Abstract
Study design: Prospective cohort study.
Objective: The aim of the study was to compare clinical results and to determine differences in outcomes between anterior cervical discectomy and fusion (ACDF) and disc arthroplasty in patients treated for symptomatic cervical degenerative disc disease.
Methods: Forty patients with cervical degenerative disc disease were treated with ProDisc-C disc arthroplasty and 40 patients with fusion using an intervetebral spacer with integrated fixation (Cervios chronoOS) implants without additional anterior fixation. Fifty disc prostheses were placed in the first group and 52 intervertebral spacers were implanted in the second group. Clinical outcomes were assessed before and 12 months following the procedure using the neck disability index (NDI) and visual analog scale (VAS) for neck and arm pain, with 15% improvement in NDI and 20% in VAS defined as a clinically significant.
Results: Eighty patients with cervical degenerative disc disease with a mean age of 49.7 years were included in the study with a minimum follow-up of 12 months. The groups were similar at baseline both clinically and statistically (P > .05) except for age and VAS for arm pain. Both groups had a statistically significant improvement in NDI and VAS for neck and arm pain (P < .05) and the arthroplasty group had a better improvement according to NDI (74.3% of patients in the arthroplasty group achieved ≥15% improvement in NDI versus 65.7% of patients in ACDF group).
Conclusions: Both ProDisc C and Cervios chronoOS prostheses resulted in significant pain reduction and functional outcome for the patients with slightly better results in the group treated with disc arthroplasty 12 months after the surgery.
| Methods evaluation and class of evidence (CoE) | |
|---|---|
| Methodological principle: | |
| Study Design | |
| RCT | |
| Cohort study | • |
| Case control | |
| Case series | |
| Methods | |
| Concealed allocation (RCT) | |
| Intent to treat (RCT) | |
| Blinded/independent evaluation of primary outcome | • |
| Follow-up ≥85% | • |
| Adequate sample size | • |
| Control for confounding | |
| Evidence class: | III |
The definiton of the different classes of evidence is available on page 83.
Study Rationale and Context
Cervical anterior discectomy and fusion (ACDF) is a standard treatment for symptomatic cervical degenerative disc disease in the patients where conservative treatment has failed. Since fusion may be associated with progressive degeneration of adjacent motion segments, disc arthroplasty, which preserves segmental motion and improves load transfer to the adjacent levels, has been introduced in hopes of achieving improved pain and function without adjacent segment disease.1,2
Objective
To compare clinical results 12 months after surgery using the neck disability index (NDI) and visual analog scale (VAS) for neck and arm pain between a standard (ACDF) group and a disc arthroplasty group in patients presenting with symptomatic cervical degenerative disc disease. The secondary objective was to determine the complications after each procedure.
Methods
Study design: Prospective cohort study.
Inclusion criteria: All symptomatic patients with one or two level soft disc herniations and/or degenerative changes of the cervical spine, not responding to conservative treatment from January 2006 to January 2008.
Exclusion criteria (Fig. 1): Patients with concomitant conditions which could confound outcomes assessment (eg, previous cervical spinal surgery), contraindications to surgery, refusal to participate or inability to complete interviews were excluded. Additionally, patients with cervical instability, osteoporosis, malignant disease, infection, spondylodiscitis, traumatic spine injury, known allergy to foreign material, myelopathy, were also excluded from the study.
Fig. 1.
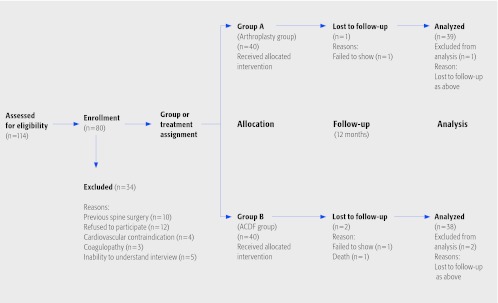
Patient sampling and selection
Patient population and interventions compared (Fig. 1):
One-hundred-and-fourteen patients were approached to participate in the study. Twenty two did not meet study criteria and 12 refused to participate leaving a total of 80 patients who completed the informed consent.
Treatment was assigned based on instrumentation availability or surgeon preference but was not related to factors that may have an influence on the outcome.
Enrollment concluded with 40 patients treated with ProDisc-C disc arthroplasty and 40 patients underwent fusion using Cervios chronoOS implants without additional anterior fixation.
Arthroplasty group: The complete anterior cervical discectomy was performed in conventional fashion under magnification with neural element decompression using high-speed drills and other neural dissection tools. The proper implant bed was prepared under the fluoroscopic x-ray control and an appropriately sized ProDisc-C artificial disc was then implanted.
