Abstract
Objectives
Steroids are a mainstay of treatment in orthotopic liver transplantation (OLT) and are associated with significant morbidity. This trial was conducted to assess the efficacy of steroids avoidance.
Methods
Patients undergoing OLT between June 2002 and April 2005 were entered into a prospective, randomized trial of complete steroids avoidance and followed until November 2011. Recipients received either standard therapy (n = 50) or complete steroids avoidance (n = 50). Analyses were performed on an intention-to-treat basis. The mean follow-up of all recipients was 2095 ± 117 days. Sixteen (32%) recipients randomized to the steroids avoidance group ultimately received steroids for clinical indications.
Results
Incidences of diabetes and hypertension prior to or after OLT were similar in both groups, as was the incidence of rejection. Patient and graft survival rates at 1, 3 and 5 years were lower in the steroids avoidance group than in the standard therapy group (patient survival: 1-year, 80% versus 86%; 3-year, 68% versus 76%; 5-year, 60% versus 72%; graft survival: 1-year, 76% versus 76%; 3-year, 64% versus 74%; 5-year, 56% versus 72%), but the differences were not statistically different.
Conclusions
Complete steroids avoidance provides liver transplant recipients with minimal benefit and appears to result in a concerning trend towards decreased graft and recipient survival. The present data support the use of at least a short course of steroids after liver transplantation.
Introduction
As a result of their efficacy in the prevention and treatment of rejection, corticosteroids have been a key factor in the induction and maintenance of immunosuppression since the early days of liver transplantation.1 However, the longterm use of steroids is associated with a multitude of adverse side-effects, such as hypertension, diabetes, infections and hypercholesterolaemia.2 Steroid use has also been associated with increased severity of recurrent hepatitis C virus (HCV) infection post-transplant3,4 and steroid boluses that are administered for acute rejection are associated with the greatest level of risk.5 Furthermore, although steroids may reduce the incidence of early rejection post-transplant, the importance of preventing mild rejection may be lower in liver recipients than in recipients of other solid organs. By contrast, mild rejection has been associated with improved longterm graft and patient survival in liver recipients without HCV infection.5,6 Newer immunosuppressants have allowed steroid use to be reduced or potentially avoided altogether, causing uncertainty about the role of steroids in liver transplant recipients.
Multiple studies examining early withdrawal of steroids following liver transplantation did not demonstrate a negative impact on the incidence of rejection, nor on patient and graft survival, but did show a potential benefit by reducing the incidence of steroid-related side-effects.7–17 Other studies have also demonstrated a reduction in the severity of HCV recurrence when the use of steroids after liver transplant is minimized.18–21 Many small retrospective and pilot studies have examined complete steroids avoidance using several different immunosuppression regimens, and have demonstrated the safety of these regimens without impact on patient or graft survival, but have shown variable success in limiting morbidity associated with steroids.6,22–27
The effect of complete steroids avoidance (i.e. no steroids for the induction or maintenance of immunosuppression) on outcomes after liver transplantation remains unclear. Therefore, 100 primary adult liver transplant recipients were prospectively randomized to either standard therapy with steroids or standard therapy with complete steroids avoidance.
Materials and methods
The University of Michigan Institutional Review Board for Human Subject Research approved this study. Written informed consent was obtained from patients. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
Study population
All consecutive, consenting candidates undergoing liver transplantation at the University of Michigan between June 2002 and May 2005 were enrolled into a prospective, open-label, randomized controlled trial (RCT) to evaluate the effects of complete steroids avoidance. Enrolled candidates were randomized to either the ‘steroids’ or ‘no-steroids’ groups using a closed-envelope system. Only candidates for a primary liver allograft were eligible for the study. Exclusion criteria refused participation to candidates aged <18 years, multiple organ recipients and patients who required post-transplant steroid therapy for an indication other than the prevention of rejection, such as autoimmune hepatitis or inflammatory bowel disease. Patient and graft survival were then recorded to 2011.
Immunosuppression and treatment of rejection
Patients randomized to the steroids group received a standard immunosuppression regimen consisting of tacrolimus, mycophenolate mofetil (MMF), and one dose of dexamethasone 50 mg intraoperatively followed by a 3–6-month taper of prednisone. Patients randomized to the no-steroids group received the same standard immunosuppression therapy as did patients in the steroids group except that induction and maintenance steroids were not administered. Both groups were given the same target levels for tacrolimus (postoperative days 0–30, 12–15 ng/ml; postoperative days 31–60, 8–12 ng/ml; >60 days postoperatively, 4–8 ng/ml). Basiliximab, an interleukin-2 receptor inhibitor, was administered on the day of transplant and on post-transplant day 4 for liver transplant recipients with acute renal insufficiency or oliguria. Tacrolimus was withheld for up to 4 days postoperatively in patients who received basiliximab.
Clinically suspected rejection was evaluated by liver biopsy and, when confirmed, treated with high-dose methylprednisolone (250 mg daily for 3 days). A second course of high-dose steroids was administered to patients who did not respond. Steroid-resistant rejection was treated with antibody therapy (OKT3 or polyclonal antithymocyte globulin). There were no protocol biopsies in this trial.
