Abstract
Glycated proteins are important biomarkers for age-related disorders, however their analysis is challenging because of the complexity of the protein-carbohydrate adducts. Here we report a method that enables the detection and identification of individual glycated proteins in complex samples using fluorescent boronic acids in gel electrophoresis. Using this method we identified glycated proteins in human serum, insect hemolymph and mouse brain homogenates, confirming this technique as a powerful proteomics tool that can be used for the identification of potential disease biomarkers.
Protein glycation, also known as non-enzymatic glycosylation, has been implicated in various disease states1,2 and is therefore an important biomarker for ageing and age-related chronic diseases such as diabetes, cardiovascular diseases, autoimmune diseases, cancer, and Alzheimer's disease (AD)3,4,5,6,7,8,9,10,11. This process whereby reducing sugar molecules such as glucose react with the amino groups of lysine, arginine or N-terminal amino acid residues of proteins ultimately leads to the formation of complex and stable advanced glycation endproducts (AGEs). This modification affects the folding, function and stability of long-lived proteins. The analysis of these non-enzymatic protein-carbohydrate adducts is challenging because of their complexity and variability. A variety of analytical techniques are currently used to analyse the protein modifications resulting from glycation, each with their advantages and limitations. Boronate affinity chromatography (BAC)12, for instance, is based on the interaction between boronic acid and cis-diol-containing carbohydrates. It can detect various types of glycated proteins and has been widely used, in low-performance agarose gel-based as well as high-performance chromatography-based systems13. It has been used for example to differentiate the HbA1c isoform as a marker for diabetes and indicator for blood sugar control. With BAC all types of glycation modifications as well as N- and O-linked glycosylation in a sample are retained and the identification and analysis of individual glycated proteins requires further separation steps. BAC has been successfully used as a sample enrichment step prior to high-performance liquid chromatography (HPLC) and mass spectrometry (MS) analyses14. Even though MS15 is an ideal technique to determine the identity of protein band in a gel, the limited availability of database information specific for glycation adducts hampers the identification of the modifications. In addition, MS only detects the most abundant proteins and if only a small percentage of a given protein species in a sample has suffered glycation damage, these adducts are likely to go undetected. Furthermore, any information regarding the specific glycation state can be lost after digestion of the protein samples prior to analysis.
In order to provide a simple, cost-effective detection and analysis tool for glycated proteins, we recently developed an electrophoresis based method using polyacrylamide incorporated with the carbohydrate affinity ligand methacrylamido phenylboronic acid (MPBA)16. We first tested this technique on simple carbohydrates and showed that by using the same basic principle as BAC, exploiting the reversible covalent interaction between boronic acid and cis-diols17,18, the MPBA-incorporated acrylamide gels enabled the improved separation of saccharides, and the differentiation between monosaccharides and dissacharides. Later we adapted this boronate-assisted saccharide electrophoresis (BASE) method to allow the separation of glycated from non-glycated proteins by incorporating MPBA into polyacrylamide gels for sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) analysis19,20. This method, coined mP-AGE, showed that under electrophoresis conditions the polyacrylamide incorporated MPBA is specific for fructosamine modified proteins, via interactions with the cis-1,2-diol-containing fructosamine adducts and by further stabilisation through an electrostatic interaction between the protonated amino group and the negatively charged boronate moiety19. This method enables the differentiation between early and late glycated adducts and has now been successfully used to analyse glycated human serum albumin (HSA) in serum from diabetes sufferers21.
Whilst mP-AGE aids the detection and separation of glycated proteins, it is only suitable for the analysis of samples with limited complexity. In this paper we present a novel application to detect and identify glycated proteins in a variety of complex protein samples, using fluoroscent phenylboronic acid gel electrophoresis (Flu-PAGE) and Eastern blotting (Flu-BLOT).
