Abstract
Objectives
This study describes changes in the survival of patients with hepatocellular carcinoma (HCC) registered with the Scottish Cancer Registry between 1985 and 2008.
Methods
Data on patients diagnosed with HCC were extracted from the Scottish Cancer Registry, along with linked data on treatment and risk factors for liver disease. One-, 3- and 5-year relative survival rates were calculated for each time period and a Cox regression model was used to assess the impact of prior admissions on survival.
Results
The incidence of HCC increased between 1985 and 2008. The proportion of patients with prior alcohol-related admissions rose over the time period studied from 16.0% to 27.1%. Five-year relative survival increased in women between 1985–1989 and 2005–2007 from 0.5% [95% confidence interval (CI) 0.0–3.7] to 10.6% (95% CI 5.2–18.1). In men, 5-year relative survival increased from 0.4% (95% CI 0.2–2.2) to 4.4% (95% CI 1.5–9.9). Regression analysis showed that older age, history of alcohol-related admissions and deprivation were associated with lower survival, and hospitalization for viral hepatitis was associated with higher survival.
Conclusions
Against the background of an increasing incidence of HCC in Scotland, survival times have increased substantially.
Introduction
The annual incidence of and mortality associated with primary hepatic malignancy in the UK has increased over the past two decades.1,2 This is thought to have resulted largely from increasing hepatitis C infections and rising rates of harmful alcohol consumption, both of which exist in the context of an increasing obesity problem.3 Certainly, primary hepatic malignancy is an increasingly important public health problem and has become one of the 20 most frequent cancers in the UK and the 10th most common cause of death by cancer in men in Scotland.1,2 Rising trends in primary liver cancer are not exclusive to the UK. In the USA, liver and bile duct cancer rose from 14th place in 1990 to ninth in 2005 in terms of its contribution to annual cancer-related deaths.4
Prior analyses have shown that survival in primary liver cancer is low; 1-year survival in Scotland was 10% between 1985 and 1990.5 Two major changes in clinical practice may have affected survival in primary liver cancer over the past two decades: an increasing range of treatments for unresectable disease, and increased surveillance for early-stage disease.6 Advances in chemoembolization, ablation and surgical resection techniques have been shown to improve the prospects of surviving this disease considerably.7,8 Although curative rates for these treatments are still low, these procedures have improved the ability to retain liver tissue and thus function. Furthermore, transplant surgery has advanced significantly and the identification of those patients most likely to benefit from liver replacement has resulted in better outcomes.9
This study reviews Scottish cancer registrations of hepatocellular carcinoma (HCC) linked with hospital admissions and death data to determine trends in survival and factors influencing survival.
Materials and methods
Cases of cancer in Scotland are registered through a comprehensive system that collects data from several sources. These are combined to create a single case registration based on the primary cancer type. Data are collected from pathology, radiotherapy, oncology, neuro-oncology and haematology departments. Further data are collected from the national databases of hospital admissions, screening programmes, prospective cancer audits and death registrations. These records are linked by probability matching and merged to create a single record for each cancer diagnosis, which is linked to hospital admissions. Patients seen in private care are also registered on this system.
Individuals aged > 20 years who were registered with a diagnosis of primary liver cancer between 1 January 1985 and 31 December 2008 were selected. These included all patients coded with ICD-9 code 155.0, ICD-10 code C22.0 and corresponding morphology codes ICD-O(1–3) 8170–8176.10 Patients with cholangiocarcinoma [ICD-9 155.1, ICD-10 C22.1 and morphology codes ICD-O(1–3) 8050, 8140–8141, 8160–8161, 8260, 8440, 8480–8500, 8570–8572] were excluded from the analysis of survival, as were patients with unknown morphology in view of the high risk for misclassifying secondary tumours. Patients who were identified by death certificate only (DCO) were also excluded. Patients without histological verification were included in the analysis.