Data collection
Donor and recipient variables collected prospectively included recipient demographic data [body mass index (BMI), cholesterol profiles, use of pre-transplant insulin and anti-hypertensive medications], donor demographics, and intraoperative variables. In addition, recipient BMI, cholesterol profiles, use of insulin and anti-hypertensive medications were evaluated at 6 months and 12 months post-transplant. Those enrolled in the steroids-free arm undergoing retransplantation received standard steroid-based induction and maintenance therapy. Graft survival was defined as the survival of the initial liver transplant until either retransplantation or death. Postoperative complications were assessed, including acute cellular rejection, chronic ductopenic rejection, hepatitis C recurrence, infections at 30 days postoperatively, primary non-function, postoperative bleeding requiring reoperation, hepatic artery thrombosis, biliary leak and/or stricture, hepatic vein or inferior vena cava stenosis, renal failure requiring haemodialysis, and retransplantation. Superficial skin infection was defined as an operative site infection that required opening of the wound. Pneumonia, bloodstream infections, peritonitis, urinary tract infections and other infections were counted only when clinical suspicion was confirmed by culture. Hepatitis C recurrence was defined as a persistent elevation of liver enzymes to ≥1.5 times the normal level for 3 months consecutively that was not explained by any other diagnosis28 and detectable HCV RNA levels in post-transplant serum, and confirmed by biopsy. Diabetes was defined by the requirement for either oral hypoglycaemic medications or insulin. Hypertension was defined as the need for anti-hypertensive medication to control blood pressure.
Primary endpoints for this study were patient and graft survival, as well as biopsy-proven rejection. Secondary endpoints were hepatitis C recurrence, and postoperative metabolic, infectious and transplant-specific complications.
Statistical analysis
Outcomes in the two study arms were compared on an intention-to-treat basis. All univariate comparisons were unpaired, and all tests of significance were two-tailed. For univariate analyses, continuous variables were compared by Student's t-test with equal or unequal variance as determined by F-test analysis of the variability of each variable. Categorical data were compared using chi-squared analysis. Values are expressed as the mean ± standard deviation (for continuous variables) or as a percentage of the group from which they were derived (for categorical variables). Patient and graft survival rates were compared using Kaplan–Meier analysis curves with log-rank test to determine significance. P-values of <0.05 were considered to indicate statistical significance. StatView Version 5.0.1 (SAS Institute, Inc., Cary, NC, USA) was used for statistical analysis.
Results
During the period from June 2002 to May 2005, a total of 190 potential subjects were assessed (Fig. 1). Of these, 135 met the study inclusion criteria, but 35 refused to participate. Overall, 100 consecutive, consenting patients who underwent primary liver transplantation were enrolled in this study. Each group included 50 patients. Mean follow-up was 2095 ± 117 days. Donor and recipient characteristics are summarized in Table 1. The two groups of recipients were well matched with respect to gender, age, race, cause of liver failure, comorbidities, Model of End-stage Liver Disease (MELD) score, renal function and ischaemic times. Sixteen of 50 recipients (32%) in the steroids-avoidance group required steroids for biopsy-proven rejection (n = 10), adrenal insufficiency (n = 1), treatment of sepsis (n = 1), calcineurin inhibitor toxicity (n = 1), autoimmune thrombocytopenia (n = 1), physician decision (n = 1) or suspected post-transplant autoimmune hepatitis (n = 1). The two study groups were also similar in donor characteristics with respect to gender, age, race, cause of death, and cytomegalovirus (CMV) positivity.
Figure 1.
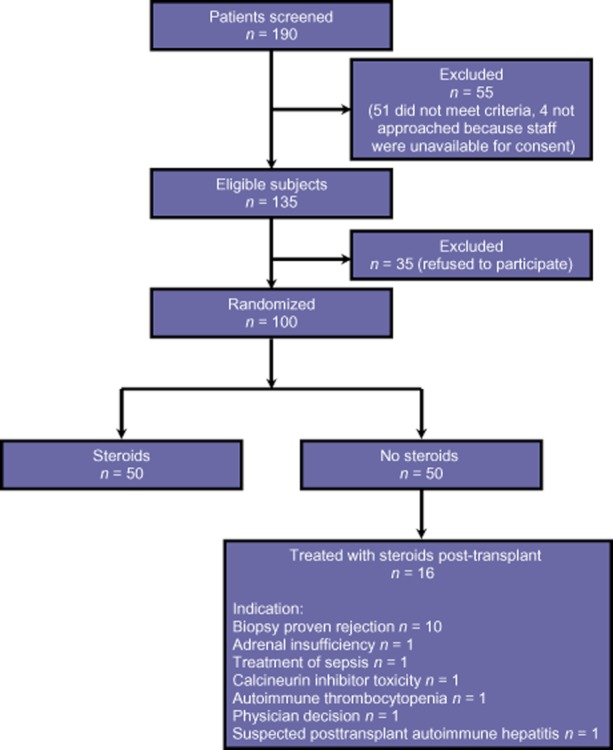
Flow diagram illustrating study enrolment and disposition of participants
Table 1.