Results
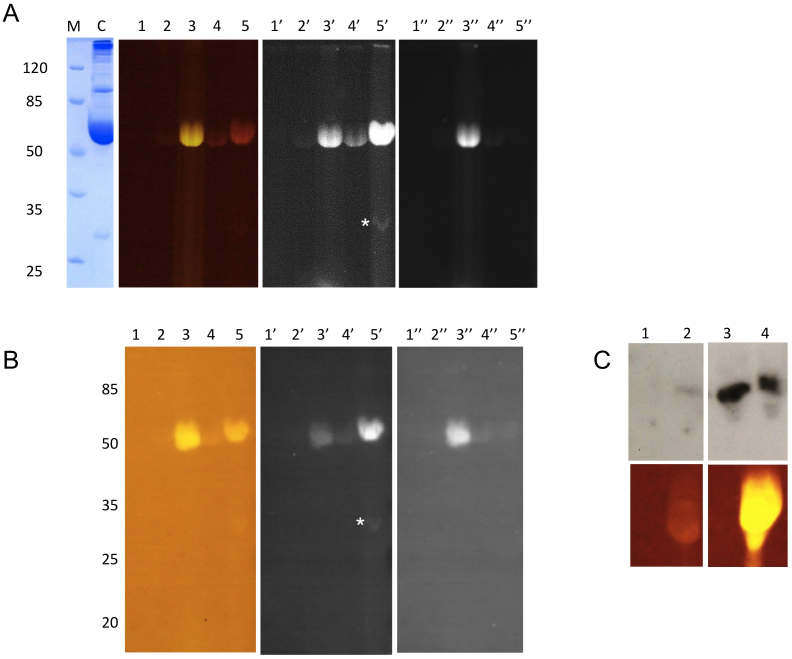
Glycated proteins present in normal human serum can be visualised after incubation of serum with fluorophore-appended boronic acids in SDS-PAGE (Flu-PAGE) and Eastern blotting (Flu-BLOT) (Fig. 1). Incubation of normal human serum with fluorescein, fluorescein-boronic acid, rhodamine and rhodamine-boronic acid (Fig. 1A; structures of these compounds are shown in Supplementary Fig. S1) results in specific fluorescent staining of glycated HSA in SDS-PAGE, only in samples containing fluorescein- or rhodamine-appended boronic acid. No known glycosylated or non-glycosylated serum proteins are labelled using this method indicating that, similar to mP-AGE, the fluorescent boronic acids used in Flu-PAGE specifically interact with fructosamine modified proteins. Glycated proteins labelled with both fluorophores show identical electrophoretic migration properties and can be readily transferred from the gels after electrophoresis (shown in Fig. 1A) to a polyvinyl difluoride (PVDF) membrane and directly visualised with visible blue and UV light (Fig. 1B). This Flu-BLOT method can be used in conjunction with standard Western blotting methods, as shown in Fig 1C. MALDI-TOF analysis of an additional fluorescent protein band in the rhodamine-boronate labelled human serum, observed in both Flu-PAGE and Flu-BLOT (Fig. 1A and 1B, lane 5′, indicated by a white asterisk) was identified as Apolipoprotein A-I (ApoA-I).
Figure 1. Flu-PAGE and Flu-Blot analyses of human serum.
Normal human serum (lanes 1, 1′ and 1″) and serum samples labelled with fluorescein (lanes 2, 2′ and 2″), fluorescein-boronic acid (lanes 3, 3′ and 3″), rhodamine (lanes 4, 4′ and 4″) and rhodamine-boronic acid (lanes 5, 5′ and 5″) in a 12% non-denaturing polyacrylamide gel (A) and Eastern blot (B). The gels and blots were imaged with Dark Reader® (lanes 1–5), UV (365 nm, with orange filter (595 nm); lanes 1′–5′) and UV (365 nm, with green filter (537 nm); lanes 1″–5″). The Coomassie stained control lane is shown in the left panel, labelled as C. The asterisks indicate an extra band present in the rhodamine-boronic acid labelled sample (lane 5′) on both the Flu-PAGE and Flu-Blot. (C) shows the Western blot analysis of glucose incubated HSA after 0 (lanes 1 and 2) and 28 days (lanes 3 and 4) using anti-AGE antibodies. Lanes 1 and 3 correspond to unlabelled samples, whereas lanes 2 and 4 are labelled with fluorescein-boronic acid.
To demonstrate that the Flu-PAGE method can be used to follow the glycation of human serum proteins over time, we subsequently incubated normal human serum with various reducing sugars, including glucose, fructose, mannose, maltose, and galactose. After 7 days of incubation with these sugars the HSA bands clearly show an increased fluorescence intensity in Flu-PAGE in the presence of fluorescein-boronic acid, especially in the sera that were incubated with glucose and mannose (Fig. 2). After 10 days of incubation, a significant increase in fluorescein-boronic acid labelling of HSA can be observed in the serum samples incubated with glucose, mannose and galactose, indicating increased levels of glycation by these sugars. A variety of other fluorescent protein bands can be observed, two of which were identified as serotransferrin and Ig γ chain constant region, using MALDI-TOF MS. These results are in agreement with the fluorescein-boronic acid labelling of sera from type I diabetes patients (Supplementary Fig. S3), showing a clear increase in fluorescence intensity of HSA in two of the diabetic sera. One of the diabetic sera displays a level of HSA fluorescence that is comparable to control serum, however, this sample has a distinct additional fluorescent band at the 50 kDa position in the gel, indicative of IgG heavy chain glycation.
Figure 2. Flu-PAGE analysis of in vitro glycated serum.
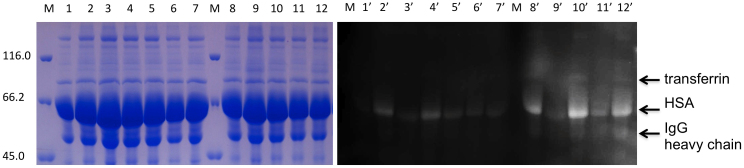
SDS-PAGE of human serum glycated with 50 mM sugar at 37°C for 7 and 10 days, lanes 2–7 and 8–12 respectively. Shown are non-incubated control (lane 1) and samples incubated with glucose (lanes 2 and 8), fructose (lanes 3 and 9), mannose (lanes 4 and 10), maltose (lanes 5 and 11), galactose (lanes 6 and 12), sucrose (lane 7). The left panel shows Coomassie stained gel (samples 1–12) and the right panel shows the gel visualised with UV prior to staining (lanes 1′–12′). The arrows indicate fluorescent proteins identified by MS.