Information on tumour morphology, registration type, age at diagnosis, gender and postcode was extracted from the cancer registry data. Data on hospital admissions (within the 5 years prior to diagnosis) for liver cirrhosis, primary biliary cirrhosis, haematochromatosis, viral hepatitis, diabetes and ischaemic heart disease were extracted from the electronic database of hospital admissions (SMR01). Data on diabetes and ischaemic heart disease were extracted to give a measure of the impact of non-alcoholic fatty liver disease (NAFLD) on disease risk. Alcohol-related diseases were selected according to the predefined list of conditions used by the Information Services Division's substance misuse team, which have been published elsewhere.11 The cancer registry has no information on tumour staging of liver cancers and thus a proxy for disease severity was devised; patients with an admission to hospital for metastatic disease (ICD-10 codes C77, C78, C79 and ICD-9 codes 196, 197, 198) within 4 weeks of diagnosis were defined as having more severe disease. At present, no data on primary liver malignancies are collected from screening or prospective cancer audits by the Scottish Cancer Registry. Socioeconomic deprivation was measured using the Scottish Index of Multiple Deprivation, a geographic indicator based on 37 indicators across income, employment, health, education, skills and training, geographic access, crime and housing.12
The hospital admissions database (SMR01) contained information on procedures and operations. These data were extracted to identify those individuals who had undergone resection or transplantation. The coding was unable to distinguish between patients treated with ablation and chemoembolization, respectively, and these were coded into a category referred to as ‘non-resection treatments’.
The cohort of patients with HCC was divided according to periods of diagnosis and the proportions of patients with each risk factor were calculated within these categories.
Age-standardized incidence rates were calculated by sex using the European standard population. Date and cause of death were extracted from the linked data on death certifications. Relative survival rates were calculated for 1 year, 3 years and 5 years for the population diagnosed between 1985 and 31 December 2007 using the ‘strel’ program developed by the London School of Hygiene and Tropical Medicine (LSHTM) for calculating relative survival with the statistical analysis program stata (StataCorp LP, College Station, TX, USA).13,14 Relative survival is a measure of survival based on the ratio of observed to expected survival for that age group and location. Expected mortality rates are based on actuarial life tables compiled by the General Register Office for Scotland (now part of National Records of Scotland) and converted into a stata format by the LSHTM. Right-hand censor date was limited to the end of 2007 because life tables for the strel program were not available for the years 2008 and 2009. stata Version 11 was used for all other statistical analyses.
The relationship between risk factors and cancer-specific survival was calculated using Cox regression for patients diagnosed between the years 1997 and 2008. These years were selected as data on treatment were available from 1997, when regional cancer registries in Scotland were centralized. The proportional hazards assumption was tested using the sphtest program based on Schoenfield residuals, and violation of the proportional hazards assumption was assumed at P < 0.05. Survival dates were right-hand censored at 31 December 2008. Cancer-specific deaths were those defined as any death by neoplasm (ICD-9 codes 140–239 and ICD-10 codes C00–D48). All deaths by other causes were treated as right-hand censored at the date of death.
Results
Of the initial dataset of 5846 primary cancers, 264 (4.5%) were DCO diagnoses and were excluded from analysis. During the 23-year study period, 2802 patients were diagnosed with HCC as defined by topography and morphology code. The proportion of histologically verified registrations of HCC was 80.6%, but this fell to 42.5% over the study period (Table 1). The remainder of the registrations referred to cholangiocarcinoma, were of non-specific morphology or had a mixed topography and morphology (i.e. topography of biliary cancer with a morphology code for HCC).
Table 1.
