Abstract
Objectives
Enhanced recovery after surgery (ERAS) protocols are coming to represent the standard of care in many surgical procedures, yet data on their use following hepatic surgery are scarce. The aim of this study was to review outcomes after the introduction of an ERAS programme for patients undergoing open hepatic resection.
Methods
A retrospective review of patients undergoing open hepatic resection from March 2005 to June 2011 was carried out. The primary outcome measure was total hospital length of stay (LoS) (including readmissions). Principles associated with enhanced recovery after surgery were documented and analysed as independent predictors of hospital LoS.
Results
A total of 120 patients underwent 128 consecutive hepatic resections, 84 (65.6%) of which were performed in patients with underlying colorectal metastases and 64 (50.0%) of which comprised major hepatic resections. The median hospital LoS was reduced from 6 days to 3 days from the first to the fourth quartiles of the study population (P = 0.021). The proportion of patients suffering complications (26.6%) remained constant across the series. Readmissions increased from the first quartile (none of 32 patients) to the fourth quartile (seven of 32 patients) (P = 0.044). Following multivariate analysis, only the development of a complication (P < 0.001), total postoperative i.v. fluid (P = 0.003) and formation of an anastomosis (P = 0.006) were independent predictors of hospital LoS.
Conclusions
An ERAS programme can be successfully applied to patients undergoing open hepatic resection with a reduction in hospital LoS, but an increase in the rate of readmissions.
Introduction
Enhanced recovery after surgery (ERAS) programmes following surgical interventions are now within the standard of care for patients undergoing colorectal surgery. High-level evidence exists to support their use.1 The magnitude of benefit to be derived from such programmes in open surgery is greater than the effect of conversion to laparoscopic surgery alone.2 However, data supporting the use of ERAS programmes after hepatic resection are relatively scarce. A recent systematic review3 of ERAS programmes for hepatic resections identified only three cohort studies4–6 of sufficient quality to meet inclusion criteria for further analysis. The results of two of these studies4,5 showed significant reductions in hospital length of stay (LoS) of 2–3 days and, in addition, one study5 showed a cost reduction in association with an ERAS programme. No studies4–6 showed an increase in rates of readmissions, complications or mortality. However, there are several problems associated with these studies in that whether the available data are sufficiently powered to detect differences in the particular outcome measures used is questionable. Moreover, no assessment of the effect on quality of life of ERAS programmes was possible from the included studies.3 The authors of the systematic review also noted significant heterogeneity in the descriptions of the methodologies of the ERAS programmes, which indicates that these results must be interpreted with caution.3
Unlike clinical trials, in which samples of patients are randomized to two or more interventions in order to resolve a specific question, real-time clinical practice often involves evolution over time as new evidence becomes available and alterations in practice take time to embed. Thus, the aims of this study were to review the introduction of an ERAS programme into a single surgeon's practice of hepatic resection over a 6-year period and, specifically, to determine whether the gradual implementation of ERAS principles reduces hospital LoS.
Materials and methods
Patients undergoing hepatic resection were identified from a prospective database of hepatic resections within a single surgeon's practice that had been compiled since the surgeon began working at this centre. This surgeon was the major provider of non-transplant hepatic surgery for patients normally resident in the upper South Island of New Zealand; surgeries were undertaken at Christchurch Hospital. A retrospective patient note review was then performed. Standard demographic data and information on diagnosis, intraoperative variables (blood loss and blood transfused) and hospital LoS were collected prospectively. Other intraoperative variables (use of nasogastric tubes, drains, use of regional analgesia such as epidural or intrathecal morphine, volume of i.v. fluids infused, use of continuous or intermittent wound catheters), and postoperative variables (volume of i.v. fluids infused, complications, non-use of steroids) were obtained retrospectively from patient notes. The volume of i.v. fluids infused was totalled at 24 h (excluding fluid given intraoperatively), 48 h and 72 h postoperatively and for the entire admission. An anastomosis was defined as any entero-enteric or hepaticojejunostomy. Total hospital LoS was defined as the LoS in days from the initial admission plus the LoS of any readmission that occurred within 30 days of initial discharge. Readmission data collected included time to readmission, reason for readmission and duration of readmission. Readmission was defined as any hospital readmission within 30 days of discharge; data were taken from the electronic database used in the hospital and its surrounding districts. There was only one hospital to which patients could be readmitted. Postoperative death was defined as any death within 90 days or within the same hospital admission or readmission. Hepatic resections were coded as sequential hepatic resections starting at one. Each admission for a hepatic resection was treated as an independent event irrespective of whether the patient had undergone a previous hepatic resection.
