described previously.7,8
Briefly, this incision extended from the lower end of the xiphoid process to above the umbilicus. The vertical midline laparotomy was performed before a Kent retractor frame (Takasago Medical Industry Co., Tokyo, Japan) was installed to keep the operative field wide open. To mobilize the right liver, which is the key and difficult part of this technique, the left retractor blade was fastened a little higher than the right blade and the upper and lateral ligaments were dissected before the lower ligaments so that the right liver could be easily displaced into the left upper quadrant of the abdomen (Fig. 2).
Figure 2.
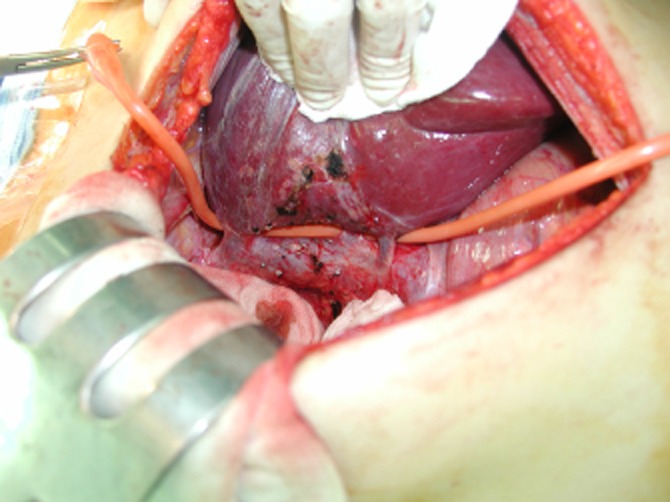
After right liver mobilization saving two sizable right inferior hepatic veins, a Nelaton catheter for the hanging manoeuvre is placed along the anteromedian surface of the inferior vena cava with its upper end between the right and middle hepatic veins and its lower end on the left side of the two right inferior hepatic veins
Other than the incision, the remaining components of the surgery (Fig. 3) were performed in line with the conventional open technique previously reported.10 Liver parenchymal transection was performed using the ultrasonic dissection device. For right hepatectomy, the right liver was mobilized before the parenchymal transection in all living donors and most tumour patients. However, in patients with tumours measuring 4–5 cm and located near the liver surface, parenchymal transection was performed before liver mobilization using an anterior approach to avoid tumour rupture.
Figure 3.

Hilar dissection into the three Glisson's pedicles (right anterior, right posterior, left) under an upper midline incision above the umbilicus. Each Glisson's pedicle was taped
Statistical analysis
Continuous variables are presented as medians and ranges.
Results
From September 2006 to September 2010, this UMI was used successfully in 308 liver resections in a conventional open-surgery technique in 160 patients with tumours of ≤ 5 cm in size and 148 consecutive living liver donors without any extension of the incision. The median length of incision was 16.4 cm (range: 12–20 cm).
The preoperative characteristics of patients and donors are shown in Table 1. Operative outcomes, including operation time, blood loss, intraoperative blood transfusion, hospital stay and days of analgesia use, are provided in Table 2. Most of the resections (n = 304, 98.7%) were anatomical and used the hanging manoeuvre previously applied in various liver resections.10
Table 1.