Risk factors and demographics in patients diagnosed with hepatocellular carcinoma (HCC) in Scotland during 1985–2008 (n = 2802)
| 1985–1989 | 1990–1994 | 1995–1999 | 2000–2004 | 2005–2008 | |
|---|---|---|---|---|---|
| HCC diagnoses, n | 355 | 395 | 562 | 669 | 821 |
| Age, years, mean ± SD | 68.59 ± 11.27 | 67.63 ± 11.80 | 67.75 ± 10.92 | 68.49 ± 10.62 | 68.37 ± 10.74 |
| Male, n (%) | 265 (74.6%) | 297 (75.2%) | 421 (74.9%) | 511 (76.6%) | 644 (78.4%) |
| Deprivation quintile 1a, n (%) | 130 (36.6%) | 113 (28.6%) | 164 (29.2%) | 166 (24.9%) | 238 (29.0%) |
| Deprivation quintile 2, n (%) | 79 (22.3%) | 91 (23.0%) | 130 (23.1%) | 171 (25.6%) | 194 (23.6%) |
| Deprivation quintile 3, n (%) | 57 (16.1%) | 76 (19.2%) | 104 (18.5%) | 132 (19.8%) | 151 (18.4%) |
| Deprivation quintile 4, n (%) | 42 (11.8%) | 62 (15.7%) | 76 (13.5%) | 90 (13.5%) | 145 (17.7%) |
| Deprivation quintile 5, n (%) | 47 (13.2%) | 53 (13.4%) | 88 (15.7%) | 109 (16.3%) | 93 (11.3%) |
| Metastasesb, n (%) | 28 (8.0%) | 36 (9.3%) | 60 (10.7%) | 71 (10.6%) | 90 (11.1%) |
| Viral hepatitis, n (%) | 5 (1.4%) | 14 (3.6%) | 45 (8.0%) | 48 (7.2%) | 67 (8.2%) |
| Liver cirrhosis, n (%) | 95 (27.1%) | 112 (28.9%) | 161 (28.8%) | 130 (19.4%) | 202 (24.8%) |
| Alcoholic liver cirrhosisc, n (%) | 37 (10.5%) | 49 (12.6%) | 81 (14.5%) | 67 (10.0%) | 115 (14.1%) |
| Non-alcoholic liver cirrhosisd, n (%) | 58 (16.3% | 63 (15.9%) | 80 (14.2%) | 63 (9.4%) | 87 (10.6%) |
| Biliary cirrhosis, n (%) | 8 (2.3%) | 14 (3.6%) | 18 (3.2%) | 22 (3.3%) | 27 (3.3%) |
| Haemochromatosis, n (%) | 18 (5.1%) | 15 (3.9%) | 23 (4.1%) | 31 (4.6%) | 43 (5.3%) |
| Alcohol-related admission, n (%) | 56 (16.0%) | 73 (18.8%) | 131 (23.4%) | 158 (23.7%) | 220 (27.1%) |
| Diabetes, n (%) | 37 (10.5%) | 57 (14.7%) | 83 (14.8%) | 132 (19.7%) | 214 (26.3%) |
| Hypertension, n (%) | 12 (3.4%) | 21 (5.4%) | 49 (8.8%) | 92 (13.8%) | 189 (23.2%) |
| Ischaemic heart disease, n (%) | 28 (8.0%) | 43 (11.1%) | 78 (13.9%) | 89 (13.3%) | 125 (15.4%) |
| Transplant, n (%) | 0 | 4 (1.0%) | 14 (2.5%) | 32 (4.8%) | 34 (4.1%) |
| Histological verification, n (%) | 286 (80.6%) | 304 (77.0%) | 378 (67.3%) | 376 (56.2%) | 349 (42.5%) |
Deprivation quintile 1 represents the most deprived.
Admitted with metastases within 4 weeks of diagnosis.
Individuals with a diagnosis of liver cirrhosis and an alcohol-related admission.
Individuals with a diagnosis of liver cirrhosis and no alcohol-related admission.
SD, standard deviation.
The annual age-standardized incidence of HCC increased between 1985 and 2008 (Fig. 1). In 1997, a temporary rise in registrations coincided with the centralization of the regional registries into a single national registry. The rate of increase rose sharply following the year 2000 to the present. This principally reflected rising numbers of male registrations. The rate of incidence in males was approximately three times greater than that in women. The relative increase was similar in both groups; however, males account for an increasing proportion of registrations as a result of their high baseline rate (Fig. 1).