Clinical pathway
Clinical care outside clinical trials is not a static or dichotomous event and practice evolves over time. During the time period reviewed in this study, the principles of an ERAS protocol were gradually introduced. The time-points at which these various principles were introduced are documented for the purpose of this study in Table 1. A full ERAS programme was then in place by December 2009 (following hepatic resection number 79).
Table 1.
Changes in the standard of care with the gradual implementation of an enhanced recovery after surgery (ERAS) protocol
| Standard of care at commencement of study | Changes to care by quartile |
|---|---|
| Preoperative care | No changes |
| Admission on day of surgery | |
| Oral carbohydrate loading | |
| No bowel preparation or routine use of premedication | |
| Prophylactic antibiotics on induction and at closure if the duration of surgery exceeds 180 min | |
| Intraoperative care | Quartile 1 |
| Anaesthesia with volatile anaesthetic, short-acting opioids and anti-emetics | From hepatic resection 14: switch from epidural analgesia to intrathecal morphine because of inability to provide an appropriate post-epidural care package18 |
| Intravenous fluids: titrated against central venous pressure (central venous pressure targeted at <5 cmH2O intraoperatively, then 8 cmH2O in the early postoperative period) | From hepatic resection 27: introduction of bolus delivery (intermittent) wound catheters with 40 ml 0.2% ropivacaine every 6 h for 3 days with a 3-mg/kg loading dose at the time of closure |
| Intraoperative hypothermia was minimized by use of upper and lower body warming devices | Quartile 3 |
| All resections were performed by open surgery using transverse incisions | From hepatic resection 79: commercial continuous delivery system of 0.2% ropivacaine |
| Not used routinely: drains, nasogastric tubes | |
| Postoperative care | Quartile 1 |
| Deep vein thrombosis prophylaxis (20–40 mg enoxaparin subcutaneously from the evening of surgery) | From hepatic resection 8: routine use of NSAIDs with omeprazole cover at 24 h postoperatively |
| Analgesia: paracetamol 1 g q.i.d. | |
| Opioid-based patient-controlled analgesia | |
| Anti-emetics as required | |
| Not used routinely: laxatives, diuretics, immunonutrition, gabapentin19 | |
| Eat and drink as tolerated without restriction from day 0 | |
| Early mobilization | |
| Urinary catheter removed at day 1 | |
NSAIDs, non-steroidal anti-inflammatory drugs.
Preoperative patient counselling was delivered by the surgeon and nurses in the outpatient department and standard pre-admission clinic. No additional media were used. Plans for discharge were discussed at these visits and included an expected day of discharge.
Opioid-based, patient-controlled analgesia was used until the patient felt comfortable about taking oral analgesia. Analgesia was administered at the discretion of a dedicated pain relief team.
In the postoperative period, patients routinely returned to a high-dependency unit in which one nurse was dedicated to two patients. Patients were able to eat and drink without restriction on return to this ward. Within 24 h patients returned to the normal ward setting. Patients ideally returned to the home ward, but were not co-located with other ERAS programme patients.
The management of i.v. fluids within the postoperative period has evolved. Initially, fluid was often used to counteract epidural-induced hypotension. After the switch to intrathecal morphine, the consultant surgeon made a concentrated effort to minimize the use of i.v. fluids. On their return to the ward, patients were charted 60–80 ml/h of crystalloid until they were drinking well. This was slowed to 20 ml/h within 12 h, as per the patient-controlled analgesia protocol. Urine outputs of 10–20 ml/h were tolerated. Nursing and junior medical staff were discouraged from simply increasing i.v. fluid intake in response to low urine outputs, and encouraged to make a complete assessment and use fluid boluses with reassessment. This process took time to embed. The overuse of 0.9% saline was discouraged. Routine weighing and diuretics were not used. The consultant surgeon or a hepatopancreatobiliary fellow routinely made ward rounds twice per day.
Patients were deemed fit for discharge when their findings were normal (including clinical assessment for hepatic dysfunction) and pain was controlled with oral analgesia. They were also required to demonstrate tolerance of an oral diet and to show that appropriate social supports were in place. A bowel motion or passage of flatus was not deemed to be a requirement for discharge. The biochemical and haematologic presence of jaundice, elevated C-reactive protein, elevated prothrombin time and low haemoglobin were not contraindications to discharge per se, but trends in these factors and evidence of stability were considered important.
To help alleviate patient concerns at the time of discharge and to ensure that these patients had direct access to the surgical service, a laminated credit card-sized card was developed. On one side, this listed symptoms patients should look out for and gave details of staff to contact in the event that they became concerned (i.e. the surgeon's mobile number, the direct line to the ward, the on-call surgical registrar's number). The other side of the card provided advice to emergency doctors or general practitioners about which blood tests to perform and who to contact if problems arose.