Preoperative characteristics of 148 living donors and 160 tumour patients undergoing resection using an upper midline incision
| Living donors (n = 148) | Patients (n = 160) | |
|---|---|---|
| Gender, male : female | 101:47 | 110:50 |
| Age, years | ||
| Mean ± SD | 31.3 ± 9.8 | 54.2 ± 10.7 |
| Median | 30 | 55 |
| Range | 17–60 | 27–79 |
| Body weight, kg | ||
| Mean ± SD | 66.1 ± 9.7 | 65.2 ± 11.0 |
| Median | 65.4 | 65.5 |
| Range | 42.0–99.0 | 42.0–98.8 |
| Body mass index, kg/m2 | ||
| Mean ± SD | 24.4 ± 3.8 | 25.3 ± 4.1 |
| Median | 24.3 | 25.7 |
| Range | 15.2–36.0 | 16.2–38.0 |
| Benign diseases, n (%) | 2 (1.4%) | 5 (3.1%) |
| Cyst | 1 (0.7%) | 1 (0.6%) |
| Haemangioma | 1 (0.7%) | 1 (0.6%) |
| Adenoma | 0 | 1 (0.6%) |
| Intrahepatic duct stone | 0 | 2 (1.3%) |
| Malignant diseases | 0 | 155 (96.9%) |
| Hepatocellular carcinoma | 0 | 108 (67.5%) |
| Colorectal liver metastasis | 0 | 34 (21.3%) |
| Cholangiocarcinoma | 0 | 7 (4.4%) |
| Gall bladder cancer | 0 | 5 (3.1%) |
| Haemangioendothelioma | 0 | 1 (0.6%) |
| Child–Pugh class | ||
| A | 148 (100%) | 157 (98.1%) |
| B | 0 | 3 (1.9%) |
| Liver cirrhosis | 0 | 101 (63.1%) |
| Previous abdominal operation | 3 (2.0%) | 20 (12.5%) |
| Cholecystectomy | 1 (0.7%) | 1 (0.6%) |
| Appendectomy | 2 (1.4%) | 1 (0.6%) |
| Pancreaticoduodenectomy | 0 | 1 (0.6%) |
| Liver resection | 0 | 10 (6.3%) |
| Colorectal resection | 0 | 6 (3.8%) |
| Gastrectomy | 0 | 1 (0.6%) |
SD, standard deviation.
Table 2.
Operative outcomes in 148 living donors and 160 tumour patients undergoing resection using an upper midline incision
| Living donors (n = 148) | Patients (n = 160) | |
|---|---|---|
| Types of liver resection, n (%) | ||
| Right hepatectomy without MHV | 138 (93.2%) | 65 (40.6%) |
| Right hepatectomy with MHV | 8 (5.4%) | 5 (3.1%) |
| Right posterior sectionectomy without RHV | 0 | 15 (9.4%) |
| Right posterior sectionectomy with RHV | 0 | 13 (8.1%) |
| Right anterior sectionectomy | 0 | 4 (2.5%) |
| Left medial sectionectomy | 0 | 1 (0.6%) |
| Central bisectionectomy | 0 | 2 (1.3%) |
| Right trisectionectomy | 0 | 3 (1.9%) |
| Left hepatectomy without MHV | 0 | 22 (13.8%) |
| Left hepatectomy with MHV | 1 (0.7%) | 9 (5.6%) |
| Left hepatectomy with caudate lobe and MHV | 1 (0.7%) | 3 (1.9%) |
| Left lateral sectionectomy | 0 | 5 (3.1%) |
| Caudate lobectomy | 0 | 2 (1.3%) |
| Bisegmentectomy (segments V and VI) | 0 | 7 (4.4%) |
| Tumorectomy within one segment | 0 | 4 (2.5%) |
| Operation time, min | ||
| Mean ± SD | 213.2 ± 37.7 | 170.2 ± 37.7 |
| Median | 208 | 168 |
| Range | 156–305 | 54–260 |
| Blood loss, ml | ||
| Mean ± SD | 311.4 ± 145.5 | 552.4 ± 215.5 |
| Median | 300 | 575 |
| Range | 100–600 | 100–1000 |
| Intraoperative blood transfusion, n | 0 | 0 |
| Intensive care unit stay, days | ||
| Mean ± SD | 0.1 ± 0.5 | 0.2 ± 0.6 |
| Median | 0 | 0 |
| Range | 0–2 | 0–4 |
| Postoperative hospital stay, days | ||
| Mean ± SD | 7.9 ± 1.7 | 8.3 ± 1.9 |
| Median | 7 | 8 |
| Range | 7–17 | 6–17 |
| Period of analgesia use, days | ||
| Mean ± SD | 3.1 ± 1.9 | 2.9 ± 2.2 |
| Median | 3 | 3 |
| Range | 1–8 | 2–9 |
MHV, middle hepatic vein; RHV, right hepatic vein; SD: standard deviation.