Figure 1.
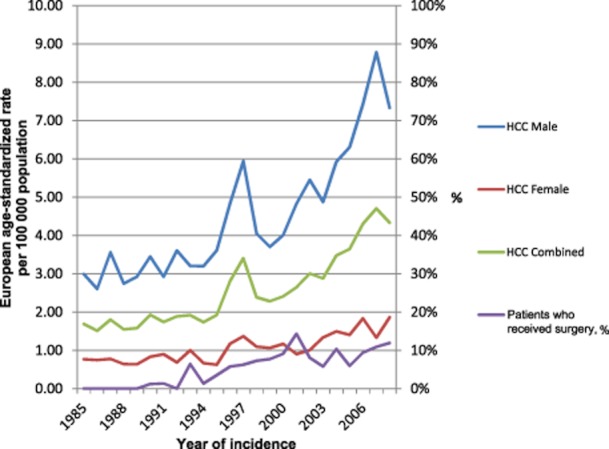
European age-standardized rates of hepatocellular carcinoma (HCC) per 100 000 population in Scotland by year of incidence
The mean age at presentation of patients with HCC remained the same through all five periods observed. The inequalities in HCC distribution amongst the deprivation quintiles fell across the time periods observed. The absolute difference between the proportion of registrations from the most and least deprived quintiles fell from 23.4% to 17.7% through the study period (Table 1).
The proportion of patients with a prior admission of viral hepatitis rose sharply after 1990, but reached a plateau in the 2000s. The proportion of patients with prior alcohol-related admissions rose consistently throughout the period under study, whereas the proportion of patients with a diagnosis of alcoholic cirrhosis was at its highest in the late 1990s.
The absolute numbers of patients with an admission for primary biliary cirrhosis (PBC) rose through the 5-year periods, although this did not result in a large increase in the proportion of total registrations of HCC with PBC. The frequency of haemochromatosis also rose within the context of a rising incidence of HCC and thus proportions remained relatively stable (Table 1).
The number of patients with measured comorbidities rose in each time period. The proportions of patients with diabetes, hypertension or ischaemic heart disease rose throughout the study period (Table 1).
Over the period under study, the proportion of patients admitted to hospital with metastases within 4 weeks of diagnosis rose slightly from 1985–1989 to 2005–2008 (Table 1).
Although incidence increased dramatically during this study period, there have been a host of innovations in treatment. The Scottish Liver Transplant Unit was first established in 1992 and has since undertaken over 1000 successful liver transplants. This is reflected in the rise in the proportion of patients recorded as undergoing liver transplantation (Table 1).
Relative survival was calculated using 2590 patients with HCC diagnosed between 1 January 1985 and 31 December 2007. It was not possible to calculate age-standardized relative survival as a result of low sample numbers. Relative survival increased significantly during the study period (Table 2). One-year survival among males during 2005–2007 was five times greater than that in 1985–1989. Three-year survival among males also increased significantly. Five-year survival in male patients also increased, although not significantly. Relative 1-, 3- and 5-year survival among females increased significantly. Cox survival analysis was performed on the 1836 patients diagnosed between 1997 and the end of 2008. The model adjusted for all recorded risk factors, cardiovascular comorbidities and surgical treatments, including transplant and resection, and a combined variable for extirpation and ablation, which were inseparable in the coding. Each additional year of age at diagnosis, the presence of metastatic disease, and alcohol-related admissions all independently reduced survival (Table 3).
Table 2.