Patients referred to the tertiary centre from peripheral hospitals were discharged to commercial facilities (at the expense of the referring hospital) or to the care of relatives or friends within the city if they did not require extra nursing care. They were asked to remain within the city for a further week. All patients were reviewed at 6–8 days after discharge in an outpatient clinic, at which point their histology was communicated to them.
Statistical analysis
Continuous variables are described as medians (ranges) and categorical variables as numbers and percentages as appropriate. Univariate analysis was performed using the Mann–Whitney U-test. Multiple linear regression was used to determine whether specific perioperative factors were able to independently predict LoS. To normalize the dependent variable (LoS), natural log transformation was performed. Dummy variables were created for the nominal variables. Stepwise multiple linear regression was also performed to check for any anomalies in the data. Variables to be included within the model were based on factors found to have P-values of < 0.20 on univariate analysis. A P-value of < 0.05 was considered to indicate statistical significance.
Results
Between March 2005 and June 2011, 129 patients were identified from a prospectively collated database. Nine (7.0%) patients were excluded from further analysis because they had undergone minor laparoscopic liver resection (n = 4), the proposed hepatic resection had been abandoned when extrahepatic malignant disease was found at laparotomy (n = 3), unexpected cirrhosis had been identified (n = 1) or they had undergone radiofrequency ablation without hepatic resection (n = 1). The 120 (93.0%) patients included in the study represented a total of 128 hospital admissions for hepatic resection; these admissions comprised the final dataset. Eight (6.7%) patients underwent two hepatic resections. Demographics and pre- and intraoperative data are shown in Table 2. Postoperative data are shown in Table 3. Overall, 34 of 128 (26.6%) hepatic resections were associated with the occurrence of complications. These consisted of primary medical complications (n = 12, 9.4%), including perioperative myocardial infarction (n = 3), cardiac arrhythmia (n = 3), cardiac arrest (n = 1), dehydration (n = 1), asthma exacerbation (n = 1), allergic reaction (n = 1) and urinary tract infection (n = 1), atelectasis (n = 1), hepatic dysfunction (n = 4, 3.1%), bile leak (n = 5, 3.9%), haemorrhage (n = 4, 3.1%), intra-abdominal abscess or collection (n = 4, 3.1%), anastomotic leak (n = 1, 0.8%), hernia (n = 1, 0.8%), ileus (n = 1, 0.8%) ischaemic gut (n = 1, 0.8%) and portal vein thrombosis (n = 1, 0.8%). Two patients died in the postoperative period, resulting in a mortality rate after hepatic resection of 1.6%. In both patients death occurred following prolonged hospitalization (at 14 and 111 days, respectively) and resulted from multi-organ failure; the initial complication was portal vein thrombosis in the first patient and ischaemic gut in the second. Overall, 15 patients (11.7%) were readmitted and required a median of 3 days of additional hospitalization. Patients were readmitted for primary medical reasons (n = 5, 3.9%), including lower respiratory tract infection (n = 2) and asthma exacerbation (n = 1), anaemia (n = 1), ascites (n = 1), bile leak (n = 3, 2.3%), wound infection (n = 1, 0.8%), ileus (n = 1, 0.8%), intra-abdominal collection (n = 3, 2.3%) and non-specific reasons in which no cause for readmission was identified other than that the patient felt safer in hospital (n = 2, 1.6%).
Table 2.
Demographics and preoperative data for patients undergoing hepatic resection during March 2005 to June 2011
| Variable (n = 128 resections) | Value |
|---|---|
| Age, years, median (range) | 63 (35–82) |
| Cirrhosis, n (%) | 4 (3%) |
| ASA score of 1 or 2, n (%) | 104 (81%) |
| Diagnosis, n (%) | |
| Colorectal metastases | 84 (66%) |
| Cholangiocarcinoma | 11 (9%) |
| Hepatocellular carcinoma | 9 (7%) |
| Gastrointestinal stromal tumour metastases | 3 (2%) |
| Carcinoid metastases | 4 (3%) |
| Other: benign/malignant | 10/8 (8%/6%) |
| Intraoperative factors, n (%) | |
| Major hepatectomy | 64 (50%) |
| Anastomosis | 10 (8%) |
| Epidural/intrathecal morphine/neither | 9/111/8 (7%/87%/6%) |
| Wound catheter: nil/continuous/intermittent | 28/51/49 (22%/40%/38%) |
| Nasogastric tube | 8 (6%) |
| Drain | 25 (20%) |
| Blood loss, <0.5 l/0.5–1.0 l/>1.0 l | 86/33/9 (67%/26%/7%) |
| Intraoperative transfusiona, units, median (range) | 0 (0–5) |
Fifteen of 128 (11.7%) patients who underwent a hepatic resection received a transfusion intraoperatively.