Overall complications according to the Clavien system of classification are shown in Table 3. The total complication rate was 8.1% (n = 25). The most common complication was wound infection (2.3%, n = 7), which required no antibiotic treatment (Grade I). Three patients showed asymptomatic biliary leakage, which was successfully treated with conservative management (Grade I). Three patients (0.9%) developed postoperative bleeding, which was immediately controlled by re-entry through the same incision (Grade IIIb) or for which they required blood transfusion (Grade II). The bleeding points were the dissected wall of the common bile duct in one patient and the dissected perihepatic ligaments in two. One 70-year-old patient with a pre-existing heart problem required a postoperative blood transfusion (Grade II). One patient underwent mechanical ileus that resolved with bowel rest and hydration (Grade II). Asymptomatic bile collection in two patients was detected in follow-up computed tomography (CT) at 1 month after discharge and resolved with percutaneous catheter drainage (Grade IIIa). One donor underwent video-assisted thoracoscopic surgery as a result of lung collapse caused by pleural effusion that had lasted for 2 months after living donor right hepatectomy (Grade IIIb). One donor underwent a hepaticojejunostomy under the same incision as a result of biliary stricture that failed endoscopic intervention at 2 months after living donor right hepatectomy (Grade IIIb). One patient (0.3%) died from heart failure at 6 days after extended right hepatectomy for hepatocellular carcinoma (Grade V). All other patients fully recovered from this smaller-than-conventional incision and returned to their previous level of activity.
Table 3.
Complications during the median follow-up of 31 months (range: 20–68 months) in 148 living donors and 160 tumour patients undergoing resection using an upper midline incision, graded using the Clavien system
| Grade | Living donors (n = 148), n (%) | Patients (n = 160), n (%) | Total (n = 308), n (%) |
|---|---|---|---|
| I | 4 (2.7%) | 8 (5.0%) | 12 (3.9%) |
| Wound infection, n = 3 | Wound infection, n = 4 | Wound infection, n = 7 | |
| MHV partial thrombosis, n = 1 | Biliary leakage, n = 3 | Biliary leakage, n = 3 | |
| Pleural effusion, n = 1 | MHV partial thrombosis, n = 1 | ||
| Pleural effusion, n = 1 | |||
| II | 2 (1.4%) | 3 (1.9%) | 5 (1.6%) |
| Transfusion, n = 2 | Transfusion, n = 2 | Transfusion, n = 4 | |
| Mechanical ileus, n = 1 | Mechanical ileus, n = 1 | ||
| IIIa | 0 | 2 (1.3%) | 2 (0.6%) |
| Biloma, n = 2 | Biloma, n = 2 | ||
| IIIb | 4 (2.7%) | 1 (0.6%) | 5 (1.6%) |
| Bleeding, n = 2 | Bleeding, n = 1 | Bleeding, n = 3 | |
| Pleural effusion, n = 1 | Pleural effusion, n = 1 | ||
| Biliary stricture, n = 1 | Biliary stricture, n = 1 | ||
| IVa | 0 | 0 | 0 |
| IVb | 0 | 0 | 0 |
| V | 0 | 1 (0.6%) | 0 |
| Heart failure, n = 1 | |||
| Total | 10 (6.8%) | 15 (9.4%) | 25 (8.1%) |
MHV, middle hepatic vein.
The median postoperative follow-up in the 148 donors and five patients with benign disease was 28 months (range: 20–40 months), during which all patients remained well.
The median postoperative follow-up in the 155 patients with malignant disease was 32 months (range: 20–68 months), during which 37 patients developed recurrence in the liver (n = 21), lung (n = 12), bone (n = 3) or lymph node (n = 1). Seven patients died as a result of recurrence.