Next we tested the possible use of fluorescent boronic acid labelling of glycated proteins as a proteomics tool in 1) an insect model system that is currently in development as an animal model for diabetes (Manduca sexta) and 2) a murine model for AD.
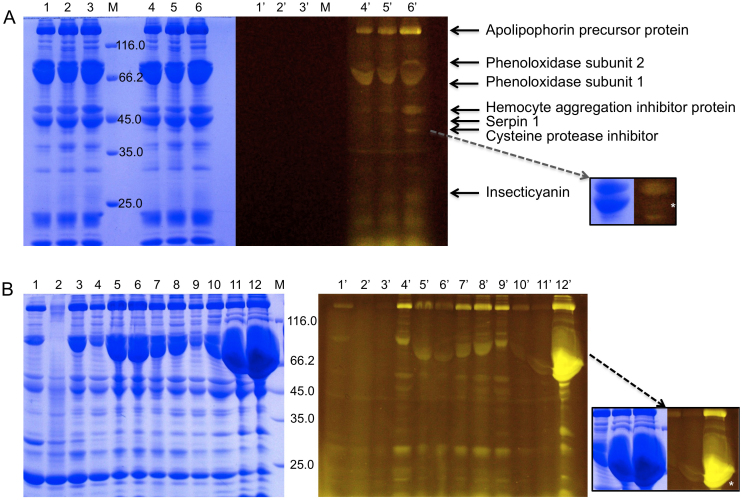
Insects are becoming increasingly popular as protein glycation model systems and have been applied for identification of biomarkers in ageing in Drosophila22, and for testing anti-diabetic drugs in a hyperglycemic silk worm model23. Incubation of Manduca sexta hemolymph with fluorescein-boronic acid resulted in labelling of at least 10 different protein bands in Flu-PAGE (Fig. 3A). It is interesting to note that the majority of protein bands seen in the Coomassie stained gel are also labelled in the Flu-PAGE analysis, indicating that a large majority of the Manduca sexta hemolymph proteins are glycated. However, not all distinct fluorescent bands correspond to a high level of protein staining, signifying that some highly glycated proteins are present in low concentrations in the hemolymph samples. Conversely, some highly expressed proteins show low levels of glycation (as indicated in Figs. 3A and 3B, insets; see also Supplementary Fig. S4). Six protein bands, with approximate molecular weights: >200, 80, 45, 40 and 25 kDa were analysed by MALDI-TOF and identified using the Manduca sexta proteome database (Agricultural Pest Genomics Resource Database: www.agripestbase.org). The most prominent fluorescent protein band observed in the gels, indicating high levels of glycation, corresponds to Apolipophorin precursor protein a lysine- and arginine-rich 367 kDa protein (see Supplementary Table S1) that constitutes the major component of lipophorin, which mediates transport for various types of lipids in hemolymph. Other glycated proteins identified in Manduca hemolymph include: phenoloxidase subunits 1 and 2, two copper-containing oxidases (79 and 80 kDa, respectively) that function in the formation of pigments such as melanins and other polyphenolic compounds; hemocyte aggregation inhibitor protein precursor (48 kDa); putative C1A cysteine protease precursor (38 kDa) and insecticyanin, a 23 kDa protein synthesised in the caterpillar epidermis and secreted into the hemolymph.
Figure 3. Flu-PAGE analysis of Manduca sexta hemolymph.
(A) Flu-PAGE analysis of fifth instar Manduca sexta hemolymph. Lanes 1–3 are unlabelled hemolymph and lanes 4–6 are samples labelled with fluorescein-boronic acid. The left panel shows the Coomassie stained samples (lanes 1–6) whilst the right panel shows gel visualised with Dark Reader® prior to protein staining (lanes 1′–6′). The arrows indicate fluorescent bands analysed by MALDI-TOF and proteins identified using the Manduca sexta proteome database. Inset: comparison of Coomassie stained and Flu-PAGE stained section of the SDS-PAGE gel showing the differences in protein concentration and levels of glycation between three of the identified proteins. Indicated (white asterisk) is the position of Serpin 1, showing high levels of expression in hemolymph in the Coomassie stained gel and low levels of glycation with Flu-PAGE. (B) Flu-PAGE analysis of fluorescein-boronic acid labelled hemolymph taken from larvae at different developmental stages. Lanes 1–12 correspond to hemolymph extracted from larvae at 4, 6, 8, 10–18 days after hatching respectively. The left panel shows Coomassie stained gel (lanes 1–12) and the right panel shows visualisation using Dark Reader® prior to protein staining (lanes 1′–12′). Inset: glycation levels of some proteins vary dramatically during development. Phenoloxidase subunit 1A (indicated with white asterisk), a highly expressed protein in Manduca sexta hemolymph, shows low levels of glycation on days 16 and 17 and high levels of glycation on day 18, on advancement to pupa stage.