Relative survival (%) at 1, 3 and 5 years in patients diagnosed with hepatocellular carcinoma in Scotland during 1985–2008 (n = 2802)
| 1-year survival, % (95% CI) | 3-year survival, % (95% CI) | 5-year survival, % (95% CI) | ||||
|---|---|---|---|---|---|---|
| Women | ||||||
| 1985–1989 | 10.7 | (5.6–17.7) | 2.4 | (0.6–6.9) | 0.5 | (0.0–3.7) |
| 1990–1994 | 14.6 | (8.6–22.0) | 5.4 | (2.1–11.0) | 5.4 | (2.1–11.0) |
| 1995–1999 | 16.3 | (10.8–22.8) | 9.7 | (5.5–15.4) | 7.1 | (3.6–12.2) |
| 2000–2004 | 28.2 | (21.3–35.5) | 11.6 | (7.0–17.5) | 8.7 | (4.7–14.2) |
| 2005–2007 | 32.1 | (24.1–40.4) | 12.6 | (7.2–19.6) | 10.6 | (5.2–18.1) |
| Men | ||||||
| 1985–1989 | 5.2 | (3.1–8.0) | 0.7 | (0.2–2.2) | 0.4 | (0.2–2.2) |
| 1990–1994 | 9.2 | (6.5–12.6) | 3.2 | (1.6–5.7) | 2.2 | (0.9–4.5) |
| 1995–1999 | 18.4 | (14.9–22.3) | 7.9 | (5.5–10.8) | 5.5 | (3.5–8.3) |
| 2000–2004 | 26.7 | (22.9–30.7) | 11.0 | (8.3–14.1) | 8.7 | (6.2–11.6) |
| 2005–2007 | 26.8 | (22.8–30.8) | 11.8 | (8.9–15.2) | 4.4 | (1.5–9.9) |
95% CI, 95% confidence interval.
Table 3.
Cox regression of risk factors against survival time adjusted for all risk factors and surgical treatment (transplant, resection, extirpation, ablation) in patients diagnosed with hepatocellular carcinoma in Scotland during 1985–2008 (n = 2802)
| Risk factor | HR | HR (adj) | SE | P-value | 95% CI |
|---|---|---|---|---|---|
| Age | 1.02 | 1.01 | 0.002 | 0.000c | 1.01–1.02 |
| Sexa | 1.04 | 1.03 | 0.066 | 0.612 | 0.91–1.17 |
| Metastases | 2.39 | 1.96 | 0.156 | 0.000c | 1.67–2.29 |
| Viral hepatitis | 0.50 | 0.71 | 0.082 | 0.003c | 0.57–0.89 |
| Primary biliary cirrhosis | 0.82 | 0.90 | 0.133 | 0.455 | 0.67–1.20 |
| Haemochromatosis | 0.72 | 0.81 | 0.107 | 0.120 | 0.63–1.05 |
| Diabetes | 1.04 | 0.95 | 0.064 | 0.433 | 0.83–1.08 |
| Ischaemic heart disease | 1.17 | 1.03 | 0.081 | 0.742 | 0.88–1.20 |
| Alcohol-related admission | 0.99 | 1.23 | 0.102 | 0.013c | 1.05–1.45 |
| Alcoholic cirrhosis | 0.90 | 0.88 | 0.097 | 0.263 | 0.71–1.10 |
| Year of incidence | 0.98 | 0.98 | 0.008 | 0.039c | 0.97–0.99 |
| Deprivation quintile 1b | 1.00 | ||||
| Deprivation quintile 2 | 0.84 | 0.91 | 0.066 | 0.194 | 0.79–1.05 |
| Deprivation quintile 3 | 0.88 | 0.93 | 0.073 | 0.355 | 0.80–1.08 |
| Deprivation quintile 4 | 0.72 | 0.76 | 0.065 | 0.001c | 0.64–0.90 |
| Deprivation quintile 5 | 0.75 | 0.83 | 0.072 | 0.030c | 0.70–0.98 |
Female baseline.
Deprivation quintile 1 = most deprived.
P < 0.05.
HR, hazard ratio; SE, standard error; 95% CI, 95% confidence interval.