ACDF group: Neural element decompression was carried out using the same technique described above. An appropriately sized Cervios chronoOS implant filled with artificial cancellous bone was inserted using fluoroscopic guidance. No further anterior fixation (such as plate and screw fixation) was used.
Cervical collars were not used postoperatively in either group. NSAIDs were used during the first 2 weeks selectively for patients with severe postoperative neck pain in both groups.
Outcomes:
Primary outcomes: Clinical outcomes included the neck disability index (NDI) and a 10-point visual analog scale (VAS) for neck and arm pain at baseline and 12 months after surgery. Measurements were conducted by study personal blinded to the surgical intervention according to a structured protocol.
Secondary outcomes: Incidence of complications related to device and surgical approach. Radiographic outcomes included assessment of hardware loosening and displacement, malalignment, heterotopic ossification and hardware failure.
Improvement was measured by calculating the change in NDI and VAS scores from baseline (preoperative) to the 12 month follow-up. A clinically significant change was considered a 15% improvement in NDI score and a 20% improvement in VAS score.
A brief description of the measures and statistical methods are provided in the web appendix at www.aospine.org/ebsj.
Analysis:
Changes from preoperative to postoperative in NDI and VAS scores were compared within treatment groups using a paired t-test and between treatment groups using an unpaired t-test. Differences in baseline continuous variable (eg, age) were tested using unpaired t-tests. Differences in categorical baseline variables (eg, gender) were tested using a chi-square test. PASW Statistics 18 software was used to provide all the statistical data.
Results
The patients in both groups had similar baseline characteristics (Table 1) with the exception of age and baseline NDI and VAS scores. Patients in the ACDF group were slightly older and had slightly lower baseline scores. The 12-month follow-up rate was 97.5% and 95% for the arthroplasty group and ACDF groups, respectively.
There was significant functional, neck and arm pain reduction in both groups from baseline to 12 months after surgery (P < .001)
The mean percent improvement was greater in the arthroplasty group compared to the fusion group in each outcome; however, these differences were not statistically significant(Table 2).
The proportion of patients achieving clinically significant improvement with each outcome was higher in the arthroplasty group compared to the fusion group (Table 2). These differences were not statistically significant.
One major complication (posterior epidural abscess) occurred in the arthroplasty group, requiring removal of the prosthesis; otherwise, there were no device related complications (such as loosening, migration of the implant, material failure, allergic reaction) and no approach related complications (dural tears or leaks, hematomas, esophageal or tracheal injuries, laryngeal nerve dysfunction).
Table 1. Demographic and baseline characteristics of treatment groups.
| Arthroplasty group n = 40 |
ACDF group n = 40 | P-value* | |
|---|---|---|---|
| Age (mean ± SD) | 48.1 ± 8.1 | 51.3 ± 8.1 | 0.02 |
| Female (n, %) | 27 (67.5%) | 2 (70%) | 0.82 |
| Male (n, %) | 13 (32.5%) | 12 (30%) | 0.82 |
| Signs and symptoms duration (mean weeks ± SD) | 45.5 ± 31.7 | 42.2 ± 38.7 | 0.68 |
| Baseline NDI (mean ± SD) | 67.4 ± 9.6 | 61.7 ± 15.1 | 0.10 |
| Baseline VAS neck (mean ± SD) | 7.1 ± 1.2 | 6.7 ± 1.5 | 0.30 |
| Baseline VAS arm (mean ± SD) | 6.9 ± 0.7 | 6.1 ± 1.4 | 0.004 |
| Number of implants used (n) | 50 | 52 | 0.64 |
Categorical variables compared using chi-square test and continuous variables with unpaired t-test.
Table 2. Summary of NDI and VAS change scores.