We also used the Flu-PAGE to follow protein glycation during the development of the Manduca sexta caterpillar. The results in Fig. 3B show that whilst the levels of most hemolymph proteins remain constant during development, concentrations of proteins such as phenoloxidase vary dramatically and its expression appears to follow a wave pattern, peaking just before pupation. Glycation levels of the protein appear to be much lower at the beginning of an expression wave and much higher at the end of a wave, especially when expression levels peak before reaching pupation. A very striking example of this can be observed in the case of phenoloxidase subunit 1. Whilst a significant increase in the level of expression of this protein can be observed in the Coomassie stained gel on day 17 (Fig. 3B, lane 11), hardly any fluorescence is detected in the Flu-PAGE analysis. On day 18, just before entering pupation, the protein has clearly become glycated as evidenced by the dramatic increase in fluorescence in the Flu-PAGE gel.
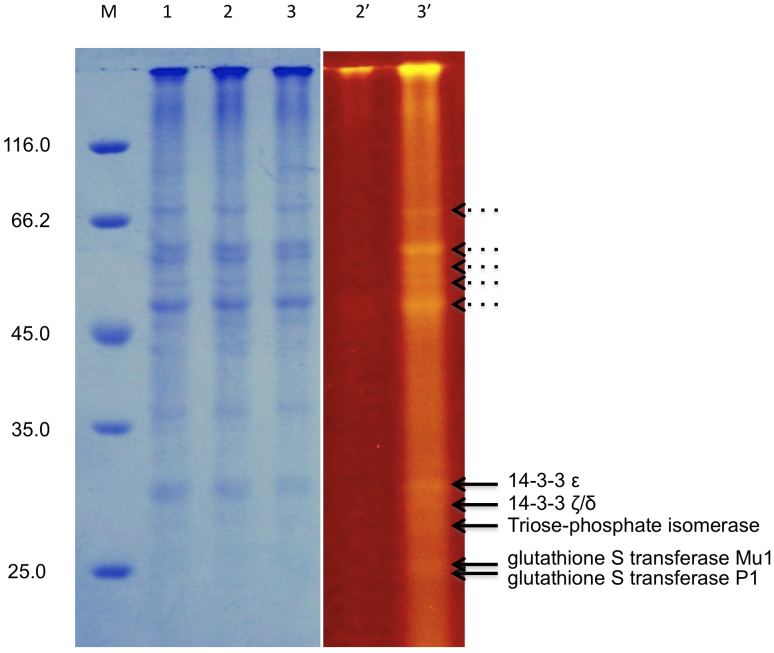
For the analysis of protein glycation in an AD mouse model, we used brain homogenates from transgenic mice that develop extensive amyloid β (Aβ) plaque pathology and normal controls. Brain homogenates were prepared from the cortices of 5-month-old heterozygote transgenic mice overexpressing both the hAPP695swe and the presenilin-1 M146V mutations (TASTPM24), and from age matched wild type C57BL/6 control animals. The results from the Flu-PAGE analysis of the brain samples are shown in Fig. 4 and Supplementary Fig. S5, where a UV image of the SDS-PAGE gel is compared with normal Coomassie stained gel. Although many bands are visible in the Coomassie stained gel, no other distinct fluorescent bands can be observed in the control cortex sample, with the exception of a strong band at approximately 120 kDa and a weaker low molecular weight band of about 15 kDa. In the TASTPM sample a large number of distinct fluorescent bands can be observed. Two groups of prominent bands can be seen between the 66 and 45 kDa molecular weight markers and between 35 and 25 kDa (Fig. 4). Five fluorescent bands between 35 and 25 kDa that are absent in the control mouse cortex sample were identified by MALDI-TOF analysis as 14-3-3 proteins (ε and ζ/δ, with respective masses of 29 and 28 kDa), triosephosphate isomerase (27 kDa), glutathione S-transferases Mu1 (26 kDa) and P1 (23 kDa), respectively.
Figure 4. Flu-PAGE analysis of TASTPM mouse cortex homogenates.
Flu-PAGE gel profile of TASTPM cortex homogenates, visualised with Dark Reader® (right panel, lanes 2′ and 3′) prior to Coomassie staining (left panel, lanes 1–3). The solid arrows indicate the positions of the proteins that have been identified using MALDI-TOF analysis. The dotted arrows represent higher molecular weight fluorescent proteins to be investigated.
Discussion
Glycated proteins are important biomarkers for age-related disorders, such as diabetes, cardiovascular diseases, autoimmune diseases, cancer, and AD. Biomarkers identifying biological and physiological entities associated with such diseases are becoming increasingly important for drug discovery. In this paper we show that glycated proteins can be visualised and identified in a variety of complex biological samples, including human serum, Manduca sexta hemolymph and mouse brain cortex homogenates, using fluorescent boronic acids in Flu-PAGE and Flu-BLOT.