Relative to patients in the most deprived quintile, being in the two least deprived quintiles was associated with a significant improvement in survival. As reflected in the increasing relative survival seen in Table 2, year of incidence was also significantly associated with an increase in survival. Finally, patients with an admission for viral hepatitis had a significantly lower hazard ratio than those without.
Discussion
This work has confirmed the increase in risk for primary liver cancer that has occurred in Scotland over the past 25 years, but has also shown apparent associated improvements in survival. Annual incidences of HCC have doubled since 1985 in both men and women, although male incidence rates are several times higher than those of females. The rate of 1-year survival in women has increased three-fold and that in men has increased by over five-fold since 1985–1989. There has also been an improvement in 5-year survival rates, which have risen from < 1% to 10.6% in women and 4.4% in men. Risk factors for HCC, such as alcohol-related admissions, alcohol-related cirrhosis admissions and viral hepatitis admissions all rose throughout the study period. However, the presence of a viral hepatitis admission was not associated with a reduction in survival. Viral hepatitis admissions, being in the two least deprived quintiles, and diagnosis in more recent years were all associated with improved survival. Metastatic disease, increasing age and prior alcohol-related admissions all reduced survival.
This study was based on national cancer registry data covering the whole of Scotland. The Scottish Cancer Registry has high-quality data capture and coding.15 In the most recent report by the International Agency for Research on Cancer (IARC), DCO records for liver cancer were reported to have fallen to < 1%.16 However, falling rates of histological verification make the veracity of case ascertainment increasingly difficult to assess. Nonetheless, this fall is not unexpected. The dramatic technical advances in liver transplantation and hepatic resection over the time period reviewed have led to pressure to change diagnostic standards to reduce metastatic tumour seeding in patients who undergo curative surgery. In these cases, the only possibility for a biopsy would be during surgery or at postmortem. Modern clinical guidelines indicate that a firm diagnosis can be made in the absence of biopsy if satisfactory imaging in two modalities, with or without a raised serum alpha fetoprotein level, are present. Thus, lesions are not biopsied initially as patients are considered for surgery and, although most patients do not fulfil criteria for curative surgery, their diagnosis is established and there is no requirement for histological confirmation.17
The combination of morphological and topographical codes improves the specificity of diagnoses and reduces the likelihood that misclassified secondary tumours will be inadvertently included. The cancer registry does not collect detailed clinical data or measures of severity. A proxy measure of severity was based on the presence of an admission for metastatic disease within 4 weeks of diagnosis. This method has effectively identified patients with poor survival previously.18 It may have limitations in HCC specifically as this tumour metastasizes uncommonly and late, and the severity of illness reflects liver failure rather than tumour bulk. Nevertheless, it was an independent predictor of prognosis in the present study.
Rising rates of HCC in the UK have been reported previously.19 In Scotland, the combination of high rates of alcohol abuse in the general population and high hepatitis C prevalences in the drug-using population have contributed heavily to the current increase in liver disease and subsequent development of primary liver cancers.20 One in four of those diagnosed with HCC since 2005 had a prior alcohol-related admission to hospital. However, in recent years the rise in alcohol-related mortality appears to have reached a plateau.21 The high prevalence of alcohol misuse may influence survival in two main ways: it may delay patients from seeking treatment, or it may encourage poor tolerance of and adherence to treatment.
Understanding of the relationship between HCC incidence, viral hepatitis and cirrhosis, along with the development of curative treatments, has led to a widespread increase in the practice of screening at-risk patients for early disease. This appears to have led to improvements in survival which may, in part, reflect ‘lead-time bias’, but may also result from the greater susceptibility to intervention of early-stage tumours.6 It should also be noted that during the past 15 years, the provision of liver transplantation has expanded rapidly in Scotland.22,23 Newer non-surgical invasive techniques have been shown to improve short-term survival prospects in early-stage disease. Radiofrequency ablation and percutaneous ethanol injection have both been shown to treat patients with small tumours (< 2 cm in diameter) effectively. However, at presentation, the majority of patients are found to be unsuitable for curative treatment because of either tumour size or location, or underlying liver failure. Therefore, the majority of chemoembolization and ablation procedures are performed with palliative intent and a minority of patients derive prolonged survival. This is consistent with the present study's finding of more striking absolute increases over time in 1-year compared with 5-year survival.