ASA, American Society of Anesthesiologists.
Table 3.
Postoperative data for patients undergoing hepatic resection during March 2005 to June 2011
| Variable (n = 128 resections) | Value |
|---|---|
| Total i.v. fluids in first 24 h, l, median (range) | 3 (0.25–10) |
| Total i.v. fluids in first 48 h, l, median (range) | 1 (0–7) |
| Total i.v. fluids in first 72 h, l, median (range) | 0 (0–4) |
| Total i.v. fluids, l, median (range) | 4.6 (1–58) |
| Reintroduction of diet, day, median (range) | 0 (0–15) |
| Patients using NSAIDs, n (%) | 70 (55%) |
| Length of stay, days, median (range) | 4 (2–111) |
| Patients readmitted, n (%) | 14 (11%) |
| Time from discharge to readmission, days, median (range) | 3 (1–21) |
| Duration of readmission, days, median (range) | 3 (1–16) |
| Reason for readmission, n (%) | |
| Medical | 4 (3%) |
| Bile leak | 3 (2%) |
| Non-specific | 2 (2%) |
| Wound infection | 1 (1%) |
| Ileus | 1 (1%) |
| Intra-abdominal collection/abscess | 3 (2%) |
NSAIDs, non-steroidal anti-inflammatory drugs.
As Fig. 1 shows, median hospital LoS decreased across the series, but the proportion of patients requiring readmission increased as LoS reduced. The frequency of complications remained constant throughout the series (Fig. 1). The median total hospital LoS was 8 days (range: 2–111 days) in patients with complications who were not readmitted and 12 days (range: 5–28 days) in those who were readmitted; however, this difference was not statistically significant (P = 0.086). Univariate analysis (Table 4) revealed 11 factors as statistically important in determining LoS. A further two factors had P-values of < 0.20 and were included in a multivariate model. On multivariate analysis, three factors remained as independent predictors of LoS: development of a complication [coefficient: 1.84, 95% confidence interval (CI) 1.48–2.28; P < 0.001]; total volume of i.v. fluid received (coefficient: 1.68, 95% CI 1.39–2.02; P = 0.003), and presence of an anastomosis (coefficient: 1.83, 95% CI 1.29–2.61; P = 0.006). Because both the development of a complication and total i.v. fluid received are likely to exhibit collinearity and occur post-resection, and the presence of an anastomosis was also associated with an increased LoS, the analysis was re-performed for only those resections (n = 90) in which no complications or anastomoses were noted so that factors directly related to the ERAS protocol could be analysed in more detail (Table 5). From this, nine factors were identified for inclusion in multivariate analysis and none of these were shown to be independent predictors of hospital LoS.
Figure 1.
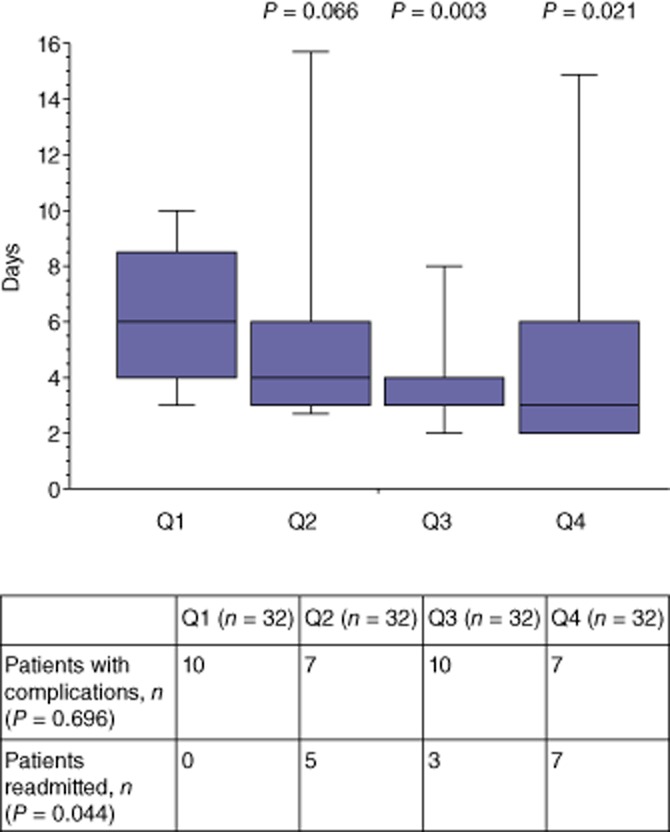
Median hospital length of stay and proportion of patients requiring readmission by quartile (n = 32 patients/quartile) in patients undergoing open hepatic resection during March 2005 to June 2011 (n = 128)
Table 4.