In samples of human serum, specific labelling of glycated HSA was observed in Flu-PAGE and Flu-BLOT and detection was most effective with fluorescein-boronic acid as it resulted in specific labelling of the glycated HSA band with all the wavelengths and filter combinations used (conventional visible blue light with 530 nm filter, and UV light sources with 595 and 537 nm filters). Glycated proteins labelled with rhodamine-boronic acid could be visualised best using UV with a 595 nm (orange) filter, however some aspecific labelling of HSA was observed in the rhodamine control lane. At this wavelength another glycated protein band was observed in the rhodamine-boronic acid labelled serum sample (Fig. 1A). The identified ApoA-I protein plays an important role in the transport of phospholipids and free cholesterol to the liver and its glycation has been linked to atherosclerosis and shown to contribute to its impaired action25. Analysis of human serum samples incubated with different reducing saccharides (Fig. 2), using Flu-PAGE, further identified serum proteins serotransferrin and IgG heavy chain as glycation targets. All of these high-abundance plasma proteins are well-known Amadori-modified proteins in type 2 diabetes and are linked with vascular complications26,27.
The Flu-PAGE results observed with saccharide-incubated serum samples are consistent with those found when analysing HSA glycation using mP-AGE19, showing strongest interactions between boronic acid and cis-1,2-diol-containing fructosamine adducts (resulting from glycation with glucose, mannose and galactose), stabilised by an electrostatic interaction between the protonated amino group and the negatively charged boronate moiety. When using Flu-PAGE in conjunction with mP-AGE analysis of the serum sample, the highest fluorescence intensity can be observed in the glycated HSA fraction that is retained in its electrophoretic mobility via interaction with the gel-incorporated MPBA (Supplementary Fig. S2).
Many proteins in Manduca sexta hemolymph appear to be affected by glycation in Flu-PAGE analysis. The most prominent fluorescent protein band corresponds to Apolipophorin precursor protein, which has a function similar to Apo A-I, identified as a glycated protein in human serum (Fig. 1A), indicating that Manduca sexta could also be a useful model system for diseases such as atherosclerosis and AD. Some of the fluorescent bands in the hemolymph Flu-PAGE pattern do not correspond to clear Coomassie stained protein bands, implying that some highly glycated proteins are present in low concentrations. During the development of Manduca sexta the levels of most hemolymph proteins remain constant in all larval stages (Fig. 3B), however, concentrations of proteins such as phenoloxidase vary dramatically between larval stages and its expression appears to follow a wave pattern, peaking just before pupation. Our Flu-PAGE analysis shows that levels of protein glycation appear to be very low during the early stages of development and increase towards later stages. Interestingly, the fluorescein-boronic acid labelling of phenoloxidase also follows a wave-like pattern that is out of phase with its expression levels. The glycation of this protein appears to reach its peak in the days close to pupation. This corresponds with high hemolymph glucose levels at this stage of development (16 mM) when compared to feeding larvae (1 mM)28. This makes Manduca sexta an ideal model for following the effects of glycation during a hyperglycemic episode.
With this example we show that fluorescent boronic acid gel electrophoresis can be used to specifically identify glycated proteins in complex samples for normal development in non-disease states and in concert follow their temporal expression and glycation patterns. It should be noted that these insects are reared on a very high sugar diet, which may explain the overall high levels of glycation seen in their blood relative to the mammalian samples. Remarkably, there is a clear correlation between fluorescence intensity of the identified hemolymph proteins and the percentage of predicted glycated lysines in these proteins (Supplementary Fig. S4, also see Supplementary Table S1).
Similar to the Manduca sexta hemolymph samples, some of the distinct fluorescent bands seen in the Flu-PAGE analysis of TASTPM cortex homogenates do not show clear protein bands in the corresponding Coomassie stained gels, indicating that these proteins are present in low concentrations in the brain samples. MALDI-TOF analysis of the low molecular weight band (~15 kDa) present in both control and TASTPM homogenates identified this protein as hemoglobin, a well-known glycation marker in diabetes. Although the hemoglobin band is present in both control and TASTPM homogenates it appears brighter in the transgenic samples, indicating a higher level of glycation (Supplementary Fig. S5). The identified 14-3-3 ε and ζ/δ proteins have previously been linked with AD and shown to be present in the neurofibrillary tangles of AD brains29. It is interesting to note that of all the glycated proteins identified in this study, 14-3-3 ζ/δ has the highest percentage of predicted glycated lysines (Supplementary Table S1). Both of the GST proteins, also discovered in the Flu-PAGE analysis of the TASTPM cortex homogenates, belong to a class of enzymes that function in the detoxification of hydrophobic electrophilic compounds, including carcinogens, therapeutic drugs, environmental toxins and products of oxidative stress, by conjugation with reduced glutathione. Glycation of these enzymes could affect their function and increase susceptibility to environmental toxins and carcinogens. Oxidative stress is strongly implicated in the pathology of AD and a recent study has shown that pro-oxidant conditions increase amyloidogenic processing of amyloid precursor protein (APP) and therefore might contribute to disease progression30.