Comparison of donor and recipient characteristics in groups of liver transplant recipients randomized to either standard immunosuppression (steroids) or complete avoidance of steroids (no steroids)
| Steroids group (n = 50) | No-steroids group (n = 50) | P-value | |
|---|---|---|---|
| Recipient characteristics | |||
| Age, years, mean ± SD | 54 ± 1 | 56 ± 1 | 0.23 |
| Male, % | 76 | 76 | 1.00 |
| White, % | 92 | 86 | 0.34 |
| Basiliximab inductiona, % | 24 | 26 | 0.82 |
| Primary diagnosisb, % | |||
| Hepatitis C virus | 62 | 46 | 0.11 |
| Alcohol-related | 38 | 46 | 0.42 |
| Hepatocellular carcinoma | 18 | 22 | 0.62 |
| PBC/PSC | 2 | 10 | 0.09 |
| Cryptogenic cirrhosis | 16 | 14 | 0.78 |
| Body mass index, kg/m2, mean ± SD | 29 ± 1 | 30 ± 1 | 0.42 |
| On preoperative anti-hypertensive, % | 74 | 72 | 0.82 |
| Preoperative diabetes mellitus, % | 24 | 40 | 0.09 |
| Preoperative CAD, % | 6 | 10 | 0.46 |
| Preoperative haemodialysis, % | 2 | 6 | 0.31 |
| Laboratory MELD score, mean ± SD | 16 ± 1 | 18 ± 1 | 0.17 |
| Liver warm ischaemic time, min, mean ± SD | 64 ± 7 | 54 ± 3 | 0.19 |
| Liver cold ischaemic time, min, mean ± SD | 518 ± 34 | 518 ± 24 | 0.99 |
| Donor characteristics | |||
| Age, years, mean ± SD | 38 ± 3 | 37 ± 2 | 0.90 |
| Male, % | 62 | 75 | 0.18 |
| White, % | 78 | 82 | 0.62 |
| Death from stroke, % | 51 | 51 | 1.00 |
| Cytomegalovirus-positive, % | 70 | 64 | 0.54 |
Administered only to patients with acute renal failure or oliguria at the time of transplantation.
Some recipients had more than one diagnosis.
SD, standard deviation; PBC, primary biliary cirrhosis; PSC, primary sclerosing cholangitis; CAD, coronary artery disease; MELD, Model for End-stage Liver Disease.
Postoperative complications were compared between the two groups (Table 2). There was no difference in the incidence of rejection or the time to the first episode of acute rejection. All rejection episodes responded to pulsed high-dose steroid therapy. No differences were seen in occurrences of primary non-function, hepatic artery thrombosis, hepatic vein/inferior vena cava stenosis, biliary complications (leak and/or stricture), renal failure or re-exploration for bleeding. There was a statistically significant increase in retransplantation in the no-steroids group, in which six patients underwent a second transplant and two of these required a third transplant, whereas no patients in the steroids group were retransplanted. The reasons for retransplantation included: primary non-function (n = 2); hepatic artery thrombosis (n = 2); early rejection (n = 1) which was successfully treated, but the patient subsequently developed intrahepatic cholangiopathy, and venous obstruction and chronic ductopenic rejection (n = 1). The reasons for the third transplants were primary non-function (n = 1) and venous occlusive problems and intrahepatic biliary stricturing (n = 1). There was no significant difference in the occurrence of infectious complications between the steroids and no-steroids groups, including in wound infections (30% and 18%, respectively; P = 0.16).
Table 2.
Comparison of complications occurring in the first year post-transplant in liver transplant recipients randomized to either standard immunosuppression (steroids) or complete avoidance of steroids (no steroids)
| Steroids group (n = 50) | No-steroids group (n = 50) | P-value | |
|---|---|---|---|
| Acute cellular rejection, % | 14 | 20 | 0.42 |
| Time to first episode, days, mean ± SD | 68 ± 20 | 56 ± 32 | 0.76 |
| Ductopenic rejection, % | 8 | 2 | 0.17 |
| HCV recurrence, % | 48 | 30 | 0.07 |
| Primary non-function, % | 4 | 6 | 0.65 |
| Hepatic artery thrombosis, % | 4 | 4 | 1.00 |
| Hepatic vein/IVC stenosis, % | 6 | 16 | 0.11 |
| Biliary complications, % | 46 | 48 | 0.84 |
| Postoperative acute renal failure, % | 24 | 36 | 0.19 |
| Postoperative chronic renal failure, % | 10 | 22 | 0.10 |
| Duration of HD, days, mean ± SD | 273 ± 165 | 302 ± 109 | 0.88 |
| Reoperation for bleeding, % | 16 | 16 | 1.00 |
| Retransplantation, % | 0 | 12 | 0.01 |
| Infectious complications | |||
| Surgical site infection, % | 30 | 18 | 0.16 |
| Pneumonia (%) | 4 | 14 | 0.08 |
| Urinary tract infection, % | 8 | 14 | 0.34 |
| Bloodstream infection, % | 2 | 6 | 0.31 |
| Peritonitis, % | 4 | 8 | 0.40 |
| Any infection, % | 44 | 52 | 0.42 |
SD, standard deviation; HCV, hepatitis C Virus; IVC, inferior vena cava; HD, hospital days.
Metabolic outcomes in the two groups were compared (Table 3). Baseline patient characteristics demonstrated that cholesterol levels (157 ± 14 versus 125 ± 6; P = 0.04) and low-density lipoprotein (LDL) levels (94 ± 13 mg/dl versus 65 ± 4 mg/dl; P = 0.04) were higher in the no-steroids group than in the steroids group. The two groups were otherwise well matched with respect to BMI, high-density lipoprotein (HDL), triglycerides, creatinine, frequency of hypertension, number of anti-hypertensive medications, diabetes mellitus, and administration of insulin. At 6 months and 1 year following transplantation, the baseline differences in cholesterol and LDL levels disappeared. At 6 months following liver transplantation, the only significant difference between the two groups referred to the increased serum creatinine level in the no-steroids group (1.60 ± 0.14 mg/dl versus 1.24 ± 0.11 mg/dl; P = 0.05). Although both groups required fewer anti-hypertensive medications at 1 year following transplantation, the no-steroids group required significantly less (0.50 ± 0.10 versus 0.86 ± 0.15; P = 0.05).