Univariate analysis of variable versus length of stay for patients undergoing hepatic resection during March 2005 to June 2011
| Variable (n = 128 resections) | Days, median (range) | P-value |
|---|---|---|
| Age, years, median (range) | ||
| Quartile 1: 48 (35–54) | 4 (2–30) | |
| Quartile 2: 60 (54–64) | 4.5 (2–111) | 0.204 |
| Quartile 3: 66 (63–69) | 4 (2–34) | 0.555 |
| Quartile 4: 76 (70–82) | 5 (2–22) | 0.113 |
| ASA score: 1 or 2 (n = 104) | 4 (2–34) | |
| 3 or 4 (n = 24) | 6.5 (2–11) | <0.001 |
| Epidural (n = 9) | 8.5 (5–30) | |
| Intrathecal morphine (n = 111) | 4 (2–111) | 0.003 |
| Blood loss: <0.5 l (n = 86) | 4 (2–25) | |
| 0.5–1.0 l (n = 33) | 4 (2–111) | 0.148 |
| >1.0 l (n = 9) | 8 (5–30) | 0.005 |
| Hepatectomy: minor (n = 64) | 4 (2–24) | |
| Major (n = 64) | 4 (2–111) | 0.159 |
| Anastomosis: No (n = 118) | 11 (4–30) | |
| Yes (n = 10) | 4 (2–111) | <0.001 |
| Use of drain: No (n = 103) | 4 (2–111) | |
| Yes (n = 25) | 8 (3–30) | <0.001 |
| NSAIDs use: No (n = 70) | 5 (2–111) | |
| Yes (n = 58) | 4 (2–34) | 0.001 |
| Wound catheter use: None (n = 28) | 4 (2–111) | |
| Intermittent (n = 49) | 4 (2–34) | 0.159 |
| Continuous (n = 51) | 5 (2–30) | 0.035 |
| Postoperative i.v. fluid in first 24 h, median (range) | ||
| Quartile 1: 1 l (0–2 l) | 4 (2–24) | |
| Quartile 2: 2 l (2–3 l) | 3 (2–25) | 0.641 |
| Quartile 3: 3 l (3–4 l) | 3 (2–10) | 0.850 |
| Quartile 4: 5 l (4–9 l) | 6 (2–34) | 0.001 |
| Total i.v. fluid, median (range) | ||
| Quartile 1: 2 l (0–3 l) | 3 (2–22) | |
| Quartile 2: 3 l (3–4 l) | 3 (2–10) | 0.200 |
| Quartile 3: 5 l (4–7 l) | 5 (2–24) | 0.001 |
| Quartile 4: 10 l (7–58 l) | 6 (3–11) | <0.001 |
| Presence of complications | ||
| No (n = 94) | 4 (2–24) | |
| Yes (n = 34) | 8 (2–111) | <0.001 |
| Hepatic resection number | ||
| Quartile 1 | 6 (2–30) | |
| Quartile 2 | 4 (2–111) | 0.066 |
| Quartile 3 | 4 (2–34) | 0.003 |
| Quartile 4 | 3 (2–24) | 0.021 |
ASA, American Society of Anesthesiologists; NSAIDs, non-steroidal anti-inflammatory drugs.
Table 5.