In conclusion we show that fluorescent boronic acids can be used in gel electrophoresis and Eastern blotting for the detection and identification of individual glycated proteins in complex samples. The advantages of the Flu-PAGE and Flu-BLOT technology are summarised below:
Direct visualisation of glycated proteins. Flu-PAGE and Flu-BLOT are the only methods that can directly visualise glycated proteins in SDS-PAGE and blots. This labelling method does not affect the electrophoretic migration properties of the proteins, nor does it hinder their subsequent identification by MS.
Detection of glycated proteins in complex samples. This method is unique in that it detects glycated proteins in complex protein mixtures without the need of additional enrichment or purification techniques such as BAC. This method also enables the study of glycated proteins in the context of other non-affected proteins in fluid (human serum and insect hemolymph) and solid (mouse brain cortex homogenates) biosamples.
Detection for early glycation adducts. The fluorescent boronic acids used in Flu-PAGE specifically interact with fructosamines, enabling the detection of early glycation adducts. With current methods to analyse glycated proteins using boronic acids (such as BAC) all types of glycation modifications in a sample are retained, including N- and O-linked glycans. Anti-AGE antibodies, used to detect glycated protein in western blotting methods, only recognise late stage glycation end products. Most of these antibodies lack specificity because they have been raised against a mixture of AGEs.
Flu-PAGE and Flu-Blot can be used in conjunction with other protein analysis tools. We show in this paper that Flu-PAGE can be used in combination with an earlier developed method (mP-AGE) to label and separate glycated from non-glycated proteins. Flu-Blot can also be used in conjunction with antibody detection in western blots to enable multiple labelling of proteins.
Using a combination of Flu-PAGE and Flu-Blot we have i) identified differences in glycation between normal human serum and that of patients suffering from type 1 diabetes, ii) discovered novel glycated proteins in hemolymph from Manduca sexta, a new animal model system for diabetes, and iii) unveiled proteins that are affected by glycation in an AD animal model. This easy-to-use method enables the study of protein glycation patterns in complex samples over time and in the context of development and disease. These results signify that fluorescent boronic acid gel electrophoresis (Flu-PAGE) and Eastern blotting (Flu-BLOT) provide a powerful and cost-effective first-stage proteomics tool for the identification and analysis of glycated protein biomarkers in ageing and age-related diseases and for probing novel glycation inhibitors and other anti-AGE therapies.
Methods
Human sera
Human serum was obtained from Lonza, and serum albumin from Sigma-Aldrich. In vitro glycated human serum was obtained by incubating human serum with 50 mM glucose, fructose, mannose, maltose, galactose or sucrose (in the presence of 0.1% azide), in a dry heating block at 37°C for 7 or 10 days under aseptic conditions.
Type 1 diabetes human sera (from patients diagnosed using glutamic acid dehydrogenase (GAD) and insulin autoimmune antibodies (IAA and IA-2)) were obtained from SunnyLab UK.
TASTPM mouse brain homogenates
Cortex from heterozygote transgenic mouse over-expressing hAPP695swe and presenilin-1 M146V mutations (TASTPM) were obtained from GlaxoSmithKline, along with age-matched wild type C57BL/6, as previously reported24,30. All animal studies were ethically reviewed and carried out in accordance with Animals (Scientific Procedures) Act 1986 and the GSK Policy on the Care, Welfare and Treatment of Animals. The cortex samples were mechanically homogenised in 0.5 mL 50 mM Tris-EDTA buffer pH 7.4 with protease inhibitor (Roche), and spun at 2,000 g for five minutes to obtain crude homogenates.
Manduca sexta hemolymph
Manduca sexta larvae were kept individually on a wheat germ based artificial diet at 25°C with 17 h light: 7 h dark photoperiod. The larvae were chilled on ice for 30 minutes, and bled in sterile tubes after brief sterilisation with 70% ethanol and cutting the ‘tail' near the tip. 5 μL of saturated phenoloxidase inhibitor 1-phenyl-2-thiourea (PTU, approx. 20 mM PTU in PBS) were added to 300 μL of hemolymph to prevent melanisation.
Synthesis of fluorescent boronic acid compounds
Fluorescein-boronic acid (3-(3-(3′,6′-Dihydroxy-3-oxo-3 H-spiro[isobenzofuran-1,9′-xanthene]-5-yl)thioureido)phenylboronic acid, structure shown in Supplementary Fig. S1A right) was synthesised as previously reported31. Briefly, 3-aminobenzeneboronic acid (0.35 g, 2.57 mmol) was added to a solution of fluorescein isothiocyanate (1.00 g, 2.57 mmol) in DMF (5 mL), stirred at room temperature for 12 h then poured into methanol (10 mL). The solvents were removed in vacuo and residue re-dissolved in the minimum amount of fresh methanol. Chloroform was added and the product was obtained as a bright orange precipitate (920 mg, 68% yield).
Rhodamine-boronic acid (structure shown in Supplementary Fig. S1B right) was synthesised as follows. Triethylamine (3 eq. 0.56 mmol, 80 μL) was added to a solution of Rhodamine B isothiocyanate (100 mg, 0.18 mmol) and 3-aminobenzene boronic acid (1.1 eq, 0.21 mmol, 28 mg) in dry DMF (5 mL) under N2. The reaction mixture was stirred overnight, then the solvent removed in vacuo. The reaction mixture was passed through a short pad of silica eluting with DCM, DCM/methanol (1% to 10%). The solvent was removed to give the product as a dark purple-red solid (65 mg, 58% yield).