| NDI | VAS neck | VAS arm | ||||
|---|---|---|---|---|---|---|
| Arthroplasty (n = 38) | ACDF (n = 38) | Arthroplasty (n = 39) | ACDF (n = 38) | Arthroplasty (n = 39) | ACDF (n = 38) | |
| Baseline (mean ± SD) | 67.4 ± 9.6 | 61.7 ± 15.1 | 7.1 ± 1.2 | 6.7 ± 1.5 | 6.9 ± 0.7 | 6.1 ± 1.4 |
| 12 months (mean ± SD) | 34.9 ± 18.5 | 38.7 ± 16.1 | 4.3 ± 1.8 | 4.7 ± 1.8 | 3.3 ± 2.2 | 3.5 ± 1.8 |
| Change (mean ± SD) | 32.1 ± 17 | 22.9 ± 16.9 | 2.8 ± 1.6 | 2 ± 1.8 | 3.5 ± 2.3 | 2.6 ± 1.6 |
| Improvement | ||||||
| Mean % | 48.2% | 37.3% | 39.4% | 29.8% | 52.2% | 42.6% |
| ≥15% in NDI | 74.3% | 65.7% | ||||
| ≥20% in VAS | 65.8% | 48.6% | 71.2% | 56.7% | ||
| Within treatment P-value* | P < .001 | P < .001 | P < .001 | P < .001 | P < .001 | P < .001 |
| Between treatment P-value† | P = .43 | P = .38 | P = .85 | |||
P-value associated with change from baseline to 12 months within each group.
P-value comparing baseline to 12 months changes between arthroplasty and ACDF groups.
Discussion
There was significant functional, neck and arm pain reduction in both groups 12 months postoperatively but these differences were not statistically significant between the arthroplasty and ACDF groups. This finding is consistent with other studies.6,7
Strengths: this is a prospective cohort study with a 96% follow-up rate at 12 months using blinded assessment of validated patient reported outcomes measures.
Limitations: The study was limited by a short follow-up period. Future studies should follow these patients for several more years. Since this was not a randomized trial, we cannot be certain that both groups were similar with respect to all baseline factors that may introduce confounding of the comparison. The variables we did collect demonstrated two relatively similar groups; however, future studies should consider other important baseline factors such as American Spinal Injury Association (ASIA) score, smoking status, disability claims, etc.
Clinically, the cervical disc arthroplasty challenges the surgeon to more precise hardware placement. We found no correlation of a potentially more complex procedure due to the occurrence of only one complication. Our study findings, including occurrence of complications, were remarkably similar to previous publications.2,6,7
The concern of fusion (ACDF) resulting in progressive degeneration of adjacent segments while disc arthroplasty potentially preserves integrity of adjacent motion segment could not be answered in our limited follow-up time.3,4,5
Studies with longer follow-up (10 years) are necessary to better evaluate the comparative effectiveness, safety and long term survival of disc arthroplasty compared to fusion.
Summary and Conclusions
Both treatments relieve patient's pain and improve functional outcome.
Despite no statistically significant between group differences in the primary outcomes, there were slightly better results in the disc arthroplasty group compared to the fusion group 12-months postoperatively.
Footnotes
The authors have no financial relationships to disclose.The ProDisc-C prostheses and Cervios chronoOS implants (Synthes) presented in the study are approved for clinical use.
References
- 1.Philips F M, Garfin S R. Cervical Disc Replacement. Spine. 2005;30:S27–33. doi: 10.1097/01.brs.0000175192.55139.69. [DOI] [PubMed] [Google Scholar]
- 2.Nabhan A, Ahlhelm F, Shariat K. et al. The Prodisc-C Prothesis. Spine. 2007;32:1935–1941. doi: 10.1097/BRS.0b013e31813162d8. [DOI] [PubMed] [Google Scholar]
- 3.DiAngelo D, Foley K T, Morrow B R. et al. In vitro biomechanics of cervical disc arthroplasty with the Prodisc-C total disc implant. Neurosurgical Focus. 2004;17(3):44–54. [PubMed] [Google Scholar]
- 4.Bertagnoli R, Yue J J, Pfeiffer F. et al. Early results after Prodisc-C cervical replacement. J Neurosurg: Spine. 2005;2:403–410. doi: 10.3171/spi.2005.2.4.0403. [DOI] [PubMed] [Google Scholar]
- 5.Chang U K, Kim D H, Lee M C. et al. Range of motion change after cervical arthroplasty with Prodisc-C artificial discs compared with anterior cervical discectomy and fusion. J Neurosurg: Spine. 2007;7:40–46. doi: 10.3171/SPI-07/07/040. [DOI] [PubMed] [Google Scholar]
- 6.Kim S W, Shin J H, Arbatin J J. et al. Effects of a cervical disc prosthesis on maintaining sagittal alignment of the functional spinal unit and overall sagittal balance of the cervical spine. Eur Spine J. 2008;17:20–29. doi: 10.1007/s00586-007-0459-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nabhan A, Ahlhelm F, Pitzen T. et al. Disc replacement using Prodisc-C versus fusion: a prospective randomized and controlled radiographic and clinical study. Eur Spine J. 2007;16:423–430. doi: 10.1007/s00586-006-0226-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