Univariate analysis of variables by length of stay for those patients who did not have an anastomosis or a postoperative complication following hepatic resection during March 2005 to June 2011
| Variable (n = 90) | Days, median (range) | P-value |
|---|---|---|
| Age, years, median (range) | ||
| Quartile 1: 49.5 (35–54) | 3 (2–6) | |
| Quartile 2: 60 (55–63) | 4 (2–24) | 0.276 |
| Quartile 3: 66 (63–69) | 3 (2–21) | 0.919 |
| Quartile 4: 74.5 (64–80) | 4 (2–10) | 0.280 |
| ASA score: 1 or 2 (n = 80) | 3 (2–24) | |
| 3 or 4 (n = 10) | 4.5 (2–21) | 0.036 |
| Epidural (n = 5) | 6 (5–10) | |
| Intrathecal morphine (n = 78) | 3 (2–24) | 0.003 |
| Blood loss: <0.5 l (n = 62) | 3 (2–24) | |
| 0.5–1.0 l (n = 24) | 4 (2–17) | 0.114 |
| >1.0 l (n = 4) | 6 (5–10) | 0.005 |
| Hepatectomy: minor (n = 48) | 3 (2–24) | |
| Major (n = 42) | 4 (2–21) | 0.161 |
| Use of drain: No (n = 82) | 3 (2–24) | |
| Yes (n = 8) | 5 (4–10) | 0.004 |
| NSAIDs use: No (n = 35) | 4 (2–21) | |
| Yes (n = 8) | 3 (2–24) | 0.204 |
| Wound catheter use: None (n = 20) | 4 (2–10) | |
| Intermittent (n = 33) | 3 (2–6) | 0.300 |
| Continuous (n = 37) | 3 (2–24) | 0.013 |
| Postoperative i.v. fluid in first 24 h, median (range) | ||
| Quartile 1: 1 l (0–2 l) | 4 (2–24) | |
| Quartile 2: 2 l (2–3 l) | 3 (2–5) | 0.099 |
| Quartile 3: 3.5 l (3–8 l) | 3 (2–10) | 0.449 |
| Quartile 4: 4 l (3–9 l) | 4.5 (2–21) | 0.162 |
| Hepatic resection number | ||
| Quartile 1 | 4 (2–10) | |
| Quartile 2 | 3 (2–6) | 0.082 |
| Quartile 3 | 3 (2–10) | 0.015 |
| Quartile 4 | 3 (2–24) | 0.091 |
ASA, American Society of Anesthesiologists; NSAIDs, non-steroidal anti-inflammatory drugs.
Discussion
This study indicates that the introduction of an ERAS programme for patients undergoing hepatic resection results in a significant reduction in median LoS from 6 days at the commencement of the study period to 3 days at the end of the study period. This magnitude in reduction is consistent with previous reports of outcomes of fast-track hepatic resections.3 The median hospital LoS of 3 days is, however, significantly less than that reported by previous studies,4–6 including studies which referred to patients undergoing laparoscopic hepatic resection.6 A small series by Stoot et al.6 of 26 patients undergoing laparoscopic hepatic resection showed a non-significant reduction in median LoS from 7 days to 5 days following the introduction of an ERAS protocol. This raises the issue of whether greater focus should be given to optimizing the non-technical aspects of an ERAS programme than to the institution of minimally invasive surgery. This would seem particularly important given that the similarities in economic costs between laparoscopic and open liver resection reported by several studies7,8 primarily derive from the decreased LoS, and because laparoscopic resection is technically demanding and imposes a long learning curve on the surgeon.9 It should, however, be acknowledged that the current study did not assess other important issues, such as return to work or normal activities, for which laparoscopic surgery is likely to be of significant benefit compared with open surgery, as has been seen in patients undergoing segmental colectomy,10 anti-reflux surgery11 and cholecystectomy.12
In the two larger assessments of patients undergoing open hepatic resection,4,5 median hospital LoS was 6 days and 7 days, respectively. Again, the current study would appear to have significantly improved on these figures, but this improvement comes at the cost of an increased readmission rate, which began to climb once median LoS fell below 4 days and reached levels as high as 21.9%. Despite this, the median total LoS (which includes readmission days) remained significantly lower than the median LoS recorded earlier in the study period. This highlights the importance of early after-care and follow-up for patients in an ERAS programme. Ensuring that patients are seen by medical teams who are aware of potential postoperative complications following hepatic resection is crucial to the success of such a programme and to minimizing the costs of readmission. Despite the increase in readmissions, the rate of complications did not increase and remained stable throughout the series. Previous studies13,14 have reported both an increasing readmission rate and decreasing complication rate in association with ERAS programmes. Whether there is an optimal duration of hospital stay that minimizes readmissions but maintains the reduced hospital LoS cannot be determined by this study. It may also be possible that significant cultural differences exist between the current study and previous reports with respect to discharge practice. For example, Lin and colleagues5 reported a pre-planned discharge date at 6 days post-surgery, which may have caused their patients to believe that discharge prior to this date was too early. A further possibility, although this is difficult to determine in a retrospective analysis, is that the behaviour of the surgical team changed over time, resulting in earlier discharge. However, it should be noted that the discharge criteria remained the same throughout the period of study.
The introduction of the ERAS programme at this centre required an evolution in clinical practice over time. It has taken time to implement and embed changes such as those pertaining to the use of intrathecal morphine, the routine use of wound catheters, non-steroidal anti-inflammatory drugs (NSAIDs) and omeprazole, and the limiting of postoperative i.v. fluids. These are now implemented as standard practice. The protocols of ERAS programmes are multifaceted and it is not clear which factors are crucial to their success. In fact, although the present study found an overall reduction in the duration of hospitalization, multivariate analysis excluding data for patients in whom postoperative complications were noted did not reveal any individual factor as an independent predictor of hospital LoS. These results further support the suggestion that it is the package of care that is of most benefit, rather than any of the individual factors. Whether further improvements can be achieved by the addition of postoperative diuretics to maintain preoperative weight or the use of opioid-sparing analgesics remains to be determined.