Labelling and electrophoresis of glycated proteins with fluorescent boronic acids
1 μL of 10-fold diluted human serum was incubated with 0.5 mM fluorescent boronic acid at room temperature for 1 h. Less stable samples can be labelled overnight at 4°C. Control samples were labelled with Fluorescein (Sigma-Aldrich; structure shown in Supplementary Fig. S1A left) or Rhodamine B (Sigma-Aldrich; structure shown in Supplementary Fig. S1B left).
Gel electrophoresis was performed using Xcell surelock mini-cell (Invitrogen) and Power Pac 300 (Bio-Rad). Proteins were blotted onto a 0.45 μm Immobilon-P PVDF membrane (Millipore) in transfer buffer (25 mM tris, 192 mM glycine) at 15 V for 50 minutes, using a Trans blot SD Semi-dry transfer cell (Bio-Rad). The gels were visualised prior to protein staining with UV light (AlphaImager 3400 gel imaging system, Alpha Innotech; wavelength 365 nm, with orange (595 nm) or green (537 nm) filters) or Dark Reader® (Clare Chemicals Research Inc.; wavelength range 420–520 nm, with amber (~530 nm) filter). Contrast has been optimised for the gel and blot images.
After blotting, the membrane was blocked using TBST buffer (50 mM Tris, 150 mM NaCl, 0.05% Tween 20, pH 7.6) with 5% milk powder at room temperature for 1 h. HRP-conjugated anti-AGE monoclonal antibody (1:800 dilution) was then applied to the blot at room temperature for 1 h. After five 30 min washes, the blot was incubated with peroxidase substrate for enhanced chemiluminescence (ECL, Pierce) at room temperature for 1 min before developing on film.
Monoclonal anti-AGE antibody (anti-AGE-BSA mAb clone 6D12, Cosmo Bio Co Ltd.) was provided by Dr Emmanuel Bourdon (Groupe d'Etude sur l'Inflammation Chronique et l'Obésité, Faculté des Sciences, La Réunion, France).
Author Contributions
T.D.J. and J.M.H.v.d.E. conceived of the project. J.M.H.v.d.E. planned and supervised the project. M.P.P.M. conducted the experiments, with assistance from D.M. T.D.J. and S.E.F. designed, synthesised and analysed the fluorescent boronic acids. R.J.W. isolated the TASTPM brain tissues, prepared the homogenates and contributed to the analysis of the MS data. N.R.W. provided the Manduca sexta hemolymph and analysed the MS data using the Manduca sexta proteome database. C.J.C. contributed to the Eastern blot (Flu-Blot) development. M.P.P.M. and J.M.H.v.d.E. wrote the manuscript with editorial input from T.D.J., N.R.W. and R.J.W.
Supplementary Material
Supplementary methods
Acknowledgments
We are indebted to Alzheimer's Research UK (ARUK-PPG2011B-17), Bath Ventures and the University of Bath Research Development & Support Office for funding. GlaxoSmithKline is thanked for providing the TASTPM brain samples.
References
- Vlassara H. Advanced glycation in health and disease: role of the modern environment. Ann N Y Acad Sci 1043, 452–60 (2005). [DOI] [PubMed] [Google Scholar]
- Vlassara H. & Uribarri J. Glycoxidation and diabetic complications: modern lessons and a warning? Rev Endocr Metab Disord 5, 181–8 (2004). [DOI] [PubMed] [Google Scholar]
- Kurien B. T. & Scofield R. H. Autoimmunity and oxidatively modified autoantigens. Autoimmun Rev 7, 567–73 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells-Knecht M. C., Thorpe S. R. & Baynes J. W. Pathways of formation of glycoxidation products during glycation of collagen. Biochemistry 34, 15134–41 (1995). [DOI] [PubMed] [Google Scholar]
- Rahbar S. Novel inhibitors of glycation and AGE formation. Cell Biochem Biophys 48, 147–57 (2007). [DOI] [PubMed] [Google Scholar]
- Unoki H. & Yamagishi S. Advanced glycation end products and insulin resistance. Curr Pharm Des 14, 987–9 (2008). [DOI] [PubMed] [Google Scholar]
- Vitek M. P. et al. Advanced glycation end products contribute to amyloidosis in Alzheimer disease. Proc Natl Acad Sci U S A 91, 4766–70 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heijst J. W., Niessen H. W., Hoekman K. & Schalkwijk C. G. Advanced glycation end products in human cancer tissues: detection of Nepsilon-(carboxymethyl)lysine and argpyrimidine. Ann N Y Acad Sci 1043, 725–33 (2005). [DOI] [PubMed] [Google Scholar]
- Smith M. A., Monnier V. M., Sayre L. M. & Perry G. Amyloidosis, advanced glycation end products and Alzheimer disease. Neuroreport 6, 1595–6 (1995). [DOI] [PubMed] [Google Scholar]
- Smith M. A., Sayre L. M., Monnier V. M. & Perry G. Oxidative posttranslational modifications in Alzheimer disease. A possible pathogenic role in the formation of senile plaques and neurofibrillary tangles. Mol Chem Neuropathol 28, 41–8 (1996). [DOI] [PubMed] [Google Scholar]