It was not possible to estimate age-adjusted relative survival from this dataset as even broad age groups were underpowered. The relative survival analysis allows adjustment for background mortality. This is important because in Scotland cardiovascular disease mortality, obesity and alcohol abuse, as well as mortality from certain cancers, are higher than in most other European countries.24 Survival measures which do not adjust for this are likely to underestimate survival as deaths which should be attributed to other causes are attributed to the cancer of interest.
Viral hepatitis is recorded in this dataset as an admission to hospital with viral hepatitis. This is more likely in patients with more severe disease and the dataset does not represent a comprehensive record of all patients with hepatitis C or B in Scotland. However, the obvious increase in patients with a diagnosis of viral hepatitis across time periods reflects the increase in testing for hepatitis C that has occurred since the early 1990s.
In conclusion, rates of HCC are increasing in Scotland, most likely as a result of increasing levels of alcohol abuse in the general population and viral hepatitis among i.v. drug users. Despite this, there is hope for survival that was not possible 25 years ago. Survival has risen significantly, no doubt partly as a result of the earlier diagnosis facilitated by improved surveillance, but also potentially as a result of the increased availability of several novel treatments and interventions, including aggressive surgical resection, ablation and transplantation. Further research correcting for severity at presentation will be required to quantify the impacts of surveillance and treatment, respectively, on changing rates of survival. Given the current plateau in alcohol-related mortality in Scotland, this burden of liver cancer may even decline in the medium term if current initiatives set to reduce the burden of alcohol-related harm continue to be supported and new initiatives are put in place.
Acknowledgments
The authors would like to acknowledge and thank Matthew Armstrong, Susan Jensen and Diane Stockton, Scottish Cancer Registry, Information Services Division, NHS National Services Scotland, Edinburgh, UK, for their help in extracting, preparing and analysing the data in this work. All data used for this study were extracted from the cancer registry and hospital admissions databases of National Health Service (NHS) Scotland. These data are available upon request from the Information Services Division (ISD) of NHS Scotland. However, data are provided in an anonymized format, in line with the data protection and disclosure control policies of the ISD.
Conflicts of interest
None declared.
References
- 1.Cancer Research UK. Cancer incidence for common cancers. 2010. Available at http://info.cancerresearchuk.org/cancerstats/incidence/commoncancers/#Twenty (last accessed 31 May 2012)
- 2.Information Services Division, NHS Scotland. Cancer in Scotland. Edinburgh: ISD, NHS Scotland; 2010. [Google Scholar]
- 3.Scottish Government. Health in Scotland 2007. Edinburgh: Scottish Government; 2008. Annual Report of the Chief Medical Officer. [Google Scholar]
- 4.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 5.Faivre J, Forman D, Estève J, Obradovic M, Sant M. Survival of patients with primary liver cancer, pancreatic cancer and biliary tract cancer in Europe. EUROCARE Working Group. Eur J Cancer. 1998;34:1103–1109. doi: 10.1016/s0959-8049(98)00330-x. [DOI] [PubMed] [Google Scholar]