The current results appear to indicate that not much more can be gained in terms of reducing hospital LoS as it would seem unlikely that day-case hepatic resection would be considered sensible, given the potential for significant haemorrhage. Perhaps further studies should focus on measuring and improving data for quality of life in the intermediate postoperative period. Laparoscopic resection may have a role of significant benefit in this area. Additionally, early identification (prior to discharge) of patients who are likely to develop complications would be useful and would allow for the tailoring of hospital LoS.
The limitations of this study include the fact that it was retrospective, but it is unlikely that any readmissions or complications were missed because patients in this area can be readmitted to only one hospital and patients were followed up in clinic prior to discharge to their primary doctors. In addition, this hospital and its surrounding districts now use a single electronic health record, which makes data review more reliable. It should also be recognized that this population of patients consisted almost exclusively of fit patients with normal underlying hepatic parenchyma, although it did include patients with hilar cholangiocarcinoma, in whom morbidity and mortality have traditionally been high.15 Because of the study's retrospective nature, it did not assess quality of life or return to normal activities, which would seem to be important factors to be included in future research. Another factor that is hard to account for retrospectively concerns the change in patient expectations that develops as the surgeon becomes more experienced and thereby more confident of achieving a shortened LoS. Furthermore, because the return of gastrointestinal function, as heralded by the passage of flatus, was not a requirement for discharge, the effect of return of gastrointestinal function on LoS was not examined. However, Hendry and colleagues16 undertook a randomized controlled trial in which they examined the effects of laxatives, preoperative carbohydrate loading and postoperative oral nutritional supplements on the return of gastrointestinal function following open hepatic resection. They found that those in the laxative group passed bowel motions sooner at a median of 4 days versus 5 days in patients who did not receive laxatives. However, despite this earlier return of gastrointestinal function, Hendry et al. did not show a reduction in the duration of hospitalization.16 A further consideration refers to the role of perioperative i.v. fluids in the return of gut function. Although no studies of this factor in patients undergoing liver resection were identified, data from studies of bowel surgery indicate that liberal fluid prescription results in delayed return of gut function because it promotes gut oedema.17 Although the present study showed an apparent increase in LoS with higher volumes of i.v. fluids infused, this disappeared when those patients who developed complications were excluded. However, because the present study did not collect data on the passage of flatus or return of bowel function, whether there was an effect on gut function remains unknown.
In conclusion, the introduction of an ERAS programme following open hepatic resection has resulted in a reduction in hospital LoS with no evidence of an increase in complications. Readmissions increase as hospital LoS reduces. No single factor within the ERAS package would appear to be an independent predictor of LoS.
Acknowledgments
The authors wish to acknowledge Khadeeja Mohamed, Statistician, Department of Medicine, Christchurch Hospital, Christchurch, New Zealand, for her statistical advice in the preparation of this manuscript.
Conflicts of interest
None declared.
References
- 1.Lassen K, Soop M, Nygren J, Cox PB, Hendry PO, Spies C, et al. Consensus review of optimal perioperative care in colorectal surgery: ERAS group recommendations. Arch Surg. 2009;144:961–969. doi: 10.1001/archsurg.2009.170. [DOI] [PubMed] [Google Scholar]
- 2.Gouvas N, Tan E, Windsor A, Xynos E, Tekkis PP. Fast-track vs. standard care in colorectal surgery: a meta-analysis update. Int J Colorectal Dis. 2009;24:1119–1131. doi: 10.1007/s00384-009-0703-5. [DOI] [PubMed] [Google Scholar]
- 3.Spelt L, Ansari D, Sturesson C, Tingstedt B, Andersson R. Fast-track programmes for hepatopancreatic resections. HPB. 2011;13:833–838. doi: 10.1111/j.1477-2574.2011.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Dam RM, Hendry PO, Coolsen MM, Bemelmans MH, Lassen K, Revhag A, et al. Initial experience with a multi-modal enhanced recovery programme in patients undergoing liver resection. Br J Surg. 2008;95:969–975. doi: 10.1002/bjs.6227. [DOI] [PubMed] [Google Scholar]