Table 3.
Comparison of metabolic outcomes in liver transplant recipients randomized to either standard immunosuppression (steroids) or complete avoidance of steroids (no steroids)
| Steroids group (n = 50) | No-steroids group (n = 50) | P-value | |
|---|---|---|---|
| Body mass index, kg/m2, mean ± SD | |||
| Pre-transplant | 29 ± 1 | 30 ± 1 | 0.42 |
| 6 months post-transplant | 27 ± 1 | 26 ± 1 | 0.57 |
| 1 year post-transplant | 28 ± 1 | 29 ± 1 | 0.67 |
| Cholesterol, mg/dl, mean ± SD | |||
| Pre-transplant | 125 ± 6 | 157 ± 14 | 0.04 |
| 6 months post-transplant | 154 ± 8 | 172 ± 16 | 0.33 |
| 1 year post-transplant | 167 ± 11 | 148 ± 8 | 0.18 |
| High-density lipoprotein, mg/dl, mean ± SD | |||
| Pre-transplant | 44 ± 3 | 44 ± 4 | 0.95 |
| 6 months post-transplant | 42 ± 3 | 40 ± 3 | 0.69 |
| 1 year post-transplant | 45 ± 3 | 45 ± 3 | 0.99 |
| Low-density lipoprotein, mg/dl, mean ± SD | |||
| Pre-transplant | 65 ± 4 | 94 ± 13 | 0.04 |
| 6 months post-transplant | 81 ± 7 | 101 ± 15 | 0.24 |
| 1 year post-transplant | 88 ± 11 | 76 ± 6 | 0.37 |
| Triglycerides, mg/dl, mean ± SD | |||
| Pre-transplant | 81 ± 8 | 97 ± 7 | 0.14 |
| 6 months post-transplant | 147 ± 12 | 148 ± 17 | 0.96 |
| 1 year post-transplant | 174 ± 16 | 142 ± 11 | 0.10 |
| Creatinine, mg/dl, mean ± SD | |||
| Pre-transplant | 1.26 ± 0.13 | 1.31 ± 0.14 | 0.79 |
| 6 months post-transplant | 1.24 ± 0.11 | 1.60 ± 0.14 | 0.05 |
| 1 year post-transplant | 1.42 ± 0.16 | 1.67 ± 0.15 | 0.24 |
| Anti-hypertensive medications, mean ± SD | |||
| Pre-transplant | 1.76 ± 0.16 | 1.70 ± 0.17 | 0.91 |
| 6 months post-transplant | 0.61 ± 0.13 | 0.72 ± 0.14 | 0.58 |
| 1 year post-transplant | 0.86 ± 0.15 | 0.50 ± 0.10 | 0.05 |
| Insulin-dependent, % | |||
| Pre-transplant | 18 | 22 | 0.62 |
| 6 months post-transplant | 22 | 27 | 0.58 |
| 1 year post-transplant | 23 | 27 | 0.71 |
| Pre-transplant diabetes mellitus, % | 24 | 40 | 0.09 |
| Post-transplant diabetes mellitus, % | 37 | 43 | 0.54 |
| On pre-transplant anti-hypertensive, % | 74 | 72 | 0.82 |
| On post-transplant anti-hypertensive, % | 47 | 55 | 0.42 |
SD, standard deviation.
Thirty-one of 50 patients (62%) in the steroids group and 23 of 50 patients (46%) in the no-steroids group had HCV infection. Incidences of recurrent HCV were similar between the no-steroids and standard therapy groups (30% versus 48%, respectively; P = 0.07). However, increased graft fibrosis was noted in recipients in the no-steroids group compared with the steroids group (Ishak scores: 2.6 ± 0.5 versus 1.5 ± 0.2, respectively; P = 0.05).
Although the difference between the groups in graft survival was not statistically different, Kaplan–Meier survival analysis demonstrated a possible decrease in graft survival in the no-steroids group. Rates of graft survival in the no-steroids and steroids groups, respectively, at 1, 3 and 5 years following transplantation were 76% versus 76%, 64% versus 74%, and 56% versus 72%, respectively (P = 0.09 with a 9-year follow-up) (Fig. 2). Overall patient survival also showed an insignificant trend towards decreased survival in the no-steroids group. Rates of recipient survival in the no-steroids and steroids groups, respectively, at 1, 3 and 5 years following transplantation were 80% versus 86%, 68% versus 76% and 60% versus 72%, respectively (P = 0.11 with a 9-year follow-up) (Fig. 3).
Figure 2.