No incisional hernia was detected by physical examination or CT.
Discussion
In this series of 148 living donors and 160 tumour patients, various liver resections, including right hepatectomy, were successfully performed using a UMI above the umbilicus and a standard open-surgery technique. Furthermore, the UMI was performed in all living donors and all patients with tumours of ≤ 5 cm, irrespective of cirrhosis or previous abdominal, including liver, surgery. Moreover, in this study population, in which BMI ranged from 15.2 kg/m2 to 38.0 kg/m2, almost all types of liver resection were possible. Using only the usual lateral retractor placed through a UMI, it was possible to maintain an adequate surgical field around the right adrenal gland, the hepatocaval junctions of the right, middle and left hepatic veins, and the inferior vena cava. This longitudinal incision runs parallel with various planes of transection for anatomic liver resections and thus the hanging manoeuvre can be applied easily. The essential prerequisite for liver resection is liver mobilization within a limited operative field, which was achieved through coordination between the operator and assistant, and by adjusting the strength and direction of the retracting force on each side of the UMI.
The actual length of the UMI ranged from 12 cm to 20 cm. The minimum size of the abdominal incision depends on the size of resected liver. A larger patient may require a longer incision, but can be operated through a UMI above the umbilicus. The length of this UMI may be somewhat greater than those reported for incisions in patients undergoing laparoscopy-assisted liver resection. However, given that additional incisions for three to five ports are required in the laparoscopic approach, the two techniques show little difference in the total length of the incisions required. In addition, most liver surgeons are more accustomed to operating using an open rather than a laparoscopic technique. Therefore, if the abdomen must be opened to deliver the resected liver and the total length of incisions are comparable in both techniques, the issue of whether the laparoscopic approach or standard open surgery using a midline or transverse incision is superior remains a subject for further study in which the respective outcomes of the two approaches should be compared in terms of feasibility, simplicity, expenditure, intensity of abdominal pain and breadth of application.
Over the last decade, high-quality surveillance facilitated by technological advances in liver imaging techniques has made the early detection of small liver lesions more feasible, especially in hepatocellular carcinoma,11 and liver resection as a curative treatment modality is now more likely to be performed in the majority of patients with liver mass. In view of the present experience of 308 consecutive surgeries performed using a UMI above the umbilicus and current technical progress in liver resection, this UMI is likely to become more widely used in hepatic surgery.
The present results revealed a low rate of wound-related complications such as wound infection (2.3%) and incisional hernia (0%), which is lower than those reported by Togo et al1 (5.4%) or Chang et al2 (10.9%) in patients operated using a reverse L incision. This probably reflects the relative shortness of the straight-line incision used in the UMI.
In the present study, the wide range in operating times in living donors reflected unexpectedly lengthy waiting times for difficult recipient hepatectomy, especially in patients who had a history of previous liver resection, rather than any factors associated with the donor hepatectomy itself. The shortest operating time was 54 min and applied to a patient with hepatic malignancy who underwent left lateral sectionectomy. However, in 11 patients who had undergone previous liver resection or pancreaticoduodenectomy, the additional adhesiolysis around the liver extended operating times up to 260 min.
The present authors were encouraged to use this UMI by the potential learning effect to be derived from experience and recent interest in minimally invasive surgery. Unlike the traditional incisions, the UMI above the umbilicus is one of the most common and familiar incisions in abdominal surgery: it is short, fast, easy to open and close, and spares the nerves supplying the skin and the rectus muscles, and thus is able to reduce operative time and avoid morbidity such as skin numbness, muscle atrophy and postoperative pain caused by right- or left-sided extension. Therefore, the UMI may represent an optimal way to begin a liver resection because it will allow an extension to the right lateral side to be made if necessary.