- 6.Sangiovanni A, Del Ninno E, Fasani P, De Fazio C, Ronchi G, Romeo R, et al. Increased survival of cirrhotic patients with a hepatocellular carcinoma detected during surveillance. Gastroenterology. 2004;126:1005–1014. doi: 10.1053/j.gastro.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 7.Schwarz RE, Abou-Alfa GK, Geschwind JF, Krishnan S, Salem R, Venook AP. Non-operative therapies for combined modality treatment of hepatocellular cancer: expert consensus statement. HPB. 2010;12:313–320. doi: 10.1111/j.1477-2574.2010.00183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jarnagin W, Chapman WC, Curley S, D'Angelica M, Rosen C, Dixon E, et al. Surgical treatment of hepatocellular carcinoma: expert consensus statement. HPB. 2010;12:302–310. doi: 10.1111/j.1477-2574.2010.00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duffy JP, Vardanian A, Benjamin E, Watson M, Farmer DG, Ghobrial RM, et al. Liver transplantation criteria for hepatocellular carcinoma should be expanded: a 22-year experience with 467 patients at UCLA. Ann Surg. 2007;246:502–599. doi: 10.1097/SLA.0b013e318148c704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parkin DM, Whelan SL, Ferlay J, Storm H. Cancer Incidence in Five Continents. VIII. Lyon: International Agency for Research on Cancer; 2002. IARC Scientific Publication No. 155. [Google Scholar]
- 11.Information Services Division Scotland. Alcohol statistics Scotland. 2007. Available at http://www.isdscotland.org/isd/4602.html (last accessed 2 March 2011)
- 12.Scottish Government. Scottish index of multiple deprivation. 2009. Available at http://www.scotland.gov.uk/Topics/Statistics/SIMD/ (last accessed 2 March 2011)
- 13.StataCorp LP. Statistical Software: Release 7.0. College Station, TX: StataCorp LP; 2001. [Google Scholar]
- 14.London School of Hygiene and Tropical Medicine. Tools for Cancer Survival Analysis. 2011. Available at http://www.lshtm.ac.uk/eph/ncde/cancersurvival/tools/ (last accessed 28 June 2011)
- 15.UK Association of Cancer Registries. UKACR Quality and Performance Indicators 2010. UKACR; 2011. [Google Scholar]
- 16.Curado MP, Edwards B, Shin HR, Storm H, Ferlay J, Heanue M, et al. Cancer Incidence in Five Continents. IX. Lyon: IARC; 2007. IARC Scientific Publications No. 160. [Google Scholar]
- 17.El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134:1752–1763. doi: 10.1053/j.gastro.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 18.Parks RW, Bettschart V, Frame S, Stockton DL, Brewster DH, Garden OJ. Benefits of specialization in the management of pancreatic cancer: results of a Scottish population-based study. Br J Cancer. 2004;91:459–465. doi: 10.1038/sj.bjc.6601999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.West J, Wood H, Logan RF, Quinn M, Aithal GP. Trends in the incidence of primary liver and biliary tract cancers in England and Wales 1971–2001. Br J Cancer. 2006;94:1751–1758. doi: 10.1038/sj.bjc.6603127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDonald SA, Hutchinson SJ, Bird SM, Robertson C, Mills PR, Dillon JF, et al. A record-linkage study of the development of hepatocellular carcinoma in persons with hepatitis C infection in Scotland. Br J Cancer. 2008;99:805–810. doi: 10.1038/sj.bjc.6604563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beeston C, Robinson M, Craig N, Graham L. Monitoring and Evaluating Scotland's Alcohol Strategy. Setting the Scene: Theory of Change and Baseline Picture. Edinburgh: NHS Health Scotland; 2011. [Google Scholar]
- 22.Bathgate AJ, Garden OJ, Forsythe JR. The outcome of the first 165 orthotopic liver transplants in Scotland. Scott Med J. 1999;44:9–10. doi: 10.1177/003693309904400104. [DOI] [PubMed] [Google Scholar]
- 23.David S, Copley L, van der Meulen J. London: RCS Clinical Effective Unit; 2009. UK Liver Transplant Audit in patients who received a liver transplant between 1 March 1994 and 31 March 2009. Annual Report to the National Commissioning Group (NCG), Clinical Effectiveness Unit, Royal College of Surgeons of England. [Google Scholar]
- 24.Whyte B. Edinburgh: Scottish Public Health Observatory (ScotPHO); 2006. Scottish Mortality in a European Context: 1950–2000. An analysis of comparative mortality trends. [Google Scholar]