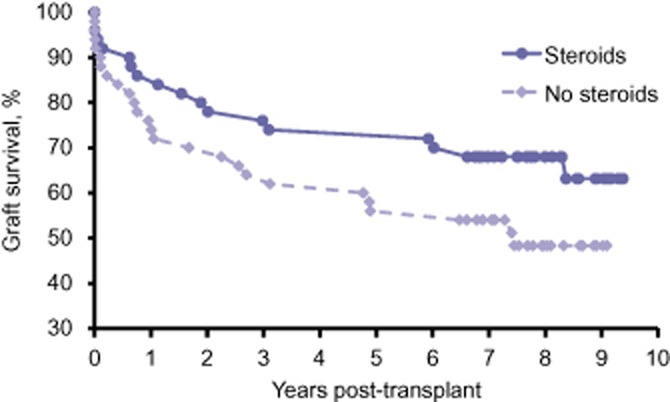
Graft survival in liver transplant recipients randomized to either standard immunosuppression (steroids) or complete avoidance of steroids (no steroids). P = 0.09 with 9-year follow-up
Figure 3.
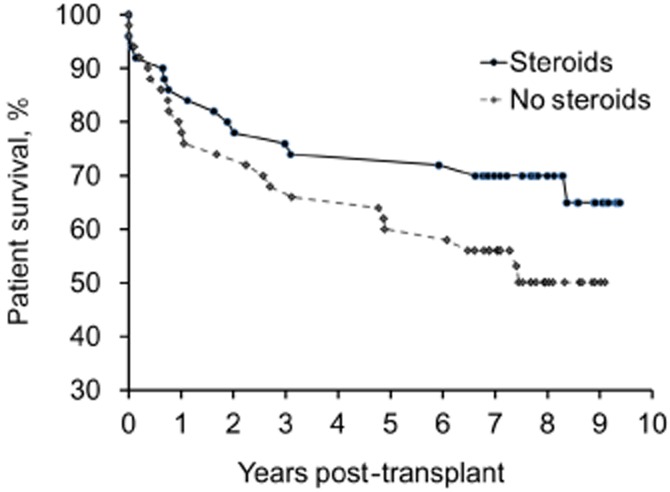
Patient survival in liver transplant recipients randomized to either standard immunosuppression (steroids) or complete avoidance of steroids (no steroids). P = 0.11 with 9-year follow-up
Discussion
Steroids remain a mainstay of treatment following liver transplantation but their administration is associated with considerable morbidity. In the present study, the potential benefit of complete steroids avoidance was prospectively investigated. Although recipient and graft survival rates at 1, 3 and 5 years did not differ significantly, there was a concerning trend towards decreased patient and graft survival in the steroids-free arm of the trial compared with the group receiving standard immunosuppression. Although there were no differences between the groups in incidences of acute rejection, chronic rejection and recurrent HCV, need for retransplant, extent of graft fibrosis and mean 6-month post-transplant serum creatinine were significantly worse in the no-steroids group. Contrary to this study hypothesis, recipients receiving steroids required more anti-hypertensive medication at 1 year post-transplant. Overall, complete steroids avoidance seemed to have minimal clinical benefit, but findings indicate that it may lead to potentially higher morbidity and mortality.
To minimize the longterm consequences of chronic steroid use, steroid withdrawal within the first year of liver transplantation has become standard.8,9 Several studies, using many different immunosuppression regimens, have investigated either complete steroids avoidance22,25,29 or the induction of immunosuppression without steroids after the administration of perioperative steroids.8,9,19,20 Some of these studies have also substituted steroids for a different immunosuppressant medication. A single-arm prospective study in which subjects were given a single intraoperative dose of steroids followed by daclizumab, MMF and tacrolimus demonstrated this to be a safe regimen and achieved patient survival of 96% at 6 months.19 A small trial in which all patients received two perioperative doses of steroids and were then randomized to tacrolimus and steroids or tacrolimus and MMF without steroids found no difference in safety and effectiveness between the two protocols.20 Pirenne et al. compared a group of 21 liver transplant recipients who received no steroids with a historical group of controls and found no difference in rejection or 3-year patient and graft survival.25 One study replaced steroid induction and maintenance with rabbit antithymocyte globulin and found no difference in graft and patient survival or rates of rejection at 1 year.22 Meta-analyses and meta-regression of 19 randomized trials resulted in inconclusive data as a result of the extreme heterogeneity of the trials themselves.29 Follow-up meta-analyses of liver transplant recipients for any indication in 21 RCTs showed no differences between steroids-free protocols and protocols including steroids for most of the parameters analysed.30 Furthermore, steroids avoidance protocols were recently examined in nine non-renal transplant studies. Seven of these studies investigated the impact of steroid withdrawal on liver recipients and found no difference in rates of acute rejection or patient and graft survival.31 To date, no significant difference in patient or graft survival has been noted in any study examining steroids avoidance, including the present RCT.
Prior studies investigating the mitigation of the potential side-effects of steroids have resulted in mixed findings. Steroids have been associated with increased incidences of hypertension,32 post-transplant diabetes18,27,32 and steroid-resistant rejection,18 and higher cholesterol levels.27 The advantages of steroids avoidance demonstrated in the present study included a decreased requirement for anti-hypertensive medication at 1 year post-transplant and the maintenance of relatively stable cholesterol levels during the post-transplant period. However, there was no difference in insulin use between the steroids and no-steroids groups. Of concern, liver recipients in the steroids-free arm of the trial had elevated serum creatinine at 6 months after transplant compared with the standard immunosuppression group. Although prior studies did not demonstrate a difference in either post-transplant patient or graft survival,18,27,32 the results of the present study are concerning because they indicate a potentially unacceptable decrease in patient and graft survival associated with complete steroids avoidance.