This study is weakened by its failure to compare outcomes of surgery using the UMI with those of surgery using the conventional reverse L incision (Fig. 1). Initially, a prospective randomized comparison study had been intended, but when the study subjects were preoperatively informed of the smaller-than-usual UMI, they indicated their preference for this incision and refused to be enrolled in a comparison study. However, the large size of the present cohort (n = 308) of liver resections may make up for the shortcomings of this single-arm study.
Last but not least, this use of a conventional open-surgery technique under a UMI above the umbilicus demonstrated good outcomes. At present, the UMI may represent the optimal choice of incision in liver surgery; however, as liver surgery inherently involves an ongoing quest for better modes of incision, the UMI cannot be definitively described as the optimal technique.
Conclusions
The present authors’ experience indicates that the UMI can be used safely and effectively in conventional open surgery in various liver resections and should therefore be considered as the first-line incision of choice, especially in living liver donors and patients with tumours of ≤ 5 cm.
Conflicts of interest
None declared.
References
- 1.Togo S, Nagano Y, Masumoto C, Takakura H, Matsuo K, Takeda K, et al. Outcome of and risk factors for incisional hernia after partial hepatectomy. J Gastrointest Surg. 2008;12:1115–1120. doi: 10.1007/s11605-008-0469-z. [DOI] [PubMed] [Google Scholar]
- 2.Chang SB, Palavecino M, Wray CJ, Kishi Y, Pisters PW, Vauthey JN. Modified Makuuchi incision for foregut procedures. Arch Surg. 2010;145:281–284. doi: 10.1001/archsurg.2010.7. [DOI] [PubMed] [Google Scholar]
- 3.Cherqui D, Soubrane O, Husson E, Barshasz E, Vignaux O, Ghimouz M, et al. Laparoscopic living donor hepatectomy for liver transplantation in children. Lancet. 2002;359:392–396. doi: 10.1016/S0140-6736(02)07598-0. [DOI] [PubMed] [Google Scholar]
- 4.Kurosaki I, Yamamoto S, Kitami C, Yokoyama N, Nakatsuka H, Kobayashi T, et al. Video-assisted living donor hemihepatectomy through a 12-cm incision for adult-to-adult liver transplantation. Surgery. 2006;139:695–703. doi: 10.1016/j.surg.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Koffron AJ, Kung R, Baker T, Fryer J, Clark L, Abecassis M. Laparoscopic-assisted right lobe donor hepatectomy. Am J Transplant. 2006;6:2522–2525. doi: 10.1111/j.1600-6143.2006.01498.x. [DOI] [PubMed] [Google Scholar]
- 6.Nitta H, Sasaki A, Fujita T, Itabashi H, Hoshikawa K, Takahara T, et al. Laparoscopy-assisted major liver resections employing a hanging technique: the original procedure. Ann Surg. 2010;251:450–453. doi: 10.1097/SLA.0b013e3181cf87da. [DOI] [PubMed] [Google Scholar]
- 7.Kim SH, Cho SY, Lee KW, Park SJ, Han SS. Upper midline incision for living donor right hepatectomy. Liver Transpl. 2009;15:193–198. doi: 10.1002/lt.21677. [DOI] [PubMed] [Google Scholar]
- 8.Kim SH, Lim SB, Ha YH, Han SS, Park SJ, Choi HS, et al. Laparoscopic-assisted combined colon and liver resection for primary colorectal cancer with synchronous liver metastases: initial experience. World J Surg. 2008;32:2701–2706. doi: 10.1007/s00268-008-9761-z. [DOI] [PubMed] [Google Scholar]
- 9.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim SH, Park SJ, Lee SA, Lee WJ, Park JW, Hong EK, et al. Various liver resections using hanging manoeuvre by three Glisson's pedicles and three hepatic veins. Ann Surg. 2007;245:201–205. doi: 10.1097/01.sla.0000245516.10349.c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sherman M. Hepatocellular carcinoma: epidemiology, surveillance, and diagnosis. Semin Liver Dis. 2010;30:3–16. doi: 10.1055/s-0030-1247128. [DOI] [PubMed] [Google Scholar]