- Smith M. A. et al. Advanced Maillard reaction end products are associated with Alzheimer disease pathology. Proc Natl Acad Sci U S A 91, 5710–4 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenk D. C. et al. Determination of glycosylated hemoglobin by affinity chromatography: comparison with colorimetric and ion-exchange methods, and effects of common interferences. Clin Chem 28, 2088–94 (1982). [PubMed] [Google Scholar]
- Zhang Q., Ames J. M., Smith R. D., Baynes J. W. & Metz T. O. A Perspective on the Maillard Reaction and the Analysis of Protein Glycation by Mass Spectrometry: Probing the Pathogenesis of Chronic Disease. Journal of Proteome Research 8, 754–769 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q. et al. Enrichment and analysis of nonenzymatically glycated peptides: boronate affinity chromatography coupled with electron-transfer dissociation mass spectrometry. J Proteome Res 6, 2323–30 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery H., Tanaka K. & Belgacem O. Glycation pattern of peptides condensed with maltose, lactose and glucose determined by ultraviolet matrix-assisted laser desorption/ionization tandem mass spectrometry. Rapid Communications in Mass Spectrometry 24, 841–848 (2010). [DOI] [PubMed] [Google Scholar]
- Jackson T. R. et al. Boronate affinity saccharide electrophoresis: A novel carbohydrate analysis tool. Electrophoresis 29, 4185–91 (2008). [DOI] [PubMed] [Google Scholar]
- Fluckiger R. & Gallop P. M. Measurement of nonenzymatic protein glycosylation. Methods Enzymol 106, 77–87 (1984). [DOI] [PubMed] [Google Scholar]
- Gould B. J. & Hall P. M. m-Aminophenylboronate affinity ligands distinguish between nonenzymically glycosylated proteins and glycoproteins. Clin Chim Acta 163, 225–30 (1987). [DOI] [PubMed] [Google Scholar]
- Pereira Morais M. P. et al. Analysis of protein glycation using phenylboronate acrylamide gel electrophoresis. Proteomics 10, 48–58 (2010). [DOI] [PubMed] [Google Scholar]
- Pereira Morais M. P., Fossey J. S., James T. D. & van den Elsen J. M. Analysis of protein glycation using phenylboronate acrylamide gel electrophoresis. Methods Mol Biol 869, 93–109 (2012). [DOI] [PubMed] [Google Scholar]
- Guerin-Dubourg A., Catan A., Bourdon E. & Rondeau P. Structural modifications of human albumin in diabetes. Diabetes Metab 38, 171–8 (2012). [DOI] [PubMed] [Google Scholar]
- Jacobson J. et al. Biomarkers of aging in Drosophila. Aging Cell 9, 466–77 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y., Sumiya E., Sugita T. & Sekimizu K. An invertebrate hyperglycemic model for the identification of anti-diabetic drugs. PLoS One 6, e18292 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett D. R. et al. Cognitive correlates of Abeta deposition in male and female mice bearing amyloid precursor protein and presenilin-1 mutant transgenes. Brain Res 1017, 130–6 (2004). [DOI] [PubMed] [Google Scholar]
- Park K. H., Jang W., Kim K. Y., Kim J. R. & Cho K. H. Fructated apolipoprotein A-I showed severe structural modification and loss of beneficial functions in lipid-free and lipid-bound state with acceleration of atherosclerosis and senescence. Biochem Biophys Res Commun 392, 295–300 (2010). [DOI] [PubMed] [Google Scholar]
- Jaleel A. et al. Identification of Amadori-modified plasma proteins in type 2 diabetes and the effect of short-term intensive insulin treatment. Diabetes Care 28, 645–52 (2005). [DOI] [PubMed] [Google Scholar]
- Dolhofer R. & Wieland O. H. Increased glycosylation of serum albumin in diabetes mellitus. Diabetes 29, 417–22 (1980). [DOI] [PubMed] [Google Scholar]
- Siegert K. J. Carbohydrate metabolism during the pupal molt of the tobacco hornworm, Manduca sexta. Archives of Insect Biochemistry and Physiology 28, 63–78 (1995). [Google Scholar]
- Layfield R. et al. Neurofibrillary tangles of Alzheimer's disease brains contain 14-3-3 proteins. Neurosci Lett 209, 57–60 (1996). [DOI] [PubMed] [Google Scholar]
- Choudhry F., Howlett D. R., Richardson J. C., Francis P. T. & Williams R. J. Pro-oxidant diet enhances beta/gamma secretase-mediated APP processing in APP/PS1 transgenic mice. Neurobiol Aging 33, 960–968 (2012). [DOI] [PubMed] [Google Scholar]
- Elfeky S. A. et al. Diol appended quenchers for fluorescein boronic acid. Chem Asian J 5, 581–8 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary methods