The incidence of rejection in the present study was similar in both groups and none of the patients were steroid-resistant. Two thirds of the patients in the no-steroids group were never given steroids for either the induction or maintenance of immunosuppression. A prospective trial in which patients were randomized to receive tacrolimus and either dacluzimab or steroids after an intraoperative dose of steroids showed no difference in the incidence of rejection, but a lower incidence of steroid-resistant rejection in recipients who had not been given postoperative steroids.18 In a small single-centre trial, a 6-year follow-up of steroids-free management with tacrolimus and dacluzimab induction showed no difference in patient or graft survival between the groups receiving induction treatment with tacrolimus or steroids, respectively.33 A randomized study of 45 patients who underwent protocol biopsies showed no significant difference in 2-year rejection rates.27 Other studies in which protocol biopsies were performed at 1 week showed high rejection rates of 70% in steroids-free patients.6,26 It is unclear whether these biopsy findings are clinically significant.
Steroids play a role in the recurrence of HCV following liver transplantation. The present study defined HCV recurrence as a persistent elevation of liver enzymes to ≥1.5 times the normal level for 3 months consecutively that could not be explained by any other diagnosis,28 and found a non-significant increased trend towards recurrent disease in the steroids group. When transplant liver biopsies were performed, liver recipients in the no-steroids group demonstrated a higher Ishak score for graft fibrosis despite their decreased rate of HCV recurrence. By contrast, a prospective randomized trial of steroids avoidance in HCV-positive patients showed higher rates of HCV recurrence with steroids.23 In addition, a recent randomized, multicentre European study showed no difference in HCV viral load between groups using and not using steroids avoidance protocols and higher recurrence rates in the steroids arm. However, the results of this study were inconclusive because protocol completion rates were poor.34 The dynamics of HCV RNA replication have been found to be faster in liver allograft recipients receiving steroids than in those who are not.27 By contrast with the present study, other studies have described more severe HCV recurrence in recipients on steroids. For example, a prospective trial in which all patients received an intraoperative dose of steroids and were then randomized to receive tacrolimus alone or tacrolimus with steroids found less severe recurrent HCV in patients who did not receive postoperative steroids.21 Furthermore, a small study of hepatitis C patients randomized to steroids or no steroids showed a trend towards advanced fibrosis at 1 year in the steroids-free group.24 The influence of maintenance steroids on HCV recurrence remains unclear.
This single-centre, prospective randomized trial has the advantage of longterm follow-up and suggests evidence that contrasts with the findings of many recent clinical trials investigating steroids avoidance. The principal strength of this study, compared with other similar trials, refers to the complete absence of any use of steroids in the steroids-free arm. Many steroids avoidance protocols reviewed here include the administration of single doses or short tapers of steroids in the intraoperative or immediate postoperative periods. However, this study is limited by its small sample size and the fact that 32% of patients in the steroids-free arm received steroids for various clinic reasons. Additionally, protocol biopsies were not employed in this study. Finally, although the differences that emerged between the groups in this study did not reach statistical significance, they do demonstrate a concerning trend. Statistical significance may not have been achieved as a result of the low power of the study and type II error. Given that the null hypothesis shows no difference between the arms of the study but may be false, the statistics presented here may actually accept the null in error, given the power of the study.
Complete avoidance of steroids appears to provide liver transplant recipients with minimal benefit. Furthermore, complete steroids avoidance during liver transplantation was associated with worse post-transplant renal function, a concerning trend towards increased graft loss, accelerated allograft fibrosis related to recurrent HCV, need for retransplantation and a possible increase in recipient mortality rates. The extreme heterogeneity of studies conducted to date makes any conclusive finding difficult to interpret. However, the findings presented in the present study suggest that following liver transplantation, at least a short course of induction or maintenance steroids should be used.
Acknowledgments
This study was supported by a grant from Astellas Pharma, Inc., Deerfield, IL, USA.
Conflicts of interest
None declared.