- 5.Lin DX, Li X, Ye QW, Lin F, Li LL, Zhang QY. Implementation of a fast-track clinical pathway decreases postoperative length of stay and hospital charges for liver resection. Cell Biochem Biophys. 2011;61:413–419. doi: 10.1007/s12013-011-9203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stoot JH, van Dam RM, Busch OR, van Hillegersberg R, De Bor M, Olde Damink SW, et al. The effect of a multimodal fast-track programme on outcomes in laparoscopic liver surgery: a multicentre pilot study. HPB. 2009;11:140–144. doi: 10.1111/j.1477-2574.2009.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polignano FM, Quym AJ, de Figueiredo RS, Henderson NA, Kulli C, Tait IS. Laparoscopic vs. open liver segmentectomy: prospective, case-matched, intention-to-treat analysis of clinical outcome and cost-effectiveness. Surg Endosc. 2008;22:2564–2570. doi: 10.1007/s00464-008-0110-y. [DOI] [PubMed] [Google Scholar]
- 8.Vanounou T, Steel JL, Nguyen KT, Tsung A, Marsh JW, Geller DA, et al. Comparing the clinical and economic impact of laparoscopic versus open liver resection. Ann Surg Oncol. 2010;17:998–1009. doi: 10.1245/s10434-009-0839-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vigano L, Laurent A, Tayar A, Tomatis M, Ponti A, Cherqui D. The learning curve in laparoscopic liver resection: improved feasibility and reproducibility. Ann Surg. 2009;250:772–782. doi: 10.1097/SLA.0b013e3181bd93b2. [DOI] [PubMed] [Google Scholar]
- 10.Vlug MS, Wind J, Hollman MW, Ubbink DT, Cense HA, Engel AF, et al. Laparoscopy in combination with fast-track multimodal management is the best perioperative strategy in patients undergoing colonic surgery: a randomized clinical trial (LAFA-study) Ann Surg. 2011;254:868–875. doi: 10.1097/SLA.0b013e31821fd1ce. [DOI] [PubMed] [Google Scholar]
- 11.Peters MJ, Mukhtar A, Yunus RM, Khan S, Pappalardo J, Memon B, et al. Meta-analysis of randomized controlled trials comparing open and laparoscopic anti-reflux surgery. Am J Gastroenterol. 2009;104:1548–1561. doi: 10.1038/ajg.2009.176. [DOI] [PubMed] [Google Scholar]
- 12.Keus F, Gooszen HG, van Laarhoven CJHM. Open, small-incision, or laparoscopic cholecystectomy for patients with symptomatic cholecystolithiasis. An overview of Cochrane Hepato-Biliary Group reviews. Cochrane Database Syst Rev. 2010;(1) doi: 10.1002/14651858.CD008318. CD008318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varadhan KK, Neal KR, Dejong CHC, Fearon KCH, Ljungqvist O, Lobo DN. The enhanced recovery after surgery (ERAS) pathway for patients undergoing major elective open colorectal surgery: a meta-analysis of randomized controlled trials. Clin Nutr. 2010;29:434–440. doi: 10.1016/j.clnu.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Hendry PO, Hausel J, Nygren J, Lassen K, Dejong CH, Ljungqvist O, et al. Enhanced Recovery after Surgery Study Group. Determinants of outcome after colorectal resection within an enhanced recovery programme. Br J Surg. 2009;96:197–205. doi: 10.1002/bjs.6445. [DOI] [PubMed] [Google Scholar]
- 15.Cannon RM, Brock G, Buell JF. Surgical resection for hilar cholangiocarcinoma: experience improves resectability. HPB. 2012;14:142–149. doi: 10.1111/j.1477-2574.2011.00419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hendry PO, van Dam RM, Bukkems SFFW, McKeown DW, Parks RW, Preston T, et al. Randomized clinical trial of laxatives and oral nutritional supplements within an enhanced recovery after surgery protocol following liver resection. Br J Surg. 2010;97:1198–1206. doi: 10.1002/bjs.7120. [DOI] [PubMed] [Google Scholar]
- 17.Lobo DN, Bostock KA, Neal KR, Perkins AC, Rowlands BJ, Allison SP. Effect of salt and water balance on recovery of gastrointestinal function after elective colonic resection: a randomised trial. Lancet. 2002;359:1812–1818. doi: 10.1016/S0140-6736(02)08711-1. [DOI] [PubMed] [Google Scholar]
- 18.Sakowska M, Docherty E, Linscott D, Connor S. A change in practice from epidural to intrathecal morphine analgesia for hepato-pancreato-biliary surgery. World J Surg. 2009;33:1802–1808. doi: 10.1007/s00268-009-0131-2. [DOI] [PubMed] [Google Scholar]
- 19.Koea JB, Young Y, Gunn K. Fast-track liver resection: the effect of a comprehensive care package and analgesia with single-dose intrathecal morphine with gabapentin or continuous epidural analgesia. HPB Surg. 2009 doi: 10.1155/2009/271986. 271986. Epub 2009 Dec 15. [DOI] [PMC free article] [PubMed] [Google Scholar]