References
- 1.Starzl TE, Marchioro TL, Vonkaulla KN, Hermann G, Brittain RS, Waddell WR. Homotransplantation of the liver in humans. Surg Gynecol Obstet. 1963;117:659–676. [PMC free article] [PubMed] [Google Scholar]
- 2.Lerut JP. Avoiding steroids in solid organ transplantation. Transpl Int. 2003;16:213–224. doi: 10.1007/s00147-003-0546-x. [DOI] [PubMed] [Google Scholar]
- 3.Lake JR. The role of immunosuppression in recurrence of hepatitis C. Liver Transpl. 2003;9(Suppl):63–66. doi: 10.1053/jlts.2003.50264. [DOI] [PubMed] [Google Scholar]
- 4.Sheiner PA, Schwartz ME, Mor E, Schluger LK, Theise N, Kishikawa K, et al. Severe or multiple rejection episodes are associated with early recurrence of hepatitis C after orthotopic liver transplantation. Hepatology (Baltimore, MD) 1995;21:30–34. [PubMed] [Google Scholar]
- 5.Charlton M, Seaberg E. Impact of immunosuppression and acute rejection on recurrence of hepatitis C: results of the National Institute of Diabetes and Digestive and Kidney Diseases Liver Transplantation Database. Liver Transpl Surg. 1999;5(4 Suppl. 1):107–114. doi: 10.1053/JTLS005s00107. [DOI] [PubMed] [Google Scholar]
- 6.Reggiani P, Arru M, Regazzi M, Gatti S, Molinaro MD, Caccamo L, et al. A ‘steroid-free’ tacrolimus and low-dose mycophenolate mofetil primary immunosuppression does not prevent early acute rejection after liver transplantation. Transplant Proc. 2005;37:1697–1699. doi: 10.1016/j.transproceed.2005.02.111. [DOI] [PubMed] [Google Scholar]
- 7.Padbury RT, Toogood GJ, McMaster P. Withdrawal of immunosuppression in liver allograft recipients. Liver Transpl Surg. 1998;4:242–248. doi: 10.1002/lt.500040309. [DOI] [PubMed] [Google Scholar]
- 8.Punch JD, Shieck VL, Campbell DA, Bromberg JS, Turcotte JG, Merion RM. Corticosteroid withdrawal after liver transplantation. Surgery. 1995;118:783–786. doi: 10.1016/s0039-6060(05)80050-9. discussion 786–788. [DOI] [PubMed] [Google Scholar]
- 9.Stegall MD, Wachs ME, Everson G, Steinberg T, Bilir B, Shrestha R, et al. Prednisone withdrawal 14 days after liver transplantation with mycophenolate: a prospective trial of cyclosporine and tacrolimus. Transplantation. 1997;64:1755–1760. doi: 10.1097/00007890-199712270-00023. [DOI] [PubMed] [Google Scholar]
- 10.Fung J, Abu-Elmagd K, Jain A, Gordon R, Tzakis A, Todo S, et al. A randomized trial of primary liver transplantation under immunosuppression with FK 506 vs cyclosporine. Transplant Proc. 1991;23:2977–2983. [PMC free article] [PubMed] [Google Scholar]
- 11.Tchervenkov JI, Tector AJ, Cantarovich M, Tahta SA, Asfar A, Naimi J, et al. Maintenance immunosuppression using cyclosporine monotherapy in adult orthotopic liver transplant recipients. Transplant Proc. 1996;28:2247–2249. [PubMed] [Google Scholar]
- 12.Gomez R, Moreno E, Colina F, Loinaz C, Gonzalez-Pinto I, Lumbreras C, et al. Steroid withdrawal is safe and beneficial in stable cyclosporine-treated liver transplant patients. J Hepatol. 1998;28:150–156. doi: 10.1016/s0168-8278(98)80214-6. [DOI] [PubMed] [Google Scholar]
- 13.Everson GT, Trouillot T, Wachs M, Bak T, Steinberg T, Kam I, et al. Early steroid withdrawal in liver transplantation is safe and beneficial. Liver Transpl Surg. 1999;5(4 Suppl. 1):48–57. doi: 10.1053/JTLS005s00048. [DOI] [PubMed] [Google Scholar]
- 14.Trotter JF, Wachs M, Bak T, Trouillot T, Stolpman N, Everson GT, et al. Liver transplantation using sirolimus and minimal corticosteroids (3-day taper) Liver Transpl. 2001;7:343–351. doi: 10.1053/jlts.2001.23012. [DOI] [PubMed] [Google Scholar]
- 15.Greig P, Lilly L, Scudamore C, Erb S, Yoshida E, Kneteman N, et al. Early steroid withdrawal after liver transplantation: the Canadian tacrolimus versus microemulsion cyclosporin A trial: 1-year follow-up. Liver Transpl. 2003;9:587–595. doi: 10.1053/jlts.2003.50102. [DOI] [PubMed] [Google Scholar]
- 16.Belli LS, de Carlis L, Rondinara G, Alberti AB, Bellati G, De Gasperi A, et al. Early cyclosporine monotherapy in liver transplantation: a 5-year follow-up of a prospective, randomized trial. Hepatology (Baltimore, MD) 1998;27:1524–1529. doi: 10.1002/hep.510270609. [DOI] [PubMed] [Google Scholar]
- 17.Pageaux GP, Calmus Y, Boillot O, Ducerf C, Vanlemmens C, Boudjema K, et al. Steroid withdrawal at day 14 after liver transplantation: a double-blind, placebo-controlled study. Liver Transpl. 2004;10:1454–1460. doi: 10.1002/lt.20291. [DOI] [PubMed] [Google Scholar]
- 18.Boillot O, Mayer DA, Boudjema K, Salizzoni M, Gridelli B, Filipponi F, et al. Corticosteroid-free immunosuppression with tacrolimus following induction with daclizumab: a large randomized clinical study. Liver Transpl. 2005;11:61–67. doi: 10.1002/lt.20307. [DOI] [PubMed] [Google Scholar]
- 19.Figueras J, Prieto M, Bernardos A, Rimola A, Suarez F, de Urbina JO, et al. Daclizumab induction and maintenance steroid-free immunosuppression with mycophenolate mofetil and tacrolimus to prevent acute rejection of hepatic allografts. Transpl Int. 2006;19:641–648. doi: 10.1111/j.1432-2277.2006.00326.x. [DOI] [PubMed] [Google Scholar]
- 20.Langrehr JM, Neumann UP, Lang M, Muller AR, Jonas S, Settmacher U, et al. First results from a prospective randomized trial comparing steroid-free induction therapy with tacrolimus and MMF versus tacrolimus and steroids in patients after liver transplantation for HCV. Transplant Proc. 2002;34:1565–1566. doi: 10.1016/s0041-1345(02)03024-5. [DOI] [PubMed] [Google Scholar]
- 21.Margarit C, Bilbao I, Castells L, Lopez I, Pou L, Allende E, et al. A prospective randomized trial comparing tacrolimus and steroids with tacrolimus monotherapy in liver transplantation: the impact on recurrence of hepatitis C. Transpl Int. 2005;18:1336–1345. doi: 10.1111/j.1432-2277.2005.00217.x. [DOI] [PubMed] [Google Scholar]
- 22.Eason JD, Nair S, Cohen AJ, Blazek JL, Loss GE., Jr Steroid-free liver transplantation using rabbit antithymocyte globulin and early tacrolimus monotherapy. Transplantation. 2003;75:1396–1399. doi: 10.1097/01.TP.0000062834.30922.FE. [DOI] [PubMed] [Google Scholar]
- 23.Filipponi F, Callea F, Salizzoni M, Grazi GL, Fassati LR, Rossi M, et al. Double-blind comparison of hepatitis C histological recurrence rate in HCV+ liver transplant recipients given basiliximab + steroids or basiliximab + placebo, in addition to cyclosporine and azathioprine. Transplantation. 2004;78:1488–1495. doi: 10.1097/01.tp.0000140881.07208.4e. [DOI] [PubMed] [Google Scholar]
- 24.Kato T, Yoshida H, Sadfar K, Martinez E, Nishida S, Moon J, et al. Steroid-free induction and pre-emptive antiviral therapy for liver transplant recipients with hepatitis C: a preliminary report from a prospective randomized study. Transplant Proc. 2005;37:1217–1219. doi: 10.1016/j.transproceed.2004.12.042. [DOI] [PubMed] [Google Scholar]
- 25.Pirenne J, Aerts R, Koshiba T, Van Gelder F, Roskams T, Schetz M, et al. Steroid-free immunosuppression during and after liver transplantation – a 3-year follow-up report. Clin Transplant. 2003;17:177–182. doi: 10.1034/j.1399-0012.2003.00017.x. [DOI] [PubMed] [Google Scholar]
- 26.Tisone G, Angelico M, Orlando G, Palmieri GP, Strati F, Di Paolo D, et al. Retrospective analysis of 30 patients who underwent liver transplantation without use of steroids. Transplant Proc. 1999;31:2908–2909. doi: 10.1016/s0041-1345(99)00611-9. [DOI] [PubMed] [Google Scholar]
- 27.Tisone G, Angelico M, Palmieri G, Pisani F, Anselmo A, Baiocchi L, et al. A pilot study on the safety and effectiveness of immunosuppression without prednisone after liver transplantation. Transplantation. 1999;67:1308–1313. doi: 10.1097/00007890-199905270-00003. [DOI] [PubMed] [Google Scholar]
- 28.Pelletier SJ, Iezzoni JC, Crabtree TD, Hahn YS, Sawyer RG, Pruett TL. Prediction of liver allograft fibrosis after transplantation for hepatitis C virus: persistent elevation of serum transaminase levels versus necroinflammatory activity. Liver Transpl. 2000;6:44–53. doi: 10.1002/lt.500060111. [DOI] [PubMed] [Google Scholar]
- 29.Segev DL, Sozio SM, Shin EJ, Nazarian SM, Nathan H, Thuluvath PJ, et al. Steroid avoidance in liver transplantation: meta-analysis and meta-regression of randomized trials. Liver Transpl. 2008;14:512–525. doi: 10.1002/lt.21396. [DOI] [PubMed] [Google Scholar]
- 30.Sgourakis G, Radtke A, Fouzas I, Mylona S, Goumas K, Gockel I, et al. Corticosteroid-free immunosuppression in liver transplantation: a meta-analysis and meta-regression of outcomes. Transpl Int. 2009;22:892–905. doi: 10.1111/j.1432-2277.2009.00893.x. [DOI] [PubMed] [Google Scholar]
- 31.Knight SR, Morris PJ. Steroid sparing protocols following non-renal transplants; the evidence is not there. A systematic review and meta-analysis. Transpl Int. 2011;24:1198–1207. doi: 10.1111/j.1432-2277.2011.01335.x. [DOI] [PubMed] [Google Scholar]
- 32.Llado L, Xiol X, Figueras J, Ramos E, Memba R, Serrano T, et al. Immunosuppression without steroids in liver transplantation is safe and reduces infection and metabolic complications: results from a prospective multicentre randomized study. J Hepatol. 2006;44:710–716. doi: 10.1016/j.jhep.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 33.Foroncewicz B, Mucha K, Ryszkowska E, Ciszek M, Ziolkowski J, Porowski D, et al. Safety and efficacy of steroid-free immunosuppression with tacrolimus and daclizumab in liver transplant recipients: 6-year follow-up in a single centre. Transplant Proc. 2009;41:3103–3106. doi: 10.1016/j.transproceed.2009.07.082. [DOI] [PubMed] [Google Scholar]
- 34.Neumann U, Samuel D, Trunecka P, Gugenheim J, Gerunda GE, Friman S. A randomized multicentre study comparing a tacrolimus-based protocol with and without steroids in HCV-positive liver allograft recipients. J Transplant. 2012;2012:894215. doi: 10.1155/2012/894215. [DOI] [PMC free article] [PubMed] [Google Scholar]


